Abstract
The lipid metabolite diacylglycerol (DAG) is required for transport carrier biogenesis at the Golgi, although how cells regulate its levels is not well understood. Phospholipid synthesis involves highly regulated pathways that consume DAG and can contribute to its regulation. Here we altered phosphatidylcholine (PC) and phosphatidylinositol synthesis for a short period of time in CHO cells to evaluate the changes in DAG and its effects in membrane trafficking at the Golgi. We found that cellular DAG rapidly increased when PC synthesis was inhibited at the non-permissive temperature for the rate-limiting step of PC synthesis in CHO-MT58 cells. DAG also increased when choline and inositol were not supplied. The major phospholipid classes and triacylglycerol remained unaltered for both experimental approaches. The analysis of Golgi ultrastructure and membrane trafficking showed that 1) the accumulation of the budding vesicular profiles induced by propanolol was prevented by inhibition of PC synthesis, 2) the density of KDEL receptor-containing punctated structures at the endoplasmic reticulum-Golgi interface correlated with the amount of DAG, and 3) the post-Golgi transport of the yellow fluorescent temperature-sensitive G protein of stomatitis virus and the secretion of a secretory form of HRP were both reduced when DAG was lowered. We confirmed that DAG-consuming reactions of lipid synthesis were present in Golgi-enriched fractions. We conclude that phospholipid synthesis pathways play a significant role to regulate the DAG required in Golgi-dependent membrane trafficking.
Keywords: Diacylglycerol, Golgi, Lipids, Membrane Trafficking, Phosphatidylcholine
Introduction
Processes such as membrane budding, cargo selection, and vesicle fission constitute the basis for the formation of transport carriers (vesicles and tubules) at the Golgi. A plethora of proteins, such as coat-forming, signaling, and cytoskeleton components, ensure the specificity of the process and the cellular destination of the vesicles (1, 2). The lipid composition of the membrane, including the amount of metabolic intermediates, is also involved in this process (3–6).
DAG5 is needed at the trans-Golgi network for the recruitment of protein kinase D and post-Golgi protein transport (7) and at the cis-Golgi for the biogenesis of coat protein complex I-coated vesicles (8, 9). Several pharmacological agents were used to show this requirement of DAG and also to examine its source. First, the effects of propanolol, a phosphatidic acid (PA) phosphatase inhibitor (10), suggested a role for PA-derived DAG (8). In this context, the PA produced by phospholipase D1 (PLD1) could be the source of DAG because PLD1 is a candidate to mediate the formation of vesicles stimulated by ARF1 at the Golgi (11). Second, the results of experiments with U73122, an inhibitor of agonist-induced phospholipase C activation (12), suggested a contribution of phospholipase C to the production of the DAG required at the cis-Golgi (8). Third, the results obtained with fumonisin B1, an inhibitor of ceramide synthase (13), suggested a role for the DAG produced by sphingomyelin synthase at the trans-Golgi network (7). Of these three lipid-metabolizing enzymes, only sphingomyelin synthase is widely believed to localize to the Golgi. PLD localization at this organelle is controversial (14–16), and phospholipase C action has not been widely studied (17).
Phospholipid transfer proteins have also been implicated in vesicular transport at the Golgi. The PI transfer protein (PITP) Nir2 participates in the secretory pathway at the Golgi in mammalian cells (18), as demonstrated previously for the PITP Sec14 in yeast (19). In both studies, the silencing of PC synthesis precluded the blockage of secretion produced by the silencing of their respective PITPs, which suggested that PITPs maintain partial inhibition of PC synthesis and regulate the homeostasis of DAG required for the production of secretory transport carriers at the Golgi. Recently, it has been shown that PITPβ is needed for the vesicular transport at the Golgi (20). In this case, the authors suggested that PITPβ is needed to bring PI to the Golgi for the synthesis of phosphatidylinositol 4-phosphate, which is involved in vesicular trafficking at the Golgi (21).
Pharmacological and silencing experimental strategies have concluded that the metabolic intermediate DAG is required for the transport carrier biogenesis at the Golgi. However, the particular metabolic steps producing or consuming DAG that are regulated under physiological conditions to ensure the proper lipid composition of Golgi membranes in the time interval required for vesicular trafficking are not well understood.
Phospholipid synthesis is strictly regulated in most cell types throughout the cell cycle (22). There is also evidence that both endogenously and exogenously triggered cellular signaling can quickly turn phospholipid synthesis pathways on and off in physiological contexts other than membrane synthesis and assembly (23–25). During a quick and short tuning of the rate of phospholipid synthesis, the amount of metabolic intermediates such as DAG is more likely to be altered than the amount of the end products of these pathways, such as major phospholipids (23, 24). Thus, cells may take advantage of these regulatory mechanisms to rapidly and finely regulate the DAG at the Golgi, as has been pointed out (26, 27). This is the hypothesis we pursued in this study. We modified DAG levels by regulating PC and PI synthesis within a short period of time (from 30 min to 3 h) by two experimental approaches: 1) regulating the rate of PC synthesis in a mutant cell line with the temperature-sensitive regulatory enzyme for the pathway, CTP:phosphocholine cytidylyltransferase; and 2) controlling the availability of substrates for PI and PC synthesis. We knew from our previous results that DAG was required for vesicle formation at the Golgi (8). We now show that the tuning of PC and PI synthesis for a short time regulates DAG homeostasis of the whole cell, including Golgi complex, without modifying the levels of major phospholipid classes or triacylglycerol. The changes of DAG produced in this way correlate with alterations in the structure and dynamics of secretory membrane trafficking at the Golgi.
EXPERIMENTAL PROCEDURES
Materials
Rabbit polyclonal antibody anti-KDEL receptor (KDELr) was kindly provided by H. D. Söling (University of Göttingen). Anti-GM130 and anti-VSV-G (ectodomain) were purchased from Sigma. Alexa 488-conjugated secondary antibodies were from Molecular Probes-Invitrogen. Secondary antibodies conjugated to Cy2 or Cy3 were from Jackson InmunoResearch Laboratories (West Grove, PA). Plasmid encoding FLAG-ssHRP was kindly provided by V. Malhotra (Centre de Regulació Genòmica, Barcelona, Spain). Plasmid encoding ts045VSV-G into pEYFP was kindly provided by K. Simons (Max Planck Institute, Dresden, Germany). Plasmid encoding EGFP-C1b-PKCθ was from I. Merida (Consejo Superior de Investigaciones Científicas, Madrid). DMEM, F-12 Ham culture medium, Opti-MEM, and fetal bovine serum were from Invitrogen. CHO-MT58 mutant cell line was kindly provided by E. Claro (Autonomous University of Barcelona). DAG measurement reagents (1,2-dioleoyl-sn-glycerol standards, cardiolipin, N-octyl glucoside, and diethylenetriaminepentaacetic acid) were purchased from Sigma. Recombinant DAG kinase from Escherichia coli was from Calbiochem. [γ-32P] ATP (10 Ci/mmol), ortho-[32P]phosphoric acid (850–912 Ci/mmol), [3H]choline (50–62 mCi/mmol), [14C]acetate (45–60 mCi/mmol), and myo-[3H]inositol (10–25 Ci/mmol) were from PerkinElmer Life Sciences. HPTLC silica gel-60 plates (10 × 10 cm) were from Merck. Organic solvents used for lipid extraction were of analytical grade. Lipofectamine 2000TM transfection reagents were from Invitrogen. ECL detection reagents were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell Culture
Chinese hamster ovary cell lines CHO-K1 (wild type) and CHO-MT58 (28), expressing a temperature-sensitive mutant of CTTα, which catalyzes the generation of CDP-choline in the rate-limiting step of PC synthesis (29), were grown in F-12 Ham medium containing 7.5% FBS, 1 mm sodium pyruvate, 10 mm l-glutamine, penicillin (100 units/ml), and streptomycin (100 μg/ml) in a humidified incubator at 33 °C and 5% CO2. Normal rat kidney (NRK) and Vero (kidney of normal green monkey) cells were grown in DMEM containing 10% FCS, 1 mm sodium pyruvate, 10 mm l-glutamine, penicillin (100 units/ml), and streptomycin (100 μg/ml) in a humidified incubator at 37 °C and 5% CO2.
Experimental Conditions to Regulate the Synthesis of PC and PI
For the first experimental approach, CHO-K1 and the mutant line CHO-MT58, with a temperature-sensitive enzyme, were grown at 33 °C. At the beginning of the experiment, cells were incubated at the permissive temperature (33 °C) or non-permissive temperature (40 °C) for different times (from 30 min to 3 h). For the second experimental strategy, cells grown in culture medium were washed and incubated with choline (200 μm) and inositol (5 mm) for up to 1 h in HEPES saline-buffered solution (137 mm NaCl, 1 mm CaCl2, 2 mm MgCl2-6H2O, 3 mm KCl, 5.6 mm d-glucose, 20 mm HEPES, pH 7.4). Lithium (5 mm) was added when choline and inositol were not present. For ultrastructural analysis, cells were treated with 60 μm propanolol during the last 15 min of the treatment when indicated.
Isolation of Golgi-enriched Fractions
Golgi fractions from Vero cells were prepared at 4 °C following a modification of a method reported elsewhere (30). Cells were harvested, washed twice in PBS and twice in homogenization buffer (250 mm sucrose in 10 mm Tris-HCl, pH 7.4), and pelleted at 1500 g for 10 min. Cells were then resuspended in 4 volumes of homogenization buffer and homogenized using the Ball-Balch device. The homogenate was brought to a sucrose concentration of 37% by the addition of 62% sucrose in 10 mm Tris-HCl, pH 7.4, and EDTA (1 mm final concentration). Twelve milliliters of this solution were placed at the bottom of a centrifuge tube and carefully overlaid with 15 ml of sucrose at 35% and 9 ml of 29% sucrose in 10 mm Tris-HCl (pH 7.4). Gradients were centrifuged at 100,000 × g (25000 rpm) for 2.5 h with the swing rotor SW28 on a L7–55 Beckmann ultracentrifuge. The Golgi-enriched fraction was recovered at the 35%/29% sucrose interface and subsequently frozen in liquid nitrogen and stored in aliquots at −80 °C until use. Protein concentration was determined using the Lowry assay. Golgi-enriched fractions from rat liver were prepared as described previously (31).
Measurement of CDP-choline:Diacylglycerol Phosphotransferase Activity in Isolated Golgi Membranes
CDP-choline:diacylglycerol phosphotransferase activity was measured in the presence of CMP to force the reaction to produce DAG and CDP-choline at the expense of endogenous Golgi membrane PC, which is the reverse of the reaction that takes place under physiological conditions. Golgi membranes (50 μg of protein) isolated from Vero cells and rat liver were incubated in 100 mm Tris/HCl buffer containing 5 mm EDTA, 5 mm DTT, 10 mm MgCl2, pH 8.2 (32), and, when indicated, 10 mm CMP. Incubations were stopped by adding 1 volume of chloroform/methanol (1:2, v/v), and samples were processed as indicated below for whole cell lipid extracts.
Determination of DAG in Lipid Extracts
For DAG determination in lipid extracts cells were grown in 60-cm2 plates to 80% confluence. Cells grown to preconfluence (corresponding to 1.2 mg of protein) were used as individual samples for the different conditions performed in duplicate in each experiment. After incubation cells were placed on ice, incubation medium was aspirated, and 1 volume of methanol was added (2 ml for 60-cm2 culture dishes). Cells were then scraped and collected into glass test tubes containing 0.5 volumes of chloroform. After 5 min on ice, phases were separated by adding 0.65 volumes each of chloroform and distilled water. Tubes were vigorously vortexed and centrifuged at 3000 × g. The lower organic phase was washed in water/methanol (1:1) and evaporated under a stream of N2. DAG levels were measured as described previously (33). The lipid contents of each sample or 1,2-dioleoyl-sn-glycerol standards (in a range of 250–8000 pmol) were resuspended by sonication in 20 μl of a mixture of cardiolipin (5 mm), N-octyl glucoside (7.5%), and DETAPAC (1 mm). The lipid suspension was then incubated at 25 °C for 1 h in a final volume of 100 μl of the reaction buffer (100 mm imidazole-HCl, 50 mm NaCl, 12.5 mm MgCl2, 1 mm EGTA, 0.5 mm DETAPAC, and 2 mm DTT) in the presence of DAG kinase from Escherichia coli (0.022 unit) and 1 μCi of [32P]ATP (2 mm). The reaction was stopped by the addition of 0.6 ml of the mixture chloroform/methanol/HCl (100:200:1, v/v/v). After 15 min at room temperature, 0.25 ml of chloroform and 0.25 ml of water were added to separate two phases. The tubes were then shaken vigorously and centrifuged at 3000 × g for 5 min. The upper aqueous phase was removed, and the lower organic phase containing 32P-lipids was washed in 0.75 ml of methanol/H2O (1:1, v/v). The washed organic phase was evaporated under a stream of N2. To separate [32P]phosphatidic acid (PA) from the rest of 32P-lipids, the lipid pellets were resuspended in 20 μl of chloroform/methanol (4:1, v/v) and spotted onto silica gel plates (HPTLC silica gel-60 plates, 10 × 10 cm), which were developed with chloroform/acetone/methanol/acetic acid/H2O (10:4:3:2:1, v/v/v/v/v). The area corresponding to [32P]PA was identified by comigration with standards, counted for radioactivity with a PhosphorImager (Typhon TRIO, Amersham Biosciences), and analyzed with ImageQuant software (Amersham Biosciences). Standard values were plotted as counts of [32P]PA versus pmol of 1,2-dioleoyl-sn-glycerol (250–8000 pmol), and the amount of DAG in each sample was interpolated after linear regression of the data. The measurements were always performed in duplicate, giving S.D. values no greater than of 10–15% of the value, which indicated that no internal control of total phospholipids present in the sample was needed. Then, the results for the different conditions in each experiment were referred to the value of a condition taken as control. The total amounts of DAG in lipid extracts measured with this method were comparable with those obtained with mass spectrometry (not shown).
Determination of Major Phospholipid Classes and Triacylglycerol
CHO-K1 and CHO-MT58 cells were radiolabeled with [3H]choline (0,5 μCi/ml), [3H]myo-inositol (0,5μCi/ml), [14C]acetate (0,25 μCi/ml), or [3H]orthophosphoric acid (5 μCi/ml) for 24 h in F-12 Ham culture medium at the permissive growing temperature. Then cells were extensively washed to remove the radiolabeled precursors and incubated at different conditions. After 90 min, lipid extracts were obtained as described above. Individual lipid classes were separated by TLC, identified with authentic standards, and counted for radioactivity. [3H]PC in [3H]choline-labeled lipid extracts and [3H]PI in myo-[3H]inositol-labeled lipid extracts were separated in one-dimensional TLC with the mobile phase, chloroform/methanol/acetone/acetic acid/water (10:2:4:2:1 by volume). Major phospholipid classes (i.e. phosphatidylethanolamine (PE), PC, sphingomyelin (SM), PI, phosphatidylserine (PS), and PA in 32P-labeled lipid extracts were separated in two-dimensional TLC with the following mobile phases: first, chloroform/methanol/NH2OH (65:35:10 by volume); second, chloroform/methanol/acetone/acetic acid/water (10:2:4:2:1 by volume). [14C]triacylglycerol in [14C]acetate-labeled lipid extracts was separated by one-dimensional TLC with the mobile phase, hexane/diethyl ether/acetic acid (70:30:1 by volume). For the 3H- and 14C-labeled lipids, the area corresponding to authentic standards on the TLC was scraped and counted for radioactivity. The 32P-labeled phospholipids were counted for radioactivity with a PhosphorImager as described above.
Analysis of the Density of KDELr-containing Transport Carriers
Cells grown on coverslips were incubated under different conditions as indicated, fixed at room temperature with 4% paraformaldehyde in PBS, rinsed, and permeabilized with 0.1% saponin containing 1% BSA (in PBS) to block the nonspecific binding sites. Cells were then incubated with anti-KDELr antibody diluted at 1:1000 in 1% BSA for 1 h followed by Alexa 488-conjugated secondary antibody. Immunostained coverslips were mounted on microscope slides using Mowiol. Microscopy and imaging were performed with a Leica TCS-NT confocal microscope (Heerbrugg, Switzerland). Five sections per image were processed using ImageJ software. To quantify cytoplasmic fluorescent punctate structures containing KDELr, 8-bit gray scale nonsaturated images were set at an arbitrary threshold value of 70. For each cell, the number of stained structures between 2 and 200 pixels over the threshold was scored covering the total cytoplasmic area, except for the Golgi area, which was identified by GM130 staining. Then the number of stained structures was expressed as a ratio of the cell area measured in pixels.
YFP-ts045VSV-G Transport Assay
CHO-K1 cells were detached and transfected with YFP-ts045VSV-G using Lipofectamine 2000TM transfection reagents. For each well of a 6-well plate, 3 μl of Lipofectamine and 2 μg of DNA were mixed with 100 μl of Opti-MEM for 30 min and added to 3 ml of cell suspension in F-12 Ham's medium without antibiotics. Cells were seeded on coverslips and incubated in this medium for 6 h. Then the medium was replaced by a complete F-12 Ham's culture medium, and cells were left at 40 °C overnight. After this period, YFP-ts045VSV-G remained accumulated at the ER. For the transport assay, cells were placed at 32 °C after washing the growth medium in HEPES buffer and adding 200 μm choline and 5 mm inositol or adding 5 mm LiCl in the absence of choline and inositol. Cells were fixed with 4% paraformaldehyde at the indicated times, stained for GM130 as a Golgi marker, and mounted onto microscope slides. The percentage of cells with YFP-ts045VSV-G at the Golgi or at the plasma membrane was analyzed under the fluorescence microscope. Fifty cells were analyzed in duplicate coverslips for each experiment. To calculate the ratio between the YFP-ts045VSV-G at the plasma membrane and the total YFP-ts045VSV-G per cell, coverslips holding cells incubated with or without choline and inositol at 32 °C for 60 min were fixed and incubated with anti-VSV-G ectodomain without permeabilization to label only the YFP-ts045VSV-G present at the plasma membrane. Microscopy and imaging were performed with an Olympus BX60 epifluorescence microscope equipped with a cooled Olympus CCD camera (Lake Success, NY).
Secretion Assay of a Secretory Form of Horseradish Peroxidase (ssHRP)
CHO-K1 cells were transfected with ssHRP using Lipofectamine 2000TM transfection reagents, according to the manufacturer's instructions, for 18–24 h. The secretion assay started after washing the growth medium in HEPES buffer and adding 200 μm choline and 5 mm inositol or adding 5 mm LiCl in the absence of choline and inositol. Aliquots of incubation buffer were collected at the indicated times and centrifuged for 5 min at 1000 × g, and the enzymatic activity of HRP present in the extracellular medium was measured with ECL detection reagents that produce a chemifluorescent signal at 440 nm, which was quantified with a Luminometer (Synergy 2 from Biotek).
Electron Microscopy
For transmission electron microscopy, CHO-K1 and CHO-MT58 cells were rapidly fixed with 1.25% glutaraldehyde in PIPES buffer (0.1 m, pH 7.4) containing sucrose (2%) and Mg2SO4 (2 mm) for 60 min at 37 °C. Cells were then gently scraped, pelleted at 100 × g for 10 min, rinsed three times in PIPES buffer, and postfixed with 1% (w/v) OsO4, 1% (w/v) K3Fe(CN)6 in PIPES buffer for 1 h at room temperature in the dark. Cells were then treated for 5 min with tannic acid (0.1%) in PIPES buffer, rinsed in distilled water, block-stained with 1% uranyl acetate in 70% ethanol for 1 h, dehydrated with graded ethanol solutions, and finally embedded in Epon plastic resin (EMS, Hatfield, PA). Ultrathin sections (50–70 nm thick) were stained with lead citrate and observed on a JEOL 1010 electron microscope (Peabody, MA). Micrographs of randomly selected areas were obtained with a Gatan Bioscan digital camera (Pleasanton, CA) at the same final magnification (×50,000) and analyzed using point-counting procedures. The minimum sample size of each stereological parameter was determined by the progressive mean technique with a confidence limit of 5% (34).
Statistical Analysis
GraphPad Prism 4 software was used for statistical analysis. To compare the means of two groups of data, we performed a two-tailed unpaired t test. For multiple comparisons, we used one-way analysis of variance followed by the Bonferroni test. Statistical significance was established at p ≤ 0.05 in both cases. Non-linear regression shown in Fig. 4C was also performed with GraphPad Prism 4 software. The data were fitted to a one-binding site rectangular hyperbola (y = a·x/(b + x)).
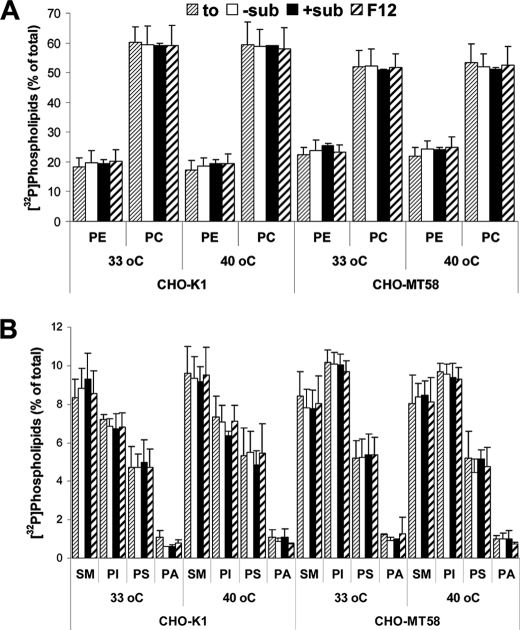
FIGURE 4.
The regulation of phospholipid synthesis for a short time does not affect the levels of 32P-labeled major phospholipid classes. CHO-K1 and CHO-MT58 cells were labeled with [32P]orthophosphoric acid for 24 h. Cells were then washed and incubated for 1 h in HEPES buffer with 200 μm choline and 5 mm inositol (+sub), without these substrates in the presence of 5 mm LiCl (−sub), or with F-12 (Ham's) growth medium (F12) at the temperature indicated (33 or 40 °C). Samples at the starting point of the assay (to) were also monitored. Lipids were extracted, separated by TLC, and counted for radioactivity. Results are mean ± S.D. (error bars) of two experiments performed in duplicate. A, PE and PC; B, SM, PI, PS, and PA.
RESULTS
DAG Levels Can Be Controlled by Regulating PC and PI Synthesis
We set up two experimental approaches to control DAG by tuning for a short period of time the synthesis of PC and PI (see diagram in Fig. 1A). First, we used wild-type CHO-K1 and the mutant line CHO-MT58, with a temperature-sensitive CTP:phosphocholine cytidylyltransferase, the enzyme that catalyzes the production of CDP-choline in the rate-limiting step of PC synthesis. When CHO-MT58 cells are transferred from a permissive temperature (33 °C) to a non-permissive temperature (40 °C), the newly synthesized CTP:phosphocholine cytidylyltransferase is not properly folded and remains inactive (28). Then the DAG consumed in the last step of PC can accumulate due to the lack of CDP-choline. As expected, the amount of DAG increased in lipid extracts from CHO-MT58 cells shifted from 33 to 40 °C (Fig. 1B). The increase was already observed at 30 min and reached a plateau from 60 min to 3 h. In contrast, the levels of DAG in CHO-K1 cells remained constant for 3 h after the shift of temperature. Our second experimental strategy consisted in controlling the presence or absence of the substrates required for the DAG- or CDP-DAG-consuming reactions in PC and PI synthesis: choline and inositol, respectively. Because the growth medium of CHO cells contains a 100 μm concentration of each substrate, the medium was replaced by a saline-buffered solution. When choline and inositol were not present, lithium was added to inhibit inositol monophosphatases and thus to reduce the amount of endogenous inositol available (35, 36). The total amount of DAG was higher in lipid extracts from NRK and CHO-K1 cells incubated for 1 h in the absence of the substrates than in their presence (Fig. 1C), which indicates that the presence of exogenous choline and inositol for a short time induced the consumption of the endogenous DAG through PI and PC synthesis and reduced its total amount.
FIGURE 1.
The cellular content of DAG can be regulated by PC and PI synthetic pathways. A, enzyme-catalyzed reactions for the synthesis of PC and PI from DAG and the exogenous substrates choline and inositol. The metabolites and the enzymes involved for the experimental strategies used in this work are indicated in rectangular or eliptical boxes, respectively. B, CHO-K1 and CHO-MT58 cells were grown at 33 °C and then shifted to 40 °C for the indicated times. Results are from one experiment representative of three experiments. C, CHO-K1 and NRK cells were incubated for 1 h in HEPES buffer with 200 μm choline and 5 mm inositol (filled bars) or without these substrates in the presence of 5 mm LiCl (open bars). Results are mean ± S.E. (error bars) of three experiments performed in duplicate. PIS, phosphatidylinositol synthase.
DAG Is Regulated by Phospholipid Synthesis in Golgi-enriched Membrane Fractions
We next examined whether the DAG variation we had measured in cell lipid extracts could take place, at least to some extent, at the Golgi. This organelle is particularly enriched in DAG in CHO cells: 70 ± 17 nmol of DAG/mg of protein in Golgi-enriched fractions versus 1.3 ± 0.4 nmol of DAG/mg of protein in whole cell homogenates. We wanted to know if this pool of DAG at the Golgi was sensitive to the phospholipid synthesis manipulation. For this purpose, we used Golgi-enriched fractions from Vero cells and from rat liver. In both cases, the DAG content increased 2-fold in the presence of 10 mm CMP (Fig. 2, A and B). The presence of CMP in this assay reverts the last step of PC synthesis catalyzed by CDP-choline:diacylglycerol phosphotransferase (Fig. 1A), which consumes the PC present in the membranes, giving rise to DAG. The reverse reaction of phosphatidylinositol synthase could also contribute to DAG production in this assay, although in this case, the main metabolite produced is CDP-DAG (see Fig. 1A). These results indicate that DAG-consuming reactions of phospholipid synthesis occur in Golgi membranes, in agreement with previously published studies (25, 37, 38).
FIGURE 2.
The content of DAG in Golgi membranes can be regulated by phospholipid synthetic pathways. DAG production induced by CMP in isolated Golgi membranes from Vero cells (A) and rat liver (B). Golgi membranes (25 μg of protein) were incubated in Tris-HCl buffer for 20 min in the absence or presence of 10 mm CMP. Lipids were extracted, and DAG was determined. Results are mean ± S.D. (error bars) of two experiments performed in duplicate.
The Blockage of Phospholipid Synthesis for a Short Time Does Not Affect the Levels of Major Phospholipids and Triacylglycerol
To ensure that the effects produced by our experimental conditions were mainly attributable to the changes of the metabolite intermediate DAG, we measured the levels of major phospholipid classes (PE, PC, SM, PI, PS, and PA) and the neutral lipid TAG for the conditions and the timing used in this work. To do so, cellular lipids were radiolabeled with [3H]choline, [3H]myo inositol, [32P]orthophosphate, or [14C]acetate for 24 h to achieve isotopic equilibrium, and then cells were incubated at different conditions for 90 min. Neither major phospholipid class (neither PA (Figs. 3 (A and B) and 4 (A and B)) nor TAG (Fig. 3C)) was altered by the conditions that were demonstrated to change DAG levels in whole cell lipid extracts. We observed that in CHO-MT58 cells, PC, TAG, and SM levels were lower, whereas PE and PI levels were higher in comparison with those of CHO-K1 cells. Of note, these differences remained constant in all conditions tested.
FIGURE 3.
The regulation of phospholipid synthesis for a short time does not affect the levels of [3H]PC, [3H]PI, or [14C]TAG. CHO-K1 and CHO-MT58 cells were labeled with [3H]choline (A), [3H]inositol (B), or [14C]acetate (C) for 24 h. Cells were then washed and incubated for 1 h in HEPES buffer with 200 μm choline and 5 mm inositol (+s), without these substrates in the presence of 5 mm LiCl (−s), or with F-12 (Ham's) growth medium (F12) at the temperature indicated (33 or 40 °C). Samples at the starting point of the assay (to) were also monitored. Lipids were extracted, separated by TLC, and counted for radioactivity. Results are mean ± S.D. (error bars) of two experiments performed in triplicate (A and B) or duplicate (C).
The Inhibition of PC Synthesis in CHO-MT58 Cells at 40 °C Prevents the Propanolol-induced Increase in Budding Vesicular Profiles at the Golgi
We previously demonstrated that propanolol decreases the levels of DAG at the Golgi and enhances the budding vesicular profiles generated by this organelle (8). This result indicated that DAG was required for the fission of COPI-coated transport carriers from Golgi cisternae. Here we examined whether the blockage of PC synthesis could counteract these ultrastructural effects of propanolol, which would reflect a restoration of control DAG levels at the Golgi. As shown in Fig. 5A, CHO-MT58 cells incubated at 33 or 40 °C showed the characteristic structural organization of the Golgi, consisting of a pile of flattened cisternae surrounded by COPI- or clathrin-coated vesicles. After propanolol treatment for 15 min, CHO-MT58 cells incubated at 33 °C showed numerous budding vesicular profiles that remained attached to the cisternae (Fig. 5A, inset), in accordance with our previous results in other cell types (8). Conversely, in propanolol-treated CHO-MT58 cells incubated at 40 °C, the Golgi architecture was indistinguishable from that of non-treated cells, indicating that the effect of propanolol was counteracted by the blockage of PC synthesis. These observations were confirmed by quantitative analysis (Fig. 5B).
FIGURE 5.
Inhibition of PC synthesis prevents the increase of Golgi-associated buds induced by propanolol. A, representative ultrastructural images for CHO-MT58 cells incubated at 33 or 40 °C for 1 h with or without propanolol (60 μm) during the last 15 min. Bar, 200 nm. B, quantitative analysis of Golgi-associated budding vesicular profiles per μm2.
The Density of KDELr-containing Transport Carriers at the ER-Golgi Interface Is Dependent on DAG Regulated by PC and PI Synthesis
To investigate whether the DAG consumed in phospholipid synthesis participates in membrane trafficking at the ER-Golgi interface, we examined the subcellular distribution of the KDELr. Luminal ER-resident proteins containing the KDEL sequence at the C terminus bind to the KDELr, either at the Golgi or at the ER-Golgi intermediate compartment. The complex formed is returned to the ER by COPI-coated vesicles. Once in the ER, the KDELr and the luminal KDEL-containing protein split, and the KDELr then returns to the Golgi (39). Thus, the subcellular staining pattern of the KDELr is representative of the membrane cycling state at the ER/Golgi interface.
The amount of fluorescent punctate structures containing the KDELr per cell area (hereafter, density of KDELr particles) was measured in NRK and CHO-K1 cells. The comparison between these two cell types was of interest because they show different basal steady-state subcellular distribution of the KDELr under control conditions. In NRK cells, KDELr is localized at the ER, Golgi, and cytoplasmic punctate structures, whereas in CHO-K1 cells, KDELr is mostly visualized at the Golgi and cytoplasmic punctate structures (Fig. 6A). In both cell types, the presence of the substrates for PC and PI synthesis, choline and inositol, which facilitates the consumption of DAG, reduced the density of KDELr particles in comparison with cells incubated in the absence of both substrates, when DAG is increased. Representative images of these experiments are shown in Fig. 6A, and the quantitative analysis is shown in Fig. 6B. In addition, the density of KDELr particles was higher in CHO-MT58 cells incubated at 40 °C for 1 h, when PC synthesis was compromised and DAG levels increased, as compared with CHO-MT58 cells maintained at 33 °C. However, the density of KDELr particles in CHO-K1 cells at 33 or 40 °C remained unaltered after 1 h (Fig. 6C). We postulated that DAG could interact with an effector protein to exert its effect on the KDELr trafficking, as reported for the role of protein kinase D in the effect of DAG at the trans-Golgi network (7). If this were also the case at the ER-Golgi interface, then KDELr particles would follow a rectangular hyperbolic kinetic curve when plotted against the amount of DAG. For this purpose, we extended the quantitative analysis of DAG and the density of KDELr particles in a series of eight different experimental conditions. CHO-K1 and CHO-MT58 cells were incubated for 1 h in HEPES saline buffer with or without the substrates for the synthesis of PC and PI at 33 or 40 °C. Golgi morphology was not affected by any of the conditions tested. The profile for the amount of DAG in the eight conditions tested (Fig. 7A) was qualitatively similar to the profile for the density of KDELr particles (Fig. 7B) under the same conditions. We then plotted the density of KDELr particles against the amount of DAG for each condition tested (Fig. 7C) to characterize the correlation between the two parameters. The data points were fitted to a rectangular hyperbola for one binding site, which indicates that the density of KDELr-containing transport carriers (COPI-coated) depends on the amount of DAG consumed in phospholipids synthesis, in a manner that is in accordance with an interaction of DAG with an effector molecule.
FIGURE 6.
The density of KDELr particles is reduced by the inhibition of PC and PI synthesis. A, representative images for KDELr staining in NRK and CHO-K1 cells in the presence or absence of choline and inositol. Bar, 10 μm. B, NRK and CHO-K1 cells were incubated in HEPES buffer for 1 h with or without 200 μm choline and 5 mm inositol in the presence of 5 mm LiCl. C, CHO-K1 and CHO-MT58 cells were incubated for 1 h in growth medium at permissive (33 °C) or at non-permissive temperature (40 °C) for the PC-synthesis in CHO-MT58. Results are mean ± S.E. (error bars) of three experiments performed in duplicate.
FIGURE 7.
The density of KDELr particles correlates with the DAG content. Shown are DAG levels (A) and density of KDELr particles (B) from a representative experiment. C, density of KDELr particles plotted against DAG for each different experimental condition tested in CHO-K1 and -MT58 cell lines incubated in HEPES buffer for 1 h. Data from individual experiments were normalized (the value for CHO-K1 cells at 33 °C in the absence of substrates was taken as 100%) and represented are means ± S.E. (error bars) of three experiments performed in duplicate. Data points were fitted by non-linear regression to a one-binding site hyperbola (r = 0.896).
The Transport of YFP-ts045VSV-G from the Golgi to the Plasma Membrane and the Secretion of ssHRP Are Both Dependent on the DAG Regulated by PC and PI Synthesis
To further investigate the role of DAG used in PC and PI synthesis along the secretory pathway, we first measured how the alterations in DAG produced by changes in choline and inositol availability affected the secretory transport from the ER to the plasma membrane of a transiently transfected membrane protein construct YFP-ts40VSV-G. In this assay, transfected cells were cultured overnight at 40 °C. At this non-permissive temperature, the newly synthesized YFP-ts045VSV-G is not properly folded and accumulates at the ER. When cells are transferred to 32 °C, VSV-G-protein correctly refolds and exits the ER to reach first the Golgi and then the plasma membrane. It is important to highlight that this experimental procedure is not compatible with the use of mutant CHO-MT58 cells because culturing them overnight at 40 °C would block the synthesis of PC for too long, and maintaining the 40 °C in the assay to block PC synthesis would prevent YFP-ts045VSV-G from exiting the ER. Therefore, the transport assay was only tested in CHO-K1 cells. Representative images of this experiment are shown in Fig. 8A. YFP-ts045VSV-G reached the Golgi at the same rate in the presence and in the absence of PC and PI synthesis substrates (Fig. 8B). Afterward, at 30 and 60 min, the percentage of cells with YFP-ts045VSV-G at the Golgi remained higher in the presence of choline and inositol than in the absence of the substrates, indicating an alteration in post-Golgi transport. Accordingly, the percentage of cells with YFP-ts045VSV-G at the plasma membrane was less in the presence of substrates than in their absence (not shown). We then determined the amount of VSV-G at the plasma membrane with an antibody against the VSV-G ectodomain, after 60 min of incubation at 32 °C, as a ratio of the total amount of YFP-ts045VSV-G inside the cell. In this case, the ratio was lower in the presence of choline and inositol than in their absence, which confirms a reduced arrival of VSV-G at the plasma membrane when DAG levels are low (Fig. 8C). On the other hand, the release of a transiently transfected ssHRP into the incubation medium was used as a model for the secretion of a luminal protein for the same experimental conditions. The amount of ssHRP secreted (measured as HRP activity) was lower in the presence of choline and inositol than in their absence, when DAG in CHO-K1 cells was low and high, respectively (Fig. 9A). The time course of ssHRP secretion was linear for up to 2 h, and the difference between the two conditions tested was significant. This result was extended by the analysis of internal ssHRP at the initial point of the assay and at 60 min, measured by peroxidase activity (Fig. 9B) and the amount of protein by Western blot (Fig. 9C). Both measurements showed that at the starting point of the assay, the amount of internal ssHRP was the same for the two conditions tested. Moreover the remaining ssHRP in the cells was higher in the presence of choline and inositol than in the absence of the substrates after 60 min.
FIGURE 8.
Post-Golgi transport of YFP-ts045VSV-G is altered by the regulation of PC and PI synthesis. CHO-K1 cells transiently transfected with YFP-ts045VSV-G were incubated overnight at 40 °C to accumulate the unfolded YFP-ts045VSV-G at the ER. Cells were then shifted to 32 °C and incubated in HEPES buffer with or without 200 μm choline and 5 mm inositol in the presence of 5 mm LiCl for the indicated times. A, representative images for the two conditions. Bar, 10 μm. B, percentage of cells with YFP-ts045VSV-G at the Golgi for the times indicated. Results are mean ± S.E. of three experiments performed in duplicate. C, cells incubated for 60 min as in B were then labeled with anti-VSV-G ectodomain antibody without permeabilization. Results represent the ratio between the staining of VSV-G at the plasma membrane and the total cellular YFP-ts045VSV-G. Results are mean ± S.E. of five experiments performed in triplicate.
FIGURE 9.
ssHRP secretion is altered by the regulation of PC and PI synthesis. CHO-K1 cells with transiently transfected ssHRP were incubated for the times indicated in HEPES buffer with (black bars) or without (white bars) 200 μm choline and 5 mm inositol in the presence of 5 mm LiCl. ssHRP in the incubation buffer (A) and in cell lysates was determined by a luminescent enzymatic assay (B) or Western blot (C), as indicated. Results are mean ± S.E. (error bars) of eight experiments performed in duplicate in A and mean ± S.D. of two experiments performed in duplicate in B and C. A.U., arbitrary units.
DISCUSSION
In the present work, we postulate that the DAG-consuming steps of phospholipid synthetic pathways present at the Golgi contribute to the regulation of DAG needed for membrane traffic at this organelle.
The experimental conditions used in this work, consisting of regulating phospholipid synthesis, were chosen with the aim to produce a fast modification of DAG, including that at the Golgi, without inducing changes in other lipids. We succeeded in modifying DAG without major changes in other lipids, but we were unable to resolve any change of DAG at the Golgi in our experimental context. Any attempt to isolate Golgi membranes after our treatments will not ensure the preservation of induced DAG alterations. Moreover, the relatively small changes in cellular DAG measured most likely remain below the sensitivity of any fluorescent construct with a DAG binding domain. In this context, although we observed that the expressed construct EGFP-C1b-PKCθ localized mainly at the Golgi, we were not able to detect any significant change in fluorescence intensity at this organelle. Only the ratio between the fluorescence intensity at the Golgi and the whole cell showed a tendency, although not significant, to decrease in the presence of phospholipid substrates as compared with its absence (not shown). Thus, what we demonstrate is that our experimental conditions regulate the total levels of the lipid metabolic intermediate DAG, which have functional and structural effects in the Golgi complex.
The stronger evidence we have to demonstrate that DAG at the Golgi is altered by our experimental conditions is that the inhibition of PC synthesis in CHO-MT58 cells prevents the increase in budding profiles produced by propanolol (8). This result indicates that DAG at the Golgi is indeed increased when we block PC synthesis and compensates for the reduction of DAG produced by propanolol.
The experimental conditions used in this work alter the rate of PC and PI synthesis, and this could eventually produce changes in the levels of major phospholipid classes and neutral lipids. In this context, CHO-MT58 cells have different amounts of phospholipids and TAG than CHO-K1 cells (see Figs. 3 and 4), which is in accordance with published results (40). We therefore wanted to make sure that the time we had chosen for our conditions did not significantly alter the levels of phospholipids and TAG, previously radiolabeled to isotopic equilibrium with a variety of metabolic precursors. For the major phospholipid classes or TAG, which were not modified by our experimental conditions, it is easy to understand that its high cellular amount should take longer than 1 or 2 h to show a significant change. Nonetheless, the results obtained for phosphatidic acid were unexpected. Because phosphatidic acid is a lipid intermediate metabolite present at much lower levels than other phospholipids and a direct product of diacylglycerol kinase action on DAG, one would expect an increase of phosphatidic acid concomitant to the DAG accumulation. Although we have no clear explanation for this result, we suggest two possibilities: the phosphatidic acid is rapidly consumed to other metabolic pathways, or the particular DAG accumulated is not a good substrate for diacylglycerol kinases.
It is widely accepted that the major site for PC and PI synthesis is the ER. Thus, under our conditions, DAG would be expected to accumulate at this compartment. Of note, it is becoming accepted that DAG-consuming steps of phospholipid synthesis are also relevant at the Golgi (41). In accordance with this, we observed a robust increase of DAG in the presence of CMP in Golgi-enriched fractions, which was unlikely to be due to ER contamination.
Our first functional observation was the alteration of subcellular distribution of the KDELr at the ER-Golgi interface, where it is continuously cycling. The density of KDELr particles concomitantly increased with the DAG content following a hyperbola-shaped correlation. Initially, this result suggested that both the anterograde transport (ER-to-Golgi) and the retrograde transport (Golgi-to-ER) were altered by our experimental conditions. This could be attributed to an alteration in DAG pools at both the ER and the Golgi, as a result of the regulation of PC and PI synthesis. Unexpectedly, the ER-to-Golgi transport of the YFP-ts045VSV-G was unaltered by our experimental conditions. We have two explanations for this apparent contradiction: 1) the exit of KDELr from the Golgi to ER, but not from the ER to Golgi, is sensitive to the changes in DAG induced in this work; 2) the regulation of PC and PI synthesis by our experimental approaches affects the DAG pool at the Golgi but not at the ER. In this context, we think that the chance for DAG to accumulate upon inhibition of phospholipid synthesis is higher at the Golgi than in the ER because DAG can more easily be diverted to neutral lipid synthesis at the ER (42) than at the Golgi. Altogether, the results concerning KDELr subcellular distribution, YFP-ts045VSV-G transport, and Golgi ultrastructural analysis, discussed above, support the conclusion that only the retrograde transport is primarily altered at the ER-Golgi interface by our experimental conditions. Nonetheless, we cannot rule out any effect of DAG in the ER-to-Golgi membrane pathway because it is not unambiguously established that the KDELr and the YFP-ts045VSV-G share the same transport carriers to move from the ER to the Golgi.
The post-Golgi trafficking to the plasma membrane of YFP-ts045VSV-G was reduced in experimental conditions that lowered DAG levels by regulating PC and PI synthesis pathways. The secretion of ssHRP was also significantly reduced. We do not know the precise subcellular location along the secretory pathway where the delay of ssHRP secretion took place. However, because the transport assay of YFP-ts045VSV-G indicates that DAG regulated by PC and PI synthesis does not affect the ER-to-Golgi protein transport, this could also be extrapolated to ssHRP.
The membrane cycling at the ER-Golgi interface was analyzed by the density of KDELr particles. The correlation between the DAG content and the density of KDELr particles supports the hypothesis that DAG recruits proteins involved in the fission of Golgi-derived transport carriers, as has already been described for protein kinase D binding to DAG at the trans-Golgi network (7). However, it does not rule out a role for DAG as a membrane component that facilitates the curvature needed for the generation of transport carriers (43).
In this study, we were able to regulate DAG levels by changing the availability of cell growth medium components, such as the essential substrates for PC and PI synthesis, choline and inositol. Alternatively, we regulated DAG by knocking down CTP:phosphocholine cytidylyltransferase, the regulatory enzyme for PC synthesis, for a short time. Interestingly, a temperature shift to 40 °C in CHO-MT58 for longer times than those used here leads to a PC mass reduction or TAG increase (40, 44). In the first case, the authors observed a decrease in secretion and suggested that PC was needed for protein transport at the Golgi. Of note, such a long incubation of CHO-MT58 cells at 40 °C was demonstrated to reduced the DAG (45). In a similar study, the secretion was slowed down after blocking PC synthesis in macrophages. In this case, the authors also concluded that PC synthesis was needed for secretion (25). The striking point here is that the levels of the metabolic intermediate DAG as well as the effects on protein transport depend on the duration of the blockage of PC synthesis. We therefore suggest caution when results generated by silencing experiments are interpreted in terms of levels of lipid intermediate metabolites because the long times required for silencing experiments might not be the most appropriate to investigate the involvement of metabolite intermediates in a particular cellular process.
Interestingly, it has recently been shown that an intracellular Ca2+ signal, elicited by an exogenous agonist through purinergic receptors or by thapsigargin, increases the DAG at the Golgi (46). Because PC synthesis can be inhibited by an intracellular Ca2+ signal (41, 47), we postulate that this synthetic pathway may be responsible for this rapid increase in DAG observed at the Golgi.
Finally, our results do not exclude a role for phospholipases (11), sphingomyelin synthase (7), or phospholipid transfer proteins (18–20) in membrane traffic at the Golgi. In the Introduction, we have already mentioned the works and the rationales that support their contribution to this cellular process. All of these enzymes, together with the synthesis of phospholipids, contribute to the metabolic network that maintains DAG homeostasis. We report here that the pathways for PC and PI synthesis control both DAG levels and secretory processes. We suggest that cells take advantage of their regulatory mechanisms for the PC and PI synthesis to rapidly and finely tune the DAG needed for the transport carrier formation at the Golgi complex. This is something that has been hypothesized (26, 27) but scarcely addressed.
Acknowledgments
We thank Maite Muñoz for technical support, Enrique Claro for advice during the first DAG measurements, Sergi Marco for help during preliminary experiments, members of the laboratory for critical reading of the manuscript and for helpful discussions, and Robin Roycroft for editorial assistance.
This work was funded by Ministerio de Ciencia e Innovación Grant BFU2009-07186.
- DAG
- diacylglycerol
- TAG
- triacylglycerol
- PC
- phosphatidylcholine
- PA
- phosphatidic acid
- PE
- phosphatidylethanolamine
- PI
- phosphatidylinositol
- SM
- sphingomyelin
- PS
- phosphatidylserine
- ssHRP
- secretory form of horseradish peroxidase
- YFP-ts045VSV-G
- yellow fluorescent temperature-sensitive G protein of stomatitis virus
- ER
- endoplasmic reticulum
- PITP
- PI transfer protein
- KDELr
- KDEL receptor
- NRK
- normal rat kidney.
REFERENCES
- 1. Beck R., Rawet M., Wieland F. T., Cassel D. (2009) FEBS Lett. 583, 2701–2709 [DOI] [PubMed] [Google Scholar]
- 2. Pfeffer S. R. (2007) Annu. Rev. Biochem. 76, 629–645 [DOI] [PubMed] [Google Scholar]
- 3. Lippincott-Schwartz J., Phair R. D. (2010) Annu. Rev. Biophys. 39, 559–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huijbregts R. P., Topalof L., Bankaitis V. A. (2000) Traffic 1, 195–202 [DOI] [PubMed] [Google Scholar]
- 5. van Meer G., Voelker D. R., Feigenson G. W. (2008) Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang J. S., Gad H., Lee S. Y., Mironov A., Zhang L., Beznoussenko G. V., Valente C., Turacchio G., Bonsra A. N., Du G., Baldanzi G., Graziani A., Bourgoin S., Frohman M. A., Luini A., Hsu V. W. (2008) Nat. Cell Biol. 10, 1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baron C. L., Malhotra V. (2002) Science 295, 325–328 [DOI] [PubMed] [Google Scholar]
- 8. Fernández-Ulibarri I., Vilella M., Lázaro-Diéguez F., Sarri E., Martínez S. E., Jiménez N., Claro E., Mérida I., Burger K. N., Egea G. (2007) Mol. Biol. Cell 18, 3250–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asp L., Kartberg F., Fernandez-Rodriguez J., Smedh M., Elsner M., Laporte F., Bárcena M., Jansen K. A., Valentijn J. A., Koster A. J., Bergeron J. J., Nilsson T. (2009) Mol. Biol. Cell 20, 780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. (1989) J. Biol. Chem. 264, 17069–17077 [PubMed] [Google Scholar]
- 11. Ktistakis N. T., Brown H. A., Sternweis P. C., Roth M. G. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 4952–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vickers J. D. (1993) J. Pharmacol. Exp. Ther. 266, 1156–1163 [PubMed] [Google Scholar]
- 13. Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H., Jr. (1991) J. Biol. Chem. 266, 14486–14490 [PubMed] [Google Scholar]
- 14. Du G., Huang P., Liang B. T., Frohman M. A. (2004) Mol. Biol. Cell 15, 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freyberg Z., Bourgoin S., Shields D. (2002) Mol. Biol. Cell 13, 3930–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarri E., Pardo R., Fensome-Green A., Cockcroft S. (2003) Biochem. J. 369, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saini D. K., Karunarathne W. K., Angaswamy N., Saini D., Cho J. H., Kalyanaraman V., Gautam N. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 11417–11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Litvak V., Dahan N., Ramachandran S., Sabanay H., Lev S. (2005) Nat. Cell Biol. 7, 225–234 [DOI] [PubMed] [Google Scholar]
- 19. Simon J. P., Morimoto T., Bankaitis V. A., Gottlieb T. A., Ivanov I. E., Adesnik M., Sabatini D. D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvou N., Holic R., Li M., Futter C., Skippen A., Cockcroft S. (2010) J. Cell Sci. 123, 1262–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haynes L. P., Thomas G. M., Burgoyne R. D. (2005) J. Biol. Chem. 280, 6047–6054 [DOI] [PubMed] [Google Scholar]
- 22. Jackowski S. (1996) J. Biol. Chem. 271, 20219–20222 [DOI] [PubMed] [Google Scholar]
- 23. Sarri E., Garcia-Dorado D., Abellan A., Soler-Soler J. (2006) Biochem. J. 394, 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gasull T., Sarri E., DeGregorio-Rocasolano N., Trullas R. (2003) J. Neurosci. 23, 4100–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian Y., Pate C., Andreolotti A., Wang L., Tuomanen E., Boyd K., Claro E., Jackowski S. (2008) J. Cell Biol. 181, 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kent C., Carman G. M. (1999) Trends. Biochem. Sci. 24, 146–150 [DOI] [PubMed] [Google Scholar]
- 27. Kearns B. G., McGee T. P., Mayinger P., Gedvilaite A., Phillips S. E., Kagiwada S., Bankaitis V. A. (1997) Nature 387, 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sweitzer T. D., Kent C. (1994) Arch. Biochem. Biophys. 311, 107–116 [DOI] [PubMed] [Google Scholar]
- 29. Kennedy E. P., Weiss S. B. (1956) J. Biol. Chem. 222, 193–214 [PubMed] [Google Scholar]
- 30. Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. (1984) Cell 39, 405–416 [DOI] [PubMed] [Google Scholar]
- 31. Hui N., Nakamura N., Slusarewicz P., Warren G. (1998) in Cell Biology: A Laboratory Handbook, 2nd Ed., Vol. II (Cellis J. E. ed) pp. 46–55, Academic Press, Inc., New York [Google Scholar]
- 32. Voziyan P. A., Goldner C. M., Melnykovych G. (1993) Biochem. J. 295, 757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. (1986) J. Biol. Chem. 261, 8597–8600 [PubMed] [Google Scholar]
- 34. Weibel E. R. (1979) Practical Methods for Biological Morphometry, Vol. 1, Academic Press, New York [Google Scholar]
- 35. Ragan C. I., Watling K. J., Gee N. S., Aspley S., Jackson R. G., Reid G. G., Baker R., Billington D. C., Barnaby R. J., Leeson P. D. (1988) Biochem. J. 249, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kennedy E. D., Challiss R. A., Ragan C. I., Nahorski S. R. (1990) Biochem. J. 267, 781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jelsema C. L., Morré D. J. (1978) J. Biol. Chem. 253, 7960–7971 [PubMed] [Google Scholar]
- 38. Henneberry A. L., Wright M. M., McMaster C. R. (2002) Mol. Biol. Cell 13, 3148–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffiths G., Ericsson M., Krijnse-Locker J., Nilsson T., Goud B., Söling H. D., Tang B. L., Wong S. H., Hong W. (1994) J. Cell Biol. 127, 1557–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caviglia J. M., De Gómez Dumm I. N., Coleman R. A., Igal R. A. (2004) J. Lipid Res. 45, 1500–1509 [DOI] [PubMed] [Google Scholar]
- 41. Chen B. B., Coon T. A., Glasser J. R., Mallampalli R. K. (2011) Mol. Cell Biol. 31, 1905–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coleman R. A., Lee D. P. (2004) Prog. Lipid Res. 43, 134–176 [DOI] [PubMed] [Google Scholar]
- 43. Shemesh T., Luini A., Malhotra V., Burger K. N., Kozlov M. M. (2003) Biophys. J. 85, 3813–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Testerink N., van der Sanden M. H., Houweling M., Helms J. B., Vaandrager A. B. (2009) J. Lipid Res. 50, 2182–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Sanden M. H., Houweling M., Duijsings D., Vaandrager A. B., van Golde L. M. (2004) Biochim. Biophys. Acta 1636, 99–107 [DOI] [PubMed] [Google Scholar]
- 46. Kunkel M. T., Newton A. C. (2010) J. Biol. Chem. 285, 22748–22752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matozaki T., Sakamoto C., Nishisaki H., Suzuki T., Wada K., Matsuda K., Nakano O., Konda Y., Nagao M., Kasuga M. (1991) J. Biol. Chem. 266, 22246–22253 [PubMed] [Google Scholar]