Abstract
Renin is essential for blood pressure control. Renin is stored in granules in juxtaglomerular (JG) cells, located in the pole of the renal afferent arterioles. The second messenger cAMP stimulates renin release. However, it is unclear whether fusion and exocytosis of renin-containing granules is involved. In addition, the role of the fusion proteins, SNAREs (soluble N-ethylmaleimide-sensitive factor attachment proteins), in renin release from JG cells has not been studied. The vesicle SNARE proteins VAMP2 (vesicle associated membrane protein 2) and VAMP3 mediate cAMP-stimulated exocytosis in other endocrine cells. Thus, we hypothesized that VAMP2 and/or -3 mediate cAMP-stimulated renin release from JG cells. By fluorescence-activated cell sorting, we isolated JG cells expressing green fluorescent protein and compared the relative abundance of VAMP2/3 in JG cells versus total mouse kidney mRNA by quantitative PCR. We found that VAMP2 and VAMP3 mRNA are expressed and enriched in JG cells. Confocal imaging of primary cultures of JG cells showed that VAMP2 (but not VAMP3) co-localized with renin-containing granules. Cleavage of VAMP2 and VAMP3 with tetanus toxin blocked cAMP-stimulated renin release from JG cells by ∼50% and impaired cAMP-stimulated exocytosis by ∼50%, as monitored with FM1–43. Then we specifically knocked down VAMP2 or VAMP3 by adenoviral-mediated delivery of short hairpin silencing RNA. We found that silencing VAMP2 blocked cAMP-induced renin release by ∼50%. In contrast, silencing VAMP3 had no effect on basal or cAMP-stimulated renin release. We conclude that VAMP2 and VAMP3 are expressed in JG cells, but only VAMP2 is targeted to renin-containing granules and mediates the stimulatory effect of cAMP on renin exocytosis.
Keywords: Cyclic AMP (cAMP), Fusion Protein, Kidney, Renal Physiology, Renin, Hypertension, SNAREs
Introduction
Renin has been a target of study for the past 100 years. It is the rate-limiting enzyme in the generation of angiotensin II (ANGII)2 and is, therefore, essential for the regulation of blood pressure and also for renal development (1, 2). In JG cells, renin is processed from a pre-pro-renin precursor (3–6) and stored in its active form in large dense core granules ranging from 0.8 to 1.2 μm size (7). Renin is released in response to low perfusion pressure or upon stimulation of JG cells with cAMP-elevating hormones (β-adrenergic receptors, prostaglandin E2) (7). Opposite to most secretory cells, renin release is stimulated by a decrease in intracellular calcium, which augments intracellular cAMP in JG cells (“calcium paradox”) (8, 9). Despite the abundant literature in understanding the processing of renin from its precursor form (6, 10), sorting into granules (3, 11, 12), expression throughout development (1, 13, 14), and hormonal regulation of release and cross-talk signaling mechanism with other kidney cells (7, 15–18), fundamental questions on how renin is released from JG cells and the proteins and molecular mechanisms involved have not been studied.
Earlier electron microscopy studies revealed that after stimulation of renin release, frequent contact areas of mature electron-dense renin-containing granules with the plasma membrane could be observed in JG cells (19). In addition, membrane capacitance and imaging studies indicated that exocytosis is stimulated in JG cells by the cAMP and protein kinase A (PKA) signaling pathway (20, 21). However, these results are indirect and direct evidence that renin release occurs via exocytosis is required.
In most secretory cells, exocytosis and membrane fusion are mediated by SNAREs (soluble N-ethylmaleimide-sensitive factor attachment proteins (SNAP) receptors) (22–25). To mediate fusion, three SNAREs families form a complex by direct interaction through their coiled-coil domains: one vesicle-associated membrane protein (VAMP), one syntaxin, and one synaptosome-associated protein (SNAP) (22, 26). In all cells studied SNARE complex zippering and exocytosis requires calcium. Each of these SNARE families is composed of several isoforms, which exhibit specific subcellular distribution (e.g. endosomal, Golgi, plasma membrane, etc.). The SNARE hypothesis proposes that a tight selectivity for their pairing between VAMPs, syntaxins, and SNAPs isoforms confer defined specificity to the intracellular trafficking events (27–29) and is specific to differential stimulatory triggers (30). Thus, identification of the SNARE isoforms involved in the different steps of granule exocytosis after agonist stimulation is essential for understanding the potential targets that regulate cell type-specific hormone release.
In the kidney, specific SNAREs isoforms are expressed, i.e. VAMP2 and VAMP3 (31), syntaxin 3 and 4 (32, 33), and SNAP-23 (34, 35). In addition, in particular nephron segments, VAMP2 and VAMP3 have been implicated in cAMP-stimulated exocytosis (25, 31, 36, 37). Despite this evidence the involvement of SNAREs in renin release may be challenged by the inhibitory effect of intracellular calcium on JG cells, which opposes the requirement of calcium for SNARE zippering and exocytosis. The expression of VAMPs and other SNAREs in JG cells and their roles in renin release have not been previously explored.
In the present study we tested whether SNAREs are present in JG cells and the specific role of VAMP2 and VAMP3 in cAMP-stimulated renin release. We found that several members of the SNARE family are present in JG cells. Specific deletion of VAMP2 or VAMP3 proteins revealed a novel and specific role for VAMP2, but not VAMP3, in stimulated renin release and exocytosis. Therefore, stimulated renin release occurs via exocytosis requiring the SNAREs fusogenic machinery with a preferential selectivity for the vesicle protein VAMP2. By implicating VAMP2 in cAMP-stimulated renin release and exocytosis, our study provides evidence that renin release in JG cells occurs via exocytosis.
EXPERIMENTAL PROCEDURES
Isolation and Primary Culture of Mouse JG Cells
Primary cultures of mouse JG cells were prepared following a protocol previously described and characterized with slight modifications (9, 38). In brief, 8–9-week-old C57/BL6 mice (The Jackson Laboratory) were sacrificed by cervical dislocation. Kidneys were removed and decapsulated, and the renal cortex was dissected. Combined cortical tissue from 4 mice was minced and then incubated with gentle stirring in a digestion buffer containing 130 mm NaCl, 5 mm KCl, 2 mm CaCl2, 10 mm glucose, 20 mm sucrose, and 10 mm HEPES (pH 7.4) along with 0.25% trypsin (Sigma) and 0.1% collagenase type A (Roche Diagnostics) at 37 °C for 45 min (9). The cell suspension was separated in 25 ml of 40% isoosmotic Percoll density gradient (Sigma) for 30 min of centrifugation at 4 °C and 27,000 × g using an SS-34 rotor/Sorvall RC 5CPlus centrifuge. Cells were maintained at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with fetal calf serum and antibiotics (9). Culture dishes were coated with a freshly prepared poly-d-lysine solution (0.1 mg/ml; Millipore). All protocols were approved by the Institutional Animal Care and Use Committee of the Henry Ford Hospital and Roswell Park Cancer Institute in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals.
Stimulation of Renin Release/Cell Treatment
Before stimulation of renin release, JG cells were serum-deprived for 2 h by replacing the medium with DMEM-serum-free medium containing 100 units/ml penicillin and 100 μg/ml streptomycin. Renin release was stimulated by increasing intracellular cAMP with forskolin (10 μm) plus IBMX (0.5 mm) for 1 h. To measure renin release, the medium was collected and centrifuged to remove cellular debris, and supernatants were stored at −20οC until processed. To measure renin content in cells, 0.5 ml of 0.1% of Triton X in phosphate-buffered saline (PBS) was added to the plates, rocked for 10 min, and then cells were scraped, collected, and spun at 16,000 × g, and supernatants were stored at −20 οC until processed.
Measurement of Renin Release and Renin Content
Samples were incubated at 37 οC for 3 h with excess rat angiotensinogen in a buffer (pH 6.5) containing 0.1 m phosphate buffer, 0.06 m disodium EDTA, 0.2% gelatin, 0.1% neomycin sulfate, and 0.1 mg/incubation of phenylmethylsulfonyl fluoride. Generated angiotensin I (ANGI) was measured using a Gamma Coat radioimmunoassay kit (Diasorin) following the manufacturer's instructions. Values for renin concentration (ng of ANGI/h of incubation) in cell culture medium (A) were normalized to renin concentration in the cell lysate (B) and expressed as a percentage of renin content (A/B ×100). Validation experiments showed that <10% of the substrate is consumed under these conditions, and the reaction maintains linearity of generated product over time.
Western Blot
To detect VAMP2 and -3, cultured JG cells were lysed in a buffer containing 150 mm NaCl, 50 HEPES (pH 7.5), 1 mm EDTA (pH 8), 2% Triton-X-100, 0.2% SDS, and a mixture of protease inhibitors (39). Protein content was measured using a colorimetric assay (BSA, Pierce). Proteins were resolved in 12% SDS-polyacrylamide gels and transferred to a PVDF membrane (Amersham Biosciences). Membranes were incubated first in blocking buffer containing 50 mm Tris, 500 mm NaCl, 0.1% Tween 20 (TBS-T), and 5% nonfat dried milk followed by primary antibody (mouse VAMP2 1/2000 or rabbit VAMP3 1/4000; Synaptic Systems) (40–42). Membranes were then washed in TBS-T and incubated with secondary antibody conjugated to horseradish peroxidase (anti-mouse or anti-rabbit 1/4000; Amersham Biosciences). The signal was detected with a chemiluminescence kit (Amersham Biosciences). As an internal loading control, membranes were re-blotted with an antibody to detect the housekeeping gene actin (Santa Cruz).
Cleavage of VAMP2/3 with Tetanus Toxin
Intact JG cells were preincubated for 19 h in DMEM-serum-free with either vehicle or 60 nm tetanus toxin (Calbiochem). After the preincubation period, cells were lysed, SDS was resolved in 12% polyacrylamide gels, and VAMP2 and -3 were detected by Western blot (mouse monoclonal antibody for VAMP2 (Synaptic System) and a rabbit polyclonal for VAMP3 (Abcam/Cambridge)). VAMP2 (∼116 amino acids) and VAMP3 (∼103 amino acids) were selectively cleaved by tetanus toxin at the C-terminal portion at position ∼76 amino acids, releasing the 1–76 N-terminal portion to the cytosol, which was then degraded (43–46). The antibody used to detect VAMP2 was directed against the N terminus (2–17 amino acids) and, therefore, did not recognize the cleaved C terminus portion of VAMP2. The antibody to detect VAMP3 was directed against amino acids 1–100. Thus, effective tetanus toxin cleavage is reflected as a decrease in VAMP2 and VAMP3 band intensity as previously reported (47–49). A second set of cells pretreated with tetanus toxin was subsequently stimulated with forskolin plus IBMX for renin release studies.
Immunofluorescence and Confocal Microscopy for VAMP2 and VAMP3 in Mouse JG Cells
JG cells were grown on poly-d-lysine-coated glass coverslips for 48 h. Four percent paraformaldehyde-fixed cells were permeabilized with 0.1% Triton X in PBS for 10 min and then blocked in TBS-T, 5% albumin. Cells were incubated with a FITC-labeled goat anti-renin antibody (Innovative Research) for 1 h followed by 1 h of incubation with an antibody for VAMP2 or -3 (Synaptic System; 1/100). After washing 3×, cells were incubated with a secondary antibody (anti rabbit IgG-Alexa Fluor 568; 1:200) washed with TBS-T, and mounted with Fluoromount-G (SouthernBiotech). Images were obtained using a laser scanning confocal imaging system (Visitech International) with excitation lasers 488 nm (renin) and 568 nm (VAMP2 and -3). Fluorescence was filtered with 525lp and 590lp emission filters, respectively. Cells were imaged with a 100× oil immersion lens (NA 1.33), 25 μm slit, at a pixel size of 0.05 μm (200× final magnification) in serial optical sections (0.3 μm) in the z axis plane of the cells. Control experiments showed null cross-bleed between 488 and 568 channels under the conditions used for imaging. Tagged image file format (TIFF) images were deconvolved using the same settings (blind deconvolution, subpixel processing, 20 iterations), and co-localization was analyzed using Autoquant software. Samples incubated in the absence of primary antibodies were used as a control for nonspecific binding. In addition, an extra sample was mounted without antibodies as control for autofluorescence.
Isolation of JG Cells from Ren-GFP Transgenic Mice by FACS
To study the gene expression of VAMP2 and VAMP3, a pure population of JG cells was prepared from mice expressing green fluorescent protein (GFP) under the control of all regulatory elements of the Ren1c gene, a transgene described in detail previously (50). Three-five adult female mice were euthanized, and kidneys were removed and stored in ice-cold Hanks' buffered salts solution (HBSS). Tissue was finely minced and suspended in 2 ml of HBSS, washed in HBSS, and resuspended in 1 ml of enzyme mix: 2 units/ml Blendzyme 3 (Roche Diagnostics), 0.25% collagenase XI (Sigma), 1.2 units/ml elastase (Roche Diagnostics), and 100 units/ml DNase I (Roche Diagnostics) in HBSS. Tubes were tumbled at 37 °C for 30 min followed by Dounce homogenization (30 strokes). The suspensions were washed 2 times with ice-cold HBSS and resuspended in ice-cold solution (RPMI 1640 mixed with 2% fetal bovine serum and 100 units/ml of DNase I) until FACS sorting. Renin-expressing cells were sorted using a BD Biosciences FACSAria cell sorter at 488-nm excitation and 530/30 bandpass emission filter. Autofluorescence was analyzed on transgene negative samples to establish limits of background fluorescence. The number of JG cells obtained was on the order of 50,000 per adult kidney pair.
Quantitative PCR
cDNA from FACS-isolated JG cells and total kidney homogenate was prepared with the High Capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's protocol. SYBR Green-based quantitative PCR was carried out using the Bio-Rad iQTMSYBR® Green Supermix kit. Reactions were performed on a Bio-Rad CFX96 Real-time PCR detection system using the preprogrammed standard 2-cycle protocol with melting curve analysis. Primers were designed using Beacon Designer 3.01 (PREMIERE Biosoft International) to span introns and yield amplification fragments of 100–200 bp (Table 1). β2-microglobulin (B2M) was performed as the reference gene. At the end of PCR cycling, melting curve analyses were performed, and representative PCR products were run on agarose gels and visualized by ethidium bromide staining. Threshold cycle values (Ct) for the internal controls B2M were subtracted from amplified gene Ct values (ΔCt). To calculate the -fold enrichment factor in JG cells versus kidney homogenates, a relative quantitation method (ΔΔCt) (51) was used, where ΔΔCt represents ((ΔCt gene studied in JG cells) − (ΔCt of gene studied in total kidney homogenate)).
TABLE 1.
Quantitative PCR primer sequence and amplicon sizes for SNAREs
Primers were designed using Beacon Designer 3.01 (PREMIERE Biosoft International) to span introns and yield amplification fragments of 100–200 bp.
| Gene | Sequence | Amplicon size (bp) |
|---|---|---|
| B2m | Forward, 5′-GACTGATACATACGCCTGCAG-3′ | 119 |
| Reverse, 5′-CAGGTTCAAATGAATCTTCAG-3′ | ||
| Renin | Forward, 5′-ATGAAGGGGGTGTCTGTGGGGTC-3′ | 194 |
| Reverse, 5′-ATGTCGGGGAGGGTGGGCACCTG-3′ | ||
| VAMP2 | Forward, 5′-CACAATCTGGTTCTTTGAGGAG-3′ | 108 |
| Reverse, 5′-AGAGACTTCAGGCAGGAATTAG-3′ | ||
| VAMP3 | Forward, 5′-CTCACCAAGGCATCAGTCTG-3′ | 135 |
| Reverse, 5′-ATTCTAAGAGCACCAGGCATC-3′ | ||
| VAMP4 | Forward, 5′-GGAGAAAGGGTAGCACACATCAG-3′ | 148 |
| Reverse, 5′-CCACATGCCATACAGAACAGAAAG-3′ | ||
| VAMP5 | Forward, 5′-CCAAAGGACTATATTTCAGGAGAC-3′ | 117 |
| Reverse, 5′-TCTGGTTTGTGGACTACTGTG-3′ | ||
| VAMP7 | Forward, 5′-GCACAACTGAAGCATCACTCTG-3′ | 138 |
| Reverse, 5′-CAGCAATTCCAACCTTTCTCCAC-3′ | ||
| VAMP8 | Forward, 5′-GGCGAAGTTCTGCTTTGAGAG-3′ | 107 |
| Reverse, 5′-ACTCCCTCCACCTCACTCTG-3′ | ||
| Munc18a | Forward, 5′-TCCTCCTGCTGCAAGATGAC-3′ | 133 |
| Reverse, 5′-AGTGGACAGACTTCTCAGATGG-3′ | ||
| Munc18b | Forward, 5′-CCACATAGCACCTACAACCTTTAC-3′ | 131 |
| Reverse, 5′-TTGCGGTATCGGATGGATGG-3′ | ||
| Munc18c | Forward, 5′-GGACTGAAGAGCGTCGTGTG-3′ | 104 |
| Reverse, 5′-TTGGTGGTAAACTCATCTAACAGC-3′ | ||
| SNAP23 | Forward, 5′-AATCCTGGGTTTAGCCATTGAGTC-3′ | 106 |
| Reverse, 5′-TTGGTCCATGCCTTCTTCTATGC-3′ | ||
| SNAP25 | Forward, 5′-GTCTTTCCTTCCCTCCCTACC-3′ | 110 |
| Reverse, 5′-AGTCAGTGGTGCTTCGTTAAAG-3′ | ||
| Syntaxin1a | Forward, 5′-GATGAGAAGACAAAGGAGGAACTG-3′ | 127 |
| Reverse, 5′-ATGAGCGGTTCAGACCTTCC-3′ | ||
| Syntaxin2 | Forward, 5′-ACAAACGACGATGGAGACACTG-3′ | 149 |
| Reverse, 5′-AGGATGATGCTGTGGTTCTTCTTC-3′ | ||
| Syntaxin3 | Forward, 5′-CTGGAAGAGATGTTGGAGAGTG-3′ | 120 |
| Reverse, 5′-CAGCCTTACGATGTCCTTGTG-3′ | ||
| Syntaxin4 | Forward, 5′-CAATGCTGGAATGGTGTCTG-3′ | 150 |
| Reverse, 5′-AACTGCTGGATCTCACTGTG-3′ |
Live Single Cell Monitoring of Exocytosis Using FM1–43
FM1–43 fluoresces only when bound to membranes but not in solution (39, 52). Therefore, the newly inserted granule membrane that occurs after exocytosis can be monitored and correlated with the increased fluorescence in JG cells bathed with FM1–43. JG cells were grown on coverglass and mounted in a 37 °C temperature-controlled chamber on an inverted confocal microscope. 4 μm FM1–43 was added to the flowing bath (cell culture media) for the duration of the experiment. FM1–43 was excited at 488 nm, and emission was collected with a 525-nm long-pass emission filter. Images were acquired with a laser scanning confocal microscopy for 30 ms, every 10s. Typically 3–5 min elapsed until fluorescence intensity at the plasma membrane reached a plateau. After a 2-min base-line period with continuous FM1–43 perfusion, forskolin plus IBMX were added to the bath, and images were recorded for a 10-min period after which FM1–43 was washed out with fresh media containing the quencher bromphenol blue (1 mm) (53). The experimental recordings obtained were analyzed with Metamorph software by designating a region of interest encompassing the plasma membrane, yielding average fluorescence intensity throughout the course of the experiment. Fluorescence intensity in each region of interest was normalized to the average base-line value set at 100 (n = total number of cells studied).
Construction of Adenoviral Vectors and Transduction of Primary Cultures of Mouse JG Cells
For adenoviral particle production encoding silencing RNA VAMP2 (VAMP2 shRNA), an oligonucleotide fragment encoding 19 nucleotides of sense VAMP2 (5′-GCTCAAGCGCAAATACTGG-3′) followed by a loop region (TTCAAGAGA) and the antisense of the 19-nucleotide fragment was subcloned into the 5′-AflII and 3′-SpeI sites of the Adenovector-pMIGHTY (Viraquest, North Liberty, IA). Oligonucleotides were generated to encode AflII and SpeI sites at the 5′ and 3′ ends, respectively, for convenient insertion into the Adenovector-pMIGHTY. The control construct (scrambled shRNA) was generated similarly using a scrambled 19-nucleotide sequence (5′-TTCTCCGAACGTGTCACGT-3′). Adenoviral particle production encoding silencing RNA VAMP3 (VAMP3 shRNA) was constructed encoding the sequence 5′-GGATCTTCTTCGAGACTTT-3′. All constructs were sequenced before viral particles were produced.
For adenoviral-mediated knockdown of VAMP2 and VAMP3, 24 h after plating primary cultures of mouse JG cells were incubated in DMEM-serum-free containing VAMP2 shRNA or VAMP3 shRNA particles (100 plaque-forming units/cell). After 3 h, fetal calf serum was added to the cultured media to reach a 5% concentration and incubated at 37 °C for another 28 h. JG cells were then stimulated with forskolin plus IBMX for 1 h as described above.
Reagents
Fetal calf serum was obtained from Hyclone, and DMEM culture medium and antibiotics were from Invitrogen. Forskolin, IBMX, Percoll, and protease inhibitors were from Sigma. Tetanus toxin was from Calbiochem, and FITC-labeled binding domain tetanus toxin was from List Biological Laboratories (San Jose, CA). We obtained poly-d-lysine from Millipore. Radioimmunoassay kits to measure ANGI were from Diasorin (Stillwater, MN). FM1–43- and Alexa Fluor-conjugated antibodies were from Invitrogen.
Statistical Analysis
Data are expressed as the mean ± S.E. and were subjected to one-way analysis of variance with multiple comparisons made by the Student-Newman-Keuls method. A value of p < 0.05 was considered significant.
RESULTS
Expression of SNARE Proteins in FACS-isolated JG Cells
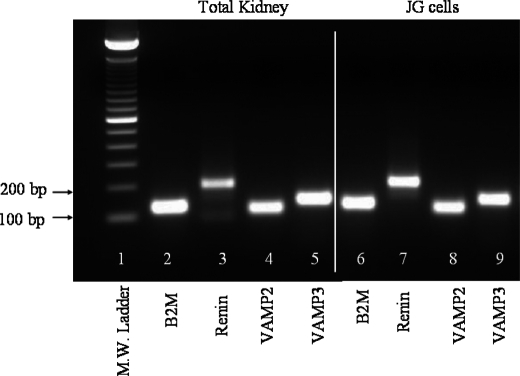
Granule exocytosis and membrane fusion are mediated by SNAREs. However, the expression of the minimal SNARE exocytic machinery in the renal vasculature and in JG cells has not been studied. The vesicle SNAREs VAMP2 and VAMP3 have been previously reported to mediate cAMP-stimulated exocytosis in renal epithelial cells (25, 31, 36, 37). Thus, we first studied their expression in a pure population of JG cells isolated from adult transgenic mice expressing green fluorescent protein (GFP) by FACS (50) and compared it to their relative abundance in total kidney homogenates. Our quantitative PCR results showed that VAMP2 and VAMP3 genes are expressed in freshly isolated JG cells and total kidney (Fig. 1). The relative mRNA abundance (-fold enrichment factor) from JG cells compared with total kidney mRNA from quantitative PCR was 6.2 ± 1-fold (VAMP2) and 3.4 ± 0.5-fold (VAMP3) (Table 2). Because renin is only expressed in JG cells under normal conditions, renin mRNA abundance was used as a control of JG cell purity. As expected, in JG cells isolated from adult mice, renin mRNA was enriched 3346 ± 345-fold when compared with total kidney homogenates. These data indicate that JG cells express VAMP2 and VAMP3 mRNAs, and their mRNA levels are relatively enriched in JG cells when compared with the rest of the kidney.
FIGURE 1.
Representative image of quantitative PCR products for VAMP2 and VAMP3. PCR products were resolved in an agarose gel stained with ethidium bromide (n = 3). Lane 1 is molecular weight ladder. Lanes 2–5 are PCR products from total kidney homogenate illustrating B2M, renin, VAMP2, and VAMP3, respectively. Lanes 6–9 correspond to PCR products from freshly isolated JG cells B2M, renin, VAMP2, and VAMP3, respectively. Some lanes not relevant to the current study have been intentionally cut out of the picture.
TABLE 2.
Real-time PCR of SNAREs in JG cells
ΔΔCt was calculated as ((ΔCt in JG cells) − (ΔCt in total kidney homogenates)), where ΔCt is the threshold value cycle (Ct) of the gene studied − Ct from the internal control B2M. –Fold enrichment factor of genes in JG cells versus total kidney was calculated as 2−ΔΔCt as previously reported (51). SNAP25 was not included in the table, as no amplification was detected. Values are the mean ± S.E.; n = 3. Negative ΔΔCt values (Syntaxin-3 and Munc18b) represent a -fold enrichment of <1, indicating a higher abundance of these genes in total kidney versus JG cells.
| Gene | ΔΔCt | -Fold enrichment factor |
|---|---|---|
| Renin | 11.7 | 3346 ± 345 |
| VAMP2 | 2.6 | 6.2 ± 1.0 |
| VAMP3 | 1.7 | 3.4 ± 0.5 |
| VAMP4 | 1.6 | 3.1 ± 0.3 |
| VAMP5 | 1.7 | 3.2 ± 0.1 |
| VAMP7 | 0.5 | 1.4 ± 0.3 |
| VAMP8 | 0.5 | 1.4 ± 0.2 |
| Syntaxin-1a | 2.4 | 6.0 ± 2.3 |
| Syntaxin-2 | 1.2 | 2.4 ± 0.3 |
| Syntaxin-3 | −1.5 | 0.4 ± 0.1 |
| Syntaxin-4a | 0.8 | 1.9 ± 0.4 |
| SNAP23 | 0.2 | 1.2 ± 0.3 |
| Munc18a | 2.8 | 7.5 ± 2.1 |
| Munc18b | −1.9 | 0.3 ± 0.0 |
| Munc18c | 1.0 | 2.1 ± 0.4 |
The expression of the minimal SNARE fusion machinery in JG cells has not been studied. As shown in Table 2, additional VAMPs isoforms (VAMP4, -5, -7, and -8) as well as several isoforms of its cognate binding partners syntaxin (Syntaxin1, -2, -3, and -4) and SNAP23 were found to be expressed in FACS-isolated JG cells. SNAP25 was not detected in JG cells or total kidney homogenates.
VAMP2 and VAMP3 Proteins Are Expressed in JG Cells and Are Differentially Localized in JG Cells
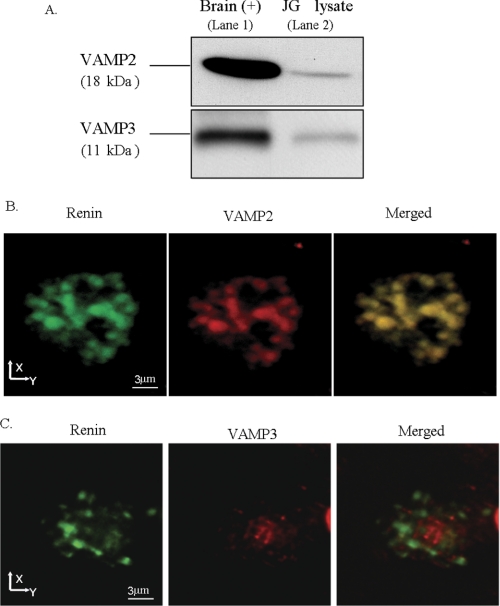
To start addressing the possible role of VAMP2 and/or VAMP3, we studied VAMP2 and VAMP3 protein expression and localization within JG cells. By Western blot analysis of primary cultures of JG cells we observed a band corresponding to the expected molecular masses of 18 kDa (VAMP2) and 11 kDa (VAMP3) (Fig. 2A, lane 2, in both panels). Brain homogenate was used as a positive control (Fig. 2A, lane 1 in both panels). To directly monitor the expression and subcellular localization of VAMP2 and VAMP3 in JG cells, we performed dual labeling immunofluorescence and confocal microscopy for renin (54) and VAMPs. As shown in Fig. 2B, we found that VAMP2 is expressed in JG cells in large granules that occupy most of the cytoplasm (middle panel) that are also stained for renin (left panel), indicating a high degree of co-localization (right panel). Different from VAMP2, VAMP3 was found in smaller vesicles and in a perinuclear area. We observed little to no co-localization of VAMP3 with renin-containing granules (Fig. 2C, right panel). Therefore, both VAMP2 and VAMP3 are expressed in JG cells, but only VAMP2 co-localized with renin-containing granules.
FIGURE 2.
Expression and subcellular localization of VAMP2 and VAMP3 in JG cells. A, a representative Western blot shows expression of VAMP2 (18 kDa) and VAMP3 (11 kDa) in a JG cell lysate. The top panel is VAMP2, and the bottom panel is VAMP3. Lane 1 is brain homogenate (2.5 μg) used as a positive control, and lane 2 is JG cell lysate (7.5 μg) (n = 4). B, immunofluorescence and confocal microscopy of VAMP2 and renin on a single mouse JG cell are shown. The left panel shows a representative image from five preparations of a single JG cell labeled with an antibody against renin (green); 32 confocal slices (z-step 0.3 μm) were stacked into one image projection. Large renin-containing granules that range in size from 0.8 to 1.5 μm can be observed. The middle panel shows VAMP2 labeling (red) in the same cell. The right panel shows a merged image illustrating co-localization of renin with VAMP2 as illustrated by a yellow-orange color (n = 5 different preparations). Bar, 3 μm. C, immunofluorescence and confocal microscopy of VAMP3 and renin on a single mouse JG cell are shown. The left panel shows an individual JG cell immunolabeled with renin (green); the middle panel (red) is the same JG cell labeled with VAMP3 antibody; the right panel is the merged image. No co-localization was observed between small VAMP3-labeled vesicles and renin granules.
Cleavage of VAMP2 and -3 by Tetanus Toxin Inhibits cAMP-stimulated Renin Release in JG Cells
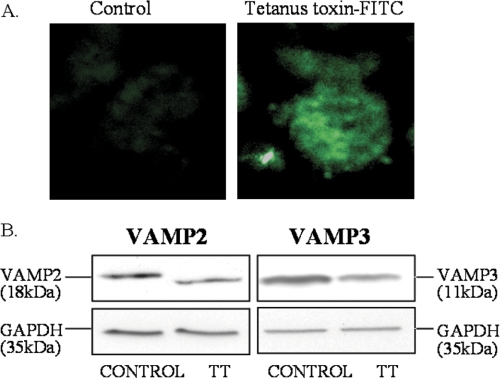
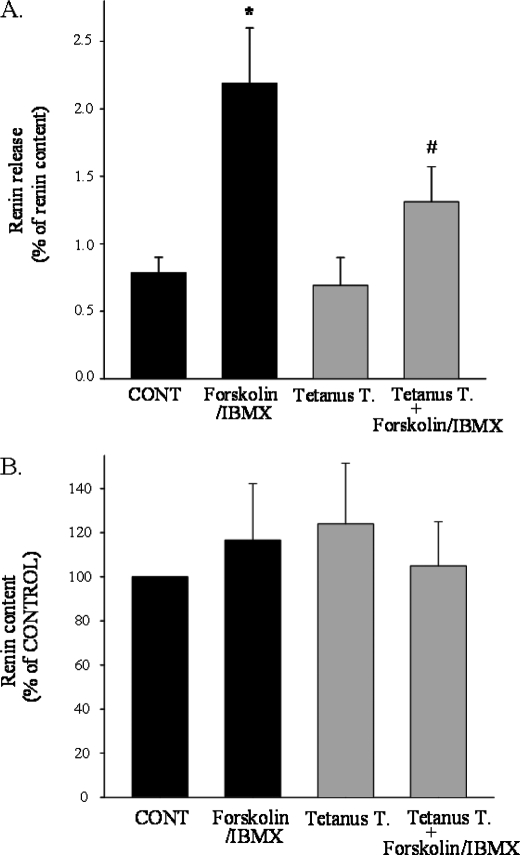
To test whether VAMP2 and/or VAMP3 are involved in cAMP-stimulated renin release, we first used tetanus toxin. Previous studies showed that cell permeabilization and treatment with the proteolytic chain of tetanus toxin (light chain) specifically cleaves and inactivates VAMP2 and -3 (44, 46, 55, 56). Maximal stimulation of JG cells results in the release of only ∼4% of the total renin content. Therefore, permeabilization induces “leakage” of renin and is not feasible in JG cells. Thus, we first tested whether tetanus toxin is efficiently internalized by intact JG cells (non-permeabilized). Incubation with a fluorescent-labeled membrane binding portion of tetanus toxin (FITC-labeled C-fragment) was sufficient to efficiently label most JG cells, indicating internalization of the toxin (Fig. 3A). We then tested whether the intact tetanus toxin efficiently cleaves VAMP2 and -3 in intact JG cells. After 19 h of incubation with tetanus toxin (60 nm) or vehicle, VAMP2 and VAMP3 protein levels were analyzed by Western blot. As shown in Fig. 3B, VAMP2 (left panel) and VAMP3 (right panel) protein was significantly decreased 40 ± 10 and 35 ± 10%, respectively (control = 1; VAMP2 = 0.6 ± 0.1; VAMP3 = 0.65 ± 0.1; n = 8; p < 0.05). Finally, we studied whether inactivation of VAMP2/3 in JG cells with tetanus toxin blocks cAMP-stimulated renin release. After pretreatment of JG cells with tetanus toxin, intracellular cAMP was increased with forskolin plus IBMX for 1 h, and renin release to the media and in cells was measured by radioimmunoassay. Under control conditions, cAMP stimulated renin release from 0.8 ± 0.1 to 2.2 ± 0.4% of renin content, a 180% increase (p < 0.01). However, in JG cells treated with tetanus toxin and, therefore, decreased VAMP2 and VAMP3 protein expression, cAMP only stimulated renin release from 0.7 ± 0.2 to 1.3 ± 0.3% of renin content, a ∼57% blockade in cAMP-stimulated renin release (p < 0.05)(Fig. 4A). Base-line levels of renin release were not affected. The blockade on renin release was not secondary to an impaired processing of renin, as tetanus toxin treatment had no effect on total renin content in JG cells (Fig. 4B). Taken together, these data indicate that VAMP2 and/or VAMP3 mediate in part cAMP-stimulated renin release from JG cells.
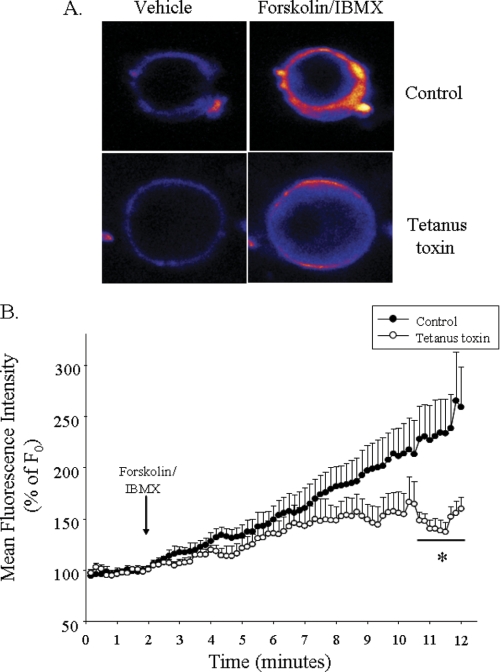
FIGURE 3.
Internalization and cleavage of VAMP2 and VAMP3 by tetanus toxin. A, tetanus toxin is efficiently internalized in intact JG cells. JG cells were incubated for 19 h with either vehicle (Control; left panel) or FITC-labeled receptor binding domain of tetanus toxin (C-fragment; List Biological Laboratories) (right panel). After fixation and mounting, incorporated fluorescence was analyzed by confocal fluorescence imaging. B, representative Western blots show efficient cleavage of VAMP2 and VAMP3 by tetanus toxin. The left panel is VAMP2, and the right panel is VAMP3 protein expression. JG cells were incubated with either vehicle (left lane in both panels) or tetanus toxin (right lane in both panels). After treatment, JG cells were lysed, and SDS-PAGE was resolved in 12% polyacrylamide gel and transferred to PVDF membranes, which were subsequently blotted with VAMP2 or VAMP3 antibodies. Note that a decrease in VAMP2 and VAMP3 band intensity reflects efficient cleavage by tetanus toxin. Membranes were re-blotted with an antibody against GAPDH as an internal loading control (Mr ∼ 35). GAPDH signal was not different between groups (p = ns).
FIGURE 4.
Effect of tetanus toxin on cAMP-stimulated renin release and total renin content. A, renin release is shown. After pretreatment of JG cells with vehicle (black bars) or tetanus toxin (gray bars) as described in Fig. 3, JG cells were serum-deprived for 2 h followed by treatment with vehicle (CONT) or forskolin (10 μm) plus IBMX (0.5 mm) for 1 h to stimulate cAMP levels as indicated in x axes. Cell culture supernatants were collected for measurement of renin release as described under “Experimental Procedures.” Attached cells in the culture dishes were lysed in 0.1% Triton X and centrifuged, and supernatants were collected for measurement of total renin content. Renin release is expressed as a percentage of the total renin content. Data are expressed as the mean ± S.E. *, p < 0.01, CONT versus forskolin/IBMX; #, p < 0.05, forskolin/IBMX versus tetanus toxin + forskolin/IBMX; n = 4. B, shown is the effect of tetanus toxin on total renin content in JG cells. Total renin content values are corrected by protein concentration (ng of ANGI/h of incubation/mg of protein). Data are expressed as the mean ± S.E. (n = 4; p = ns).
Cleavage of VAMP2 and VAMP3 with Tetanus Toxin Impairs cAMP-stimulated Exocytosis in JG Cells
We found that the SNAREs proteins VAMP2 and/or VAMP3 mediate cAMP-dependent renin release. We then tested whether VAMP2 and/or VAMP3 mediate this effect by directly affecting exocytosis. For that, we monitored the effect of forskolin plus IBMX on FM1–43 fluorescence intensity on JG cells. FM1–43 is a styryl dye that is impermeable to cell membranes and fluoresces only when bound to membranes. Therefore, its intensity is proportional to membrane surface area and is used as an index of cumulative exocytosis (51). We observed that incubation of JG cells with medium containing FM1–43 (4 μm) for 2 min resulted in a stable fluorescent intensity. After the addition of forskolin plus IBMX, we observed a rapid and sustained increase in FM1–43 fluorescence that reached a maximum of 259 ± 39% after 9–10 min (Fig. 5B). However, in JG cells pretreated with tetanus toxin, the increase in fluorescence caused by forskolin plus IBMX was slower and less pronounced increase reaching 159 ± 11% of base line, indicative of a ∼62% blockade in exocytosis. These data indicate that VAMP2 and/or VAMP3 mediate the acute stimulation of cAMP on renin release via exocytosis.
FIGURE 5.
Measurement of single-cell exocytosis using FM1–43. A, shown are representative pictures of two JG cells stained with 4 μm FM1–43. The top panels show fluorescence intensity after the addition of vehicle (2 min, basal) or forskolin plus IBMX (12 min, stimulated). The bottom panels show fluorescence intensity in a JG cell pretreated with tetanus toxin under basal (left) or cAMP-stimulated (right) conditions. Pictures were pseudocolored with rainbow scale (blue, low; red, high fluorescence intensity). B, shown are cumulative data for FM1–43 fluorescence intensity over time. Fluorescence intensity was normalized to initial intensity (F0) in JG cells treated with vehicle (basal). The arrow indicates the addition of forskolin plus IBMX. Fluorescence intensity was measured in regions of interest from n = 7 cells from 4 independent JG cell preparations. Data are expressed as the mean ± S.E.; *, p < 0.05 versus control group.
Adenoviral-mediated Knockdown of VAMP2, but Not VAMP3, Inhibits cAMP-stimulated Renin Release in Primary Cultures of Mouse JG Cells
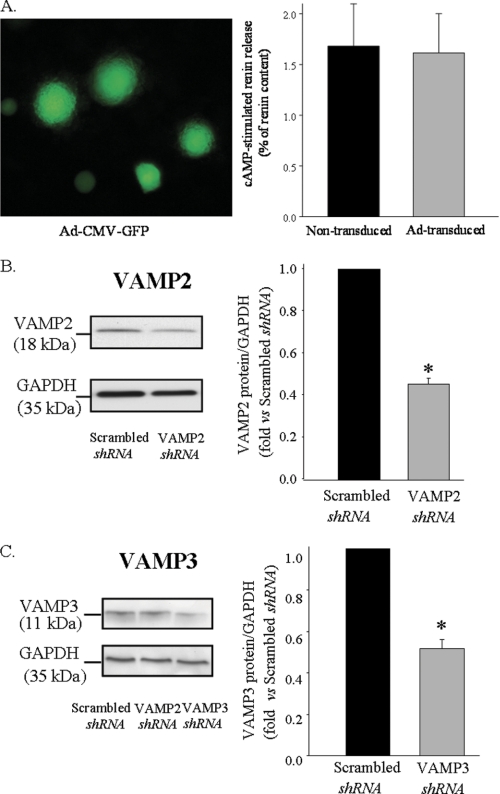
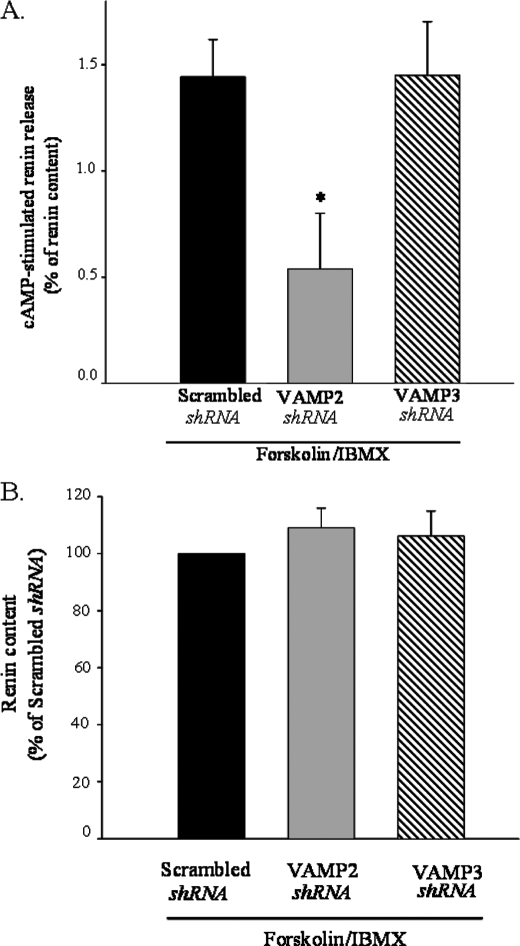
Primary cultures of JG cells are difficult to transfect and, therefore, require adenoviral delivery to enhance transfection efficiency. Visualization of JG cells transduced with an adenovirus encoding GFP driven by a cytomegalovirus (CMV) promoter (Ad-CMV-GFP) showed ∼95% transduction efficiency (Fig. 6A, left panel). Long term viral delivery did not affect cAMP-stimulated renin release when compared with non-transduced JG cells (Fig. 6A, right panel). To dissect the role of VAMP2 and VAMP3 on cAMP-stimulated renin release, we specifically knocked down VAMP2 or VAMP3. Nude siRNA sequences to target either VAMP2 or VAMP3 were shown to specifically knock down its protein expression more than 70% when tested in a mouse cell line (MS1, ATCC) (data not shown). Effective working sequences were then subcloned in an adenovector, and viral particles were produced. Transduction of JG cells with adenovirus encoding short hairpin-silencing RNA for VAMP2 (VAMP2 shRNA) or VAMP3 shRNA resulted in a ∼54 and ∼48% reduction in VAMP2 and VAMP3 protein, respectively, compared with adenovirus scrambled sequence (Scrambled shRNA) (n = 3; p < 0.05) (Fig. 6, B and C). We then tested its individual involvement on cAMP-stimulated renin release. In JG cells transduced with a scrambled sequence, cAMP stimulated renin release from 0.6 ± 0.1 to 2.1 ± 0.2% of renin content. However, in JG cells transduced with adenovirus silencing VAMP2 (VAMP2 shRNA), cAMP stimulation of renin release was significantly impaired by ∼67% (from 0.8 ± 0.2 to 1.30 ± 0.3% of renin content). Opposite to VAMP2, knocking down VAMP3 protein did not affect cAMP-stimulated renin release (Fig. 7A). Total renin content from VAMP2 and VAMP3 knockdown was unaffected when compared with the scrambled-transduced group (p = ns; n = 9) (Fig. 7B). Our results indicate that VAMP2, but not VAMP3, is partially implicated in stimulated renin release. The fact that neither VAMP2 nor VAMP3 alters total renin content indicates that neither of these VAMPs is involved in renin-containing granule to granule fusion/maturation.
FIGURE 6.
Effective adenoviral delivery and specific knockdown of VAMP2 and VAMP3 in JG cells. A, left panel, transduction efficiency in JG cells is shown. JG cells were transduced for 24 h with an adenovirus encoding GFP under the control of cytomegalovirus promoter (Ad-CMV-GFP). No fluorescence was detected in the control (non-transduced) cells. Right panel, shown is a comparison of cAMP-stimulated response in either non-transduced (black bar) or JG cells transduced with Ad-CMV-GFP (gray bar). After transduction of JG cells for up to 48 h, cells were serum-starved for 2 h and treated for 1 h with forskolin plus IBMX (10 μm/0.5 mm) or vehicle according to description under “Experimental Procedures.” Data show stimulated renin release (forskolin/IBMX stimulated renin release − basal (vehicle-treated) renin release) expressed as a percentage of renin content. Basal renin release values for non-transduced and transduced JG cells were 1.0 ± 0.1 and 0.9 ± 0.3% of renin content, respectively (n = 4; p = ns). Data are expressed as the mean ± S.E. B, shown is a representative Western blot illustrating effective silencing of VAMP2 (left panel). JG cells were transduced with adenoviral particles encoding shRNA for either a scrambled sequence or VAMP2. 28 h after transduction, JG cells were lysed, and SDS-PAGE was resolved in 12% polyacrylamide gel and transferred to PVDF membranes, which were subsequently blotted with VAMP2 antibody. Membranes were reblotted with an antibody against GAPDH as an internal loading control (Mr = ∼35 kDa). The right panel shows quantification of VAMP2 protein expression expressed as a ratio of GAPDH signal (n = 4). Scrambled shRNA was arbitrarily set to 1. C, shown is a representative Western blot illustrating effective silencing of VAMP3 (left panel, third lane) versus scrambled shRNA (first lane). Lane 2 shows JG cells transduced with shRNA for VAMP2, which does not decrease VAMP3 expression. The right panel is a quantification of VAMP3 protein expressed as a ratio of GAPDH signal (n = 3; p < 0.01).
FIGURE 7.
Effect of adenoviral delivery of short hairpin silencing for VAMP2 and VAMP3 on cAMP-stimulated renin release and total renin content in JG cells. A, forskolin plus IBMX-stimulated renin release is shown in JG cells transduced with scrambled (black bar), VAMP2 shRNA (gray bar) and VAMP3 shRNA (striped bar). After transduction of JG cells for 28 h, cells were serum-starved for 2 h and treated for 1 h with forskolin plus IBMX (10 μm/0.5 mm) or vehicle according to the description under “Experimental Procedures.” Data show stimulated renin release (forskolin/IBMX-stimulated renin release − vehicle-treated (basal) renin release) expressed as percentage of renin content. Basal renin release values were not significant different: scrambled shRNA = 0.6 ± 0.1; VAMP2 shRNA = 0.8 ± 0.2; VAMP3 shRNA = 0.7 ± 0.3; (p = ns). Data are expressed as the mean ± S.E. B, VAMP2 and VAMP3 knockdown does not affect renin content. Total renin content values are corrected by protein concentration (ng of ANGI/h of incubation/mg of protein). Renin content from scrambled shRNA was arbitrarily set to 100. Data are expressed as the mean ± S.E. (n = 4; p = ns).
DISCUSSION
Renin release from renal JG cells is essential for kidney development and the control of blood pressure. Although the hormonal and paracrine control of renin release has been extensively studied, the proteins involved and the biological mechanism mediating renin release have not been identified. Moreover, whether renin release from granules requires fusion with the plasma membrane has not been directly demonstrated. In several endocrine and secretory cells, specific SNARE proteins mediate granule exocytosis and peptide/hormone release (57–59). However, the expression and role SNAREs in renin release have not been addressed. One of the reasons why SNAREs and fusion were not explored might be the paradoxical inhibitory effect of calcium on renin release that opposes the necessary role of calcium on SNARE complex zippering. Our data show for the first time that the SNARE machinery is expressed in JG cells and the VAMP isoform VAMP2 localizes to renin-containing granules and mediates cAMP-stimulated renin release. These are the first data identifying a trafficking protein involved in renin release. More importantly, the requirement of the SNARE protein, VAMP2, on renin release implies that the renin granule undergoes exocytosis.
We observed differential localization of VAMP2 and VAMP3 in JG cells. Most VAMP2 localized to renin-containing granules, whereas VAMP3 was excluded from renin granules and present in smaller vesicles and in a perinuclear pattern. Although our data indicate that VAMP3 is not involved in constitutive or regulated renin release, VAMP3 may play additional roles in JG cells. Similar to our results, in other exocrine cells VAMP3 was found to be mainly localized to organelles involved in the biosynthetic trafficking pathway rather than in controlling final steps of vesicle or exocytosis (27).
To study the role of VAMP2 and -3 on renin release, we first used the clostridial tetanus toxin. Tetanus toxin recognizes and cleaves a sequence uniquely present in VAMP2 and -3 (44, 46, 55, 56). In intact (non-permeabilized) JG cells, tetanus toxin was efficiently internalized and cleaved ∼50% of the total pool of VAMP2 and VAMP3. This effect was sufficient to block cAMP-stimulated renin release by 50–60%. It is possible that incomplete VAMP2 or VAMP3 inactivation explains the partial effect of tetanus toxin. However, higher concentrations (100 nm) of the toxin did not further inhibit renin release (data not shown). Tetanus toxin inhibition of renin release did not affect processing of renin from its pro-form as total renin content in JG cell lysates did not change when compared with controls. The role of VAMP2 on exocytosis rather than renin processing is further supported by the inhibition of cAMP-stimulated exocytosis, as measured with FM1–43 membrane labeling.
To specifically address the role of these VAMPs, we knocked down VAMP2 and VAMP3 by shRNA. Gene silencing in primary cells is difficult; thus, we used adenoviral vectors with VAMP2 or VAMP3 shRNA sequences. Our data show that a 50–60% protein knockdown of VAMP2 inhibits cAMP-stimulated renin release by 50%, whereas silencing VAMP3 with similar efficacy had no effect. Although these data clearly support a role of VAMP2 in mediating renin release, we cannot rule out the involvement of other VAMPs. It is possible that VAMP7 or -8 (which are not cleaved by tetanus toxin) may be involved in mediating part (30–50%) of the stimulatory effect of cAMP on granule fusion with the plasma membrane. This would imply that there may be at least two pools of granules containing mature renin, perhaps with different SNARES. However, it is also possible that upon decrease or inactivation of VAMP2, other VAMP isoforms exert a compensatory role, as it occurs in other secretory cells (60). Taken together our data demonstrate that VAMP2 but not VAMP3 mediates most of the cAMP-stimulated renin release, although the partial involvement of other isoforms cannot be ruled out and will require further investigation.
Base-line (non-stimulated) renin release was not affected by either tetanus toxin or by selective knockdown of either VAMP2 or VAMP3. These data indicate that VAMP2 participates in the regulated rather than the constitutive release of renin or in its maturation process. It is likely that different VAMPs mediate the constitutive and the regulated renin pathways as reported in other exocrine cells (22, 29). In addition, we found that inactivation or knockdown of VAMP2 and VAMP3 with tetanus toxin or silencing RNA did not affect the amount of total renin in JG cells (renin content). Thus, the inhibitory effect of silencing VAMP2 is not likely to be due to a decrease in the amount of mature renin in JG cells or defective granule maturation. Rather, our data point to VAMP2 being necessary for granule-to plasma membrane fusion (exocytosis per se).
In vivo, the amount of renin released into the circulation represents a very small percentage of the total renin content (61). Renin release from JG cells in isolated afferent arteriole is ∼2–5% of the total renin content (15). For our experiments, we have used an extensively characterized preparation of primary JG cells that is at least 80% pure and responds to physiological stimuli (9). Although the percent of total renin released may seem small, our data are in excellent agreement with previous work studying JG cells in vivo or after isolation (15, 62–64). This magnitude of response is similar to most endocrine cells, which release a small fraction of it granular contents upon stimulation (24, 65). We found that culturing JG cells for up to 72 h did not result in dedifferentiation, as renin release values under constitutive or stimulated conditions were comparable with those of cells cultured for 24 or 48 h. In addition, longer incubation times and viral transduction per se did not affect renin release or renin content values.
At least two other SNARE proteins, a syntaxin and SNAP, are required to mediate granule exocytosis. The expression of these SNAREs has not been studied in JG cells. In other endocrine cells where exocytosis is stimulated by cAMP, VAMP2 pairs primarily with syntaxin-1, -3, and -4 and SNAP23. We found that syntaxin-1, -2 -3, and -4 and SNAP23 (but not SNAP25) are present in JG cells. In addition, we also found that other VAMP isoforms (VAMP4, -5, -7 and -8) as well as the accessory proteins Munc18 (Munc18a, -b, and -c) are expressed in JG cells (Table 2). Although the full fusogenic machinery seems to be present in JG cells, we did not find a differential pattern of enrichment for those SNAREs in JG cells. This is somehow predictable as it is likely that the different SNAREs are involved in different biological processes within the JG cells as well as kidney cell types. Furthermore, some of the isoforms that we found to be expressed in JG cells may be involved in renin granule maturation. Thus, it is essential to study the VAMP2-interacting SNARE proteins individually and their specific involvement on cAMP-stimulated renin granule exocytosis.
The molecular mechanisms by which cAMP increases renin release via VAMP2 are not known. Previous studies have shown that SNARE complex and exocytosis can be regulated directly by phosphorylation in other secretory cells (57, 66, 67). It is possible that cAMP via PKA could be directly responsible for phosphorylation of VAMP2 binding partners such as SNAP23 (57, 67). In addition there are several proteins that modulate SNARE-mediated exocytosis such as snapin, tomosyn, and complexins (68–70), which are targets for the cAMP-PKA pathways. Any of these may be present in JG cells and could subsequently enhance fusion of pre-docked dense core renin granules to the plasma membrane, but these have not been studied.
In most cells studied SNARE zippering and complex formation, which mediates the ultimately step in membrane fusion, requires calcium (22). In addition, in most secretory cells studied, calcium is the triggering second messenger for exocytosis (22, 71). Opposite to most cells, in JG cells a decrease in intracellular calcium stimulates renin release, whereas high calcium inhibits it, a phenomenon known as the calcium paradox (7). Although it might seem contradictory that SNAREs mediate renin release with an opposing effect of intracellular calcium on secretion, there are several potential explanations. One possible explanation is that a localized calcium spike or calcium microdomain occurs at the plasma membrane specifically near fusion sites without affecting intracellular calcium levels or stores (72). In addition, some SNAREs are less sensitive to the requirement for calcium to induce exocytosis. For example, SNAP23 is less sensitive to changes in calcium concentration than SNAP25 (73). Synaptotagmins, which are SNARE complex modulatory proteins, serve as calcium sensors (74). Another possible explanation could be that the synaptotagmin isoforms mediating renin release in JG cells are calcium-independent as described earlier (74). Thus, it is possible that the VAMP2-mediated renin release primarily involves a set of SNAREs that does not require an increase in calcium for zippering. However, this remains speculative and requires future investigation.
From all of the hypertensive patient population, less than 30% possess the expectedly low plasma renin activity (75, 76). The remaining percentage exhibits either normal or high plasma renin values, both of which are unexpected or “abnormal” as renin should be suppressed by higher blood pressure. The mechanisms that mediate these higher than normal renin levels in hypertension and whether they involve enhanced renin release and alterations in the proteins mediating renin secretion or signaling in JG cells have not been elucidated. Our findings identify for the first time that the SNARE protein VAMP2, but not VAMP3, mediates cAMP-stimulated renin release. Future studies focusing on the selective SNARE pairs from the syntaxin family and the mechanisms that modulate SNARE complex formation in JG cells would advance our understanding of renin exocytosis and may provide novel targets for inhibition of renin release.
Acknowledgments
We thank Craig Jones, MaryKay Ellsworth, and the Flow and Image Cytometry Resource at Roswell Park Cancer Institute, which is supported by NCI, National Institutes of Health-funded Cancer Center Support Grant CA016056. We also thank Dr. Pablo Ortiz for reading of the manuscript and suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grant National Research Service Awards F32 HL096346-01 (to M. M.) and 5R01HL48459-17 (to K. W. G.). This work also supported by a National Kidney Foundation fellowship grant.
- ANG
- angiotensin
- JG
- juxtaglomerular
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment (SNAP) proteins receptor
- VAMP2
- vesicle-associated membrane protein-2
- VAMP3
- vesicle-associated membrane protein-3
- B2M
- β2-microglobulin
- HBSS
- Hanks' buffered salts solution
- ns
- not significant
- IBMX
- isobutylmethylxanthine.
REFERENCES
- 1. Gomez R. A., Norwood V. F., Tufro-McReddie A. (1997) Microsc. Res. Tech. 39, 254–260 [DOI] [PubMed] [Google Scholar]
- 2. Sequeira López M. L., Pentz E. S., Nomasa T., Smithies O., Gomez R. A. (2004) Dev. Cell 6, 719–728 [DOI] [PubMed] [Google Scholar]
- 3. Lacombe M. J., Mercure C., Dikeakos J. D., Reudelhuber T. L. (2005) J. Biol. Chem. 280, 4803–4807 [DOI] [PubMed] [Google Scholar]
- 4. Methot D., Reudelhuber T. L. (2001) Curr. Hypertens. Rep. 3, 68–73 [DOI] [PubMed] [Google Scholar]
- 5. Jutras I., Reudelhuber T. L. (1999) FEBS Lett. 443, 48–52 [DOI] [PubMed] [Google Scholar]
- 6. Reudelhuber T. L., Brechler V., Jutras I., Mercure C., Methot D. (1998) Adv. Exp. Med. Biol. 436, 229–238 [DOI] [PubMed] [Google Scholar]
- 7. Hackenthal E., Paul M., Ganten D., Taugner R. (1990) Physiol. Rev. 70, 1067–1116 [DOI] [PubMed] [Google Scholar]
- 8. Beierwaltes W. H. (2010) Am. J. Physiol. Renal Physiol. 298, F1–F11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ortiz-Capisano M. C., Ortiz P. A., Harding P., Garvin J. L., Beierwaltes W. H. (2007) Hypertension 49, 162–169 [DOI] [PubMed] [Google Scholar]
- 10. Reudelhuber T. L., Ramla D., Chiu L., Mercure C., Seidah N. G. (1994) Kidney Int. 46, 1522–1524 [DOI] [PubMed] [Google Scholar]
- 11. Chu W. N., Baxter J. D., Reudelhuber T. L. (1990) Mol. Endocrinol. 4, 1905–1913 [DOI] [PubMed] [Google Scholar]
- 12. Brechler V., Chu W. N., Baxter J. D., Thibault G., Reudelhuber T. L. (1996) J. Biol. Chem. 271, 20636–20640 [DOI] [PubMed] [Google Scholar]
- 13. Hackenthal E., Schwertschlag U., Taugner R. (1983) Clin. Exp. Hypertens. A. 5, 975–993 [DOI] [PubMed] [Google Scholar]
- 14. Sequeira Lopez M. L., Gomez R. A. (2003) Methods Mol. Med. 86, 193–204 [DOI] [PubMed] [Google Scholar]
- 15. Itoh S., Carretero O. A., Murray R. D. (1985) Kidney Int. 27, 762–767 [DOI] [PubMed] [Google Scholar]
- 16. Hackenthal E., Taugner R. (1986) Mol. Cell. Endocrinol. 47, 1–12 [DOI] [PubMed] [Google Scholar]
- 17. Itoh S., Carretero O. A., Murray R. D. (1985) J. Clin. Invest. 76, 1412–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Itoh S., Carretero O. A. (1985) Hypertension 7, I49–I54 [DOI] [PubMed] [Google Scholar]
- 19. Taugner R., Bührle C. P., Nobiling R. (1984) Cell Tissue Res. 237, 459–472 [DOI] [PubMed] [Google Scholar]
- 20. Friis U. G., Jensen B. L., Aas J. K., Skøtt O. (1999) Circ. Res. 84, 929–936 [DOI] [PubMed] [Google Scholar]
- 21. Peti-Peterdi J., Fintha A., Fuson A. L., Tousson A., Chow R. H. (2004) Am. J. Physiol. Renal Physiol. 287, F329–F335 [DOI] [PubMed] [Google Scholar]
- 22. Chen Y. A., Scheller R. H. (2001) Nat. Rev. Mol. Cell Biol. 2, 98–106 [DOI] [PubMed] [Google Scholar]
- 23. Cosen-Binker L. I., Morris G. P., Vanner S., Gaisano H. Y. (2008) World J. Gastroenterol. 14, 2314–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang X., Sheu L., Tamori Y., Trimble W. S., Gaisano H. Y. (2001) Pancreas 23, 125–133 [DOI] [PubMed] [Google Scholar]
- 25. Nielsen S., Marples D., Birn H., Mohtashami M., Dalby N. O., Trimble M., Knepper M. (1995) J. Clin. Invest. 96, 1834–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jahn R., Scheller R. H. (2006) Nat. Rev. Mol. Cell Biol. 7, 631–643 [DOI] [PubMed] [Google Scholar]
- 27. Martin S., Tellam J., Livingstone C., Slot J. W., Gould G. W., James D. E. (1996) J. Cell Biol. 134, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin L. B., Shewan A., Millar C. A., Gould G. W., James D. E. (1998) J. Biol. Chem. 273, 1444–1452 [DOI] [PubMed] [Google Scholar]
- 29. Weng N., Thomas D. D., Groblewski G. E. (2007) J. Biol. Chem. 282, 9635–9645 [DOI] [PubMed] [Google Scholar]
- 30. Williams D., Pessin J. E. (2008) J. Cell Biol. 180, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ortiz P. A. (2006) Am. J. Physiol. Renal Physiol. 290, F608–F616 [DOI] [PubMed] [Google Scholar]
- 32. Mandon B., Chou C. L., Nielsen S., Knepper M. A. (1996) J. Clin. Invest. 98, 906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandon B., Nielsen S., Kishore B. K., Knepper M. A. (1997) Am. J. Physiol. 273, F718–F730 [DOI] [PubMed] [Google Scholar]
- 34. Inoue T., Nielsen S., Mandon B., Terris J., Kishore B. K., Knepper M. A. (1998) Am. J. Physiol. 275, F752–F760 [DOI] [PubMed] [Google Scholar]
- 35. Low S. H., Roche P. A., Anderson H. A., van Ijzendoorn S. C., Zhang M., Mostov K. E., Weimbs T. (1998) J. Biol. Chem. 273, 3422–3430 [DOI] [PubMed] [Google Scholar]
- 36. Alexander E. A., Shih T., Schwartz J. H. (1997) Am. J. Physiol. 273, F1054–F1057 [DOI] [PubMed] [Google Scholar]
- 37. Jo I., Harris H. W., Amendt-Raduege A. M., Majewski R. R., Hammond T. G. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1876–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. della Bruna R. D., Pinet F., Corvol P., Kurtz A. (1991) Cell. Physiol. Biochem. 1, 98–110 [Google Scholar]
- 39. Caceres P. S., Ares G. R., Ortiz P. A. (2009) J. Biol. Chem. 284, 24965–24971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gil C., Soler-Jover A., Blasi J., Aguilera J. (2005) Biochem. Biophys. Res. Commun. 329, 117–124 [DOI] [PubMed] [Google Scholar]
- 41. Pennuto M., Bonanomi D., Benfenati F., Valtorta F. (2003) Mol. Biol. Cell 14, 4909–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li G., Alexander E. A., Schwartz J. H. (2003) J. Biol. Chem. 278, 19791–19797 [DOI] [PubMed] [Google Scholar]
- 43. Pellizzari R., Rossetto O., Washbourne P., Tonello F., Nicotera P. L., Montecucco C. (1998) Toxicol. Lett. 102–103, 191–197 [DOI] [PubMed] [Google Scholar]
- 44. Matsuda M., Okabe T., Sugimoto N., Senda T., Fujita H. (1994) Ann. N.Y. Acad. Sci. 710, 94–106 [DOI] [PubMed] [Google Scholar]
- 45. Gouraud S., Laera A., Calamita G., Carmosino M., Procino G., Rossetto O., Mannucci R., Rosenthal W., Svelto M., Valenti G. (2002) J. Cell Sci. 115, 3667–3674 [DOI] [PubMed] [Google Scholar]
- 46. Turton K., Chaddock J. A., Acharya K. R. (2002) Trends Biochem. Sci. 27, 552–558 [DOI] [PubMed] [Google Scholar]
- 47. Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. (1994) J. Cell Biol. 125, 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hajduch E., Aledo J. C., Watts C., Hundal H. S. (1997) Biochem. J. 321, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Verderio C., Coco S., Rossetto O., Montecucco C., Matteoli M. (1999) J. Neurochem. 73, 372–379 [DOI] [PubMed] [Google Scholar]
- 50. Glenn S. T., Jones C. A., Pan L., Gross K. W. (2008) Physiol. Genomics 35, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Winer J., Jung C. K., Shackel I., Williams P. M. (1999) Anal. Biochem. 270, 41–49 [DOI] [PubMed] [Google Scholar]
- 52. Cochilla A. J., Angleson J. K., Betz W. J. (1999) Annu. Rev. Neurosci. 22, 1–10 [DOI] [PubMed] [Google Scholar]
- 53. Harata N. C., Choi S., Pyle J. L., Aravanis A. M., Tsien R. W. (2006) Neuron 49, 243–256 [DOI] [PubMed] [Google Scholar]
- 54. Ortiz-Capisano M. C., Ortiz P. A., Harding P., Garvin J. L., Beierwaltes W. H. (2007) Hypertension 49, 618–624 [DOI] [PubMed] [Google Scholar]
- 55. Humeau Y., Doussau F., Grant N. J., Poulain B. (2000) Biochimie 82, 427–446 [DOI] [PubMed] [Google Scholar]
- 56. Sterling H., Lin D. H., Wei Y., Wang W. H. (2003) Am. J. Physiol. Renal Physiol. 284, F510–F517 [DOI] [PubMed] [Google Scholar]
- 57. Foster L. J., Yeung B., Mohtashami M., Ross K., Trimble W. S., Klip A. (1998) Biochemistry 37, 11089–11096 [DOI] [PubMed] [Google Scholar]
- 58. Fujita-Yoshigaki J., Dohke Y., Hara-Yokoyama M., Kamata Y., Kozaki S., Furuyama S., Sugiya H. (1996) J. Biol. Chem. 271, 13130–13134 [DOI] [PubMed] [Google Scholar]
- 59. Fujita-Yoshigaki J., Dohke Y., Hara-Yokoyama M., Furuyama S., Sugiya H. (1998) Eur. J. Morphol. 36, 46–49 [PubMed] [Google Scholar]
- 60. Zhao P., Yang L., Lopez J. A., Fan J., Burchfield J. G., Bai L., Hong W., Xu T., James D. E. (2009) J. Cell Sci. 122, 3472–3480 [DOI] [PubMed] [Google Scholar]
- 61. Park C. S., Malvin R. L., Murray R. D., Cho K. W. (1978) Am. J. Physiol. 234, F506–F509 [DOI] [PubMed] [Google Scholar]
- 62. Jensen B. L., Schmid C., Kurtz A. (1996) Am. J. Physiol. 271, F659–F669 [DOI] [PubMed] [Google Scholar]
- 63. Schricker K., Kurtz A. (1995) Am. J. Physiol. 269, F64–F69 [DOI] [PubMed] [Google Scholar]
- 64. de Jong W. (1969) Proc. Soc. Exp. Biol. Med. 130, 85–88 [DOI] [PubMed] [Google Scholar]
- 65. Thurmond D. C., Gonelle-Gispert C., Furukawa M., Halban P. A., Pessin J. E. (2003) Mol. Endocrinol. 17, 732–742 [DOI] [PubMed] [Google Scholar]
- 66. Cabaniols J. P., Ravichandran V., Roche P. A. (1999) Mol. Biol. Cell 10, 4033–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hepp R., Puri N., Hohenstein A. C., Crawford G. L., Whiteheart S. W., Roche P. A. (2005) J. Biol. Chem. 280, 6610–6620 [DOI] [PubMed] [Google Scholar]
- 68. Chheda M. G., Ashery U., Thakur P., Rettig J., Sheng Z. H. (2001) Nat. Cell Biol. 3, 331–338 [DOI] [PubMed] [Google Scholar]
- 69. Butterworth M. B., Frizzell R. A., Johnson J. P., Peters K. W., Edinger R. S. (2005) Am. J. Physiol. Renal Physiol. 289, F969–F977 [DOI] [PubMed] [Google Scholar]
- 70. Baba T., Sakisaka T., Mochida S., Takai Y. (2005) J. Cell Biol. 170, 1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burgoyne R. D., Morgan A. (2003) Physiol. Rev. 83, 581–632 [DOI] [PubMed] [Google Scholar]
- 72. Becherer U., Moser T., Stühmer W., Oheim M. (2003) Nat. Neurosci. 6, 846–853 [DOI] [PubMed] [Google Scholar]
- 73. Chieregatti E., Chicka M. C., Chapman E. R., Baldini G. (2004) Mol. Biol. Cell 15, 1918–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Südhof T. C., Rizo J. (1996) Neuron. 17, 379–388 [DOI] [PubMed] [Google Scholar]
- 75. Genest J., Boucher R., Kuchel O., Nowaczynski W. (1973) Can. Med. Assoc. J. 109, 475–478 [PMC free article] [PubMed] [Google Scholar]
- 76. Laragh J. H. (1975) Johns Hopkins Med. J. 137, 184–194 [PubMed] [Google Scholar]