Abstract
Alveolar macrophages (AM) are exposed to respirable microbial particles. Similar to phagocytes in the gastrointestinal tract, AM can suppress inflammation after exposure to nonpathogenic organisms. IRAK-M is one inhibitor of innate immunity, normally suppressing pulmonary inflammation. During pneumonia, neutrophils (PMN) are recruited by chemotactic factors released by AM to produce an intense inflammation. We report that intact IRAK-M is strongly expressed in resting human AM, but is cleaved in patients with pneumonia via PMN-mediated induction of caspase 6 (CASP-6) activity. PMN contact is necessary and PMN membranes are sufficient for CASP-6 induction in macrophages. PMN fail to fully induce TNF-α in macrophages expressing CASP-6 cleavage resistant IRAK-M. Without CASP-6 expression, PMN stimulation fails to cleave IRAK-M, degrade IκBα or induce TNF-α. CASP-6−/− mice subjected to cecal ligation and puncture have impaired TNF-α production in the lung and decreased mortality. LPS did not induce or require CASP-6 activity demonstrating that TLR2/4 signaling is independent from the CASP-6 regulated pathway. These data define a central role for CASP-6 in PMN-driven macrophage activation and identify IRAK-M as an important target for CASP-6. PMN de-repress AM via CASP-6 mediated IRAK-M cleavage. This regulatory system will blunt lung inflammation unless PMN infiltrate the alveolar spaces.
INTRODUCTION
Alveolar macrophages (AM) and polymorphonuclear neutrophils (PMN) play critical roles in the innate immune response, which is the first line of host defense against infection in the lung (1). AM predominate in the alveolar space with normal bronchoalveolar lavage (BAL) containing more than 90% AM and fewer than 2% PMN (2). Microbes and inorganic particles activate AM to release IL-8 and recruit PMN into the lower respiratory tract (3). This augments phagocytic and killing activity in the lung (4, 5) but floods the alveolar space with fluid producing loss of segmental lung function and the appearance of focal infiltrates on the chest x-ray
The lower respiratory tract is subjected to respirable particles that can contain high concentrations of lipopolysaccharide (LPS). Paradoxically immune cells from children exposed to the highest inhaled LPS concentrations have the least capacity to produce cytokines after exposure to bacterial products (6). This suggests that like the gastrointestinal tract, the lung expresses counter-regulatory systems that inhibit innate immunity during exposure to nonpathogenic bacterial products. AM express transcriptional repressors induced by GM-CSF and surfactant protein A (7-10). These transcriptional repressors inhibit TNF-α maintaining the lung in a resting state (11, 12). We have observed that lymphocytes or PMN activate AM via de-repression, mimicking the state of activation produced in pneumonia (13). The de-repressing signal requires direct contact between the macrophage and the recruited inflammatory cell (13). Interestingly, membrane fractions of inflammatory cells carry the de-repressing activity observed in co-culture experiments (14). The requirement for direct contact will limit the damaging effects of inflammation to lung segments that recruit inflammatory cells.
Recent studies have revealed that macrophages and PMN interact through their cell surface receptors, modulating macrophage function (15, 16). Stimulation of CD80 on dendritic cells induces inflammatory cytokines (17). PMN cytoplasmic membrane expresses CD28 that activates macrophage CD80 leading to transcriptional de-repression and macrophage activation (14). CD80 is central to cytokine production and systemic inflammatory response to innate immune stimulation produced by sepsis (18, 19). IRAK-M binds macrophage CD80 in the resting state (19) and is released after addition of PMN membranes raising the possibility that IRAK-M regulates CD80 mediated inflammatory cytokine production. The regulatory pathway controlling contact mediated macrophage activation remains poorly understood.
IRAK-M (also known as IRAK-3) is a kinase deficient member of the Toll like receptor / IL-1 receptor associated kinase family (20). IRAK-M−/− mice produce more pro-inflammatory cytokines after bacterial infection and are more susceptible to the lethal effects of sepsis (21). IRAK-M is a negative regulator that is expressed in macrophages and binds to MyD88 and TRAF6 (22), blocking TLR receptor action by stabilizing the signaling complex (21). Like the transcriptional repressors expressed in AM, IRAK-M is induced by GM-CSF differentiation of macrophages. LPS, TREM or adiponectin also induce IRAK-M, blunting macrophages’ cytokine expression and protecting the host from the injurious effects of stimulation with bacterial products (23-25). IRAK-M is therefore an important counter regulatory molecule, but it can produce immunoparalysis following sepsis (26). It also plays an important role in reducing inflammation in the gastrointestinal tract (27, 28).
We investigated the fate of IRAK-M after PMN/macrophage contact. We observed CASP-6 mediates IRAK-M cleavage and facilitates innate immune signaling in macrophages during PMN-contact. This report describes a novel role of CASP-6 in innate immunity where cleavage of IRAK-M activates macrophages via de-repression.
MATERIALS AND METHODS
Patients
The bronchoalveolar lavage (BAL) protocol for pneumonia patients and controls without lung disease was approved by the human subjects review committees of New York University Langone Medical Center and Bellevue Hospital Center IRB protocol. Patients with pneumonia had focal infiltrates on chest radiograph and signs of infection. They received a research bronchoscopy prior to discharge from the hospital after being stabilized on antibiotics. BAL was performed with normal saline in a radiographically involved lung segment. The BAL cell differential from these patients is shown in Table 1. Control BAL was obtained from volunteers with normal chest radiographs and had a normal BAL cell differentials.
Table 1.
Pneumonia patient BAL cell differential
| Pt | AM % | PMN % | Lym % | Eo% |
|---|---|---|---|---|
| 1 | 22 | 69 | 7 | 2 |
| 2 | 18 | 82 | 0 | 0 |
| 3 | 81 | 6 | 13 | 0 |
| 4 | 80 | 12 | 5 | 3 |
| 5 | 76 | 18 | 6 | 0 |
Mice
6-8 week old female C57BL/6J (WT) and CASP-6−/− mice (B6.129S6-Casp6tm1Flv/J) were purchased from Jackson Labs. All mice were housed in SPF facility and allowed to acclimatize for 1 week prior to use. All studies were approved by Institutional Animal Care and Use Committee (IACUC).
Cecal Ligation and Puncture (CLP)
CLP was performed as previously described (19). Briefly, mice were anesthetized with 2.5% isoflourane and underwent CLP with a 19 gauge needle. Mice received 1ml of 0.9% saline subcutaneously for resuscitation. The CLP procedure was performed in as little as 10 min for each mouse by an experienced operator. At specified times after CLP, the mice were used to collect BAL cells as described (19). For survival experiments, mice were monitored for 5 days.
Cell preparation and culture
Peripheral blood from healthy donors was separated into PBMC and PMN containing RBCs by ficoll-gradient centrifuge. PMN were separated from RBCs in 3% dextran/PBS (Sigma-Aldrich®). The purified PMN were more than 97% viable by trypan blue (invitrogen™) staining. Monocyte-derived macrophages, alveolar macrophages, THP-1 cells (ATCC TIB-202) were cultured in RPMI1640 (invitrogen™) with 10% FBS (Thermo Fisher Scientific) 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C 5% CO2. THP-1 cells were incubated in the medium with 20 ng/mL of PMA (Sigma-Aldrich®) for 48 h to differentiate into macrophages. HEK 293T human embryonic kidney cell line was maintained in DMEM medium supplemented with 10% FBS and penicillin-streptomycin. Where noted, PMNs and alveolar macrophages were separated by means of a 0.4-micrometer pore cell culture insert (Corning).
Stable CASP-6 knock down in THP-1 cells
CASP-6 knockdown was established with commercially available shRNA constructs (SABiosciences™). The plasmids were transfected into THP-1 cells by electroporation using the Gene Pulser (Bio-Rad). As a negative control vector (NC), scrambled sequence with no homology to any sequence in the human genomes was also transfected. Stable transfectants were selected over several weeks in the presence of 2 μg/ml puromycin (Sigma-Aldrich®) and screened for CASP-6 knockdown by Western blot. The cells were maintained in RPMI1640 medium containing 1μg/ml puromycin.
Mutation of CASP-6 target site in IRAK-M and establishment of its stable transfectant
IRAK-M mutant described in this work was generated by site-directed mutagenesis with the QuickChange kit (Stratagene) as recommended by the manufacturer with primers containing the desired mutations. (D135EF:5′-GCCAATGTCACCGTGGAGAATGTTCTTATTCCTG3′, D135ER:5′-CAGGAATAAGAACATTCTCCACGGTGACATTGGC-3′). Constructs were sent for DNA sequencing to confirm the mutations. Stably transfected THP-1 cells with IRAK-M mutant were established by lentivirus mediated transfection (GeneCopeia™) as recommended by the manufacturer and enriched the cells expressing EGFP bicistronically via internal ribosomal entry site by MoFlo™ XDP cell sorter as previously described (29). The transfectants were maintained in RPMI1640 medium.
Reagents
The antibodies were obtained from various sources; IRAK-M (ab8116, Y278) from Abcam Inc.; CASP-6 (#9762), IkappaBalpha (L35A), phosphor-IkappaBalpha (5A5), MAPKp38(#9212), and phospho-p38 (#9211) from Cell Signaling; IRAK-M (C-20), TRAF6 (D-10) from Santa Cruz Biotechnology; anti-FLAG®M2 from Stratagene; anti-HA Tag (05-904) from Millipore; β-actin (AC-15) from Sigma-Aldrich®; Horseradish peroxidase-conjugated F(ab’)2 directed against rabbit and mouse IgG from Jackson Immuno-Research Laboratories. LPS was obtained from Sigma-Aldrich®. Lipofectamine 2000 was obtained from Invitrogen™. Recombinant active human CASP-6 was obtained from BioVision, inc. and its specific inhibitor Z-VEID-FMK was from Santa Cruz biotechnology, Inc. Unless otherwise stated, all chemicals were from Fisher-Scientific.
Detergent-resistant membrane isolation
Cells were lysed on ice in 150 μL of MNE buffer (25 mM MES [pH 6.5], 150 mM NaCl and 5 mM EDTA) with 1% of Triton-X 100,1mM PMSF, 1 mM dithiothreitol, 1 mM Na3VO4 and Protease inhibitor cocktail. The sample was mixed with the same volume of 80% sucrose as cell lysate, then overlaid with 1250 uL of 30% sucrose and 400 uL of 5% sucrose in MNE buffer and spun for 16-24h at 44,000 rpm at 4C in Beckman TLS 55 swing rotor by use of a Beckman Optima TLX ultracentrifuge. 150 uL fractions were harvested serially from the top of the gradient. The detergent resistant membrane raft fraction was usually obtained in fractions 2-6.
Immunoprecipitation and Western blot analysis
Cultured cells were lysed in lysis buffer containing 50 mM Hepes, pH 7.9, 250 mM NaCl, 1 mM PMSF, 1 mM dithiothreitol (DTT), protease inhibitor cocktail (Sigma-Aldrich®), 1 mM NaF, 1 mM Na3VO4 and 1% NP-40 buffer. Cellular debris was removed by centrifugation at 15,000 rpm for 15 min. For immuoprecipitation, the cell lysate was incubated with protein sepharose A or G beads (GE Healthcare) attached appropriate antibodies for 3 h at 4°C with gently rocking. After washing four times with lysis buffer, proteins bound to the beads were denatured by boiling in SDS sample buffer for immunoblotting. The proteins were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and blotted with the indicated antibodies. The reactive bands were visualized with horseradish peroxidase coupled to the appropriate secondary antibodies and the enhanced chemiluminescence (ECL plus) Western blot detection system (GE Healthcare).
Immunofluorescence Confocal Laser Microscopy
Cells were cultured on Lab-Tek® Chamber Slide™ (nunc) and fixed in warmed 3.7% formaldehyde for 10min. After permeabilizing in 0.5% Saponin/PBS for 30 minutes, 4 drops of Image-iT™ FX signal enhancer (Invitrogen) was applied. They were incubated with primary antibody (1:250-500) in 1% BSA/PBS-Tween 20 over night at 4 °C. Following rinsing with PBS, they were incubated with Alexa-Fluor-labeled 488 F(ab’)2 fragment rabbit anti-goat and 568 F(ab’)2 fragment goat anti-rabbit IgG (H+L) (invitrogen) (1:1000) for 30 minutes at room temperature. Nuclei were stained with 0.1μg/ml DAPI for 1 min. After a final wash, samples were mounted with Fluoromount G (Southern Biotechnology associates Inc.). Images were obtained by Zeiss LSM 510 confocal microscope and were processed with AxioVision Rel. 4.7 (Carl Zeiss).
Luminescent assay to measure caspase activity
Caspase activity was quantified by Caspase-Glo® (Promega). Briefly, after stimulation cells were lysed with caspase lysis buffer at 4°C, centrifuged at 15,000 rpm and the supernatants were mixed with each luminogenic caspase substrates, Z-VEID-aminoluciferin solution at equal volume for 1 h at room temperature. The luminescence was measured by the luminometer. Doxorubicin was used as a positive control for caspase activation.
In vitro caspase-cleavage assay
HEK293T cells were transfected with expression plasmids for Flag-tagged wild type or caspase-cleavage resistant mutants of IRAK-M by lipofection. After 48 h cell lysates were prepared and immunoprecipitated with protein G sepharose beads conjugated anti-Flag mAb. The protein bound-beads were re-suspended in Caspase cleavage buffer containing with 50 mM Hepes, pH 7.2, 50 mM NaCl, 10 mM EDTA, 5% glycerol, and 10 mM DTT. Active recombinant human CASP-6 was added and the aliquots were incubated for 2 h at 37°C. And then the samples were analyzed by Western blot using anti-FLAG antibody.
Measurement of TNF-α concentration
The concentration of TNF-α in supernatants was measured by ELISA (R&D Systems®) according to the manufacturers’ specifications.
Statistical analysis
All statistics were done with GraphPad Prism 5.0 (San Diego, CA). The significance of mean changes was determined by an unpaired Student’s t test. Survival was analyzed by Kaplan-Meier analysis. Significance was recognized when p < 0.05.
RESULTS
PMN-contact induces IRAK-M short form in human alveolar macrophages
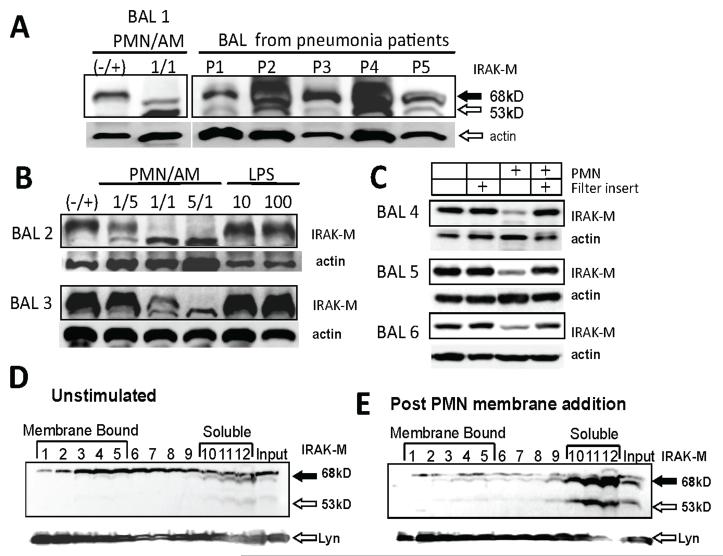
Since IRAK-M is an important inhibitor of the innate immune system, we measured IRAK-M protein expression in primary human AM. Only the full length 68 kD IRAK-M isoform occurred in AM from five volunteers without pulmonary disease (Fig. 1 panels A, B and C samples labeled BAL 1-5). AM obtained from five pneumonia patients expressed both full length and truncated IRAK-M (Fig. 1A lanes 3-7). The pneumonia patients had increased proportions of PMN and/or lymphocytes in their BAL cell differentials (table 1). The variable amounts of actin in the pneumonia patient protein extracts was likely due to variable amounts of red blood cells in the cells from these infected patients. Co-culture of AM preparations from six separate volunteers with PMN from blood of healthy donors reduced expression of full length IRAK-M and frequently produced a 53 kD isoform that co-migrated with the short isoforms observed in AM from pneumonia patients (Fig. 1 panel A lane 2, panel B lanes 2-4 and panel C lane 3). The appearance of the short 53 kD isoform increased as the ratio of PMN to AM increased (Fig 1B lanes 2-4). PMN do not express IRAK-M (data not shown) demonstrating that PMN co-culture altered IRAK-M expression in AM. Stimulation of AM with LPS did not alter IRAK-M expression (Fig 1B lanes 5 and 6).
Figure 1. PMN-contact induces short form of IRAK-M and CASP-6 activation in alveolar macrophages.
(A) Western blot probed with IRAK-M antibody. Alveolar macrophages from a volunteer without lung disease (lane 1) were cultured with PMN at a ratio of 1/1 for 4 h. After incubation, protein extract from macrophages was subjected to western blot with anti-IRAK-M antibody. BAL cells of 5 separate patients with pneumonia were processed immediately post bronchoscopy (lanes 3-7). The same membrane was re-probed with anti-β-actin as a loading control. Densitometry reveled that relative actin expression in these clinical samples with differing red blood cell contamination of pneumonia patient BAL cells was 1.9, 1.9, 1.0, 1.9 and 1.2. (B) Western blot probed with IRAK-M antibody. Alveolar macrophages from two additional volunteers without lung disease (lane 1) were cultured with differing PMN ratios for 4 h or stimulated with three concentrations of LPS, in ng/ml. The same membrane was re-probed with anti-β-actin as a loading control. (C) Western blot probed with IRAK-M antibody. Alveolar macrophages from two additional volunteers without lung disease (lane 1) are shown. A 0.4 micrometer filter was placed above the AM (lane 2). PMN at a 5/1 ratio for 4 h were added directly to the AM (lane 3) or placed in the filter insert above the AM (lane 4). The same membrane was re-probed with anti-β-actin as a loading control. (D) Western blot probed with IRAK-M antibody. Cell lysate was prepared from unstimulated THP-1 macrophages, the protein extract was separated in the sucrose gradient centrifuge and fractionated from the top of tubes (fractions 1-5 represent membrane bound protein and fractions 10-12 represent soluble unbound proteins). The same membrane was re-probed with anti-Lyn, a protein that binds to detergent resistant membranes. The Lyn signal is a control for the quality detergent resistant membrane separation by the sucrose density gradient. Representative data of three independent experiments are shown. (E) Western blot probed with IRAK-M antibody. THP-1 macrophages 1h after addition of isolated PMN membrane. Extracts were separated on a sucrose gradient as described in panel D. The Lyn re-probe of the membrane is shown as a loading control.
We then tested if soluble factors are capable of altering IRAK-M expression. PMN were separated from AM in the co-culture experiments with a 0.4-micrometer pore filter insert that prevented direct contact but permitted diffusion of soluble factors (14). When the filter insert separated PMN from AM, IRAK-M expression was not altered (Fig. 1 C).
Purified membrane fractions of PMN were added to THP-1 macrophages to confirm the conclusion from the filter transwell experiments. Resting THP-1 macrophages strongly express the full length 68 kD IRAK-M (Fig. 1 D lane 13 labeled input) while 1h after PMN membrane addition truncated IRAK-M appeared (Fig. 1 E lane 13 labeled input white arrow). This finding demonstrates that a biological activity leading to IRAK-M cleavage is contained in PMN membranes and no soluble factors are required.
Confocal microscopy suggests that a significant proportion of IRAK-M is located near the cytoplasmic membrane in resting macrophages and is released upon stimulation with PMN (19 and Fig. 4B). To further define the nature of this interaction macrophage protein extracts produced by detergent lysis were separated by sucrose gradient buoyant density centrifugation. In resting macrophages a significant proportion of the IRAK-M is present in the buoyant top fractions (Fig 1 D lanes 2-5), demonstrating it is bound to detergent resistant membranes. The adequacy of the sucrose gradient was confirmed by reprobing the western blot with Lyn antibodies, a protein that binds detergent resistant membranes (14). After addition of PMN membranes, both intact and truncated IRAK-M are present in the dense bottom fractions of the sucrose gradient (Fig. 1 E lanes 10-12), suggesting IRAK-M is no longer membrane bound. Similar results are observed with AM preparations (data not shown).
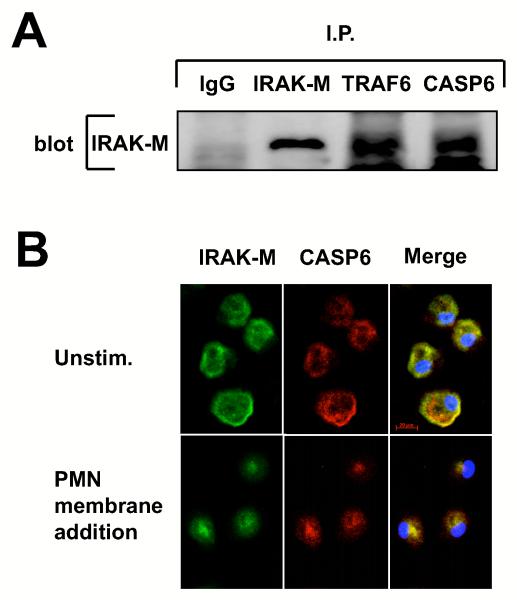
Figure 4. Co-immunoprecipitation and co-localization of IRAK-M with CASP-6.
(A) Western blot probed with IRAK-M antibody. Protein extract from THP-1 macrophages was subjected to immunoprecipitation with isotype control IgG (lane 1), anti-IRAK-M (lane 2), anti-TRAF6 (lane 3) and anti-CASP-6 (lane 4) antibody, and then the precipitants were resolved on SDS-PAGE. (B) Confocal microscopy of primary monocyte-derived macrophages cultured in the absence (upper panel) or the presence of PMN membranes (lower panel). One hr after PMN membrane addition slide chambers were fixed and slides were incubated with goat anti-IRAK-M or rabbit anti-CASP-6 and probed with the Alexa Fluor® 488 conjugated anti-goat Ig (green) and Alexa Fluor® 568 conjugated anti-rabbit Ig (red). Co-localization can be seen as yellow in the merge view. Nuclei were stained with DAPI (blue). Bar 20μm. All experiments were performed at least three times.
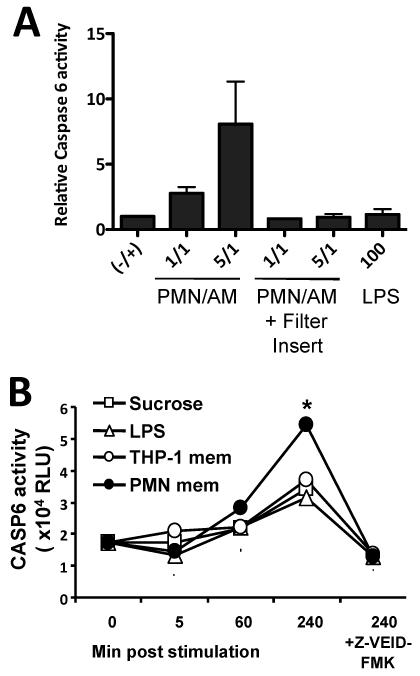
PMN contact or isolated membranes induce CASP-6 activation in macrophages
The appearance of truncated IRAK-M isoforms after co-culture of AM with PMN raised the possibility that PMN contact induced proteolysis of IRAK-M in AM. IRAK-M has a caspase recognition sequence that would produce a 53 kDa degradation product (Fig. 3A). Direct contact of PMN with AM induced CASP-6 activity in AM (Fig. 2A lanes 2 and 3). CASP-6 activity did not increase if a filter insert prevented PMN/AM contact (Fig. 2A lanes 4 and 5). LPS stimulation did not induce CASP-6 activity (Fig 2A lane 6). Isolated PMN membranes also significantly induced CASP-6 activity in macrophages, while sucrose used in the isolation gradients, THP-1 membrane preparations and LPS had minimal effect on CASP-6 activity (Fig. 2B p < 0.0001 for PMN membrane vs. LPS or THP-1 membrane). A specific CASP-6 inhibitor abolished the activity documenting the specificity of the CASP-6 assay.
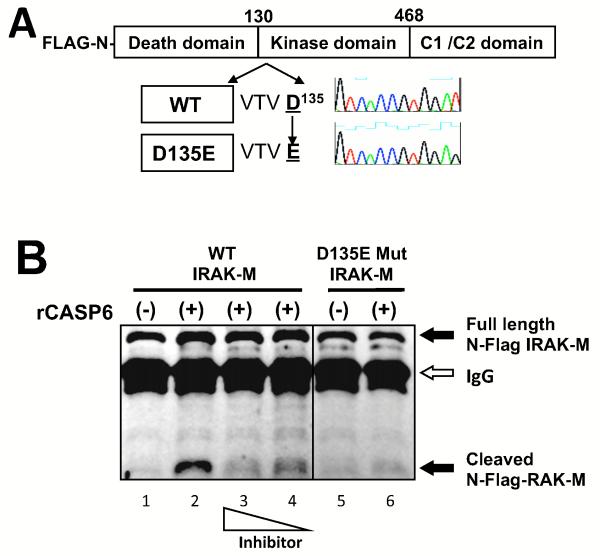
Figure 3. Active recombinant CASP-6 cleaves wild type but not mutant IRAK-M in vitro.
(A) Schema of IRAK-M (WT) and its CASP-6-target motif VTVD135 (D135E) mutant. Mammalian expression vectors of N-terminal FLAG tagged wild type were constructed by site-directed mutagenesis. (B) Western blot probed with anti-FLAG antibody. WT and mutant N-terminal flag-tagged IRAK-M were produced in 293T cells and isolated by immunoprecipitation with anti-FLAG antibody. Immunoprecipitants were incubated in the absence (−) or the presence (+) of 1U active recombinant CASP-6 (rCASP-6) and cleavage was assayed by western blot. Full length 68 kD IRAK-M or N-terminal fragment are shown by arrow respectively. The signal from IgG heavy chain used in the IP is also shown by an arrow. The casapse inhibitor Z-VEID-FMK was added with rCASP-6 at concentrations 10 or 5 μg/ml (lane 3 and 4). CASP-6-target motif VTVD135 (D135E) mutant was applied to in vitro cleavage assay (lane 5 and 6). Black lines indicated that intervening lanes have been spliced out. All experiments were performed at least three times.
Figure 2. PMN contact and isolated membranes induce CASP-6 activation in macrophages.
(A) Relative CASP-6 activity measured by luminogenic substrate with the activity of unstimulated AM defined as 1. Cell extracts were obtained four hours after the stimulus noted on the x axis. The graph represents the combined results of the five BAL cell preparations shown in panels A, B and C. p< 0.03 for the comparison of 5/1 PMN stimulation and either unstimulated control or when PMN and AM are separated by a filter insert. (B) CASP-6 activity measured by luminescent substrate. THP-1 macrophages were incubated with PMN membranes (PMN mem), 1000 ng/ml LPS or membrane fractions of THP-1 (THP-1 mem). The time post stimulation is indicated on the x axis. 10 μg/ml of Z-VEID-FMK was added as a specific inhibitor for CASP-6. Data are means ± SD. * p < 0.001. Representative data from two independent experiments are shown.
IRAK-M is cleaved by recombinant CASP-6 at a caspase recognition sequence
IRAK-M has a VTVD135 CASP-6-cleavage motif in a death domain that yields a predicted 53 kD cleavage product (Fig 3 A). Proteolysis of IRAK-M by caspase 6 is therefore a possible mechanism for generation of the 53 kD truncated IRAK-M isoform. To determine if CASP-6 cleaves IRAK-M directly, we performed in vitro cleavage reactions using isolated recombinant proteins. N-terminal FLAG-tagged IRAK-M produced in 293T cells was purified by immunoprecipitation with anti-FLAG antibody (Fig. 3B lane 1). Addition of active recombinant CASP-6 produced a degradation product consistent with cleavage at the (VTVD135) caspase recognition domain (Fig. 3B lane 2). A CASP-6 specific inhibitor reduced the cleavage in a dose dependent manner (Fig. 3B lanes 3 and 4). We then used a mutational approach to confirm that (VTVD135) was in fact the target for recombinant CASP-6. No cleavage occurred when recombinant CASP-6 was added to the D135E mutant (Fig. 3B lane 6). These data confirm that IRAK-M is a substrate for CASP-6 in vitro, cleaving the protein at the (VTVD135).
CASP-6 forms a Molecular Complex with IRAK-M and TRAF6
IRAK-M is part of a protein complex that includes TRAF6 (21). To examine if CASP-6 is another component of that molecular complex in macrophages, we performed a co-immunoprecipitation in THP-1 macrophages. The 68 kD IRAK-M was co-precipitated with antibodies to CASP-6 and TRAF6 (Fig. 4A). Other caspase family members did not bind IRAK-M (data not shown). Confocal microscopy of primary monocyte derived macrophages also demonstrated co-localization of IRAK-M (green) and CASP-6 (red) near the plasma membrane of resting macrophages (Fig. 4B upper row, merged view demonstrates yellow at the plasma membrane). PMN membrane addition produced redistribution of both IRAK-M and CASP-6 to the peri-nuclear space (Fig. 4B lower row). This finding is consistent with the result of biochemical analysis on membrane distribution of IRAK-M (Fig. 1D and E). These results demonstrate that IRAK-M and CASP-6 form the molecular complex near the plasma membrane of resting macrophages that that activation with PMN membranes disrupts this association.
A cleavage resistant IRAK-M mutant inhibits PMN stimulated TNF-α production
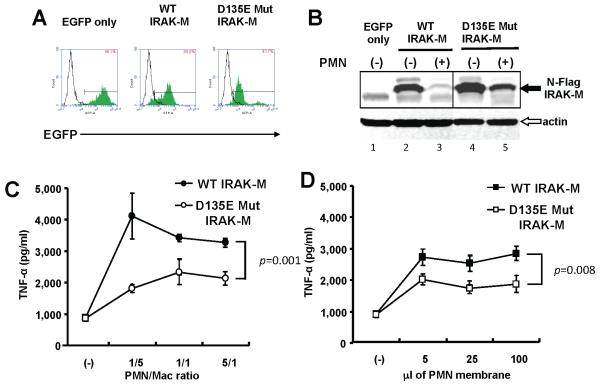
We next created THP-1 macrophages expressing mutant IRAK-M to test if cleavage resistant mutants have functional effects. We established stable transfectants of THP-1 cells expressing either wild type (WT) or mutant (D135E) HA tagged IRAK-M. The expression vector also encoded an EGFP auto-fluorescent protein so cell sorting could isolate IRAK-M expressing cells (Fig. 5A). Transduced THP-1 macrophages strongly expressed WT and mutant IRAK-M (Fig. 5B lane 2 and 4). Macrophages efficiently degraded WT IRAK-M after 3 h of PMN co-culture, (Fig. 5B lanes 2 and 3), whereas the D135E mutant was resistant to cleavage (Fig. 5B lanes 4 and 5).
Figure 5. IRAK-M mutants are resistant to PMN-contact dependent cleavage in stably transfected THP-1 macrophages.
(A) FACS of THP-1 cells stably expressing wild type (WT) or mutant (D135E) IRAK-M. C-terminus HA-tagged IRAK-M pseudotyped lentivirual expression vector transduced THP-1 cells. The expression vector produced a polycystronic mRNA that contains IRAK-M, an IRES and EGFP coding sequence and allowing isolation of transduced cells by sorting. The number in the square indicates positive ratio of EGFP in each transfectant (green histograms) compared to parental cells (open histograms). (B) Western blot probed with anti-HA antibody. THP-1 macrophages expressing WT or mutant (D135E) IRAK-M were co-cultured with equal numbers of PMN for 2hr. An anti-β-actin antibody served as loading control. (C) TNF-α expression in cell culture supernatants quantified by ELISA. THP-1 macrophages expressing WT or mutant (D135E) IRAK-M were co-cultured with PMN for 2hr. The ratio of PMN to macrophages is shown on the x axis. P value for the difference in TNF-α expression is shown. (D) TNF-α expression in cell culture supernatants. THP-1 macrophages expressing WT or mutant (D135E) IRAK-M were stimulated with PMN membranes for 2hr. The volume of isolated membrane fractions is shown on the x axis. Representative data of three independent experiments are shown.
We have observed that PMN induce significant TNF-α production in primary AM (data not shown). We therefore used TNF-α production as a measure of the biological effect of cleavage resistant mutant IRAK-M. PMN co-culture produced significantly less TNF-α in THP-1 macrophages expressing D135E mutant IRAK-M (Fig. 5C). Isolated PMN membranes induced less TNF-α in THP-1 macrophages expressing mutant IRAK-M than cells expressing WT IRAK-M (Fig. 5 D).
CASP-6 knock-down inhibits IRAK-M cleavage, IκBα degradation and TNF-α production after PMN stimulation
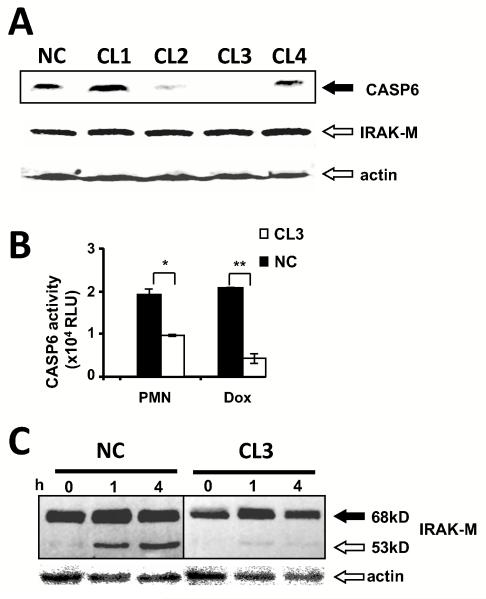
To test if CASP-6 expression is necessary for IRAK-M cleavage during PMN-macrophage contact, we developed stable transfectants of THP-1 macrophages expressing short hairpin RNA constructs designed to knock down CASP-6 (Fig. 6A, CL1 to 4). The protein level of CASP-6 in CL2 and CL3 transfectants was distinctly decreased, while the expression of IRAK-M was unaffected (Fig. 6A). CASP-6 activity induced by PMN-contact or doxorubicin was significantly decreased in CL3 compared to scrambled short hairpin RNA control (Fig. 6B, CL3 vs NC p = 0.02). CASP-6 deficient macrophages failed to cleave IRAK-M after PMN co-culture (Fig. 6C).
Figure 6. CASP-6 knockdown abolishes IRAK-M cleavage.
(A) Western blot probed with anti-CASP-6 antibody. The impact of differing shRNA construction on CASP-6 protein expression in THP-1 macrophages was determined. CASP-6 knockdown THP-1 macrophages were established by stable transfection of shRNA expressing constructs (CL1-CL4) or scramble shRNA control (NC). (B) CASP-6 activity assay. The impact of shRNA CL3 (white bar) and NC (black bar) on enzyme activity in THP-1 macrophages was determined. Macrophages were stimulated with PMN at a 1/1 ratio or 10μM Doxorubicin as shown on the x-axis. Data are mean ± SE from two independent experiments. (* p=0.02, ** p=0.005). (C) Western blot with probed with anti-IRAK-M. The impact of shRNA CL3 (white bar) and NC (black bar) on IRAK-M cleavage in THP-1 macrophages was determined. CL3 and NC macrophages co-cultured with equal numbers of PMN during the indicated time. The same membrane was re-probed with anti-β-actin as a loading control. Representative data of independent two experiments are shown.
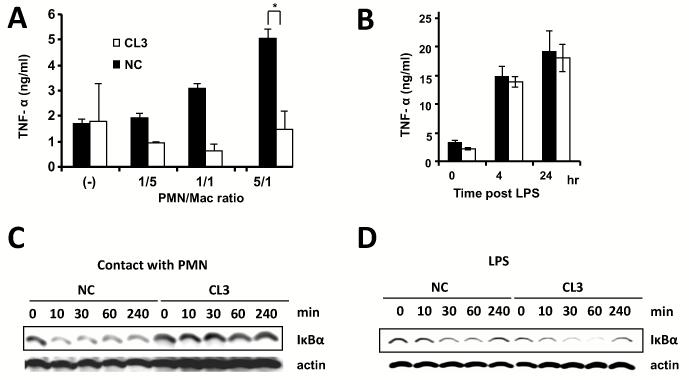
We then tested if reducing CASP-6 expression had functional consequences for innate immunity. CASP-6 knock-down CL3 macrophages produced significantly less TNF-α after PMN co-culture (Fig. 7A p < 0.05). TNF-α production after LPS stimulation was unaffected by CASP-6 knockdown (Fig. 7B). Since TNF-α is controlled by NF-κB binding to its promoter and NF-κB is activated by degradation of IκBα, we tested if CASP-6 knockdown affected IκBα degradation. PMN co-culture produced significant IκBα degradation in NC macrophages. CASP-6 deficient CL3 macrophages failed to degrade IκBα after contacting with PMN (Fig. 7C). CASP-6 knock down did not alter the effect of LPS on IκBα degradation (Fig 7D). CASP-6 deficient macrophages continued to phosphorylate p38 after both PMN and LPS stimulation (data not shown).
Figure 7. CASP-6 knockdown and blunts TNF-α production and IκBα degradation after PMN contact.
(A) TNF-α level in cell culture supernatants. CL3 CASP-6 knockdown or NC control macrophages were co-cultured with PMN at the indicated ratio for 16h (* p = 0.04, ** p = 0.01). Data are mean levels ± SE from three independent experiments. (B) TNF-α level in cell culture supernatants. CL3 or NC macrophages were stimulated with 100 ng/ml LPS during the indicated time. Data are mean levels ± SE from two independent experiments. (C)Western blot probed with anti-IκBα. CL3 and NC macrophages were co-cultured with equal cell-number of PMN to macrophages during the indicated time period. The same membrane was re-probed with anti-β-actin as a loading control. (D) Western blot probed with anti-IκBα. The macrophages were stimulated with 100 ng/ml LPS for the indicated times. The same membrane was re-probed with anti-β-actin as a loading control.
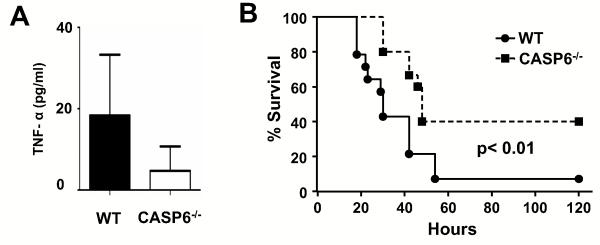
CASP-6 deficient mice have reduced pulmonary TNF-α production and prolonged survival after sepsis caused by bacterial peritonitis
To confirm the biological significance of CASP-6 in regulating the innate immune response in vivo, we employed the cecal ligation and puncture (CLP) model of sepsis in WT and CASP-6−/−mice. As the cecum is an endogenous source of bacterial contamination, perforation of the cecum results in systemic inflammatory response PMN infiltration in the lung (18). After 18h of CLP, TNF-α level in BAL fluid from CASP-6−/− mice was significantly decreased compare to similarly treated WT mice (Fig. 8A p<0.05). Bacteremia triggers septic shock and death. CASP-6−/−mice were protected from death after CLP (Fig. 8B CASP-6−/− vs WT p =0.01). These results support the validity of co-culture experiments on CASP-6 knock down macrophages and demonstrate CASP-6 is an important regulator of innate immunity in vivo.
Figure 8. CASP-6−/− mice have less BAL TNF-α production and lower mortality in polymicrobial sepsis CLP model.
(A) TNF-α level in BAL fluid. Samples were obtained 18h after CLP from CASP-6−/− (n = 8) and WT mice (n = 8). Data are represented as mean ± SE. (B) Survival after CLP. CASP-6−/− (n =15) and WT (n = 14) underwent CLP and were monitored for survival. The data are shown as a Kaplan-Meier plot with survival on the Y axis and time post CLP on the x axis.
DISCUSSION
This report demonstrates that PMN/macrophage contact activates CASP-6, producing IRAK-M cleavage and de-repression of AM. The novel conclusion that CASP-6 regulates innate immunity is supported by the observations that CASP-6 knock down macrophages have reduced TNF-α production after PMN stimulation in vitro and that CASP-6 deletion reduces TNF-α production in the lungs of septic mice in vivo. Importantly, CASP-6 deletion protects mice from death caused by bacterial peritonitis suggesting that pharmacological inhibition of CASP-6 activity may have therapeutic benefit during overwhelming infection. The data also provide evidence that IRAK-M is a significant target for the regulatory effects of CASP-6 since a cleavage resistant IRAK-M mutant blunts TNF-α production in macrophages after PMN stimulation in vitro.
CASP-6 is a member of the caspase family known to regulate B cell activation and differentiation into plasma cells (30). It is also induced in macrophages during apoptosis (31, 32). Caspases are synthesized as zymogens with a pro-domain of variable length followed by a large and a small subunit. Pro-CASP-6 has low activity and self-activation is driven by dimerization (33). The large pro-domains contain the caspase recruitment domain (CARD) or death effecter domain that allows recruitment to large protein complexes called inflammasomes. The inflammasome is an intracellular pathogen-recognition sensor that initiates inflammatory signaling by leading to caspase auto-phosphorylation and initiation of the inflammatory pathways (34). Caspases have well defined roles in inflammation, cleaving and activating pro-IL-1 beta and IL-18 (35).
A function for CASP-6 in innate immunity has not been reported. As with other caspases that regulate inflammation, CASP-6 is part of a multi-protein complex. We demonstrate that IRAK-M and TRAF6 bind CASP-6 in resting cells. This complex is present in a per-plasma membrane location. The presence of CASP-6 and IRAK-M in the same complex likely contributes to substrate specificity. PMN contact activates the CASP-6 dependent inflammatory pathway.
We confirmed the importance of CASP-6 on TNF-α production in vivo by using the CLP model in CASP-6−/− mice. In the CLP model, large numbers of neutrophils migrate into the lung during the systemic inflammatory response to overwhelming infection (36). CASP-6−/− mice have a marked reduction in TNF-α in BAL fluid reflecting decreased innate immunity that reduces mortality during overwhelming infection. Further definition of the regulatory pathway controlling contact-mediated macrophage activation will provide new insight in understanding the pathophysiology of inflammation. For example, the data from CASP-6−/− mice suggests that pharmacological inhibition of CASP-6 activity will reduce inflammation and may improve mortality in sepsis. Contact-dependent TNF-α production will limit the damaging effects of inflammation to areas of the lung where PMN are recruited.
The pathway regulated by CASP-6 in macrophages is separate from pathways controlling TLR signaling. Stimulation of macrophages with LPS does not induce CASP-6 or produce IRAK-M cleavage. Knock down of CASP-6 only prevents IκBα degradation and TNFα production after PMN co-culture; it does not alter the effects of LPS stimulation on macrophages. The innate immune function of CD80 is likely affected by CASP-6 activation. CD80 is central to the innate immune response in sepsis and CD80 binds IRAK-M in resting macrophages (18, 19). PMN stimulation disassociates this complex facilitating CD80 signaling (19). We now report that IRAK-M is also bound to CASP-6. This complex is located near the plasma membrane in resting cells and the components of this complex redistribute to the nucleus and cytoplasm following PMN stimulation. The signals that impact on the function of CASP-6 and IRAK-M in this complex will regulate signal transduction during PMN/macrophage contact.
The full length 68 kDa was the only isoform observed in resting AM obtained from volunteers without lung disease, AM from patients with pneumonia and macrophages after PMN co-culture express several truncated isoforms of IRAK-M. A second 53kDa short isoform occurred in alveolar macrophages from patients with pneumonia and after PMN stimulation in vitro. Several lines of evidence demonstrate CASP-6 is the protease that cleaves IRAK-M after PMN stimulation. First, cleavage at a predicted caspase recognition domain of IRAK-M, VTVD135, will produce a 53 kDa degradation product. Second, active recombinant CASP-6 cleaves WT IRAK-M but not a mutant IRAK-M, where D135 is substituted with E (D135E mutant). Finally, macrophages without CASP-6 fail to cleave IRAK-M upon PMN stimulation. The cleavage resistant D135E IRAK-M mutant is a dominant negative inhibitor of TNF-α production in macrophages. Cells with the D135E mutant have reduced TNF-α production after PMN stimulation even though endogenous WT IRAK-M is present. This data demonstrates that IRAK-M is an important target for CASP-6 and that cleaved IRAK-M loses inhibitory activity.
Stimulating macrophages with the membrane fractions of PMN substitutes for co-culture with intact PMN. Filter inserts that allow soluble factor diffusion but prevent direct contact between PMN and AM, abolish the ability of PMN to induce IRAK-M cleavage. Therefore, PMN membranes contain the biological activity needed to induce CASP-6 and produce IRAK-M cleavage. The membrane isolation protocol uses buoyant density centrifugation of detergent resistant membrane complexes that will separate the membranes fractions from proteins, nucleic acids and inorganic signaling molecules. These detergent resistant membrane complexes contain CD40 ligand and CD28 that mediate the de-repressing activity of PMN contact (14). In B cells CD40 stimulation activates CASP-6 (30, 37) and we have observed that CD40 stimulation produces cleaved IRAK-M (unpublished data). CD80 is required for TNF-α production in macrophages during PMN driven innate immunity. This may explain why stimulation of both CD40 and CD80 are required for the innate immune response to sepsis (9, 19).
IRAK-M is one of many inhibitors of innate immunity expressed in macrophages (7,12). Dominant negative transcription factors that inhibit TNF-α transcription are strongly expressed in resting AM and expression of these inhibitors is lost upon contact with activated lymphocytes or PMN (13,14). Expression of a redundant series of innate immune inhibitors is likely important for preventing chronic inflammation. Loss of these inhibitors may contribute to the pathophysiology of chronic inflammatory diseases where PMNs infiltrate tissues. For example, joints of patients with active rheumatoid arthritis or bowel wall in inflammatory bowel disease (38). Further understanding of PMN-contact dependent macrophage activation by de-repression may point to novel strategies to treat tissue injury caused by infectious and chronic inflammatory diseases.
ACKNOWLEDGMENTS
We thank Peter A. Lopez for flow cytometry and sorting, Yang Ruixi for DNA sequencing, the Ruth Lehmann lab for assistance in confocal microscopy.
This work was supported by National Institutes of Health (grants K24A1080298 (MDW), K23HLO84191 (AN), RO1HL0578779 (MDW), HL090316, TL1RR029892, UL1RR029893 (NYU CTSI) and T32 ES 07267 (BN), NYU Physician Scientist Training Program (LNS) and Yokohama foundation of advancement of medical science and Japan rheumatism foundation (HK)).
Glossary
Abbreviations used in this paper
- AM
Alveolar macrophages
- CASP
Caspase
- IRAK
IL-1 receptor associated kinase
- TLR
Toll-like receptor
- PAMPs
pathogen-associated molecular patterns
- TRAF
TNF receptor-associated factor
- Z-VEID-FMK
benzyloxycarbonyl (Cbz)-Val-Glu-Ile-Asp (Ome)-fluoromethylketone
- IRES
internal ribosome entry site
- CLP
Cecal ligation and puncture
REFERENCES
- 1.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AB, Rossi M. Neutrophils in normal lungs. Am Rev Respir Dis. 1983;127:S3–9. doi: 10.1164/arrd.1983.127.2P2.S3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki T, Maeda M, Hayashi H, Nakamura Y, Moriguchi H, Kamei T, Yasuoka S, Ogura T. Role of alveolar macrophages in the neutrophil-dependent defense system against Pseudomonas aeruginosa infection in the lower respiratory tract. Amplifying effect of muramyl dipeptide analog. Am Rev Respir Dis. 1989;140:1595–1601. doi: 10.1164/ajrccm/140.6.1595. [DOI] [PubMed] [Google Scholar]
- 6.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 7.Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol. 2000;165:2028–2039. doi: 10.4049/jimmunol.165.4.2028. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka N, Hoshino Y, Gold J, Hoshino S, Martiniuk F, Kurata T, Pine R, Levy D, Rom WN, Weiden M. Interleukin-10 induces inhibitory C/EBPbeta through STAT-3 and represses HIV-1 transcription in macrophages. Am J Respir Cell Mol Biol. 2005;33:406–411. doi: 10.1165/rcmb.2005-0140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold JA, Hoshino Y, Hoshino S, Jones MB, Nolan A, Weiden MD. Exogenous gamma and alpha/beta interferon rescues human macrophages from cell death induced by Bacillus anthracis. Infect Immun. 2004;72:1291–1297. doi: 10.1128/IAI.72.3.1291-1297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komuro I, Yokota Y, Yasuda S, Iwamoto A, Kagawa KS. CSF-induced and HIV-1-mediated distinct regulation of Hck and C/EBPbeta represent a heterogeneous susceptibility of monocyte-derived macrophages to M-tropic HIV-1 infection. J Exp Med. 2003;198:443–453. doi: 10.1084/jem.20022018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope RM, Leutz A, Ness SA. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda Y, Rogers L, Nakata K, Zhao BY, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med. 1998;188:1255–1265. doi: 10.1084/jem.188.7.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse DB, Shioda T, Rom WN, Weiden M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med. 2002;195:495–505. doi: 10.1084/jem.20011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino Y, Hoshino S, Gold JA, Raju B, Prabhakar S, Pine R, Rom WN, Nakata K, Weiden M. Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J Infect Dis. 2007;195:1303–1310. doi: 10.1086/513438. [DOI] [PubMed] [Google Scholar]
- 15.Magnarin M, Spessotto P, Soranzo MR, Pontillo A, Zabucchi G. Human neutrophils specifically interact with human monocyte-derived macrophage monolayers. Inflammation. 2000;24:89–98. doi: 10.1023/a:1006992126707. [DOI] [PubMed] [Google Scholar]
- 16.Venuprasad K, Banerjee PP, Chattopadhyay S, Sharma S, Pal S, Parab PB, Mitra D, Saha B. Human neutrophil-expressed CD28 interacts with macrophage B7 to induce phosphatidylinositol 3-kinase-dependent IFN-gamma secretion and restriction of Leishmania growth. J Immunol. 2002;169:920–928. doi: 10.4049/jimmunol.169.2.920. [DOI] [PubMed] [Google Scholar]
- 17.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 18.Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med. 2008;177:301–308. doi: 10.1164/rccm.200703-515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One. 2009;4:e6600. doi: 10.1371/journal.pone.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–19410. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 22.Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M, Du K, Singh S, Pike JW, Darnay BG, Choi Y, Wu H. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–447. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 23.Lagler H, Sharif O, Haslinger I, Matt U, Stich K, Furtner T, Doninger B, Schmid K, Gattringer R, de Vos AF, Knapp S. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. J Immunol. 2009;183:2027–2036. doi: 10.4049/jimmunol.0803862. [DOI] [PubMed] [Google Scholar]
- 24.Zacharioudaki V, Androulidaki A, Arranz A, Vrentzos G, Margioris AN, Tsatsanis C. Adiponectin promotes endotoxin tolerance in macrophages by inducing IRAK-M expression. J Immunol. 2009;182:6444–6451. doi: 10.4049/jimmunol.0803694. [DOI] [PubMed] [Google Scholar]
- 25.van ’t Veer C, van den Pangaart PS, van Zoelen MA, de Kruif M, Birjmohun RS, Stroes ES, de Vos AF, van der Poll T. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007;179:7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 26.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takebayashi K, Hokari R, Kurihara C, Okada Y, Okudaira K, Matsunaga H, Komoto S, Watanabe C, Kawaguchi A, Nagao S, Tsuzuki Y, Miura S. Oral tolerance induced by enterobacteria altered the process of lymphocyte recruitment to intestinal microvessels: roles of endothelial cell adhesion molecules, TGF-beta and negative regulators of TLR signaling. Microcirculation. 2009;16:251–264. doi: 10.1080/10739680802574166. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H, Hosono O, Iwata S, Kawasaki H, Kuwana M, Tanaka H, Dang NH, Morimoto C. The tetraspanin CD9 is preferentially expressed on the human CD4(+)CD45RA+ naive T cell population and is involved in T cell activation. Clin Exp Immunol. 2004;137:101–108. doi: 10.1111/j.1365-2249.2004.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe C, Shu GL, Zheng TS, Flavell RA, Clark EA. Caspase 6 regulates B cell activation and differentiation into plasma cells. J Immunol. 2008;181:6810–6819. doi: 10.4049/jimmunol.181.10.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SY, Cherla RP, Tesh VL. Simultaneous induction of apoptotic and survival signaling pathways in macrophage-like THP-1 cells by Shiga toxin 1. Infect Immun. 2007;75:1291–1302. doi: 10.1128/IAI.01700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ojcius DM, Kaufmann SH. Microbes and Infection: Past, present and future. Microbes Infect. 2009 doi: 10.1016/j.micinf.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Klaiman G, Champagne N, LeBlanc AC. Self-activation of Caspase-6 in vitro and in vivo: Caspase-6 activation does not induce cell death in HEK293T cells. Biochim Biophys Acta. 2009;1793:592–601. doi: 10.1016/j.bbamcr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Lamkanfi M, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases leave the beaten track: caspase-mediated activation of NF-kappaB. J Cell Biol. 2006;173:165–171. doi: 10.1083/jcb.200509092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 36.Mercer-Jones MA, Shrotri MS, Heinzelmann M, Peyton JC, Cheadle WG. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J Leukoc Biol. 1999;65:249–255. doi: 10.1002/jlb.65.2.249. [DOI] [PubMed] [Google Scholar]
- 37.Olson NE, Graves JD, Shu GL, Ryan EJ, Clark EA. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J Immunol. 2003;170:6065–6072. doi: 10.4049/jimmunol.170.12.6065. [DOI] [PubMed] [Google Scholar]
- 38.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]