Abstract
Lipid rafts and caveolae play a pivotal role in organization of signaling by Toll-like Receptor (TLR)4 and several other immune receptors. Beyond the simple cataloguing of signaling events compartmentalized by these membrane microdomains, recent studies have revealed the surprisingly central importance of dynamic remodeling of membrane lipid domains to immune signaling. Simple interventions upon membrane lipid, such as changes in cholesterol loading or crosslinking of raft lipids, are sufficient to induce micron-scale reordering of membranes and their protein cargo with consequent signal transduction. In this review, using TLR signaling in the macrophage as a central focus, we discuss emerging evidence that environmental and genetic perturbations of membrane lipid regulate protein signaling, illustrate how homeostatic flow of cholesterol and other lipids through rafts regulates the innate immune response, and highlight recent attempts to harness these insights towards therapeutic development.
Keywords: Lipid raft, caveolae, macrophage, Toll like Receptor, cholesterol
Since the inception of the lipid raft hypothesis in 1997 (1), a profusion of studies have reported roles for these cholesterol-enriched membrane microdomains in organization of cell signaling. As a crossroads for immunology, biophysics, and lipid science, the raft field has suffered growing pains in terminology, technique, and interpretation. Progressively refined imaging techniques continue to support the existence of lateral protein/lipid heterogeneities in biological membranes (2, 3), but the precise nature, size, and malleability of these microdomains remain a matter of debate. A burgeoning field that has catalogued an increasing number of signaling events within rafts at the same time finds itself at risk of losing sight of the implications of this localization. In this review, rather than focus on definitions of rafts/caveolae (for this the reader is referred to recent scholarly reviews (2, 4, 5)), the objective will be to synthesize and interpret emerging insights on how genetic and environmental modification of raft lipid plays a fundamental role in determining immune signaling and disease. The case will be made that dynamic remodeling of raft lipid is not only necessary in many signaling cascades, but that primary perturbations of raft lipid (e.g., cholesterol loading or unloading, raft coalescence) can also be sufficient initiating events to trigger protein signaling. Using the macrophage, and, in particular, TLR signaling in macrophages as a primary case in point, the dependence of inflammatory signaling upon cholesterol-loading conditions and on the regulatory proteins that control homeostatic intracellular trafficking of cholesterol through rafts will be highlighted.
Lipid Rafts and Caveolae
Lipid rafts are thought to be highly dynamic, nanoscale (i.e., <200 nm), cholesterol- and sphingolipid-enriched membrane microdomains, likely present in all eukaryotic cells, that compartmentalize select signaling and functional events. While it is difficult to place a lower limit on their size in the resting state, and evidence indeed exists for ‘lipid shells’ surrounding individual proteins in biological membranes (2), rafts can also be driven to coalesce into more stable, micron-range domains through lipid-lipid, protein-lipid, and protein-protein interactions. The mechanism(s) underlying raft ‘coalescence’ or ‘clustering,’ however, in many cases remain elusive. It is generally thought that the saturated acyl chains of raft sphingolipids and phospholipids exhibit tight packing in a manner analogous to the liquid-ordered (Lo)2 domains observed in model membranes, and that this may account for their resistance to solubilization by cold nonionic detergents (e.g., Triton-X-100). However, as detergent can itself induce the formation of domains in membranes (6), rafts should not be equated with ‘detergent-resistant membranes’ (DRMs); nor can identification of a protein in DRMs be taken as sufficient evidence for assigning raft localization in vivo. While good evidence supports the co-existence within cell membranes of heterogeneous populations of lipid rafts, isolation of DRMs of discrete composition with the use of different detergents should not be considered as evidence for discrete raft domains in vivo.
Caveolae are ~60–80 nm cholesterol-enriched membrane invaginations whose flask-shaped morphology derives from caveolin proteins, expression of which suffices to confer caveolar morphology (7). Caveolae are thought to represent a discrete, specialized subpopulation of membrane microdomains, and thus should not be simply equated with ‘lipid rafts’. The caveolin proteins, through direct regulatory interactions with other proteins (e.g., TLR4 (8)), are in particular thought to play a central role in signal regulation within caveolae. Of interest, while caveolae are well-studied in certain cell types (e.g., endothelial cells, fibroblasts) and thought to be absent in others (e.g., lymphocytes), their presence in macrophages is less well-defined and indeed controversial, varying by macrophage type (reviewed in (9)).
While rafts and/or caveolae promote immune receptor signaling in several pathways by serving as platforms for dynamic assembly of signaling complexes, in other cases, raft-localization suppresses signaling (e.g., TGFβ and epidermal growth factor receptors) (10-12). Moreover, in addition to concentrating signaling proteins, the lipid micro-environment of rafts may itself alter protein function (13), in some cases shaping signaling much more selectively than as just a simple binary switch. Thus, localization of the TNF receptor to raft vs. non-raft domains determines responses to TNFα, including cell fate as well as signaling events (14).
Protein localization to rafts, in many cases determined by GPI linkage or palmitoylation, is also thought to be responsive to raft cholesterol levels. Indeed, lipid-induced changes in the raft proteome likely explain reports, discussed below, that acute or chronic changes in raft cholesterol may determine protein signaling. Conversely, some proteins (e.g., NAP-22) and peptides (apolipoprotein A-I mimetic 4F) may themselves induce phase separation of cholesterol-rich and –poor domains (15), or induce raft signaling by deforming membrane lipids (16). It is also important to note that proteins, through scaffolding and other interactions, have been shown in some contexts to play dominant roles in determining membrane domains in immune cells (17, 18). Raft coalescence induced in dendritic cell membranes by physical contact of uric acid crystals (19), or in RAW 264.7 membranes by altered topography of the cell substratum (20), can also activate signaling proteins including Syk and NF-κB. Taken together, these findings suggest that protein and lipid remodeling of the membrane interact to shape domains and cell signaling, and that raft signaling may be profoundly influenced or indeed induced by ‘ligand independent’ interventions upon plasma membrane lipid.
Rafts as poised signaling units: signal initiation by microdomain coalescence
Interestingly, recent work indicates that the resting plasma membrane may be poised at the edge of a phase boundary such that simple membrane perturbations can drive large-scale phase separation of discrete protein/lipid macrodomains, thereby inducing signaling. Thus, crosslinking of the raft glycosphingolipid GM1 with cholera toxin B subunit induces cholesterol-dependent coalescence of micron-scale GM1 domains that recruit lipid-anchored raft proteins but exclude the non-raft transferrin receptor (21). Cholesterol depletion with methyl-β-cyclodextrin (mβCD) also induces micron-scale phase separation of the plasma membrane into fluid and ordered domains in living CHO cells (22), GM1-rich domains that concentrate Lck and LAT and signal to ERK activation in T cells (23), and GM1- and CD11b-rich domains in neutrophils (24). Imaging techniques with higher resolution than fluorescence microscopy will almost certainly be required to properly characterize raft co-localization and coalescence. Nonetheless, together, these findings confirm that membrane lipid remodeling is sufficient to drive cell signaling by reorganizing protein cargo, and also demonstrate, perhaps paradoxically, that cholesterol depletion can increase membrane order and coalescence of raft-like domains, a topic to which we will return below.
Notably, antibody-mediated crosslinking of several GPI-linked proteins can also coalesce/remodel rafts and induce signaling by co-patching proteins within rafts. Crosslinking of external leaflet raft proteins induces co-patching and activation of inner leaflet raft proteins such as H-ras (25), whereas crosslinking of GM1 can interestingly induce its co-patching with TLR4 (26) and CD18 (27). As oligomeric cholesterol-binding cytolysins such as listeriolysin O both cluster CD14-rich rafts (28) and activate TLR4 (29), it seems plausible that some TLR4 agonists may activate this receptor through raft-mediated receptor clustering. In this light, it is important to remember that lipopolysaccharide (LPS), the canonical TLR4 ligand, is itself a polymeric molecule that induces receptor clustering.
The two faces of rafts: signal inhibition and activation by raft-perturbing agents
Perhaps the most widely used experimental tools used to ‘disrupt’ rafts are the β-cyclodextrins, mβCD and 2-hydroxyl-β-CD, cyclic oligosaccharides that remove cholesterol from membranes. While numerous papers have used mβCD to infer that cell signals, including those induced by LPS, are raft-dependent, some caution is warranted (reviewed in (30)). Thus, mβCD depletes cholesterol to varying degrees in different cell types, may under high concentrations (i.e., >10 mM) or prolonged incubations (>30 min) also remove extra-raft cholesterol or even cause cell death, and may interact with non-sterol lipids or immobilize membrane proteins through effects on the cytoskeleton (30). Moreover, mβCD and other in vitro manipulations of cell membrane lipid may not necessarily be physiologically relevant. These concerns notwithstanding, good evidence suggests that low concentration/short incubation usage of mβCD may be selective for raft cholesterol (30–32). Moreover, multiple control strategies are available, including clamping of cell cholesterol with mβCD-cholesterol complexes, use of the structurally dissimilar cholesterol-sequestering agents filipin and nystatin, cholesterol depletion by lipoprotein-deficient serum, as well as additional raft-perturbing agents that have been described (Table I).
Table 1.
Agents reported to ‘disrupt’ raft structure and/or function and their associated effects on the cell.*
| Disruptor | Cell type | Effect | |
|---|---|---|---|
| PUFAs | EL4 cell | ↓raft coalescence, MHC I mislocalization | (82) |
| HDL | monocyte | ↓raft chol, ↓CD11b activation | (54) |
| 4F | MDM | ↓rafts, altered cell differentiation | (88) |
| LXR agonists | prostate Ca cell | ↓raft size, ↓raft Akt phosphorylation | (89) |
| SQS inhibitor | prostate Ca cell | ↓raft chol, ↓cell proliferation | (85) |
| Statins | NK cell | ↓membrane chol, ↓NK cell cytotoxicity | (84) |
| OxLDL | endothelial | ↓raft chol, ↓raft eNOS, ↓eNOS activation | (75) |
| OxPAPC | endothelial | ↓LPS-induced raft TLR4, ↓LPS response | (77) |
| Ceramide | PBMC | ↓raft Lck, ↓raft PLD1, ↑PLD1 activity | (90) |
| DPPE | CD8+ T cell | ↓MHC-induced raft proteins, ↓CTL activation | (72) |
| DPPC, surfactant | A549 | ↓LPS-induced TLR4 translocation to rafts | (73) |
| High glucose | THP-1 | ↓number and size of caveolae | (91) |
| Ethanol | Mϕ | ↓LPS-induced raft CD14 and TLR4 | (92) |
| ESeroS-GS | Mϕ | ↓LPS-induced raft CD14 and TLR4 | (80) |
Select examples are shown for each agent. Measures of raft ‘disruption’ differ among reports. For some agents (e.g., ceramide), both raft stabilization and destabilization have been reported. For others, (e.g., ethanol), inhibitory and stimulatory effects have been reported on the TLR4 pathway. Ca, cancer; chol, cholesterol; CTL, cytotoxic T lymphocyte; DPPC, dipalmitoyl-phosphatidylcholine; DPPE, dipalmitoyl-phosphatidylethanolamine; eNOS, endothelial nitric oxide synthase; HDL, high density lipoprotein; LPS, lipopolysaccharide; LXR, Liver X Receptor; Mϕ, macrophage; MDM, monocyte-derived macrophage; oxLDL, oxidized low density lipoprotein; oxPAPC, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine; PUFA, polyunsaturated fatty acid; SQS, squalene synthase; TLR4, Toll like Receptor 4; 4F, apolipoprotein mimetic peptide 4F.
While raft isolation and perturbation strategies have been used to show the requirement for raft integrity in several signaling pathways, a perhaps more intriguing chain of literature has shown that acute cholesterol depletion can itself initiate signaling cascades in a cell type-dependent fashion. CDs activate ERK in Rat-1 and RAW 264.7 cells (31, 33), p38 and Cdc42 in human neutrophils (34), and tyrosine phosphorylation in RBL-2H3 cells (35). MβCD and filipin initiate ligand-independent activation of epidermal growth factor receptor (10, 36) and Fas (37) by a mechanism involving displacement of these receptors from rafts. Conversely, cholesterol depletion may also cause a disintegrin and metalloproteinase domain-containing protein (ADAM)10- and/or ADAM17-dependent cleavage of several receptors (IL-6R, CD44, CD30, TNFR1, TNFR2) by causing their displacement from rafts (38–41). MβCD also activates NF-κB in macrophages by a mechanism involving the adaptor myeloid differentiation primary response gene 88 (MyD88)(33). Consistent with these findings with CDs, we recently reported that the physiologic cholesterol acceptor apolipoprotein (apo)A-I activates a TLR2-, TLR4-, and MyD88-dependent pathway to NF-κB in macrophages (33). While the full significance of these assorted findings is not yet clear, and multiple underlying mechanisms are likely involved, taken together, these reports suggest that native microdomains of the cell membrane may serve to maintain signal quiescence by sequestering pathway components, and that their perturbation through cholesterol removal may induce pathways the nature of which is determined by the specific signaling proteins expressed in the cell under study.
Regulation of rafts and TLR signaling by intracellular cholesterol traffic
While it is now well recognized that pharmacologic raft-perturbing agents can modify raft-dependent signaling by delocalization of proteins, the effects of cholesterol loading on raft function under more physiologic settings are perhaps less widely appreciated. In primary murine macrophages, raft levels of TLR4 and TLR9, and cell responsiveness to TLR2, TLR4, TLR7, and TLR9 ligands, are all directly associated with exogenously manipulated raft cholesterol levels (42, 43). Moreover, hypercholesterolemia increases macrophage raft cholesterol in mouse and man in vivo, increasing cell responsiveness to LPS (44, 45). Perhaps more striking are reports that indicate that acute cholesterol loading of membranes may suffice to activate TLRs. Thus, cholesterol loading of the macrophage plasma membrane induces TLR4-dependent signaling, and loading of endosomal membranes induces TLR3- and TLR4-dependent responses (46). Conversely, it is also recognized that, in other contexts (e.g., modified lipoprotein treatment) cholesterol loading can also be associated with reduced macrophage inflammatory function (47, 48). This may in part reflect the propensity of conditions to load cytosolic cholesterol ester instead of membrane cholesterol, as well as to activate nuclear receptors (e.g., Liver X Receptors, Peroxisome Proliferator-activated Receptors).
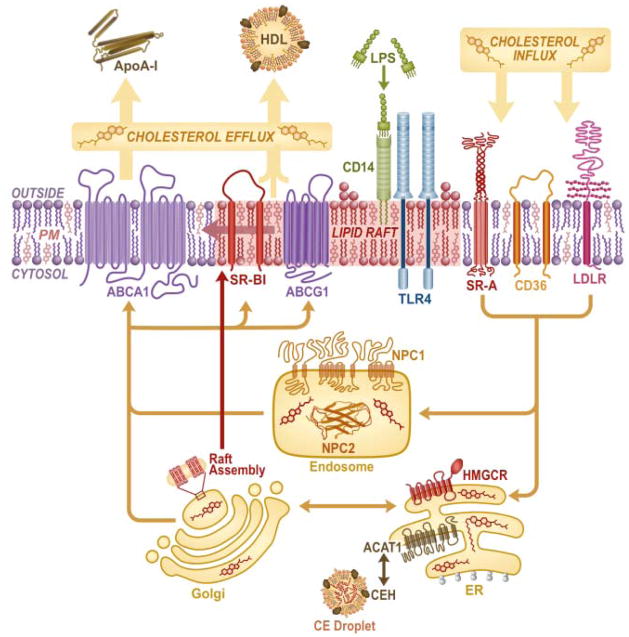
Physiologically, raft/caveolar cholesterol content is regulated by homeostatic trafficking of cholesterol through the cell (Figure 1), a topic covered in depth by recent comprehensive reviews (49). In brief, following cholesterol synthesis in the endoplasmic reticulum (ER) or endosomal recycling of internalized cholesterol to the ER/Golgi by Niemann Pick C1 (NPC1) protein, it is thought that caveolae are assembled in the Golgi and transported to the plasma membrane in a caveolin- and NPC1-dependent fashion (7, 50). Thus, NPC-deficient fibroblasts have reduced plasma membrane caveolar cholesterol (50) and late endosomal cholesterol overload with raft overcrowding (51). Raft/caveolar cholesterol is, in turn, regulated by transporter-mediated (ATP Binding Cassette [ABC]A1; ABCG1; and scavenger receptor [SR]-BI) efflux of plasma membrane cholesterol to extracellular acceptors including lipid-free apoA-I and HDL, as well as by aqueous diffusion. Overexpression of ABCA1 (52), and treatment with HDL or apoA-I (53, 54) all disrupt/deplete raft domains, inhibiting raft-dependent signaling. The effect of stimulated cholesterol efflux is quite complex, however, as apoA-I, like mβCD, can enhance responses to some stimuli such as platelet derived growth factor (55) by removing cholesterol from rafts (55, 56). Similarly, SR-BI-mediated cholesterol efflux to HDL activates eNOS (57). Moreover, apoA-I may increase caveolar cholesterol by stimulating its transfer from intracellular compartments faster than its efflux (58).
Figure 1. Intracellular cholesterol trafficking regulates macrophage rafts.
Cholesterol synthesized in the endoplasmic reticulum (ER) by HMG CoA reductase (HMGCR) or internalized via scavenger receptors (CD36, SR-A) or low density lipoprotein receptor (LDLR), is assembled into nascent rafts in the Golgi apparatus for caveolin- and Niemann Pick C1 protein (NPC1)-dependent transfer to the plasma membrane. NPC1 together with NPC2 also regulates endosomal recycling of cholesterol to the plasma membrane. In turn, cholesterol is effluxed either by simple diffusion or via transporters (ATP Binding Cassette [ABC]A1, ABCG1, SR-BI) to extracellular acceptors (apolipoprotein [apo]A-I, high density lipoprotein [HDL]), and also likely equilibrates with non-raft regions. Cholesterol esterification is regulated in the cytosol by cholesterol ester hydrolase (CEH) and in the ER by acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1). Raft cholesterol/abundance and abundance of LPS recognition proteins (CD14, TLR4) are regulated by cholesterol flux through this pathway.
Building upon earlier reports that TLR4 signaling occurs in lipid rafts (34), an exciting chain of literature has recently demonstrated profound effects of cholesterol trafficking through rafts on TLR signaling in the macrophage. ABCA1-null macrophages have enlarged, cholesterol-laden lipid rafts (42, 59) (Figure 2) containing increased TLR4 (43), and are hyperresponsive to LPS (42, 59–61) as well as to TLR-2, -7, and -9 ligands (42, 61). ABCG1-null macrophages display a similar albeit perhaps more pronounced TLR-hyperresponsive phenotype (61, 62). NPC1-null macrophages display basal activation of TLR3, -7, -8, and -4 (46), the first three of which may reflect cholesterol overloading of endosomal rafts, and the last, TLR4 accumulation in endosomes due to blocked trafficking (63). Taken together, these reports indicate an intriguing degree of overlap between the pathway for trafficking of host lipids and that for recognition of microbial lipids, perhaps even suggesting common evolutionary roots between the two. Indeed, it was recently reported that, in addition to regulating efflux of cholesterol and phospholipid, ABCA1 also regulates efflux of LPS from the macrophage (64).
Figure 2. ATP Binding Cassette (ABC)A1-deficient macrophages have enlarged lipid rafts.
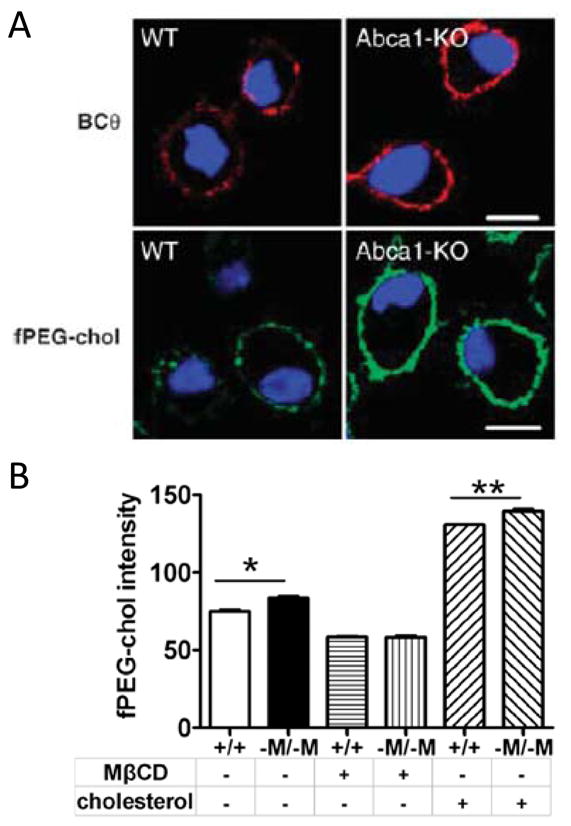
(A) Rafts were imaged in peritoneal macrophages from WT and Abca1 null mice with the use two raft cholesterol probes, BCθ toxin (red) and fPEG-chol (green). Nuclei were stained with 4’6-diaminophenylindole (blue). Reprinted from (59) with permission. (B) Peritoneal macrophages from wild type (+/+) or macrophage-specific Abca1 null (-M/-M) mice were cholesterol-depleted with mβCD or cholesterol-loaded with mβCD-cholesterol, stained with fPEG-chol, and then quantified by flow cytometry. Data are mean +/− SEM. *, p<.05; **, p<.01. Reprinted from (43).
Modification of rafts and their signaling by non-sterol lipids
Complex effects upon raft remodeling and signaling in TLR and other pathways have also been described for sphingomyelin and the product of its breakdown by sphingomyelinase, ceramide. Sphingomyelin and cholesterol promote raft formation in the Golgi via strong physical interactions (65), and ceramide indeed stabilizes rafts more effectively than cholesterol (66). On the other hand, good evidence indicates that sphingomyelinase treatment and ceramide itself both displace cholesterol from rafts (67, 68) in a manner that could realistically occur in vivo during inflammation. Indeed, acid sphingomyelinase-induced remodeling of the plasma membrane into enlarged ceramide-rich rafts during P. aeruginosa infection is critical for bacterial internalization and for successful host defense (69). Interestingly, local acid sphingomyelinase-mediated ceramide production in rafts has also been reported to be required for LPS-induced recruitment of TLR4 to rafts (70), and ceramide itself elicits TLR4-dependent signaling (71). While the full implications of these findings are not yet clear, it appears plausible that ceramide and cholesterol may ‘compete’ to form somewhat distinct rafts, and that dynamic remodeling of raft lipid and protein composition by local ceramide induction may be a critical step in TLR signaling and perhaps other pathways.
Phospholipids have also been shown to modulate raft structure and function. Dipalmitoyl-phosphatidylethanolamine (DPPE) partitions into lipid rafts, inhibiting MHC peptide-induced raft recruitment of acylated proteins in CD8+ T cells (72) and TNF-induced recruitment of its receptor to rafts in HT1080 cells (14), without displaying overt effects on raft integrity. Similarly, the surfactant phospholipid dipalmitoyl-phosphatidylcholine (DPPC) and surfactant itself both attenuate LPS signaling by inhibiting TLR4 recruitment to rafts (73), perhaps suggesting that the lipid environment of the alveolus may dampen innate immune responses through effects on rafts. A role for phospholipid metabolism in cell-intrinsic TLR4 responses is also suggested by a report that lysophosphatidylcholine acyltransferase is required for LPS-induced translocation of TLR4 to rafts (74).
Oxidized lipids present during disease have been shown to modify rafts with associated effects on signaling. Thus, oxidized low density lipoprotein (oxLDL) reduces caveolar cholesterol, displacing eNOS and caveolin (75). The major oxysterol in oxLDL, 7-ketocholesterol, partitions into rafts, depleting them of cholesterol (56) and activating Src within them (76). Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine, a major oxidized phospholipid present in oxLDL, has also been shown to disrupt rafts through a mechanism involving ceramide induction, thereby inhibiting LPS-induced translocation of TLR4 to rafts (77, 78). Reports that reactive oxygen species are not only necessary but also sufficient to induce assembly of TCR-associated proteins in T cell rafts (79) and TLR4 trafficking to rafts in macrophages (26), suggest that oxidation of membrane lipids may also serve as a critical step in innate and adaptive immune signaling through raft remodeling. Indeed, an antioxidant α-tocopherol derivative has been shown to attenuate LPS signaling by interfering with CD14 and TLR4 recruitment to rafts (80).
Emerging opportunities for targeting rafts in disease
Several strategies have shown early promise as potential therapeutic measures to modify raft signaling through intervening upon raft lipids. These include dietary polyunsaturated fatty acids (PUFAs), statins (i.e., HMG CoA reductase inhibitors), squalene synthase inhibitors, raft-targeting lipids, and edelfosine. PUFAs such as docosahexaenoic acid and eicosapentaenoic acid inhibit signaling in Jurkat T cells by incorporating into rafts and displacing acylated signaling proteins (Lck, Fyn, LAT) (81). Alternatively, it has been proposed that PUFAs do not incorporate into rafts due to their unsaturation, but rather form extra-raft domains that interfere indirectly with raft-dependent protein clustering (82, 83). Statins, increasingly studied for their anti-inflammatory actions, attenuate leukocyte function in part through membrane raft depletion (84). Inhibitors of squalene synthase, an enzyme downstream of HMG CoA reductase in the cholesterol biosynthetic pathway, selectively reduce raft cholesterol in cancer cells and induce cell death (85). In an additional raft-centric strategy for cancer therapy, adhesion and cell cycle progression of breast cancer cells was recently shown to be more effectively inhibited by targeting a Src family kinase inhibitor to rafts through palmitoylation (86). Finally, a recent study has shown that the phospholipid ether edelfosine may be an effective therapeutic for multiple myeloma by accumulating in myeloma cell rafts, thereby inducing apoptosis through co-clustering of rafts and death receptors (87). Taken together, these reports indicate the exciting potential to manipulate disease cells through several independent interventions that target raft lipids.
Conclusions
Many basic questions about the nature of rafts and caveolae remain unanswered, and many of these questions will almost certainly require high-resolution imaging techniques. Nonetheless, a convergence of independent approaches including biophysics, immunology, and lipid science has begun to indicate the fundamental importance of dynamic and chronic changes in membrane lipid to signaling in immune and other cells. Pathways for trafficking of cholesterol and other lipids through the macrophage, initially studied for their relevance to cell biology and metabolism, are now understood to be critical determinants of TLR signaling. At a basic level, these studies have also challenged the traditional paradigm of hierarchical protein signaling, showing that lateral changes in lipid domain segregation in the plasma membrane are not only necessary, but also sufficient to initiate protein signaling. A challenge for the field as it looks now to apply these insights to better understand ‘old’ diseases and to develop new therapeutics, will be to avoid conceptual constraint by the raft hypothesis itself.
Acknowledgments
The authors thank Sue Edelstein for assistance with figure design.
Footnotes
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES102005) and by HL094525 (JSP).
Abbreviations used in this paper: ABC, ATP Binding Cassette; ADAM, a disintegrin and metalloproteinase domain-containing protein; Apo, apolipoprotein; DPPC, dipalmitoyl-phosphatidylcholine; DPPE, dipalmitoyl-phosphatidylethanolamine; DRM, detergent resistant membrane; HDL, high density lipoprotein; mβCD, methyl-betacyclodextrin; MyD88, myeloid differentiation primary response gene 88; NPC1, Niemann Pick C1; oxLDL, oxidized low density lipoprotein; PUFA, polyunsaturated fatty acid; SR, scavenger receptor; TRIF, Toll-Interleukin-1 receptor-domain-containing adapter-inducing interferon-β; TRAM, TRIF-related adaptor molecule.
References
- 1.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 3.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 4.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 5.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 8.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 9.Gargalovic P, Dory L. Caveolins and macrophage lipid metabolism. J Lipid Res. 2003;44:11–21. doi: 10.1194/jlr.r200005-jlr200. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Resh MD. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J Biol Chem. 2002;277:49631–49637. doi: 10.1074/jbc.M208327200. [DOI] [PubMed] [Google Scholar]
- 11.Pike LJ, Casey L. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry. 2002;41:10315–10322. doi: 10.1021/bi025943i. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, I, Liu H, Fliesler SJ, Han X, Huang SS, Huang JS. Cholesterol suppresses cellular TGF-beta responsiveness: implications in atherogenesis. J Cell Sci. 2007;120:3509–3521. doi: 10.1242/jcs.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 15.Epand RM. Do proteins facilitate the formation of cholesterol-rich domains? Biochim Biophys Acta. 2004;1666:227–238. doi: 10.1016/j.bbamem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Min CK, Bang SY, Cho BA, Choi YH, Yang JS, Lee SH, Seong SY, Kim KW, Kim S, Jung JU, Choi MS, Kim IS, Cho NH. Role of amphipathic helix of a herpesviral protein in membrane deformation and T cell receptor downregulation. PLoS Pathog. 2008;4:e1000209. doi: 10.1371/journal.ppat.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto-Tane A, Yokosuka T, Ishihara C, Sakuma M, Kobayashi W, Saito T. T-cell receptor microclusters critical for T-cell activation are formed independently of lipid raft clustering. Mol Cell Biol. 30:3421–3429. doi: 10.1128/MCB.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harder T, Engelhardt KR. Membrane domains in lymphocytes - from lipid rafts to protein scaffolds. Traffic. 2004;5:265–275. doi: 10.1111/j.1600-0854.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 19.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterfield JD, Ali TA, Nahid F, Kusano K, Brunette DM. The effect of surface topography on early NFkappaB signaling in macrophages. J Biomed Mater Res A. 2010;95:837–847. doi: 10.1002/jbm.a.32857. [DOI] [PubMed] [Google Scholar]
- 21.Lingwood D, Ries J, Schwille P, Simons K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci U S A. 2008;105:10005–10010. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci U S A. 2001;98:13072–13077. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahammad S, Dinic J, Adler J, Parmryd I. Limited cholesterol depletion causes aggregation of plasma membrane lipid rafts inducing T cell activation. Biochim Biophys Acta. 2010;1801:625–634. doi: 10.1016/j.bbalip.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Solomkin JS, Robinson CT, Cave CM, Ehmer B, Lentsch AB. Alterations in membrane cholesterol cause mobilization of lipid rafts from specific granules and prime human neutrophils for enhanced adherence-dependent oxidant production. Shock. 2007;28:334–338. doi: 10.1097/shk.0b013e318047b893. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg S, Shvartsman DE, Ehrlich M, Henis YI. Clustering of raft-associated proteins in the external membrane leaflet modulates internal leaflet H-ras diffusion and signaling. Mol Cell Biol. 2006;26:7190–7200. doi: 10.1128/MCB.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauss K, Altevogt P. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J Biol Chem. 1999;274:36921–36927. doi: 10.1074/jbc.274.52.36921. [DOI] [PubMed] [Google Scholar]
- 28.Gekara NO, Jacobs T, Chakraborty T, Weiss S. The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol. 2005;7:1345–1356. doi: 10.1111/j.1462-5822.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 29.Park JM, V, Ng H, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuchi T, Anderson RG. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK) J Biol Chem. 1998;273:21099–21104. doi: 10.1074/jbc.273.33.21099. [DOI] [PubMed] [Google Scholar]
- 32.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smoak KA, Aloor JJ, Madenspacher J, Merrick BA, Collins JB, Zhu X, Cavigiolio G, Oda MN, Parks JS, Fessler MB. Myeloid differentiation primary response protein 88 couples reverse cholesterol transport to inflammation. Cell Metab. 2010;11:493–502. doi: 10.1016/j.cmet.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fessler MB, Arndt PG, Frasch SC, Lieber JG, Johnson CA, Murphy RC, Nick JA, Bratton DL, Malcolm KC, Worthen GS. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. J Biol Chem. 2004;279:39989–39998. doi: 10.1074/jbc.M401080200. [DOI] [PubMed] [Google Scholar]
- 35.Surviladze Z, Draberova L, Kovarova M, Boubelik M, Draber P. Differential sensitivity to acute cholesterol lowering of activation mediated via the high- affinity IgE receptor and Thy-1 glycoprotein. Eur J Immunol. 2001;31:1–10. doi: 10.1002/1521-4141(200101)31:1<1::AID-IMMU1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 36.Lambert S, Vind-Kezunovic D, Karvinen S, Gniadecki R. Ligand-independent activation of the EGFR by lipid raft disruption. J Invest Dermatol. 2006;126:954–962. doi: 10.1038/sj.jid.5700168. [DOI] [PubMed] [Google Scholar]
- 37.Gniadecki R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320:165–169. doi: 10.1016/j.bbrc.2004.05.145. [DOI] [PubMed] [Google Scholar]
- 38.Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, Rose-John S. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J Biol Chem. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 39.Murai T, Maruyama Y, Mio K, Nishiyama H, Suga M, Sato C. Low cholesterol triggers membrane microdomain-dependent CD44 shedding and suppresses tumor cell migration. J Biol Chem. 2011;286:1999–2007. doi: 10.1074/jbc.M110.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Tresckow B, Kallen KJ, von Strandmann EP, Borchmann P, Lange H, Engert A, Hansen HP. Depletion of cellular cholesterol and lipid rafts increases shedding of CD30. J Immunol. 2004;172:4324–4331. doi: 10.4049/jimmunol.172.7.4324. [DOI] [PubMed] [Google Scholar]
- 41.Tellier E, Canault M, Poggi M, Bonardo B, Nicolay A, Alessi MC, Nalbone G, Peiretti F. HDLs activate ADAM17-dependent shedding. J Cell Physiol. 2008;214:687–693. doi: 10.1002/jcp.21265. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madenspacher JH, Draper DW, Smoak KA, Li H, Griffiths GL, Suratt BT, Wilson MD, Rudel LL, Fessler MB. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J Immunol. 2010;185:1660–1669. doi: 10.4049/jimmunol.0903501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oiknine J, Aviram M. Increased susceptibility to activation and increased uptake of low density lipoprotein by cholesterol-loaded macrophages. Arterioscler Thromb. 1992;12:745–753. doi: 10.1161/01.atv.12.6.745. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton TA, Ma GP, Chisolm GM. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990;144:2343–2350. [PubMed] [Google Scholar]
- 48.Haga Y, Takata K, Araki N, Sakamoto K, Akagi M, Morino Y, Horiuchi S. Intracellular accumulation of cholesteryl esters suppresses production of lipopolysaccharide-induced interleukin 1 by rat peritoneal macrophages. Biochem Biophys Res Commun. 1989;160:874–880. doi: 10.1016/0006-291x(89)92516-3. [DOI] [PubMed] [Google Scholar]
- 49.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 50.Garver WS, Krishnan K, Gallagos JR, Michikawa M, Francis GA, Heidenreich RA. Niemann-Pick C1 protein regulates cholesterol transport to the trans-Golgi network and plasma membrane caveolae. J Lipid Res. 2002;43:579–589. [PubMed] [Google Scholar]
- 51.Lusa S, Blom TS, Eskelinen EL, Kuismanen E, Mansson JE, Simons K, Ikonen E. Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane. J Cell Sci. 2001;114:1893–1900. doi: 10.1242/jcs.114.10.1893. [DOI] [PubMed] [Google Scholar]
- 52.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J Biol Chem. 2006;281:36091–36101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- 53.Peshavariya H, Dusting GJ, Di Bartolo B, Rye KA, Barter PJ, Jiang F. Reconstituted high-density lipoprotein suppresses leukocyte NADPH oxidase activation by disrupting lipid rafts. Free Radic Res. 2009;43:772–782. doi: 10.1080/10715760903045304. [DOI] [PubMed] [Google Scholar]
- 54.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 55.Fielding PE, Russel JS, Spencer TA, Hakamata H, Nagao K, Fielding CJ. Sterol efflux to apolipoprotein A-I originates from caveolin-rich microdomains and potentiates PDGF-dependent protein kinase activity. Biochemistry. 2002;41:4929–4937. doi: 10.1021/bi012091y. [DOI] [PubMed] [Google Scholar]
- 56.Gaus K, Kritharides L, Schmitz G, Boettcher A, Drobnik W, Langmann T, Quinn CM, Death A, Dean RT, Jessup W. Apolipoprotein A-1 interaction with plasma membrane lipid rafts controls cholesterol export from macrophages. FASEB J. 2004;18:574–576. doi: 10.1096/fj.03-0486fje. [DOI] [PubMed] [Google Scholar]
- 57.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sviridov D, Fidge N, Beaumier-Gallon G, Fielding C. Apolipoprotein A-I stimulates the transport of intracellular cholesterol to cell-surface cholesterol-rich domains (caveolae) Biochem J. 2001;358:79–86. doi: 10.1042/0264-6021:3580079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1198–1205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- 61.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Draper DW, Madenspacher JH, Dixon D, King DH, Remaley AT, Fessler MB. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am J Respir Crit Care Med. 2010;182:404–412. doi: 10.1164/rccm.200910-1580OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, Hosokawa H, Davies JP, Ioannou YA, Vanier MT, Ohno K, Ninomiya H. Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci. 2007;27:1879–1891. doi: 10.1523/JNEUROSCI.5282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson PA, Gauthier KC, Varley AW, Kitchens RL. ABCA1 promotes the efflux of bacterial LPS from macrophages and accelerates recovery from LPS-induced tolerance. J Lipid Res. 2010;51:2672–2685. doi: 10.1194/jlr.M007435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van der Luit AH, Budde M, Zerp S, Caan W, Klarenbeek JB, Verheij M, Van Blitterswijk WJ. Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin-and cholesterol-deficiency in lipid rafts. Biochem J. 2007;401:541–549. doi: 10.1042/BJ20061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 67.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 68.Yu C, Alterman M, Dobrowsky RT. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. J Lipid Res. 2005;46:1678–1691. doi: 10.1194/jlr.M500060-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 70.Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier RV. Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surg Infect (Larchmt) 2007;8:91–106. doi: 10.1089/sur.2006.050. [DOI] [PubMed] [Google Scholar]
- 71.Fischer H, Ellstrom P, Ekstrom K, Gustafsson L, Gustafsson M, Svanborg C. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell Microbiol. 2007;9:1239–1251. doi: 10.1111/j.1462-5822.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 72.Legler DF, Doucey MA, Cerottini JC, Bron C, Luescher IF. Selective inhibition of CTL activation by a dipalmitoyl-phospholipid that prevents the recruitment of signaling molecules to lipid rafts. FASEB J. 2001;15:1601–1603. doi: 10.1096/fj.00-0841fje. [DOI] [PubMed] [Google Scholar]
- 73.Abate W, Alghaithy AA, Parton J, Jones KP, Jackson SK. Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J Lipid Res. 2010;51:334–344. doi: 10.1194/jlr.M000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson SK, Abate W, Parton J, Jones S, Harwood JL. Lysophospholipid metabolism facilitates Toll-like receptor 4 membrane translocation to regulate the inflammatory response. J Leukoc Biol. 2008;84:86–92. doi: 10.1189/jlb.0907601. [DOI] [PubMed] [Google Scholar]
- 75.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 76.Myers SJ, Stanley KK. Src family kinase activation in glycosphingolipid-rich membrane domains of endothelial cells treated with oxidised low density lipoprotein. Atherosclerosis. 1999;143:389–397. doi: 10.1016/s0021-9150(98)00331-1. [DOI] [PubMed] [Google Scholar]
- 77.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, Modlin RL, Lucas RM, Nakai J, Smart EJ, Vora DK, Berliner JA. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 78.Walton KA, Gugiu BG, Thomas M, Basseri RJ, Eliav DR, Salomon RG, Berliner JA. A role for neutral sphingomyelinase activation in the inhibition of LPS action by phospholipid oxidation products. J Lipid Res. 2006;47:1967–1974. doi: 10.1194/jlr.M600060-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Lu SP, Lin Feng MH, Huang HL, Huang YC, Tsou WI, Lai MZ. Reactive oxygen species promote raft formation in T lymphocytes. Free Radic Biol Med. 2007;42:936–944. doi: 10.1016/j.freeradbiomed.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 80.Duan W, Zhou J, Zhang S, Zhao K, Zhao L, Ogata K, Sakaue T, Mori A, Wei T. ESeroS-GS modulates lipopolysaccharide-induced macrophage activation by impairing the assembly of TLR-4 complexes in lipid rafts. Biochim Biophys Acta. 2011 Jan 27; doi: 10.1016/j.bbamcr.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 81.Stulnig TM, Berger M, Sigmund T, Raederstorff D, Stockinger H, Waldhausl W. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 83.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non- raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 84.Hillyard DZ, Nutt CD, Thomson J, McDonald KJ, Wan RK, Cameron AJ, Mark PB, Jardine AG. Statins inhibit NK cell cytotoxicity by membrane raft depletion rather than inhibition of isoprenylation. Atherosclerosis. 2007;191:319–325. doi: 10.1016/j.atherosclerosis.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 85.Brusselmans K, Timmermans L, Van de Sande T, Van Veldhoven PP, Guan G, Shechter I, Claessens F, Verhoeven G, Swinnen JV. Squalene synthase, a determinant of Raft-associated cholesterol and modulator of cancer cell proliferation. J Biol Chem. 2007;282:18777–18785. doi: 10.1074/jbc.M611763200. [DOI] [PubMed] [Google Scholar]
- 86.Hitosugi T, Sato M, Sasaki K, Umezawa Y. Lipid raft specific knockdown of SRC family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells. Cancer Res. 2007;67:8139–8148. doi: 10.1158/0008-5472.CAN-06-4539. [DOI] [PubMed] [Google Scholar]
- 87.Mollinedo F, de la Iglesia-Vicente J, Gajate C, Estella-Hermoso de Mendoza A, Villa-Pulgarin JA, Campanero MA, Blanco-Prieto MJ. Lipid raft-targeted therapy in multiple myeloma. Oncogene. 2010;29:3748–3757. doi: 10.1038/onc.2010.131. [DOI] [PubMed] [Google Scholar]
- 88.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am J Physiol Cell Physiol. 2010;298:C1538–1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, Baron S, Lobaccaro JM. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 90.Diaz O, Mebarek-Azzam S, Benzaria A, Dubois M, Lagarde M, Nemoz G, Prigent AF. Disruption of lipid rafts stimulates phospholipase d activity in human lymphocytes: implication in the regulation of immune function. J Immunol. 2005;175:8077–8086. doi: 10.4049/jimmunol.175.12.8077. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi T, Juliet PA, Miyazaki A, Ignarro LJ, Iguchi A. High glucose downregulates the number of caveolae in monocytes through oxidative stress from NADPH oxidase: implications for atherosclerosis. Biochim Biophys Acta. 2007;1772:364–372. doi: 10.1016/j.bbadis.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 92.Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]