Abstract
Inflammatory processes play essential roles in the pathogenesis of tendinitis and tendinopathy. These events are accompanied by catabolic processes initiated by pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). Pharmacological treatments for tendinitis are restricted to the use of non-steroidal anti-inflammatory drugs. Recent studies in various cell models have demonstrated that curcumin targets the NF-κB signaling pathway. However, its potential for the treatment of tendinitis has not been explored. Herein, we used an in vitro model of human tenocytes to study the mechanism of curcumin action on IL-1β-mediated inflammatory signaling. Curcumin at concentrations of 5–20 μm inhibited IL-1β-induced inflammation and apoptosis in cultures of human tenocytes. The anti-inflammatory effects of curcumin included down-regulation of gene products that mediate matrix degradation (matrix metalloproteinase-1, -9, and -13), prostanoid production (cyclooxygenase-2), apoptosis (Bax and activated caspase-3), and stimulation of cell survival (Bcl-2), all known to be regulated by NF-κB. Furthermore, curcumin suppressed IL-1β-induced NF-κB activation via inhibition of phosphorylation and degradation of inhibitor of κBα, inhibition of inhibitor of κB-kinase activity, and inhibition of nuclear translocation of NF-κB. Furthermore, the effects of IL-1β were abrogated by wortmannin, suggesting a role for the phosphatidylinositol 3-kinase (PI-3K) pathway in IL-1β signaling. Curcumin suppressed IL-1β-induced PI-3K p85/Akt activation and its association with IKK. These results demonstrate, for the first time, a potential role for curcumin in treating tendon inflammation through modulation of NF-κB signaling, which involves PI-3K/Akt and the tendon-specific transcription factor scleraxis in tenocytes.
Keywords: Akt PKB, Apoptosis, Inflammation, Interleukin, NF-κB, PI 3-Kinase, Curcumin, Scleraxis, Tendinopathy, Tenocytes
Introduction
The global incidence of tendon injuries has increased in conjunction with the rise in aging and inflammatory diseases (1). The etiology of tendinopathy is considered to be multifactorial, but mechanical loading, overuse injury (or association with arthritis), adverse effects of quinolone antibiotics or other drugs, degeneration, and inflammation that cause tendon injuries and rupture are the major causative factors (2–5). Tenocytes are embedded in an extensive three-dimensional network of extracellular matrix components consisting predominantly of collagen type I fibrils (>95% of the total collagen in tendon), other types of collagen (type III and type V), proteoglycans, elastin, and fibronectin (6–8). These specific matrix components give tendon its resilience and biomechanical stability.
Tendon and ligament are dense fibrous connective tissues with a very limited intrinsic potential for regeneration (7, 9). Therefore, their repair and regeneration poses a complex clinical challenge. It is important to understand the cellular and molecular mechanisms involved in tendon degeneration during the early stages of disease pathogenesis to develop new and effective treatments for tendinopathy. Subtle changes such as the release of IL-1β or other inflammatory cytokines by infiltrating macrophages/monocytes may occur (10). Moreover, like in other connective tissue injuries, tendon inflammation is accompanied by the up-regulation of pro-inflammatory cytokines such as IL-1β. In vitro studies have shown that IL-1β can induce inflammatory mediators such as COX-2, prostaglandin E2, and matrix metalloproteinases (MMP),3 all known to be involved in tendon matrix degradation (11, 12).
IL-1β is a potent pro-inflammatory cytokine that has been reported to be present in significantly increased quantities in the synovium where it enhances inflammatory reactions in injured joints (13, 14). The intracellular signaling pathways activated by IL-1β are responsible for stimulating MMP expression and COX-2 production. However, these pathways have not been explored in detail in tendon cells. Pro-inflammatory cytokines (e.g. IL-1β) induce activation of a central transcription factor known as NF-κB, which is a key regulator of gene expression (15, 16). NF-κB is present in the cytoplasm in its resting stage as a heterotrimer complex consisting of two subunits and an additional inhibitory subunit, IκBα (17). During the activation process, the inhibitory subunit IκBα is phosphorylated at Ser-32 and Ser-36 residues by IKK kinase (IκBα kinase) and is subsequently degraded. Once released, subunits of activated NF-κB translocate to the nucleus and mediate transcription of various inflammatory and catabolic gene products (16, 18). NF-κB activation has been shown to regulate the expression of more than 500 different gene products linked with inflammation, tumor cell transformation, survival, proliferation, invasion, angiogenesis, metastasis, and chemoresistance (19). Thus, inhibitors of NF-κB activation may have therapeutic potential and are actively being researched.
Non-steroidal anti-inflammatory drugs are commonly prescribed for the treatment of tendinitis (20). However, the use of non-steroidal anti-inflammatory drugs is associated with numerous side effects, which can be quite adverse. Therefore, the search is still on for safer and more selective pharmacotherapies for tendinopathy. Curcumin (diferuloylmethane) is a naturally occurring polyphenol derived from the rhizome of Curcuma longa Linn, with the potential for treatment of various diseases acting via NF-κB inhibition (21–23). Commercially available preparations of curcumin contain three major components: curcumin (77%), demethoxycurcumin (17%), and bisdemethoxycurcumin (3%), altogether referred to as the “curcuminoids” (22, 24–28). Recent studies have shown that curcumin mediates its effects by modulation of several important molecular targets, including transcription factors (e.g. NF-κB, AP-1, β-catenin, and peroxisome proliferator-activated receptor-γ), enzymes (e.g. COX-2, 5-LOX, and iNOS), pro-inflammatory cytokines (e.g. TNF-α, IL-1β, and IL-6), and cell surface adhesion molecules. Because of its ability to modulate the expression of these targets, the therapeutic potential of curcumin for treating cancer, arthritis, diabetes, Crohn disease, cardiovascular diseases, osteoporosis, Alzheimer disease, psoriasis, and other pathologies is now under investigation (24, 28, 29). Furthermore, curcumin has been studied in clinical trials for its anti-inflammatory, anti-carcinogenic, and free radical scavenger properties (22). Phase I clinical trials have indicated that human subjects can tolerate curcumin doses as high as 8–12 g/day with no adverse side effects (30, 31). Moreover, several aspects of the pharmacological properties and the use of curcumin for cancer chemoprevention have been reviewed recently (32). Although curcumin is a potent inhibitor of NF-κB, its effects on human tenocytes have not been investigated at the cellular or molecular levels.
Phosphatidylinositol 3-kinases (PI-3Ks) are a highly conserved family of kinases that catalyze the 3-position of the inositol ring of phosphoinositides to generate phosphatidylinositol 3-phosphate, phosphatidylinositol 3,4-bisphosphate, and phosphatidylinositol 3,4,5-trisphosphate (33). PI-3K is a heterodimeric lipid kinase consisting of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit that plays a pivotal role in cell movement, growth, vesicular trafficking, mitogenesis, and cell survival (34, 35). PI-3K is involved in the IL-1β signaling pathway and mediates activation and translocation of NF-κB through targeting IKK-α or phosphorylation of p65, a process that is inhibited by the PI-3K-specific inhibitor wortmannin (36, 37). Several reports suggest that PI-3K activates protein kinase B (Akt), one of the main downstream kinases in cells (33, 38). However, the PI-3K/Akt signaling pathway has not yet been implicated in the activation of NF-κB in tenocytes.
The aim of this study was to exploit an in vitro model of human tenocytes to study the mechanism of curcumin in IL-1β signaling and investigate whether curcumin might antagonize the catabolic effects of pro-inflammatory cytokines by suppressing NF-κB-activation and NF-κB-induced gene expression. We also explored the molecular mechanisms by which curcumin suppresses NF-κB activation in tenocytes, a process that was partly mediated by the PI-3K/Akt signaling pathway.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were purchased from Chemicon International, Inc. (Temecula, CA): polyclonal anti-collagen type I, polyclonal anti-collagen type III, polyclonal anti-decorin antibody, and alkaline phosphatase linked sheep anti-mouse and sheep anti-rabbit secondary antibodies for immunoblotting. Polyclonal anti-active caspase-3 was obtained from R&D Systems. Monoclonal antibody to β-actin, and protein A/G-Sepharose beads were from Sigma. Polyclonal anti-tenomodulin (sc-49325) and anti-phosphatidylinositol 3-kinase (PI-3K) p85 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-scleraxis (SCXA) (ab58655) was obtained from Abcam (Cambridge, UK). Polyclonal anti-active caspase-3 (AF835) was purchased from R&D Systems. Antibodies against phospho-specific IκBα (Ser-32/36) and against anti-phospho-specific p65 (NF-κB)/(Ser-536) were obtained from Cell Technology (Beverly, MA). Anti-IκB kinase (anti-IKK)-α and (anti-IKK)-β antibodies were obtained from Imgenex (Hamburg, Germany). All antibodies were used at concentrations and dilutions recommended by the manufacturer (dilutions ranged from 1:100 for immunomorphological experiments to 1:10,000 for Western blot analysis). Secondary antibodies for immunofluorescence were purchased from Dianova (Hamburg, Germany).
Growth Medium and Chemicals
Growth medium (Ham's F-12/Dulbecco's modified Eagle's medium (50/50) containing 10% fetal calf serum (FCS), 25 μg/ml of ascorbic acid, 50 IU/ml of streptomycin, 50 IU/ml of penicillin, 2.5 μg/ml of amphotericin B, essential amino acids, and l-glutamine) was obtained from Seromed (Munich, Germany). Trypsin/EDTA (EC 3.4.21.4) and wortmannin were purchased from Sigma. Epon was obtained from Plano (Marburg, Germany). Curcumin with a purity of greater than 95% was purchased from Indsaff (Punjab, India). Curcumin was dissolved in dimethyl sulfoxide as a stock concentration of 500 μm and stored at −80 °C. Serial dilutions were prepared in culture medium. Curcumin was diluted in dimethyl sulfoxide as a 5000 μm concentration and then further diluted in cell culture medium. IL-1β was obtained from Acris Antibodies GmbH (Herold, Germany).
Tenocyte Isolation and Culture
The peritendineum of human tendon explants (healthy finger tendon of one male middle-aged donor), obtained during tendon-rupture surgery, was carefully removed before culturing in growth medium (Ham's F-12/Dulbecco's modified Eagle's medium (50/50)) for several days (39). Tendon samples were derived from patients with full informed consent and local ethics committee approval. After 1–2 weeks, tenocytes continuously migrated from this explant and adhered to Petri dishes. Tendon cells were trypsinized and expanded in monolayers to gain a sufficient number of cells for the experiments described.
Experimental Design
Tenocyte monolayer cultures were washed three times with serum-starved medium and incubated for 1 h with serum-starved medium (3% FCS). Serum-starved human tenocytes were either left untreated or treated with 10 ng/ml of IL-1β alone for the indicated time periods or pre-treated with 5 μm curcumin or 5 μm curcumin for 4 h followed by co-treatment with 10 ng/ml of IL-1β and 5 μm curcumin for the indicated time periods. For investigation of NF-κB translocation and IκBα phosphorylation, primary tenocyte monolayer cultures were washed three times with serum-starved medium and incubated for 1 h with serum-starved medium (3% FCS). The cells were then either treated with 5 μm curcumin or 20 nm wortmannin or pre-treated with 5 μm curcumin or 20 nm wortmannin for 0, 5, 10, 20, 40, and 60 min, followed by stimulation with 10 ng/ml of IL-1β for 30 min. In separate experiments, tenocytes were preincubated with curcumin or wortmannin at the indicated concentrations for 4 h followed by co-treatment with 10 ng/ml of IL-1β and curcumin or wortmannin for 30 min. Nuclear and cytoplasmic extracts were then prepared as described later. These experiments were performed in triplicate and the results are provided as mean values from three independent experiments.
Immunofluorescence Analysis of NF-κB (p65) Localization
The effect of curcumin on the nuclear translocation of p65 was investigated by an immunocytochemical method as previously described in detail (28). Briefly, human tenocytes were cultured on glass coverslips and incubated for 24 h. Serum-starved human tenocytes were treated with 10 ng/ml of IL-1β or 5 μm curcumin, or were pre-treated with 5 μm curcumin for 4 h and then co-treated with 10 ng/ml of IL-1β and curcumin for 30 min in serum-starved medium. Glass coverslips with tenocyte monolayers were rinsed three times in Hanks' solution before methanol fixation for 10 min at ambient temperature, and rinsing with PBS. Cell and nuclear membranes of tenocytes were permeabilized by treatment with 0.1% Triton X-100 for 1 min on ice. Cells were overlaid with protease-free bovine serum albumin (BSA) for 10 min at ambient temperature, rinsed with PBS, and incubated with primary antibodies (phospho-p65) in a humid chamber overnight at 4 °C. They were gently washed several times with PBS before incubation with rhodamine red-conjugated secondary antibody for 2 h at ambient temperature and finally washed again three times with Aqua dest. Counterstaining was performed with DAPI to visualize the cell nuclei. Samples were evaluated by light microscopy (Leica, Germany) and photomicrographs were digitally captured and stored.
Western Blot Analysis
For Western blot analysis proteins were extracted from the monolayer cultures with lysis buffer (50 mm Tris-HCl, pH 7.2, 150 mm NaCl, 1% (v/v) Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium pyrophosphate, 100 mm sodium fluoride, 0.01% (v/v) aprotinin, 4 μg/ml of pepstatin A, 10 μg/ml of leupeptin, 1 mm phenylmethylsulfonyl fluoride, PMSF) on ice for 30 min as previously described (40). Total protein concentration was measured with the bicinchonic acid assay system (Uptima, Monlucon, France) using bovine serum albumin as a standard. Samples were further reduced with 2-mercaptoethanol and equal quantities of protein (500 ng/lane), separated under reducing conditions by SDS-PAGE using 5, 7.5, 10, and 12% gels and transferred onto nitrocellulose membranes using a transblot apparatus (Bio-Rad). After preincubation in blocking buffer (5% skimmed milk powder in PBS, 0.1% Tween 20) for 1 h, membranes were incubated with primary antibodies at 4 °C overnight, washed three times with blocking buffer, and then further incubated with alkaline phosphatase-conjugated secondary antibodies for 2 h at ambient temperature. After further washing in 0.1 m Tris, pH 9.5, containing 0.05 m MgCl2 and 0.1 m NaCl, specific antigen-antibody complexes were detected using nitro blue tetrazolium and 5-bromo-4-chloro-3-indoylphosphate (p-toluidine salt; Pierce).
Transmission Electron Microscopy
Electron microscopy was performed as previously described (41). Briefly, monolayer cultures were fixed for 1 h in Karnovsky fixative followed by post-fixation in 1% OsO4 solution. After dehydration in an ascending alcohol series, pellets were embedded in Epon and cut ultrathin with a Reichert-Jung Ultracut E (Darmstadt, Germany). Sections were contrasted with a mixture of 2% uranyl acetate/lead citrate and examined with a transmission electron microscope (TEM 10, Zeiss, Jena, Germany).
Isolation of Tenocyte Cytoplasmic and Nuclear Extracts
Isolation of tenocyte cytoplasmic and nuclear extracts was performed as previously described (29). Briefly, tenocytes were trypsinized and washed twice in 1 ml of ice-cold PBS. The supernatant was carefully removed. The cell pellet was resuspended in hypotonic lysis buffer containing protease inhibitors and was incubated on ice for 15 min. Then 12.5 μl of 10% Nonidet P-40 was added and the cell suspension was vigorously mixed for 15 s. The extracts were centrifuged for 1.5 min. The supernatants (cytoplasmic extracts) were frozen at −70 °C. 25 μl of ice-cold nuclear extraction buffer were added to the pellets and incubated for 30 min with intermittent mixing. Extracts were centrifuged, and the supernatant (nuclear extracts) was transferred to pre-chilled tubes for storage at −70 °C.
Immune Complex Kinase Assay
To test the effect of curcumin on IL-1β-induced IKK activation, immune complex kinase assays were performed as previously described (29). The IKK complex was immunoprecipitated from whole cell lysates with antibodies against IKK-α and IKK-β and subsequently incubated with protein A/G-agarose beads (Pierce). After 2 h of incubation, the beads were washed with lysis buffer and resuspended in a kinase assay solution containing 50 mm HEPES, pH 7.4, 20 mm MgCl2, 2 mm dithiothreitol, 10 μm unlabeled ATP, and 2 mg of substrate GST-IκBα (amino acids 1 to 54), and incubated at 30 °C for 30 min. Phosphorylation of GST-IκBα was assessed using a specific antibody against phospho-specific IκBα (Ser-32/36). To demonstrate the total amounts of IKK-α and IKK-β in each sample, whole cell lysates were transferred to a nitrocellulose membrane after SDS-PAGE under reducing conditions as described above. Detection of IKK-α and IKK-β was performed by immunoblotting with either anti-IKK-α or anti-IKK-β antibodies.
Immunoprecipitation of p65 and p65 Acetylation Assay
To examine the effect of curcumin on IL-1β-induced acetylation of p65, tenocytes were pre-treated with 5 μm curcumin for 4 h and then exposed to 10 ng/ml of IL-1β for the indicated times. The cells were washed with ice-cold phosphate-buffered saline (PBS), and lysed in a buffer containing 50 mm Tris-HCl, pH 7.2, 150 mm NaCl, 1% (v/v) Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium pyrophosphate, 100 mm sodium fluoride, 0.01% (v/v) aprotinin, 4 μg/ml of pepstatin A, 10 μg/ml of leupeptin, 1 mm phenylmethylsulfonyl fluoride (PMSF) to prepare whole cell lysates. Whole cell extracts were pre-cleared by incubating with 25 μl of either normal rabbit IgG serum or normal mouse IgG serum and protein A/G-Sepharose beads, then with primary antibodies (anti-p65 antibody) appropriately diluted in wash buffer (0.1% Tween 20, 150 mm NaCl, 50 mm Tris-HCl (pH 7.2), 1 mm CaCl2, 1 mm MgCl2, and 1 mm PMSF) for 2 h at 4 °C. After 1 h of incubation, immunocomplexes were washed with lysis buffer, boiled with SDS sample buffer for 5 min, resolved on SDS-PAGE, and subjected to Western blot analysis using an anti-acetyl-lysine antibody.
Pharmacological Experiments with Wortmannin
Tenocytes were grown in growth medium for 24 h. PI-3K inhibition experiments were carried out in serum-starved medium. Tenocytes were pre-treated for 1 h with serum-starved medium containing wortmannin (1, 10, and 20 nm) for 1 h, treated with 5 μm curcumin for 4 h, and then exposed to 10 ng/ml of IL-1β for 1 h. After these treatments, nuclear extracts were prepared and examined for NF-κB as described above.
Statistical Analysis
The optical density (specific binding) of each protein band was measured and semiquantitatively analyzed using the Quantity One software package (Bio-Rad). The results are shown as the mean ± S.D. of a representative experiment performed in triplicate.
RESULTS
Cell Culture
This study was undertaken to investigate the effect of curcumin on the PI-3K/Akt signaling pathway leading to activation of the transcription factor NF-κB signaling pathway and on NF-κB-regulated gene products in human tenocytes in an in vitro model of tendinopathy. Tenocytes treated with curcumin (5–20 μm) or wortmannin (1–40 nm) showed no signs of cytotoxic effects or any negative effects on cell viability (data not shown) at the light microscopic and ultrastructural levels. To examine the effect of curcumin on the NF-κB activation pathway, we used IL-1β because the pathway activated by this cytokine is relatively well understood.
Curcumin Inhibits IL-1β-induced Degenerative Features and Apoptosis in Tenocytes
Untreated monolayer tenocytes exhibited typically flattened shapes with small cytoplasmic processes, large mostly euchromatic nuclei with nucleoli and a well structured cytoplasm (Fig. 1A, a). Treatment of tenocyte monolayer cultures with 10 ng/ml of IL-1β for 12, 24, and 48 h led to degenerative changes such as multiple vacuoles, swelling of rough endoplasmic reticulum, and clustering of swollen mitochondria, condensed heterochromatin in the cell nuclei, and multiple autophagic cytoplasmic vacuoles. The flattened monolayer tenocytes became more and more rounded, lost their microvilli-like processes, and became apoptotic (Fig. 1A, b–d).
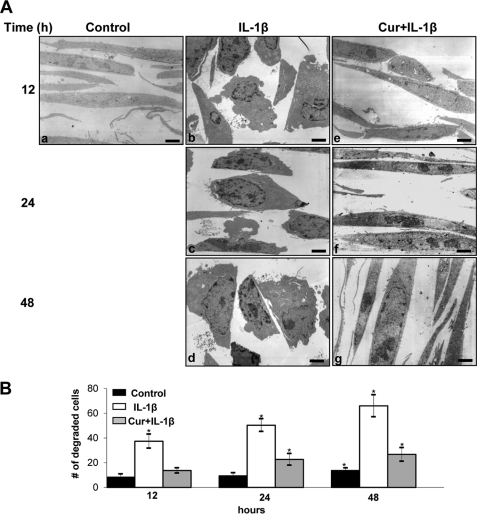
FIGURE 1.
Effect of curcumin on IL-1β-induced cellular degeneration and apoptosis in tenocytes. A, electron microscopy was performed to demonstrate the effects of curcumin on IL-1β-stimulated tenocytes in monolayer culture. Untreated control tenocytes containing mitochondria, rough endoplasmic reticulum, and many other cell organelles are in panel a. In contrast, stimulation of tenocytes with 10 ng/ml of IL-1β for 12, 24, and 48 h resulted in degenerative changes of the cells. After 12 h, tenocytes became rounded and the nucleus contained more condensed chromatin (b). After 24 h multiple vacuoles, swelling of rough endoplasmic reticulum, and clustering of swollen mitochondria was visible (c). Longer incubations of 48 h led to the formation of apoptotic bodies and cell lysis (d). However, pre-treatment of IL-1β-stimulated tenocytes with curcumin inhibited the adverse effects of IL-1β (e–g) and after 48 h treatment (g) tenocytes demonstrated large, flattened cells with numerous microvilli-like processes and mitochondria were comparable with control cultures. Bar, 1 μm. B, to quantify cellular degradation and apoptosis in these cultures, 100 cells from 20 microscopic fields were counted. The number of degraded and apoptotic cells was highest in cultures stimulated with IL-1β alone. In contrast to this, pre-treatment of IL-1β-stimulated cultures with curcumin inhibited the cellular degradation and apoptotic effects of IL-1β and the number of degraded and apoptotic cells remained significantly lower over the entire culture period (*).
Tenocytes that were pretreated with curcumin (4 h) and then co-treated with IL-1β and 5 μm curcumin for 12, 24, and 48 h showed less severe cellular degeneration at the ultrastructural level as early as 12 h after co-treatment (Fig. 1A, e–g). The tenocytes regained a flattened shape and numerous microvilli-like cytoplasmic processes.
Statistical evaluation of the data clearly highlighted changes in the number of cells with mitochondrial changes before and after IL-1β-treatment. Co-treatment with curcumin clearly decreased the number of cells with mitochondrial changes (Fig. 1B).
Effect of Curcumin on IL-1β-induced Inhibition of Extracellular Matrix and Signaling Protein Expression in Tenocytes
Serum-starved human tenocytes were cultured for 24 h and then treated with 10 ng/ml of IL-1β or 5 μm curcumin or were pre-treated with 5 μm curcumin for 4 h and then co-treated with 10 ng/ml of IL-1β and curcumin or left untreated and evaluated after 24 h. Western blotting was performed by probing whole cell lysates with antibodies against collagen types I and III, decorin, tenomodulin, and the tendon-specific transcription factor SCXA (Fig. 2). Primary human tenocytes stimulated with IL-1β alone showed a significant down-regulation of synthesis of collagen types I, III, decorin, tenomodulin, and SCXA expression (Fig. 2). In contrast, pre-treatment of tenocytes with curcumin followed by stimulation with IL-1β resulted in an inhibition of cytokine-induced effects on the above mentioned protein production (Fig. 2). Synthesis of the housekeeping protein β-actin remained unaffected (Fig. 2).
FIGURE 2.
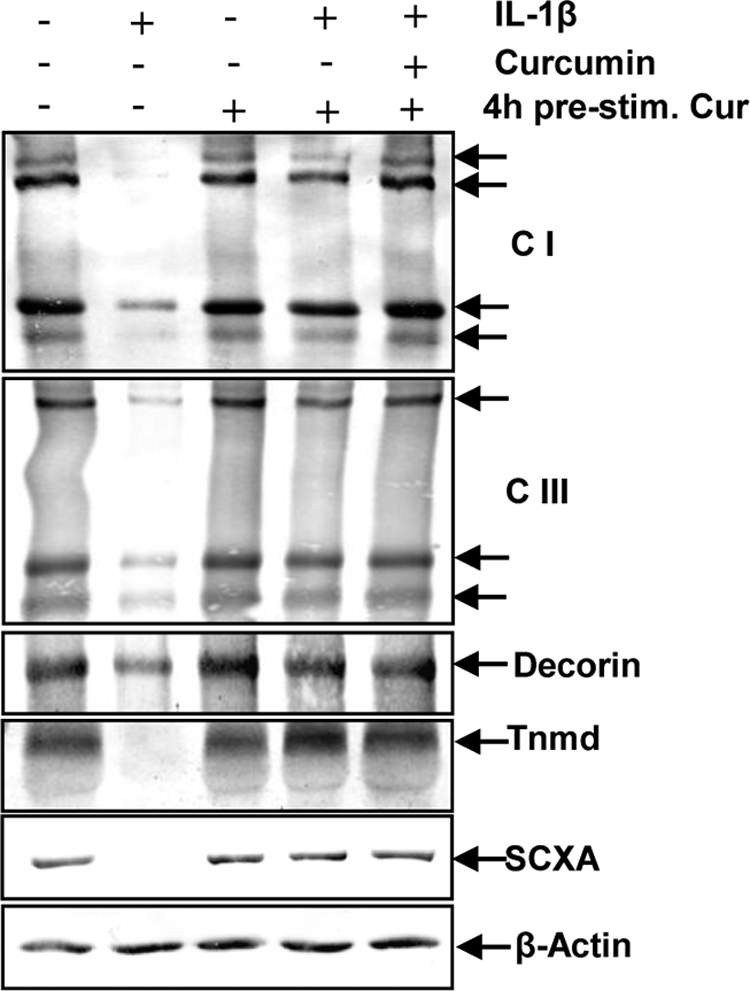
Effects of curcumin on IL-1β-induced inhibition of extracellular matrix compounds and signaling proteins expression in tenocytes. To evaluate the effects of curcumin on IL-1β-stimulated tenogenic inhibition in tenocytes, whole cell lysates (500 ng of protein/lane) were probed with antibodies to collagen type I (CI), collagen type III (CIII), decorin, tenomodulin (Tnmd), and the tenogenic specific transcription factor SCXA. Serum-starved human tenocytes (1 × 106 cells/ml) were exposed to 10 ng/ml of IL-1β alone, 5 μm curcumin alone, or were pre-treated with 5 μm curcumin for 4 h and followed either by incubation with IL-1β alone or incubation with IL-1β and curcumin, or left untreated for 24 h. Each experiment was performed in triplicate. Expression of the β-actin housekeeping gene was not affected by IL-1β and/or curcumin.
Curcumin Inhibits IL-1β-induced NF-κB-dependent Pro-inflammatory and Matrix Degradation Gene Products in Tenocytes
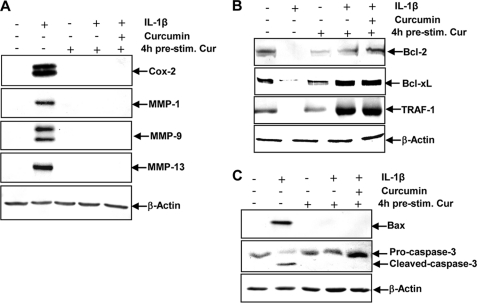
We examined whether curcumin can modulate the expression of IL-1β-induced NF-κB-regulated gene products involved in the inflammatory and degradative processes in tenocytes. Primary human tenocytes with or without pre-treatment with curcumin were examined for IL-1β-induced gene products by Western blot analysis using specific antibodies (Fig. 3A). IL-1β induced the expression of COX-2, MMP-1, MMP-9, and MMP-13, but treatment with curcumin inhibited the expression of these proteins in primary tenocytes (Fig. 3A).
FIGURE 3.
Effects of curcumin on IL-1β-induced NF-κB-dependent pro-inflammatory, anti-apoptotic, and pro-apoptotic gene products in tenocytes. A, to evaluate whether curcumin exerts effects on IL-1β-induced NF-κB-dependent expression of pro-inflammatory (COX-2) and matrix-degrading (MMP-1, -9, and -13) gene products, serum-starved tenocytes (1 × 106 cells/ml) were exposed to 10 ng/ml of IL-1β alone, 5 μm curcumin alone, or were pre-treated with 5 μm curcumin for 4 h and followed either by incubation with IL-1β alone or incubation with IL-1β and curcumin, or left untreated for 24 h. Each experiment was performed in triplicate. Expression of the β-actin housekeeping gene was not affected by IL-1β and/or curcumin. B, to determine whether curcumin treatment actively stimulates the production of anti-apoptotic gene products (Bcl-2, TRAF1, and Bcl-xL), serum-starved human tenocytes (1 × 106 cells/ml) were exposed to 10 ng/ml of IL-1β alone, 5 μm curcumin alone, or were pre-treated with 5 μm curcumin for 4 h and followed either by incubation with IL-1β alone or incubation with IL-1β and curcumin, or left untreated for 24 h. Synthesis of the β-actin housekeeping gene remained unaffected. C, whole cell lysates of serum-starved human tenocytes (1 × 106 cells/ml) were exposed to 10 ng/ml of IL-1β alone, 5 μm curcumin alone, or were pre-treated with 5 μm curcumin for 4 h and followed either by incubation with IL-1β alone or incubation with IL-1β and curcumin, or left untreated and evaluated with Western blot analysis to examine the effect on the pro-apoptotic proteins Bax and caspase-3. Expression of the housekeeping protein β-actin remained unaffected.
Effect of Curcumin on IL-1β-induced NF-κB-dependent Anti-apoptotic and Pro-apoptotic Gene Products in Tenocytes
It is known that NF-κB regulates the expression of anti-apoptotic proteins Bcl-2, Bcl-xL, and TRAF1 (TNF receptor-associated factor 1) (42, 43). We investigated whether curcumin can modulate the expression of these anti-apoptotic gene products. IL-1β-stimulated primary human tenocytes were examined by Western blot analysis with or without curcumin pre-treatment (Fig. 3B). As shown in Fig. 3B, IL-1β inhibited the expression of Bcl-2, Bcl-xL, and TRAF1 in a time-dependent manner. In contrast, curcumin stimulated the expression of these anti-apoptotic proteins (Fig. 3B). To determine whether curcumin inhibits the IL-1β-induced pro-apoptotic gene product, Bax and activated caspase-3, in the same cell cultures, tenocytes were incubated with IL-1β (10 ng/ml) alone for the indicated time or preincubated with curcumin (5 μm) for 4 h and then co-treated with IL-1β (10 ng/ml) for the indicated time. As shown in Fig. 3C, pre-treatment with curcumin significantly down-regulated the level of Bax and biologically active caspase-3 in IL-1β-stimulated cultures compared with human tenocytes stimulated with IL-1β alone.
Curcumin Suppresses IL-1β-induced Activation and Nuclear Translocation of NF-κB in a Concentration- and Time-dependent Manner in Tenocytes
To evaluate whether curcumin inhibits the IL-1β-induced activation and nuclear translocation of NF-κB, nuclear protein extracts from serum-starved tenocytes were probed for the phosphorylated form of the p65 NF-κB subunit after either the cells were stimulated with 10 ng/ml of IL-1β for the indicated times or pre-treated with 5 μm curcumin for the indicated times followed by stimulation with 10 ng/ml of IL-1β for 30 min (Fig. 4A).
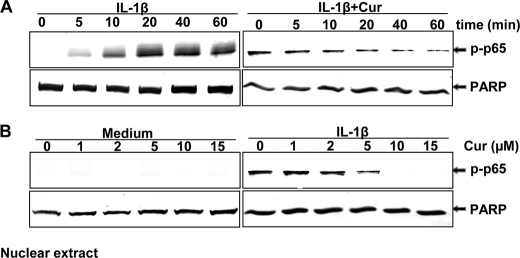
FIGURE 4.
Curcumin suppression of IL-1β-induced NF-κB activation. A, serum-starved human tenocytes (1 × 106 cells/ml) were either stimulated with 10 ng/ml of IL-1β for the indicated times or preincubated with 5 μm curcumin for 0, 5, 10, 20, 40, and 60 min, co-treated with 10 ng/ml of IL-1β for 30 min, and then probed for phospho-p65 by Western blot analysis using antibodies to phospho-specific p65 and poly(ADP-ribose) polymerase (PARP) (control). B, serum-starved human tenocytes were either incubated with curcumin at various concentrations (0, 1, 2, 5, 10, and 15 μm) for 4 h or preincubated with curcumin at various concentrations for 4 h followed by 10 ng/ml of IL-1β stimulation for 30 min. The nuclear extracts (500 ng of protein/lane) were probed for phospho-p65 by Western blot analysis using antibodies to phospho-specific p65 and PARP (control). Expression of PARP remained unaffected in the nuclear extracts. The results shown are representative of three independent experiments.
IL-1β induced p65 phosphorylation in the nuclear fraction in a time-dependent manner (Fig. 4A, left panel). Curcumin blocked IL-1β-induced translocation of p65 to the nucleus in a time-dependent manner (Fig. 4A, right panel). Tenocytes were either incubated with curcumin at various concentrations for 4 h or preincubated with curcumin at various concentrations for 4 h followed by 10 ng/ml of IL-1β stimulation for 30 min. The nuclear extracts were probed for phospho-p65 by Western blot analysis (Fig. 4B). Curcumin inhibited IL-1β-induced NF-κB activation in a concentration-dependent manner (Fig. 4B, right panel).
PI-3K Signaling Pathway Is Involved in the IL-1β-mediated NF-κB Activation in Tenocytes
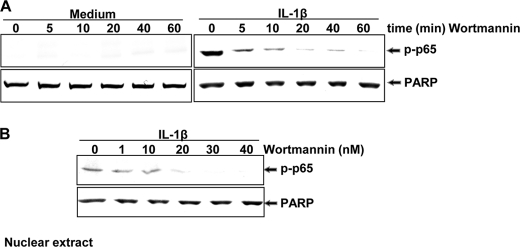
To examine a possible functional relationship between inhibition of the PI-3K pathway and suppression of NF-κB activation in response to curcumin treatment, tenocytes were either stimulated with 20 nm wortmannin, a specific inhibitor of PI-3K, for the indicated times or preincubated with 20 nm wortmannin for the indicated times followed by 10 ng/ml of IL-1β stimulation for 30 min. Tenocyte nuclear protein extracts were probed for the phosphorylated form of the p65 NF-κB subunit (Fig. 5A). In separate experiments, tenocytes were preincubated with wortmannin at the indicated concentrations for 4 h followed by co-treatment with 10 ng/ml of IL-1β and wortmannin for 30 min (Fig. 5B). As shown in Fig. 5, A and B, wortmannin substantially inhibited IL-1β-induced NF-κB activation in a time- and concentration-dependent manner, suggesting that the PI-3K signaling pathway is functionally involved in the process of IL-1β-induced activation of NF-κB. Wortmannin alone had no effect on the NF-κB activation (Fig. 5A, left panel).
FIGURE 5.
Effect of wortmannin on IL-1β-induced NF-κB activation. A, serum-starved human tenocytes (1 × 106 cells/ml) were either stimulated with 20 nm wortmannin for 0, 5, 10, 20, 40, and 60 min or preincubated with 20 nm wortmannin for 0, 5, 10, 20, 40, and 60 min, co-treated with 10 ng/ml of IL-1β for 30 min, and then probed for phospho-p65 by Western blotting using antibodies to phospho-specific p65 and poly(ADP-ribose) polymerase (PARP) (control). B, serum-starved tenocytes were preincubated with wortmannin at various concentrations (0, 1, 10, 20, 30, and 40 nm) for 1 h followed by 10 ng/ml of IL-1β stimulation for 30 min. Nuclear extracts (500 ng of protein/lane) were probed for phospho-p65 by Western blotting using antibodies to phospho-specific p65 and PARP (control). Expression of PARP remained unaffected in nuclear extracts. The results shown are representative of three independent experiments.
Curcumin Inhibits IL-1β-dependent IκBα Degradation and Phosphorylation
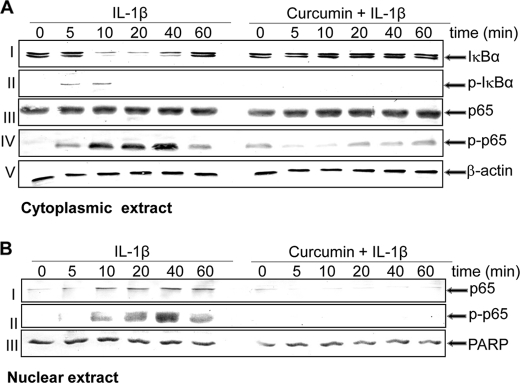
It is well known that an important prerequisite for the activation of NF-κB is the phosphorylation and degradation of IκBα, the natural blocker of NF-κB (17, 42, 44). To examine whether the inhibitory activity of curcumin was through inhibition of IκBα degradation, cells were treated with curcumin, followed by IL-1β, and subsequently probed for NF-κB expression in the nucleus and IκBα expression in the cytoplasm by Western blot analysis. IL-1β induced IκBα degradation in control cells within 10 min but not at all in curcumin-treated cells (Fig. 6A, row I). These results indicate that curcumin blocks IL-1β-induced IκBα degradation. Data shown are representative of three independent experiments.
FIGURE 6.
Effect of curcumin on the IL-1β-induced phosphorylation and degradation of IκBα and the phosphorylation and translocation of p65. Serum-starved human tenocytes (1 × 106 cells/ml) were either stimulated with 10 ng/ml of IL-1β for the indicated times or pre-treated with 5 μm curcumin for 4 h, followed by 10 ng/ml of IL-1β stimulation for the indicated times. Cytoplasmic (A) and nuclear extracts (B) were prepared, fractionated with SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed with anti-IκBα, anti-phosphospecific IκBα, anti-p65, and anti-phosphospecific p65 antibodies. The results shown are representative of three independent experiments. PARP, poly(ADP-ribose) polymerase.
Next, we examined whether inhibition of IL-1β-induced IκBα degradation was through inhibition of IκBα phosphorylation, the tenocytes were treated with curcumin and then with IL-1β and examined for IκBα phosphorylation in the cytoplasm by Western blot analysis. IL-1β-induced IκB-α phosphorylation was almost completely blocked by curcumin (Fig. 6A, row II).
Curcumin Inhibits IL-1β-induced Nuclear Translocation of NF-κB (p65)
Pro-inflammatory cytokines induce the phosphorylation of p65, which is required for NF-κB transcriptional activity. The phosphorylation of NF-κB is known to be mediated through IKK (28, 45, 46). In addition, cytoplasmic extracts were examined for expression of pan-/phospho-p65 (Fig. 6A, rows III and IV). Western blot analysis confirmed the IL-1β-induced phosphorylation of the cytoplasmic pool of p65 in a time-dependent manner, and p65 phosphorylation could be seen as early as 5 min and increased up to 40 min (Fig. 6A, row IV). Pre-treatment with curcumin inhibited the IL-1β-induced phosphorylation of cytoplasmic p65. IL-1β also induced p65 phosphorylation in the nuclear fraction in a time-dependent manner, and curcumin blocked IL-1β-induced translocation of p65 to the nucleus (Fig. 6B, rows I and II).
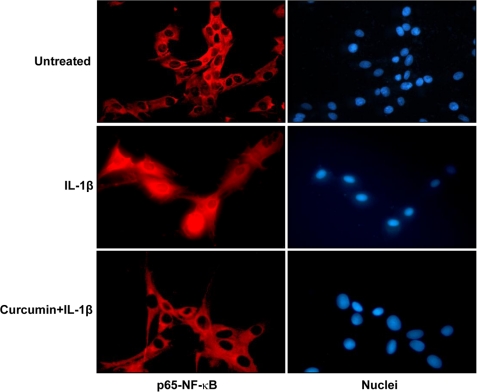
The immunocytochemical analysis confirmed the Western blot findings that curcumin blocked the translocation of p65 from the cytoplasm to the nucleus. The p65 subunit of NF-κB was localized in the cytoplasm of untreated cells. IL-1β induced nuclear translocation of p65 and curcumin blocked the translocation (Fig. 7).
FIGURE 7.
Immunocytochemical analysis of p65 localization after treatment with IL-1β as revealed by immunofluorescence microscopy. Tenocyte cultures either served as controls (not treated) or were treated with IL-1β alone for 10 min or co-treated with 5 μm curcumin and 10 ng/ml of IL-1β for 40 min before immunolabeling with phospho-p65 antibodies and rhodamine-coupled secondary antibodies. Data shown are representative of three independent experiments. Original magnification, ×160.
Effect of Curcumin on IL-1β-induced Acetylation of NF-κB in Tenocytes
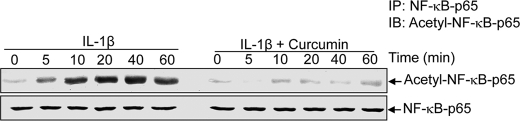
The acetylation of p65 plays an essential role in IκBα-mediated activation of NF-κB transcriptional activity (47, 48). To investigate the effect of curcumin on the acetylation of p65 by IL-1β, human tenocytes were pretreated with curcumin for 4 h and then exposed to IL-1β for the indicated times. Whole cell extracts were examined and immunoprecipitated with anti-p65 antibody, and Western blot analysis was performed using anti-acetyl-lysine antibody. As shown in Fig. 8, IL-1β induced the acetylation of p65 in a time-dependent manner, and curcumin completely suppressed the IL-1β-induced acetylation of p65 (Fig. 8).
FIGURE 8.
Effect of curcumin on IL-1β-induced acetylation of p65. Serum-starved human tenocytes (1 × 106 cells/ml) were either stimulated with 10 ng/ml of IL-1β for the indicated times or pre-treated with 5 μm curcumin for 4 h and then exposed to 10 ng/ml of IL-1β. Whole cell extracts were prepared, immunoprecipitated (IP) with anti-p65 antibody, and subjected to Western blot analysis using anti-acetyl-lysine antibody. The same blots were re-probed with anti-p65 antibody. IB, immunoblot.
Effect of Curcumin on IL-1β-induced Activation of IKK
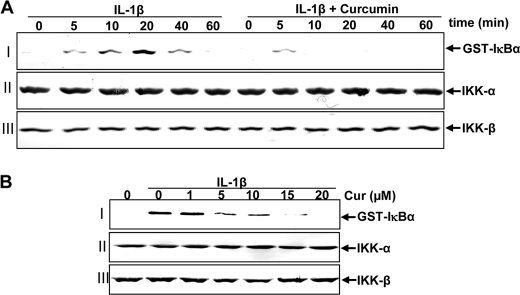
It has been shown that IKK is required for TNF-induced Phosphorylation of IκBα (44). We further evaluated the effect of curcumin on IL-1β-induced IKK activation, which is required for IL-1β-induced phosphorylation of IκBα. The results from the immune complex kinase assay showed that IL-1β induced the activation of IKK in a time-dependent manner and that curcumin blocked IL-1β-activated IKK (Fig. 9A, lane I). IL-1β or curcumin had no direct effect on the expression of IKK-α or IKK-β proteins (Fig. 9A, lanes II and III).
FIGURE 9.
A, effects of curcumin treatment on IL-1β-induced IκB kinase activation. Serum-starved primary human tenocytes were either stimulated with 10 ng/ml of IL-1β for the indicated times or pre-treated with 5 μm curcumin for 4 h and then co-treated with IL-1β (10 ng/ml) for the indicated times. Whole cell extracts were immunoprecipitated with an antibody against IκB kinase (IKK)-α and analyzed by an immune complex kinase assay. To examine the effect of curcumin on the expression level of IKK proteins, whole cell extracts (500 ng of protein/lane) were fractionated by SDS-PAGE and examined using Western blot analysis with anti-IKK-α and anti-IKK-β antibodies. Data shown are representative of three independent experiments. B, direct effect of curcumin treatment on IL-1β-induced IκB kinase activation. Serum-starved human tenocytes (1 × 106 cells/ml) were treated with 10 ng/ml of IL-1β. Whole cell extracts were prepared and immunoprecipitated with anti-IKK-α antibody. The immunocomplex kinase assay was performed in the absence or presence of curcumin at the indicated concentrations. Data shown are representative of three independent experiments.
Next, to examine further whether curcumin blocks IKK activity directly by binding IKK or indirectly by inhibition of its activation, human tenocytes were treated with IL-1β or left untreated. Whole cell extracts from untreated cells and IL-1β-stimulated cells were incubated with anti-IKK-α antibody. After precipitation with protein A/G-Sephadex beads, the immunocomplexes were exposed with various concentrations of curcumin. As shown in Fig. 8B, the immunocomplex kinase assay revealed that curcumin directly suppressed the activity of IKK (Fig. 9B, row I). IL-1β or curcumin had no direct effect on the expression of IKK-α or IKK-β proteins (Fig. 9B, rows II and III).
Curcumin Blocks IL-1β-induced Akt Stimulation
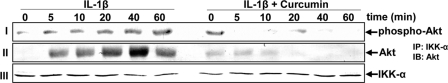
The stimulation of IKK-α is required for the phosphorylation of IκBα. Moreover, it has been reported that pro-inflammatory cytokine-mediated stimulation of IKK-α is associated with an upstream protein kinase, Akt (serine-threonine kinase, protein kinase B), and in turn Akt mediates IKK-α phosphorylation (49). To investigate whether curcumin blocks the IL-1β-induced IκBα phosphorylation due to inhibition of Akt, the serum-starved tenocytes were treated with IL-1β (10 ng/ml) for different indicated times or pre-treated with curcumin (5 μm) for 4 h and then co-treated with IL-1β for the indicated times. The whole cell extracts were analyzed by Western blotting using anti-phospho-specific-Akt antibody. As shown in Fig. 10 row I, IL-1β induced activation of Akt in a time-dependent manner. In contrast, in co-treated tenocytes, curcumin clearly inhibited the phosphorylation of Akt.
FIGURE 10.
Effect of curcumin on IL-1β-induced Akt phosphorylation. Serum-starved human tenocytes (1 × 106 cells/ml) were either stimulated with 10 ng/ml of IL-1β for the indicated times or pre-treated with 5 μm curcumin for 4 h and exposed to 10 ng/ml of IL-1β for the indicated times. Whole cell extracts were analyzed by Western blot analysis using anti-phospho-specific Akt (row I). Cell extracts were immunoprecipitated (IP) with anti-IKK-α antibody and the immunoprecipitates were separated (500 ng of protein/lane) by SDS-PAGE and analyzed by immunoblotting (IB) using anti-Akt antibody (row II) or with anti-IKK-α antibody (row III, as a loading control). Results shown are representative of three independent experiments.
Next, we explored whether curcumin affects the association of Akt with IKK. Whole cell lysates from human tenocytes pretreated with curcumin and co-treated with IL-1β were immunoprecipitated with anti-IKK-α antibody followed by Western blot analysis using anti-Akt antibody. As shown in Fig. 10, row II, IL-1β induced a combination between IKK-α and Akt in a time-dependent fashion, and this was clearly inhibited by curcumin. Taken together, these results indicate that curcumin blocks IKK-α activation through inhibition of an upstream protein kinase B (Akt).
Curcumin Modulates IL-1β-induced NF-κB Activation by Inhibition of the PI-3K/p85 Signaling Pathway in Tenocytes
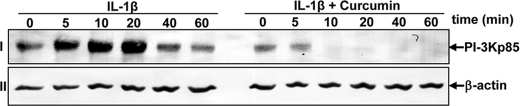
To gain a mechanistic insight into the mode of action of curcumin on IL-1β-stimulated tenocytes we studied the effects of curcumin on PI-3K activation using the Akt/IKK assay. Akt is a well known downstream protein kinase B target of PI-3K, and its activation is mainly induced by the phosphorylation of Ser or Thr residues (50). Previous work has shown that the PI-3K signaling pathway is inhibited by curcumin treatment in MCF7 cells (51). Therefore, to investigate whether the effect of curcumin on the IL-1β-induced Akt phosphorylation is mediated through inhibition of the PI-3K signaling pathway in human tenocytes, the serum-starved tenocytes were treated with IL-1β (10 ng/ml) for the times indicated or pre-treated with curcumin (5 μm) for 4 h and then co-treated with IL-1β. Whole cell extracts were probed by Western blot analysis using a PI-3K/p85 subunit antibody. As shown in Fig. 11, row I, IL-1β-induced activation of PI-3K/p85 in a time-dependent manner. In contrast, in co-treated tenocytes, curcumin substantially inhibited the activation of PI-3K/p85. Consistent with this observation, IL-1β significantly induced Akt phosphorylation, which was reduced by curcumin in a dose-dependent manner (Fig. 10). Taken together, these findings suggest that curcumin mediates its anti-inflammatory effects, at least in part, through modulation of the PI-3K/Akt signaling pathway in human tenocytes. Expression of the housekeeping protein β-actin was unaffected (Fig. 11, row II).
FIGURE 11.
Effects of curcumin treatment on IL-1β-induced PI-3K/p85. Serum-starved human tenocytes (1 × 106 cells/ml) were either stimulated with 10 ng/ml of IL-1β for the indicated times or pre-treated with 5 μm curcumin for 4 h and exposed to 10 ng/ml of IL-1β for the indicated times. Whole cell extracts (500 ng of protein/lane) were analyzed by Western blot analysis using anti-PI-3K/p85. Expression of the housekeeping protein β-actin remained unaffected. Results shown are representative of three independent experiments.
Suppression of PI-3K Signaling Supports the Inhibitory Effect of Curcumin
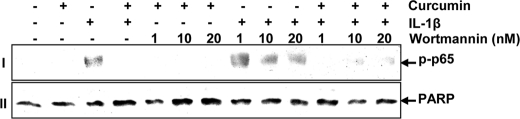
The mechanism involved in curcumin-mediated inhibition of IL-1β-induced NF-κB activation in human tenocytes was further investigated. It is known that the PI-3K pathway is required for activation of NF-κB by IL-1β and wortmannin is a blocker of PI-3K signaling (36). Pre-treatment of tenocytes with different concentrations of wortmannin (1, 10, and 20 nm) for 1 h, treated with curcumin (5 μm) for 4 h, and then treated with IL-1β for 1 h, inhibited the IL-1β-induced NF-κB activation. The inhibitory effects of wortmannin and curcumin on IL-1β-stimulated human tenocytes appeared to be synergistic (Fig. 12, row I).
FIGURE 12.
Effects of wortmannin on curcumin-mediated inhibition of NF-κB activated by IL-1β. Serum-starved human tenocytes (1 × 106 cells/ml) were pre-treated with different concentrations of wortmannin for 1 h, treated with 5 μm curcumin for 4 h, and then exposed to 10 ng/ml of IL-1β for 30 min. After these treatments, nuclear extracts were prepared and analyzed for NF-κB and poly(ADP-ribose) polymerase (PARP) (control) as described under “Experimental Procedures.”
DISCUSSION
The purpose of this study was to investigate the effects of curcumin on the IL-1β-induced NF-κB activation pathway and NF-κB-regulated gene products that influence inflammation in tendon. Using monolayer-cultured human tenocytes, we found that curcumin inhibited IL-1β-induced NF-κB activation through suppression of IκBα phosphorylation, IκBα degradation, IκBα kinase activity, and NF-κB-dependent gene products involved in inflammation (COX-2), in extracellular matrix degradation (MMPs), apoptosis (Bcl-2, Bcl-xL, and TRFA-1), and activation of apoptosis (i.e. activation of caspase-3). This inhibition was correlated with suppression of p65 phosphorylation, p65 nuclear translocation, and p65 acetylation. We also demonstrated that the PI-3K/Akt signaling pathway is activated in response to IL-1β and suppression of IL-1β-induced NF-κB activation by curcumin appears to involve the PI-3K/Akt pathway and its association with IKK.
Tendinopathy is accompanied by inflammation and degradation of the tendon extracellular matrix. At a tendon injury site, pro-inflammatory cytokines such as IL-1β may initiate a cascade of events leading to tendon destruction and loss of biomechanical structural integrity. Furthermore, besides the up-regulation of inflammatory mediators, we found that IL-1β significantly down-regulates the expression of collagen types I and III, decorin, and tenomodulin in tenocytes. Thus, IL-1β-mediated suppression of collagen type I and other tendon-specific extracellular matrix compound expression may lead to the reduced deposition of extracellular matrix and consequently, it might affect normal tissue remodeling and lead to the development of tendinopathy. The control of IL-1β secretion may be critical for protecting tendons from pathological processes. In fact, human tenocytes express IL-1β receptors so that the ligand-receptor signal is transduced via the specific and functional IL-1β receptor. Moreover, IL-1β activates numerous signal transduction systems through protein kinases and this causes induction of genes by activation and suppression of specific transcription factors such as NF-κB (52).
We found that curcumin suppresses the activation of NF-κB in human tenocytes in vitro and inhibits the expression of NF-κB-regulated gene products, including COX-2, MMPs, Bax, and caspase-3. Curcumin can both stimulate and inhibit apoptotic signaling, and the treatment time as well as the concentration may determine the effects of curcumin on various cell types (53). Although curcumin has been shown to inhibit cytokine-induced NF-κB activation in many different primary cells and cell lines of various origins (22, 25, 28, 29, 54–57), to the best of our knowledge, this is the first such report in human tenocytes.
A possible mechanism underlying the inhibition of inducible NF-κB by curcumin could be its capacity to inhibit the PI-3K and Akt signaling pathways. Previous studies using other cells have shown that curcumin inhibits the DNA binding function of NF-κB through suppression of IκBα phosphorylation (25, 29, 58). Thus, down-regulation of upstream signaling proteins, such as PI-3K/Akt, may be involved in curcumin-mediated activation of IL-1β-induced NF-κB inhibition in tenocytes. In fact it has been reported that the PI-3K pathway is required for activation of NF-κB by cytokines such as IL-1β (36). Our results demonstrate that wortmannin, a specific inhibitor of the PI-3K pathway, inhibits NF-κB activation and its translocation in the nucleus in tenocytes. These observations suggest that the PI-3K pathway may be involved in IL-1β signaling. Furthermore, IL-1β-induced activation of PI-3K/p85 and Akt could be inhibited clearly by curcumin in human tenocytes. We found that inhibition of PI-3K/p85 by curcumin, a process required for Akt activation, inhibits IKK and phosphorylation of both IκBα and p65. We have also shown that curcumin stimulates the expression of several anti-apoptotic proteins that are regulated by NF-κB, including Bcl-2, Bcl-xL, and TRAF1. Curcumin also inhibits the pro-apoptotic protein caspase-3, the matrix degrading MMPs, as well as the inflammatory enzyme COX-2. This is consistent with previous reports that have shown that NF-κB activation requires the PI-3K/Akt signaling pathway (49, 59, 60) and that curcumin suppresses the expression of pro-apoptotic proteins (Bax and caspase-3) and matrix degrading enzymes (MMPs) and mediators of inflammation (COX-2) (61, 62). Several groups have demonstrated that curcumin can also inhibit the two subunits of PI-3K (p110 and p85) and phosphorylation of the Akt signaling pathway (63, 64). These findings might explain the anti-inflammatory and anti-apoptotic effects of curcumin in tenocytes.
It is well known that the tendon-specific transcription factor SCXA is required for expression of tendon-specific extracellular matrix genes (65). We also observed a reduction in collagen type I and SCXA expression in tenocytes after treatment with IL-1β. However, curcumin pre-treatment inhibited the IL-1β-induced down-regulation of collagen type I and SCXA expression. Thus, curcumin stimulated tenocytes, at least in part, through activation of the tenogenic transcription factor scleraxis, enhancing transcription of tendon-associated collagens in a SCXA-dependent fashion.
Moreover, curcumin did not exert any toxicity on the cells. Studies on the phase I clinical trials suggest that curcumin can be orally administered safely at doses of 0.2–12 g/day with no dose-limiting toxicity, reaching peak serum concentration at 1–2 h (0.51 ± 0.11 μm at 4000 mg, 0.63 ± 0.06 μm at 6000 mg, and 1.77 ± 1.87 μm at 8000 mg) and is eliminated within 12 h (30, 31, 66, 67). Recently, a phase II study of this agent has shown that this compound has biological activity in patients with pancreatic cancer (68).
Overall, our data suggest that curcumin down-regulates NF-κB and NF-κB-regulated gene products involved in apoptosis, matrix degradation, and inflammation in human tenocytes in vitro. These effects are mediated, at least in part, through down-regulation of PI-3K/Akt signaling. This study provides additional support for designing anti-inflammatory compounds based on curcumin for diseases mediated through NF-κB activation. Therefore, curcumin might have prophylactic potential for the treatment of tendinitis.
Acknowledgments
Katharina Sperling and Ursula Schwikowski are gratefully acknowledged for excellent technical assistance.
Footnotes
- MMP
- matrix metalloproteinase
- IKK
- IκBα kinase
- SCXA
- scleraxis
- TRAF1
- TNF receptor-associated factor 1.
REFERENCES
- 1. Maffulli N., Wong J., Almekinders L. C. (2003) Clin. Sports Med. 22, 675–692 [DOI] [PubMed] [Google Scholar]
- 2. Almekinders L. C. (1998) J. Am. Acad. Orthop. Surg. 6, 157–164 [DOI] [PubMed] [Google Scholar]
- 3. Riley G. (2004) Rheumatology 43, 131–142 [DOI] [PubMed] [Google Scholar]
- 4. Sendzik J., Shakibaei M., Schäfer-Korting M., Lode H., Stahlmann R. (2010) Int. J. Antimicrob. Agents 35, 366–374 [DOI] [PubMed] [Google Scholar]
- 5. Sendzik J., Shakibaei M., Schäfer-Korting M., Stahlmann R. (2005) Toxicology 212, 24–36 [DOI] [PubMed] [Google Scholar]
- 6. Bernard-Beaubois K., Hecquet C., Houcine O., Hayem G., Adolphe M. (1997) Cell Biol. Toxicol. 13, 103–113 [DOI] [PubMed] [Google Scholar]
- 7. Kannus P. (2000) Scand. J. Med. Sci. Sports 10, 312–320 [DOI] [PubMed] [Google Scholar]
- 8. Rees S. G., Flannery C. R., Little C. B., Hughes C. E., Caterson B., Dent C. M. (2000) Biochem. J. 350, 181–188 [PMC free article] [PubMed] [Google Scholar]
- 9. Butler D. L., Juncosa N., Dressler M. R. (2004) Annu. Rev. Biomed. Eng. 6, 303–329 [DOI] [PubMed] [Google Scholar]
- 10. Tsuzaki M., Guyton G., Garrett W., Archambault J. M., Herzog W., Almekinders L., Bynum D., Yang X., Banes A. J. (2003) J. Orthop. Res. 21, 256–264 [DOI] [PubMed] [Google Scholar]
- 11. Archambault J., Tsuzaki M., Herzog W., Banes A. J. (2002) J. Orthop. Res. 20, 36–39 [DOI] [PubMed] [Google Scholar]
- 12. Tsuzaki M., Bynum D., Almekinders L., Yang X., Faber J., Banes A. J. (2003) J. Cell Biochem. 89, 556–562 [DOI] [PubMed] [Google Scholar]
- 13. Gotoh M., Hamada K., Yamakawa H., Nakamura M., Yamazaki H., Ueyama Y., Tamaoki N., Inoue A., Fukuda H. (2000) J. Rheumatol. 27, 2886–2892 [PubMed] [Google Scholar]
- 14. Gotoh M., Hamada K., Yamakawa H., Yanagisawa K., Nakamura M., Yamazaki H., Inoue A., Fukuda H. (2002) J. Orthop. Res. 20, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 15. Barnes P. J., Karin M. (1997) N. Engl. J. Med. 336, 1066–1071 [DOI] [PubMed] [Google Scholar]
- 16. Largo R., Alvarez-Soria M. A., Díez-Ortego I., Calvo E., Sánchez-Pernaute O., Egido J., Herrero-Beaumont G. (2003) Osteoarthritis Cartilage 11, 290–298 [DOI] [PubMed] [Google Scholar]
- 17. Kumar A., Takada Y., Boriek A. M., Aggarwal B. B. (2004) J. Mol. Med. 82, 434–448 [DOI] [PubMed] [Google Scholar]
- 18. Ding G. J., Fischer P. A., Boltz R. C., Schmidt J. A., Colaianne J. J., Gough A., Rubin R. A., Miller D. K. (1998) J. Biol. Chem. 273, 28897–28905 [DOI] [PubMed] [Google Scholar]
- 19. Sung B., Pandey M. K., Ahn K. S., Yi T., Chaturvedi M. M., Liu M., Aggarwal B. B. (2008) Blood 111, 4880–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J. H., Iosifidis M. I., Fu F. H. (2006) Clin. Orthop. Relat. Res. 443, 320–332 [DOI] [PubMed] [Google Scholar]
- 21. Bharti A. C., Aggarwal B. B. (2002) Ann. N.Y. Acad. Sci. 973, 392–395 [DOI] [PubMed] [Google Scholar]
- 22. Bharti A. C., Donato N., Singh S., Aggarwal B. B. (2003) Blood 101, 1053–1062 [DOI] [PubMed] [Google Scholar]
- 23. Mukhopadhyay A., Bueso-Ramos C., Chatterjee D., Pantazis P., Aggarwal B. B. (2001) Oncogene 20, 7597–7609 [DOI] [PubMed] [Google Scholar]
- 24. Aggarwal B. B., Kumar A., Bharti A. C. (2003) Anticancer Res. 23, 363–398 [PubMed] [Google Scholar]
- 25. Buhrmann C., Mobasheri A., Matis U., Shakibaei M. (2010) Arthritis Res. Ther. 12, R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatcher H., Planalp R., Cho J., Torti F. M., Torti S. V. (2008) Cell Mol. Life Sci. 65, 1631–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jurenka J. S. (2009) Altern. Med. Rev. 14, 141–153 [PubMed] [Google Scholar]
- 28. Shakibaei M., John T., Schulze-Tanzil G., Lehmann I., Mobasheri A. (2007) Biochem. Pharmacol. 73, 1434–1445 [DOI] [PubMed] [Google Scholar]
- 29. Csaki C., Mobasheri A., Shakibaei M. (2009) Arthritis Res. Ther. 11, R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng A. L., Hsu C. H., Lin J. K., Hsu M. M., Ho Y. F., Shen T. S., Ko J. Y., Lin J. T., Lin B. R., Ming-Shiang W., Yu H. S., Jee S. H., Chen G. S., Chen T. M., Chen C. A., Lai M. K., Pu Y. S., Pan M. H., Wang Y. J., Tsai C. C., Hsieh C. Y. (2001) Anticancer Res. 21, 2895–2900 [PubMed] [Google Scholar]
- 31. Sharma R. A., McLelland H. R., Hill K. A., Ireson C. R., Euden S. A., Manson M. M., Pirmohamed M., Marnett L. J., Gescher A. J., Steward W. P. (2001) Clin. Cancer Res. 7, 1894–1900 [PubMed] [Google Scholar]
- 32. Shehzad A., Wahid F., Lee Y. S. (2010) Arch. Pharm. 343, 489–499 [DOI] [PubMed] [Google Scholar]
- 33. Vanhaesebroeck B., Alessi D. R. (2000) Biochem. J. 346, 561–576 [PMC free article] [PubMed] [Google Scholar]
- 34. Coffer P. J., Geijsen N., M'rabet L., Schweizer R. C., Maikoe T., Raaijmakers J. A., Lammers J. W., Koenderman L. (1998) Biochem. J. 329, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heldin C. H., Ostman A., Rönnstrand L. (1998) Biochim. Biophys. Acta 1378, F79–113 [DOI] [PubMed] [Google Scholar]
- 36. Reddy S. A., Huang J. H., Liao W. S. (1997) J. Biol. Chem. 272, 29167–29173 [DOI] [PubMed] [Google Scholar]
- 37. Reddy S. A., Huang J. H., Liao W. S. (2000) J. Immunol. 164, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 38. Burgering B. M., Coffer P. J. (1995) Nature 376, 599–602 [DOI] [PubMed] [Google Scholar]
- 39. Schulze-Tanzil G., Mobasheri A., Clegg P. D., Sendzik J., John T., Shakibaei M. (2004) Histochem. Cell Biol. 122, 219–228 [DOI] [PubMed] [Google Scholar]
- 40. Shakibaei M., Schulze-Tanzil G., de Souza P., John T., Rahmanzadeh M., Rahmanzadeh R., Merker H. J. (2001) J. Biol. Chem. 276, 13289–13294 [DOI] [PubMed] [Google Scholar]
- 41. Shakibaei M., De Souza P., Merker H. J. (1997) Cell Biol. Int. 21, 115–125 [DOI] [PubMed] [Google Scholar]
- 42. Aggarwal B. B. (2004) Cancer Cell 6, 203–208 [DOI] [PubMed] [Google Scholar]
- 43. Aggarwal B. B., Takada Y. (2005) Cancer Treat. Res. 126, 103–127 [DOI] [PubMed] [Google Scholar]
- 44. Ghosh S., Karin M. (2002) Cell 109, (suppl.) S81–96 [DOI] [PubMed] [Google Scholar]
- 45. Ghosh S., May M. J., Kopp E. B. (1998) Annu. Rev. Immunol. 16, 225–260 [DOI] [PubMed] [Google Scholar]
- 46. Sizemore N., Lerner N., Dombrowski N., Sakurai H., Stark G. R. (2002) J. Biol. Chem. 277, 3863–3869 [DOI] [PubMed] [Google Scholar]
- 47. Chen Z. J., Parent L., Maniatis T. (1996) Cell 84, 853–862 [DOI] [PubMed] [Google Scholar]
- 48. Stancovski I., Baltimore D. (1997) Cell 91, 299–302 [DOI] [PubMed] [Google Scholar]
- 49. Ozes O. N., Mayo L. D., Gustin J. A., Pfeffer S. R., Pfeffer L. M., Donner D. B. (1999) Nature 401, 82–85 [DOI] [PubMed] [Google Scholar]
- 50. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 51. Squires M. S., Hudson E. A., Howells L., Sale S., Houghton C. E., Jones J. L., Fox L. H., Dickens M., Prigent S. A., Manson M. M. (2003) Biochem. Pharmacol. 65, 361–376 [DOI] [PubMed] [Google Scholar]
- 52. Williams D. H., Jeffery L. J., Murray E. J. (1992) Biochim. Biophys. Acta 1180, 9–14 [DOI] [PubMed] [Google Scholar]
- 53. Chan W. H., Wu H. Y., Chang W. H. (2006) Food Chem. Toxicol. 44, 1362–1371 [DOI] [PubMed] [Google Scholar]
- 54. Jang M. K., Sohn D. H., Ryu J. H. (2001) Planta Med. 67, 550–552 [DOI] [PubMed] [Google Scholar]
- 55. Kumar A., Dhawan S., Hardegen N. J., Aggarwal B. B. (1998) Biochem. Pharmacol. 55, 775–783 [DOI] [PubMed] [Google Scholar]
- 56. Plummer S. M., Holloway K. A., Manson M. M., Munks R. J., Kaptein A., Farrow S., Howells L. (1999) Oncogene 18, 6013–6020 [DOI] [PubMed] [Google Scholar]
- 57. Sandur S. K., Deorukhkar A., Pandey M. K., Pabón A. M., Shentu S., Guha S., Aggarwal B. B., Krishnan S. (2009) Int. J. Radiat. Oncol. Biol. Phys. 75, 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang C., Li B., Zhang X., Hazarika P., Aggarwal B. B., Duvic M. (2010) J. Invest. Dermatol. 130, 2110–2119 [DOI] [PubMed] [Google Scholar]
- 59. Kane L. P., Shapiro V. S., Stokoe D., Weiss A. (1999) Curr. Biol. 9, 601–604 [DOI] [PubMed] [Google Scholar]
- 60. Romashkova J. A., Makarov S. S. (1999) Nature 401, 86–90 [DOI] [PubMed] [Google Scholar]
- 61. Pahl H. L. (1999) Oncogene 18, 6853–6866 [DOI] [PubMed] [Google Scholar]
- 62. Shishodia S., Aggarwal B. B. (2002) J. Biochem. Mol. Biol. 35, 28–40 [DOI] [PubMed] [Google Scholar]
- 63. Hussain A. R., Al-Rasheed M., Manogaran P. S., Al-Hussein K. A., Platanias L. C., Al Kuraya K., Uddin S. (2006) Apoptosis 11, 245–254 [DOI] [PubMed] [Google Scholar]
- 64. Shankar S., Srivastava R. K. (2007) Int. J. Oncol. 30, 905–918 [PubMed] [Google Scholar]
- 65. Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A., Tabin C. J. (2001) Development 128, 3855–3866 [DOI] [PubMed] [Google Scholar]
- 66. Goel A., Kunnumakkara A. B., Aggarwal B. B. (2008) Biochem. Pharmacol. 75, 787–809 [DOI] [PubMed] [Google Scholar]
- 67. Sharma R. A., Euden S. A., Platton S. L., Cooke D. N., Shafayat A., Hewitt H. R., Marczylo T. H., Morgan B., Hemingway D., Plummer S. M., Pirmohamed M., Gescher A. J., Steward W. P. (2004) Clin. Cancer Res. 10, 6847–6854 [DOI] [PubMed] [Google Scholar]
- 68. Dhillon N., Aggarwal B. B., Newman R. A., Wolff R. A., Kunnumakkara A. B., Abbruzzese J. L., Ng C. S., Badmaev V., Kurzrock R. (2008) Clin. Cancer Res. 14, 4491–4499 [DOI] [PubMed] [Google Scholar]