Crystals of swine CD8α diffracted to 1.8 Å resolution and belonged to space group P3221, with unit-cell parameters a = 80.97, b = 80.97, c = 95.19 Å; they contained two molecules in the asymmetric unit. The Matthews coefficient and solvent content were calculated to be 3.23 Å3 Da−1 and 61.89%, respectively.
Keywords: CD8α, swine
Abstract
CD8αα homodimers or CD8αβ heterodimers form on the T-cell surface, where they are essential as co-receptors for MHC class I molecules in activation of the CTL response. To date, swine have been found to show the highest percentage of lymphocytes with surface expression of CD8α. Crystallographic analysis of swine CD8α (sCD8α) to 1.8 Å resolution revealed that the crystals belonged to space group P3221, with unit-cell parameters a = 80.97, b = 80.97, c = 95.19 Å. The Matthews coefficient and the solvent content were calculated to be 3.23 Å3 Da−1 and 61.89%, respectively. These results may aid further structural and functional analyses of sCD8α.
1. Introduction
CD8+ cytotoxic T lymphocytes (CTLs) recognize antigenic peptides bound to major histocompatibility complex class I (MHC I) molecules on the surface of target cells and play a pivotal role in host defence towards intracellular pathogens and tumours (van der Merwe & Davis, 2003 ▶). CD8 attaches to peptide–MHC I (pMHC I) bound to a specific T-cell receptor (TCR) and forms an immunological synapse which results in the proliferation of CTL and clearance of the target cells (O’Rourke & Mescher, 1993 ▶). CD8 is expressed on the T-cell surface as dimers in two forms: CD8αα homodimers and CD8αβ heterodimers. Both dimers are composed of an extracellular immunoglobulin-like (Ig-like) domain, a stalk region, a transmembrane domain and an intracellular tail (Chang et al., 2005 ▶; Gao & Jakobsen, 2000 ▶). Crystal structures of human and mouse CD8αα and CD8αβ show the extracellular Ig-like domain is a typical Ig variable domain and is involved in the binding to MHC I (Leahy et al., 1992 ▶; Chang et al., 2005 ▶; Gao et al., 1997 ▶). Although both CD8αα and CD8αβ can serve as co-receptors for enhancing antigen presentation, they are different in several aspects. CD8αβ is expressed mainly by αβ T cells in the thymus and peripheral blood, while CD8αα is distributed in γδ T cells, intestinal intraepithelial lymphocytes, dendritic cells (DCs) and natural killer (NK) cell subpopulations (Norment & Littman, 1988 ▶; Chang et al., 2005 ▶). These findings suggest that CD8αβ is more sensitive than CD8αα in the activation and differentiation of T cells (Wheeler et al., 1992 ▶). In addition, CD8αα also binds to the nonclassical MHC I molecule (TL) with greater affinity than to classical MHC I molecules (Liu et al., 2003 ▶).
The swine cellular immune system has many unique species characteristics, which are particularly reflected in CD8+ T cells (Gerner et al., 2009 ▶). Swine CD8+ T cells include αβ T cells, γδ T cells, NK cells and a unique subset of extrathymic CD4+CD8+ double-positive cells (Yang & Parkhouse, 1997 ▶). The frequency of double-positive cells in peripheral blood is variable, but can reach as high as 80% (Saalmüller et al., 1989 ▶; Pescovitz et al., 1985 ▶). In comparison to other species, the expression of the CD8α chain on swine lymphocytes is much higher and is mainly in the CD8αα homodimer form (Gerner et al., 2009 ▶; Saalmüller et al., 1987 ▶). Furthermore, these CD8αα homodimers may be co-expressed together with CD8αβ heterodimers on αβ T lymphocytes (Gerner et al., 2009 ▶). The abundant expression of swine CD8α (sCD8α) chain on lymphocytes indicates a possible important role for sCD8α in the immune response which has yet to be fully described. Here, we report the purification and crystallization of the swine CD8αα homodimer. These results may aid further structural and functional analyses of the role of sCD8α in the swine cellular immune system.
2. Materials and methods
2.1. Preparation of the sCD8α protein
The gene fragment encoding sCD8α mature peptide residues 1–125 (extracellular Ig-like domain) of Sus scrofa was chemically synthesized by the Shanghai Generay Biotechnology Company according to the sequence in GenBank (NM_001001907). The gene product was ligated into the pET21a vector (Novagen) using a pair of primers (forward primer, CCAACATATGagcttgttccggacgtcgccgg; reverse primer, CCGCTCGAGTTAgccgcagaaccacccgaaac). The plasmid was transformed into Escherichia coli strain BL21 (DE3). 0.5 mM IPTG was used to induce the expression of sCD8α inclusion bodies (Cole et al., 2005 ▶). The bacteria were harvested by centrifugation at 6000g for 10 min and then resuspended in cold phosphate-buffered saline (PBS). After sonication, the sample was centrifuged at 16 000g and the pellet was washed three times with a solution consisting of 20 mM Tris–HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 1 mM DTT and 0.5% Triton X-100. Finally, the inclusion bodies were dissolved in guanidinium chloride (Gua–HCl) buffer [6 M Gua–HCl, 50 mM Tris–HCl pH 8.0, 10 mM EDTA, 100 mM NaCl, 10%(v/v) glycerine, 10 mM DTT] to a concentration of 30 mg ml−1.
2.2. Protein refolding and purification
Soluble sCD8α was prepared essentially as described previously by Gao et al. (1998 ▶) with some modifications. The dissolved sCD8α inclusion bodies were gradually added to refolding buffer (100 mM Tris–HCl, 2 mM EDTA, 400 mM l-arginine–HCl, 0.5 mM oxidized glutathione, 5 mM reduced glutathione pH 7.4) to 60 mg l−1. After incubation for 24 h at 277 K, the soluble portion was concentrated and purified by chromatography on a Superdex 75 10/300 column (GE Healthcare).
2.3. Crystallization of sCD8α
Purified sCD8α was concentrated to 6 mg ml−1 in a buffer consisting of 20 mM Tris pH 7.4 and 50 mM NaCl for crystallization. After mixing it with reservoir buffer in a 1:1 ratio, the sCD8α was crystallized by the hanging-drop vapour-diffusion technique at 291 K. The Index kit (Hampton Research, Riverside, California, USA) was used for crystal screening. After 7 d, crystals of sCD8α were obtained using solution No. 32 [1.0 M ammonium sulfate, 0.1 M Bis-Tris pH 5.5, 1%(w/v) polyethylene glycol 3350].
2.4. Data collection and processing
Diffraction data were collected to 1.8 Å resolution on beamline NE3A in the KEK synchrotron facility (Tsukuba, Japan) at a wavelength of 1.0 Å using an ADSC Q270 imaging-plate detector. In each case, the crystal was first soaked in reservoir solution containing 15% glycerol as a cryoprotectant for several seconds and then flash-cooled in a stream of gaseous nitrogen at 100 K (Parkin & Hope, 1998 ▶). The collected intensities were indexed, integrated, corrected for absorption, scaled and merged using HKL-2000 (Otwinowski & Minor, 1997 ▶).
2.5. Alignment of sCD8α with human, monkey and mouse CD8α molecules
The amino-acid sequence of sCD8α was aligned with CD8α sequences from human, monkey and mouse CD8α crystal structures. Sequence alignment was performed using the DNAMAN program v.5.2.2 (Lynnon Biosoft). The GenBank accession Nos. or PDB entries of the used sequences are as follows: sCD8α, ABK30934; human CD8α, 1akj (Gao et al., 1997) ▶; monkey CD8α, 2q3a (L. Zong, Y. Chen, H. Peng, D. K. Cole, F. Gao, Y. Liu & G. F. Gao, unpublished work); mouse CD8α, 3dmm (Wang et al., 2009 ▶).
3. Results and discussion
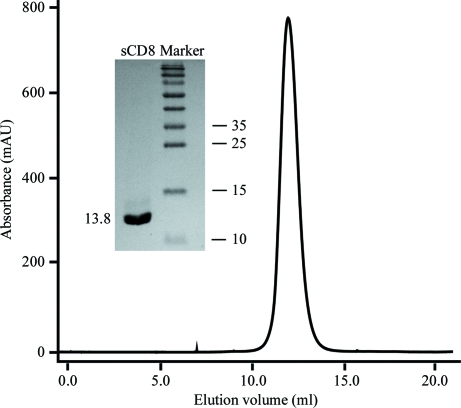
The sCD8α protein was expressed as inclusion bodies in E. coli as described previously for human and monkey CD8α (Zong et al., 2009 ▶; Gao et al., 1998 ▶). Soluble sCD8α was obtained by in vitro refolding with approximately 5% efficiency and was purified to homogeneity by Superdex 75 10/300 size-exclusion chromatography (Fig. 1 ▶). The chromatographic elution profile shows a smooth, homogeneous and symmetrical peak at 12 ml (∼28 kDa). Considering the molecular weight of sCD8α (13.8 kDa), the result shows that the soluble sCD8α exists in a homodimeric form. SDS–PAGE analysis shows a pure band corresponding to the expected molecular weight of sCD8α (see inset in Fig. 1 ▶).
Figure 1.
Purification of refolded sCD8α by FPLC Superdex 75 10/300 column chromatography (GE Healthcare). The peak represents the correctly refolded sCD8αα homodimer (∼27 kDa). Inset: reduced SDS–PAGE gel (15%) for the peak. The right column contains molecular-weight markers (labelled in kDa).
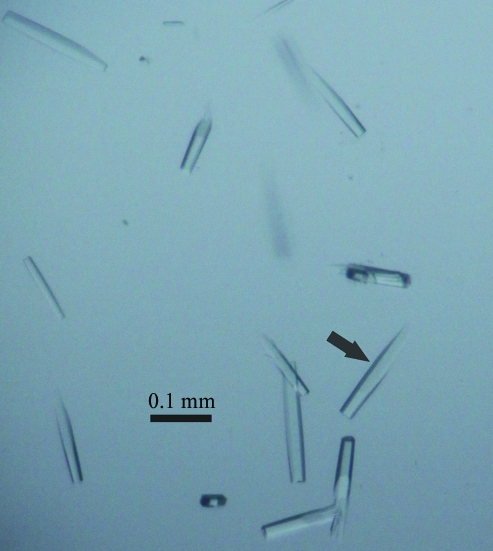
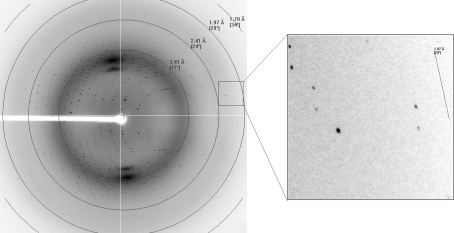
The purified sCD8α was concentrated to 6 mg ml−1 for crystal screening. Crystals (Fig. 2 ▶) appeared after 7 d under the initial conditions. The crystal used for data collection belonged to space group P3221, with unit-cell parameters a = 80.97, b = 80.97, c = 95.19 Å, and diffracted to 1.8 Å resolution (Fig. 3 ▶). The crystal was estimated to contain two sCD8α molecules in the asymmetric unit and the Matthews coefficient V M was 3.23 Å3 Da−1, with a calculated solvent content of 61.9%. Selected data statistics are shown in Table 1 ▶.
Figure 2.
Photograph of the crystals used for diffraction analysis.
Figure 3.
Diffraction pattern of sCD8α; spots corresponding to diffraction to high resolution are highlighted in the box.
Table 1. X-ray diffraction data and processing statistics.
Values in parentheses are for the highest resolution shell.
| Space group | P3221 |
| Unit-cell parameters (Å) | a = 80.97, b = 80.97, c = 95.19 |
| Resolution range (Å) | 50−1.80 (1.86–1.80) |
| Total reflections | 516532 |
| Unique reflections | 33859 |
| Completeness (%) | 99.9 (99.9) |
| Rmerge† (%) | 10.7 (58.9) |
| Average I/σ(I) | 30.24 (5.48) |
| Average multiplicity | 15.2 (14.4) |
R
merge = 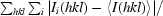
 , where Ii(hkl)is the observed intensity and 〈I(hkl)〉 is the average intensity from multiple measurements.
, where Ii(hkl)is the observed intensity and 〈I(hkl)〉 is the average intensity from multiple measurements.
A multiple amino-acid sequence alignment is shown in Fig. 4 ▶. The similarity of the residues and the identity of two cysteine residues indicate that sCD8α should contain an extracellular Ig-like domain similar to those of CD8α molecules from other species. However, sCD8α only has 57.50, 55.83 and 34.40% amino-acid sequence identity to human, monkey and mouse CD8α molecules, respectively. The low homology indicates that sCD8α could have unique structural characteristics. These results provide the basis for the further study of the functions of sCD8α in the swine cellular immune system.
Figure 4.
Amino-acid sequence alignment of sCD8α with CD8α molecules from human, monkey and mouse for which structures have been determined. The sequence identities between sCD8α and the CD8α molecules from other species are listed on the right. Dashes represent identical residues to sCD8α. Two conserved cysteines form an intramolecular disulfide bond and are highlighted by shading. GenBank accession Nos. and PDB codes are as follows: sCD8α, ABK30934; human CD8α, 1akj; monkey CD8α, 2q3a; mouse CD8α, 3dmm.
Structure determination and refinement is currently under way by molecular replacement with human CD8αα (PDB entry 1akj; Gao et al., 1997 ▶) as the search model.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (grant No. 2009ZX08009-150B) and the National Key Basic Research Program of China (973 Program, grant No. 2007CB815805). We thank Professor George F. Gao (Institute of Microbiology, Chinese Academy of Sciences) for helpful suggestions. We thank Joel Haywood for his comments on the manuscript. The authors declare no competing financial interests.
References
- Chang, H.-C., Tan, K., Ouyang, J., Parisini, E., Liu, J., Le, Y., Wang, X., Reinherz, E. L. & Wang, J. (2005). Immunity, 23, 661–671. [DOI] [PubMed]
- Cole, D. K., Rizkallah, P. J., Sami, M., Lissin, N. M., Gao, F., Bell, J. I., Boulter, J. M., Glick, M., Vuidepot, A., Jakobsen, B. K. & Gao, G. F. (2005). Acta Cryst. F61, 285–287. [DOI] [PMC free article] [PubMed]
- Gao, G. F., Gerth, U. C., Wyer, J. R., Willcox, B. E., O’Callaghan, C. A., Zhang, Z., Jones, E. Y., Bell, J. I. & Jakobsen, B. K. (1998). Protein Sci. 7, 1245–1249. [DOI] [PMC free article] [PubMed]
- Gao, G. F. & Jakobsen, B. K. (2000). Immunol. Today, 21, 630–636. [DOI] [PubMed]
- Gao, G. F., Tormo, J., Gerth, U. C., Wyer, J. R., McMichael, A. J., Stuart, D. I., Bell, J. I., Jones, E. Y. & Jakobsen, B. K. (1997). Nature (London), 387, 630–634. [DOI] [PubMed]
- Gerner, W., Käser, T. & Saalmüller, A. (2009). Dev. Comput. Immunol. 33, 310–320. [DOI] [PubMed]
- Leahy, D. J., Axel, R. & Hendrickson, W. A. (1992). Cell, 68, 1145–1162. [DOI] [PubMed]
- Liu, Y., Xiong, Y., Naidenko, O. V., Liu, J., Zhang, R., Joachimiak, A., Kronenberg, M., Cheroutre, H., Reinherz, E. L. & Wang, J. (2003). Immunity, 18, 205–215. [DOI] [PubMed]
- Merwe, P. A. van der & Davis, S. J. (2003). Annu. Rev. Immunol. 21, 659–684. [DOI] [PubMed]
- Norment, A. M. & Littman, D. R. (1988). EMBO J. 7, 3433–3439. [DOI] [PMC free article] [PubMed]
- O’Rourke, A. M. & Mescher, M. F. (1993). Immunol. Today, 14, 183–188. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Parkin, S. & Hope, H. (1998). J. Appl. Cryst. 31, 945–953.
- Pescovitz, M. D., Lunney, J. K. & Sachs, D. H. (1985). J. Immunol. 134, 37–44. [PubMed]
- Saalmüller, A., Hirt, W. & Reddehase, M. J. (1989). Eur. J. Immunol. 19, 2011–2016. [DOI] [PubMed]
- Saalmüller, A., Reddehase, M. J., Bühring, H. J., Jonjić, S. & Koszinowski, U. H. (1987). Eur. J. Immunol. 17, 1297–1301. [DOI] [PubMed]
- Wang, R., Natarajan, K. & Margulies, D. H. (2009). J. Immunol. 183, 2554–2564. [DOI] [PMC free article] [PubMed]
- Wheeler, C. J., von Hoegen, P. & Parnes, J. R. (1992). Nature (London), 357, 247–249. [DOI] [PubMed]
- Yang, H. & Parkhouse, R. M. (1997). Immunology, 92, 45–52. [DOI] [PMC free article] [PubMed]
- Zong, L., Chen, Y., Peng, H., Gao, F., Iwamoto, A. & Gao, G. F. (2009). Proteins, 75, 241–244. [DOI] [PubMed]