Abstract
Dynorphin A (Dyn A) is an endogenous ligand for kappa (κ) opioid receptors. To restrict the conformational mobility, we synthesized several cyclic Dyn A-(1-11)NH2 analogs on solid phase utilizing ring-closing metathesis (RCM) between the side chains of allylglycine (AllGly) residues incorporated in positions 2, 5 and/or 8. Cyclizations between the side chains of AllGly gave reasonable yields (56–74%) of all of the desired cyclic peptides. Both the cis and trans isomers were obtained for all of the cyclic peptides, with the ratio of cis to trans isomers depending on the position and stereochemistry of the AllGly. Most of the cyclic Dyn A-(1-11)NH2 analogs examined exhibit low nanomolar binding affinity for κ opioid receptors (Ki = 0.84–11 nM). In two of the three cases the configuration of the double bond has a significant influence on the opioid receptor affinity and agonist potency. All of the peptides inhibited adenylyl cyclase (AC) activity in a concentration-dependent manner with full or close to full agonist activity. These potent Dyn A analogs are the first ones cyclized by RCM.
Introduction
Potent clinically used narcotic analgesic agents, such as morphine and its analogs, mainly act as mu (μ) opioid receptor agonists. However, their use is associated with serious side effects, such as respiratory depression, physiological and psychological dependence, and constipation.1 Therefore considerable effort has focused on the development of κ selective opioid agonists, especially those acting in the periphery, as potential analgesics without the side effects associated with morphine and other μ opioid receptor agonists.2, 3 Besides their roles in analgesia, κ opioid receptor agonists may also have other therapeutic applications, which include the treatment of cocaine dependence,4 as neuroprotective and anticonvulsant agents,5 and the treatment of HIV-1 and HIV-1 related encephalopathy. 6, 7 Ligands for κ opioid receptors are very useful for studying the functions of κ opioid receptors at the molecular level, which in turn could be very important in the development of new therapeutic agents.
Dynorphin A (Dyn A, Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln), a heptadecapeptide first isolated from porcine pituitary,8 is an endogenous ligand for κ opioid receptors and is thought to be involved in a variety of physiological functions.9 Dyn A has an identical N-terminal tetrapeptide sequence (the “message” sequence, Tyr-Gly-Gly-Phe)10 as most other mammalian opioid peptides and a C-terminal sequence (the “address” sequence)10 which is unique to Dyn A. Dyn A-(1-13) and Dyn A-(1-11) exhibit similar κ opioid receptor activity to Dyn A, 10 and therefore these two shorter peptides have often served as parent structures for structure-activity relationship (SAR) studies and for the development of analogs with improved κ affinity, selectivity, potency, and/or altered efficacy.
Like most linear peptides, Dyn A can adopt numerous conformations, and because of this, the biologically active conformations are not yet clear.11–16 This inherent conformational flexibility may be one of the reasons that Dyn A also exhibits significant affinity for μ and δ opioid receptors, resulting in low selectivity for κ opioid receptors.
Conformational constraint by cyclization is one approach that can be used to restrict the flexibility of peptides, and therefore is a valuable approach to study topographical requirements of receptors.17–20 Cyclization of peptides can provide potent and selective ligands for receptors when appropriate conformational constraints are incorporated,18 because a well-fit pre-organized conformation decreases the entropy penalty for receptor binding.19 Furthermore, cyclic peptides are often more stable to peptidases,21–23 and therefore they can have improved pharmacokinetic profiles and represent promising lead compounds for further development.
Conformational constraint by cyclization has been successfully employed in the development of several potent opioid peptides. Several cyclic Dyn A analogs have been synthesized and evaluated for their biological activity.1, 19, 24–31 Our laboratory previously reported several cyclic Dyn A analogs where either the “message” or “address” sequence 10 was constrained. cyclo[D-Asp2,Dap5]Dyn A-(1-13)NH2 (Dap = 2,3-diaminopropionic acid) exhibits high affinity for both κ and μ opioid receptors.29 cyclo[D-Asp5,Dap8]Dyn A-(1-13)NH2 shows modest affinity for κ opioid receptors compared with the linear peptide Dyn A-(1-13)NH2, but it shows increased selectivity for κ over μ and δ opioid receptors compared to Dyn A-(1-13)NH2.31 Cyclic Dyn A analogs have also been prepared by other research groups utilizing either disulfide25–28 or amide24, 30 bonds to constrain the peptides.
We utilized ring-closing metathesis (RCM) in the design and synthesis of new cyclic Dyn A analogs. RCM has emerged as a very useful method for making cyclic organic compounds as well as cyclic peptides.32–35 Compared with peptides cyclized by amide or disulfide bond formation, there are some advantages of using RCM. The resulting carbon-carbon bond is more stable than a disulfide or an amide bond.34, 36 Furthermore, in contrast to cyclization via amide or disulfide bond, side chain functionalities can be maintained by appropriate choice of the amino acid side chains for cyclization by RCM. In addition, cyclization by RCM can potentially stabilize different conformations of a peptide compared to a disulfide or amide as a result of the geometry of the linkage, The C-S-S-C dihedral angle for disulfide linkages is approximately ± 90°. While the amide bond in a lactam linkage can potentially be either cis (0°) or trans (180°), the conformation of this bond for secondary amides is predominantly trans, except in selected cases involving a small ring that favor the cis conformation due to ring strain in the trans conformation. Also the two conformations of the amide bond cannot be isolated from one another. In contrast, in many cases both the cis and trans isomers of the double bond are obtained from RCM and can often be separated from one another by careful choice of chromatographic conditions (see below). This permits the examination of the effect of a cis double bond on the biological activity of the peptide compared to that of the trans isomer, a comparison that isn’t possible with cyclization via a lactam.
The application of RCM to the cyclization of opioid peptides has been very limited. To date there have been only three reports of RCM cyclic analogs of short opioid peptides, namely pentapeptide enkephalin analogs and tetrapeptides related to dermorphin.37–39 These analogs were cyclized between D-allylglycine (D-AllGly) in position 2 and D- or L-AllGly in position 4 in the tetrapeptides or position 5 in the enkephalin derivatives. Some of these cyclic analogs showed potent activity at μ and δ opioid receptors; as expected, the enkephalin derivatives exhibited low affinity for κ opioid receptors (the receptor affinities of the tetrapeptides were not reported). In contrast, there have been no reports of longer opioid peptides (e.g. Dyn A analogs) cyclized through RCM., or of peptides that preferentially interact with κ opioid receptors. Here we describe our results for incorporating a cyclic constraint via RCM in the “address” (C-terminal) sequence as well as the “message” (N-terminal) sequence of Dyn A-(1-11)NH2. and the effects of the double bond configuration on opioid receptor affinity and potency.
Results and discussion
Analog design
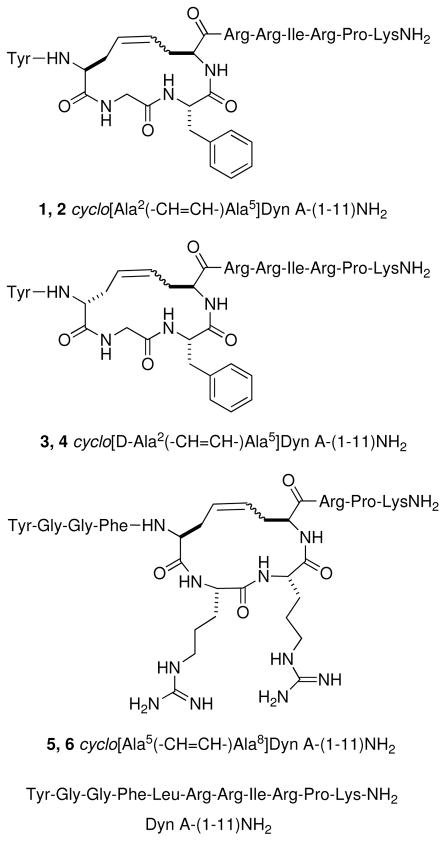
Cyclic [2,5] and [5,8] Dyn A-(1-11)NH2 analogs (Figure 1) were chosen to evaluate RCM for cyclizing Dyn A analogs and to examine the effects of these cyclizations on κ opioid receptor affinity, selectivity, efficacy, and potency. Substitution of a D-amino acid in position 2 for Gly in the linear peptides is well tolerated by κ opioid receptors; however, this modification in Dyn A analogs can greatly increase μ receptor affinity, resulting in compounds that are either nonselective or selective for μ opioid receptors.40 Substitution of this position with an L-amino acid decreases binding affinity for all three types of opioid receptors. However, the κ opioid receptor is more tolerant of the L-configuration at this position than the other opioid receptors, and therefore the selectivity for κ opioid receptors can be increased by substitution with an L-amino acid.40 Based on these observations, in the [2,5] cyclic analogs (Figure 1) both L- and D-allylglycine (AllGly) were introduced in position 2 to evaluate their effects on affinity, selectivity, potency, and efficacy at κ opioid receptors. Leu5 in Dyn A is not important for opioid activity,41 and therefore this position can be used for cyclization. Similarly, Leu5 and Ile8 were substituted with AllGly and then cyclized by RCM to yield the [5,8] cyclic analogs (Figure 1). Cyclization by RCM maintains the hydrophobic nature of these residues (Figure 1).
Figure 1.
Structural comparison of cyclo[L/D-Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2 (1–4) and cyclo[Ala5(-CH=CH-)Ala8]Dyn A-(1-11)NH2 (5, 6) with Dyn A-(1-11)NH2
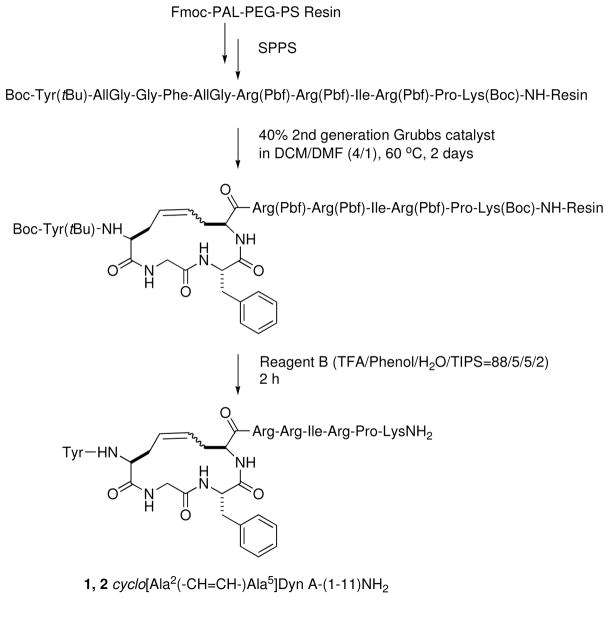
Synthesis
The peptides were synthesized by solid phase synthesis using Fmoc-protected (Fmoc = 9-fluorenylmethoxycarbonyl) amino acids (Scheme 1). L/D-AllGly was incorporated in appropriate positions in the linear precursor peptides, and upon completion of assembly of the peptide chains, second generation Grubbs’ catalyst was used to cyclize the peptides on the solid support. A mixture of dichloromethane (DCM) and N,N-dimethylformamide (DMF) (4/1, v/v) was used as the solvent for the RCM reaction. The addition of a small amount of DMF has several advantages; DMF is compatible with both the hydrophilic peptide chain and resin and allows a higher temperature to be used for the reaction. The position or stereochemistry of the AllGly residue did not have much influence on the yield of the desired cyclic peptides. Cyclizations between the side chains of AllGly generally gave reasonable yields of the desired cyclic peptides (56–74%, Table 1). Both the cis and trans isomers were obtained for all of the cyclic peptides, with the ratio of cis to trans isomers, as determined by NMR, varying from approximately 1:1.1 to 1:2.3, depending on the position and stereochemistry of the AllGly (Table 1).
Scheme 1.
Synthesis of cyclic peptides 1 and 2
Table 1.
Yields and cis/trans ratios of Dyn A(1-11)NH2 analogs cyclized by RCM
Because the cis and trans isomers have very similar retention times (within 0.7 min) in the standard high-performance liquid chromatography (HPLC) system (5–50% of MeCN with 0.1% TFA over 45 min at 1 mL/min, see Table 2), a very slow gradient (0.1% increase in solvent B/min) was used for purification. The two isomers were successfully separated and characterized by HPLC, electrospray ionization mass spectrometry (ESI-MS), and nuclear magnetic resonance (NMR) of the purified fractions (Tables 2 and 3).
Table 2.
HPLC and ESI-MS data of purified peptides 1–6
| Peptides | HPLC tR (min)a | ESI-MS (m/z) | ||
|---|---|---|---|---|
| System 1 | System 2 | Calculated | Observed | |
| 1 | 13.23 | 24.08 | [M+3H]3+=453.3 [M+4H]4+=340.2 [M+2H]2+=679.4 |
[M+3H]3+=453.3 [M+4H]4+=340.2 [M+2H]2+=679.4 |
| 2 | 13.30 | 25.38 | [M+4H]4+=340.2 [M+3H]3+=453.3 [M+2H]2+=679.4 |
[M+4H]4+=340.2 [M+3H]3+=453.3 [M+2H]2+=679.4 |
| 3 | 12.78 | 24.87 | [M+4H]4+=340.2 [M+3H]3+=453.3 [M+2H]2+=679.4 |
[M+4H]4+=340.2 [M+3H]3+=453.2 [M+2H]2+=679.4 |
| 4 | 13.49 | 26.99 | [M+4H]4+=340.2 [M+3H]3+=453.3 [M+2H]2+=679.4 |
[M+4H]4+=340.2 [M+3H]3+=453.3 [M+2H]2+=679.4 |
| 5 | 9.11 | 16.32 | [M+3H]3+=434.6 [M+4H]4+=326.2 |
[M+3H]3+=434.6 [M+4H]4+=326.2 |
| 6 | 9.63 | 17.85 | [M+3H]3+=434.6 [M+4H]4+=326.2 |
[M+3H]3+=434.6 [M+4H]4+=326.2 |
System 1: Solvent B = MeCN; system 2: Solvent B = MeOH; see the experimental section for details. The final purity of all of the peptides by both methods was greater than 98%.
Table 3.
1H-NMR data for vinyl protons of cyclic peptides 1–6
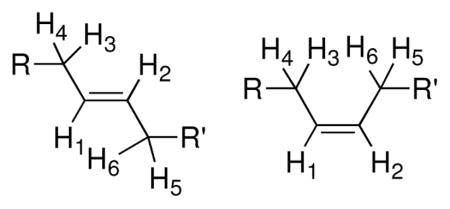 | ||
|---|---|---|
| Peptide | Chemical shifts (δ) of vinyl protons | J (Hz) |
| 1 (cis) | H1 = 5.10 | J12 = 10.4 |
| H2 = 5.34 | J21 = 12.4 | |
| 2 (trans) | H1 = 5.25 | J12 = 14.9 |
| H2 = 5.47 | J21 = 13.9 | |
| 3 (cis) | H1 = 5.05 | J12 = 11.4 |
| H2 = 5.16 | J21 = 12.1 | |
| 4 (trans) | H1 = 5.12 | J12 = 14.7 |
| H2 = 5.26 | J21 = 15.4 | |
| 5 (cis) | H1 = 5.14 | J12 = 10.5 |
| H2 = 5.41 | J21 = 12.9 | |
| 6 (trans) | H1 = 5.21 | J12 = 14.9 |
| H2 = 5.46 | J21 = 13.9 | |
The NMR J-couplings and chemical shifts were used to distinguish between the cis and trans isomers.42–44 The splitting patterns of the vinyl protons for the cis and trans isomers are very different due to the different coupling constants to the adjacent methylene protons. For the trans isomer, the coupling constants between the two vinyl protons (J12) are ~15 Hz, while the coupling constants between the vinyl protons and their corresponding adjacent methylene protons (J13, J14, J25 and J26) are around 7–8 Hz (Table 3). Because of the coupling constants, generally five peaks (approximate ratio of 1:2:2:2:1) were observed in the NMR spectra of the trans isomers (Figure 2). For the cis isomer, the coupling constants between the two vinyl protons (J12) are ~10 Hz, while the coupling constants between the vinyl protons and adjacent methylene protons are ~10 and 2 Hz. Because of the broad linewidth, only three peaks were generally observed for the cis isomers in a ratio of 1:2:1 (Figure 2). The chemical shifts of the two vinyl protons in the cyclic peptides are between 5.0 and 5.5 ppm (Table 3). The two vinyl protons in the cis isomer are slightly more shielded (upfield) than in the trans isomers (Figure 2 and Table 3).
Figure 2.
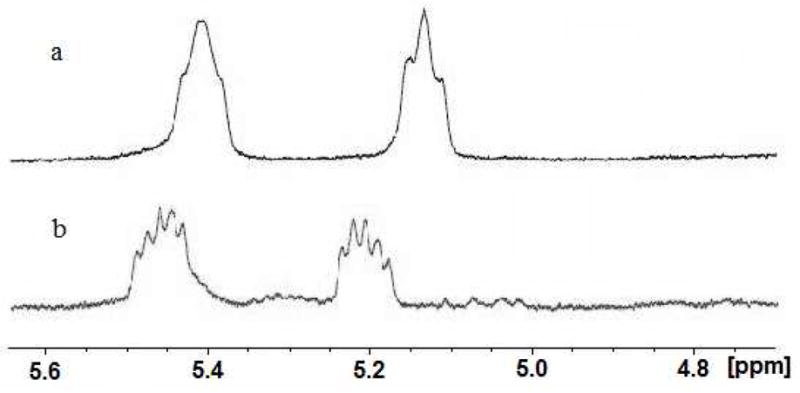
Chemical shifts and splitting patterns of the two vinyl protons of cyclic peptides 5 (a, cis) and 6 (b, trans)
Pharmacology
The cyclic peptides were evaluated for their binding affinity at κ, μ, and δ opioid receptors using radioligand binding assays31 (Table 4). Except for compound 1, all of the cyclic Dyn A analogs examined exhibit low nanomolar binding affinity for κ opioid receptors (Ki = 0.84 – 11 nM).
Table 4.
Opioid receptor binding affinities of Dyn A analogs cyclized by RCM
| Compound | Ki (nM) | Ki ratio (κ/μ/δ) | ||
|---|---|---|---|---|
| κ | μ | δ | ||
| 1 | 87.2 ± 6.9 | 763 ± 35 | 7670 ± 1030 | 1/8.8/88 |
| 2 | 9.46 ± 1.80 | 180 ± 10 | 1130 ± 98 | 1/19/119 |
| 3 | 0.84 ± 0.10 | 2.33 ± 0.20 | 9.30 ± 1.00 | 1/2.8/11 |
| 4 | 1.38 ± 0.31 | 2.33 ± 0.22 | 7.17 ± 0.55 | 1/1.7/5.2 |
| 5 | 10.9 ± 1.8 | 93.0 ± 6.0 | 1210 ± 90 | 1/8.5/111 |
| 6 | 2.46 ± 0.57 | 36.0 ± 2.1 | 460 ± 51 | 1/15/187 |
| Dyn A-(1-11)NH2a | 0.57 ± 0.01 | 1.85 ± 0.52 | 6.18 ± 1.01 | 1/3/11 |
From reference 45.
The [2,5] cyclizations involving an L-AllGly in position 2 decreased κ opioid receptor affinity. The trans isomer of cyclo[Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2 (2) exhibits modest κ opioid receptor affinity (Ki = 9.5 nM) (Table 4).45 However, the cis isomer 1 exhibits 10-fold lower affinity for κ receptors than the trans isomer 2, presumably due to the differences in the peptides’ conformations. Compared with Dyn A-(1-11)NH2, both isomers 1 and 2 exhibit much lower affinity for μ and δ receptors, resulting in higher κ opioid receptor selectivity for these compounds than Dyn A-(1-11)NH2 (Table 4). The cis isomer (1) has similar selectivity as the trans isomer (2), even though it shows about 10-fold lower affinity for κ opioid receptors. Thus while substitution of an L-amino acid in position 2 in the cyclic peptides decreases κ opioid receptor affinity compared with Dyn A-(1-11)NH2, the selectivity towards the other opioid receptors (μ and δ) can be increased, as the latter receptors are less tolerant of the introduction of an L-amino acid in position 2 compared to κ opioid receptors.
In contrast, the [2,5] cyclizations involving a D-AllGly in position 2 are well tolerated by κ opioid receptors. The two isomers of cyclo[D-Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2 (3 and 4) exhibit high κ opioid receptor affinity (Ki = 0.84 and 1.33 nM for the cis and trans isomers, respectively, Table 4). However, these two compounds also exhibit high affinity for μ and δ opioid receptors, and therefore have minimal selectivity for κ over these opioid receptors. These results are similar to those found for cyclo[D-Asp2,Dap5]Dyn A-(1-13)NH2 and cyclo[D-Asp2,Dap5]Dyn A-(1-11)NH2.29, 46
Schiller and Hruby previously reported the synthesis of several tetrapeptide and enkephalin analogs utilizing RCM.37–39 The two olefinic dicarba analogs of the enkephalin cyclic peptide H-Tyr-c[D-Cys-Gly-Phe-L-Cys]NH2 exhibit nanomolar affinity for both μ (2.40 nM and 0.616 nM for the cis and trans isomers, respectively) and δ (6.55 nM and 1.25 nM for the cis and trans isomers, respectively) opioid receptors.38 As expected, these enkephalin analogs exhibit low affinity for κ opioid receptors (200 nM and 57.6 nM for the cis and trans isomers, respectively).38 The addition of the C-terminal residues of Dyn A-(1-11)NH2 substantially increases the affinity for κ opioid receptors, while maintaining the affinity for μ opioid receptors and decreasing the affinity for δ opioid receptors. Interestingly, while the two enkephalin isomers reported previously have different affinities for all three opioid receptors, our two cyclic Dyn A isomers 3 and 4 exhibit very similar affinities for each of the receptors.
The cyclizations in the “address” sequence of Dyn A involving two AllGly residues substituted in positions 5 and 8 are also tolerated by κ opioid receptors. The κ opioid receptor affinity of the trans isomer of cyclo[Ala5(-CH=CH-)Ala8]Dyn A-(1-11)NH2 (6) (2.46 nM) is 5-fold lower than Dyn A-(1-11)NH2. However, this compound exhibits significantly higher selectivity for κ over μ and δ receptors (15- and 187-fold, respectively, Table 4) than the linear Dyn A-(1-11)NH2. Similar to the isomers 1 and 2 of cyclo[Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2, the cis isomer of cyclo[Ala5(-CH=CH-)Ala8]Dyn A-(1-11)NH2 (5) shows significantly lower affinity (4.4-fold) for κ opioid receptors (Ki = 10.9 nM) than the trans isomer 6. However, the two isomers (5 and 6) exhibit similar selectivities for κ vs μ and δ opioid receptors (Table 4).
The cyclic analogs were also examined for concentration-dependent inhibition of adenylyl cyclase (AC) (Table 5).47 All of the compounds inhibit AC activity in a concentration-dependent manner with similar efficacy (≥ 90%) to the reference agonist Dyn A-(1-13)NH2. Thus the cyclizations have little or no effect on the efficacy of these Dyn A-(1-11)NH2 analogs. The potencies (EC50) of these cyclic analogs in the AC assays (Table 5) are well correlated with their κ opioid receptor affinities. The two isomers of cyclo[D-Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2 (3 and 4) exhibit the highest potency with EC50 values (0.80 and 0.47 nM, respectively, Table 5) comparable to that of the parent peptide Dyn A-(1-11)NH2. These results are consistent with those reported for cyclo[D-Asp2,Dap5]Dyn A-(1-11)NH2.46 For the two isomers of cyclo[Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2 (1 and 2), where the configuration of position 2 is L instead of D, the potencies dropped significantly (487- and 69-fold for 1 and 2, respectively) compared with Dyn A-(1-11)NH2. Similar to the affinities for κ opioid receptors, the trans isomer (2) is about 7-fold more potent than the cis isomer (1). The two isomers of cyclo[Ala5(-CH=CH-)Ala8]Dyn A-(1-11)NH2 (5 and 6) show intermediate potency among these cyclic peptides, with the trans isomer 6 being 2.8-fold more potent than the corresponding cis isomer 5.
Table 5.
κ opioid receptor potencies and efficacies in the AC assay of Dyn A analogs cyclized by RCM
| Compound | EC50 (nM) | Maximum AC % inhibitiona |
|---|---|---|
| 1 | 190 ± 19 | 90 ± 2 |
| 2 | 27 ± 0.0 | 104 ± 4 |
| 3 | 0.80 ± 0.34 | 111 ± 6 |
| 4 | 0.47 ± 0.11 | 107 ± 4 |
| 5 | 23 ± 11 | 110 ± 10 |
| 6 | 8.3 ± 3.7 | 106 ± 6 |
| Dyn A-(1-11)NH2 | 0.39 ±0.02 | 100 |
Relative to Dyn A-(1-11)NH2 (100%).
Conclusions
Here we report the synthesis of the first Dyn A analogs cyclized by RCM and the first peptides that preferentially interact with κ opioid receptors cyclized by this methodology. Cyclizations in both the “message” and “address” sequences of Dyn A were explored to prepare potent κ opioid receptor agonists. While cyclization by RCM has been introduced into the “message” sequence in enkephalin analogs and tetrapeptides related to dermorphin, 37–39 this is the first application of this type of cyclization in the “address” sequence of an opioid peptide. Cyclization by RCM has some advantages over traditional approaches such as amide or disulfide bond formation in these Dyn A analogs. The carbon-carbon double bond in these peptides retains similar lipophilicity to the side chains of Leu and Ile found in positions 5 and 8 in Dyn A. The positions and stereochemistry of the residues involved in the cyclizations influenced the affinity and selectivity for κ opioid receptors. Both [2,5] cyclic analogs with the D-configuration in position 2 (compounds 3 and 4) retain high affinity for κ, μ, and δ receptors, indicating that the conformations which are adopted by these two peptides are compatible with all three opioid receptor types. Thus, these two peptides show minimal selectivity for κ over μ receptors and low selectivity for κ over δ receptors. The [5,8] cyclic analogs (compounds 5 and 6) show intermediate affinity for κ opioid receptors; however, their selectivity over the other opioid receptors is greater than Dyn A-(1-11)NH2. The [2,5] cyclic analogs with the L-configuration in position 2 (1 and 2) show the lowest affinity for κ opioid receptors; however, these two compounds also exhibit higher selectivity for κ receptors compared with the parent peptide Dyn A-(1-11)NH2. In the latter two cases the configuration of the double bond has a significant influence on the opioid receptor affinity and agonist potency, likely due to the double bond configuration affecting the conformation of the cyclic portion of the peptide. In both cases the peptides containing the trans double bond exhibits higher κ opioid receptor affinity, selectivity and agonist potency in the AC assay than the cis isomer. Similar to Dyn A, these cyclic Dyn A analogs all exhibit concentration-dependent agonist activity (≥ 90% efficacy) at κ opioid receptors with potencies well correlated with their affinities.
These analogs represent interesting lead compounds for further characterization of conformation-activity relationships for Dyn A at κ opioid receptors. Further studies of these and other Dyn A analogs cyclized by RCM are ongoing in our laboratory.
Experimental section
Materials
All standard Fmoc-protected amino acids were purchased from Bachem (King of Prussia, PA), Calbiochem-Novabiochem (San Diego, CA), Applied Biosystems (Foster City, CA), or Peptides International (Louisville, KY). Fmoc-AllGly-OH and Fmoc-D-AllGly-OH were purchased from NeoMPS (San Diego, CA). Fmoc-PAL-PEG-PS (PAL-PEG-PS = Peptide Amide Linker-poly(ethylene glycol)-polystyrene) resin was purchased from Applied Biosystems. Benzotriazole-1-yloxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) was purchased from Calbiochem-Novabiochem. DCM, N,N-diisopropylethylamine (DIEA), DMF, acetic acid, diethyl ether, acetonitrile, methanol, and trifluoroacetic acid (TFA) were purchased from Fisher Scientific (Hampton, NH). 1-Hydroxybenzotriazole (HOBt) and triisopropylsilane (TIPS) were purchased from Acros Chemical Co. (Pittsburgh, PA). All other chemicals including phenol, piperidine, second generation Grubbs’ catalyst and DMSO-d6 (dimethyl sulfoxide-d6) were purchased from Aldrich Chemical Co. (Milwaukee, WI).
Synthesis of cyclic Dyn A analogs
The peptides were synthesized on the Fmoc-PAL-PEG-PS resin (300 mg, 0.19–0.21 mmol/g) using a CS Bio automated peptide synthesizer, except for the couplings of Fmoc-AllGly-OH and Fmoc-D-AllGly-OH, which were performed manually. The synthesis of the peptides cyclo[Ala2(-CH=CH-)Ala5]Dyn A-(1-11)NH2 (1 and 2) is shown in Scheme 1 as an example. The desired Fmoc-protected amino acids were coupled to the growing peptide chain with PyBOP, HOBt, and DIEA (4/4/10 relative to the resin substitution) in DMF (2 mL) for 2 h.; for Fmoc-AllGly-OH and Fmoc-D-AllGly-OH 2 equiv were used for the couplings (using 2, 2, and 5 equiv of PyBOP, HOBt, and DIEA relative to the resin, respectively). The completion of the reactions was determined by the ninhydrin test.48 Following the assemble of linear precursor, the resin was mixed with 40 mol% second-generation Grubbs’ catalyst in DCM/DMF (4/1, v/v) under reflux conditions (60 °C) for 2 d (Scheme 1). The resin was then washed with DCM (10 × 5 mL) to remove the catalyst. Finally, the resin was washed with methanol and dried under vacuum. The crude cyclic peptides were cleaved from the resin by treating with 5 mL Reagent B (88% TFA, 5% phenol, 5% water, and 2% TIPS) for 2 h49 and the peptides isolated as described previously.50
Analysis of cyclic Dyn A analogs
The crude peptides were analyzed by analytical reversed-phase HPLC to determine the yields and ratios of the two isomers obtained from the RCM reaction. A linear gradient of 5–50% MeCN containing 0.1% TFA over 45 minutes, at a flow rate of 1 mL/min, was used for the analysis.
The crude peptides were purified by preparative reversed-phase HPLC using a linear gradient of 5–23% aqueous MeCN containing 0.1% TFA over 3 h (0.1% MeCN/min), at a flow rate of 20 mL/min. The purity of the final peptides was verified using two analytical HPLC systems (Table 2). For analytical HPLC, a linear gradient of 5–50% solvent B (solvent A, aqueous 0.1% TFA and solvent B, MeCN (system 1) or MeOH (system 2) containing 0.1% TFA) over 45 minutes, at a flow rate of 1 mL/min, was used. The final purity of all of the peptides by both methods was greater than 98%. Molecular weights of the peptides (Table 2) were determined by ESI-MS (Waters, Q-TOF).
The configuration of the double bond of the cyclic RCM peptides was determined by NMR analysis. 1H NMR spectra of these compounds (2–5 mg) were obtained at 25 °C in DMSO-d6 on a Bruker AVANCE DRX-500 spectrometer (500.13 MHz proton frequency) equipped with a 5 mm z-gradient Cryoprobe. 1H chemical shifts referenced to the residual DMSO signal at 2.49 ppm and coupling constants were extracted from the 1D spectra (Table 3). The 1D 1H-NMR spectra of these cyclic analogs are provided in the supporting information.
Pharmacological assays
Radioligand binding assays were performed as previously described31 using cloned rat κ, rat μ, and mouse δ opioid receptors stably expressed separately on CHO cells. [3H]Diprenorphine (0.4 nM), [3H]DAMGO ([D-Ala2,MePhe4,glyol]enkephalin, 1 nM) and [3H]DPDPE (cyclo[D-Pen2,D-Pen5]enkephalin, 0.15 nM) were used as radioligands in the binding assays for κ, μ, and δ opioid receptors, respectively. Incubations were carried out in triplicate with varying concentrations of peptides (0.1 nM to 10 μM) for 90 min at room temperature in the presence of peptidase inhibitors (10 μM bestatin, 30 μM captopril, and 50 μM L-leucyl-L-leucine) and 3 mM Mg2+. Nonspecific binding was determined in the presence of 10 μM unlabeled Dyn A-(1-13)NH2,, DAMGO or DPDPE for κ, μ and δ receptors, respectively. IC50 values were determined by nonlinear regression analysis to fit a logistic equation to the competition data using GraphPad Prism software (GraphPad Software Co., San Diego, CA). Ki values were calculated from the IC50 values by the Cheng and Prusoff equation,51 using KD values of 0.45, 0.49 and 1.76 nM for [3H]diprenorphine, [3H]DAMGO and [3H]DPDPE, respectively. The results presented are the mean ± SEM from at least three separate assays.
The peptides were also evaluated for their ability to inhibit the synthesis of cAMP by AC using cloned rat κ opioid receptors stably expressed on CHO cells as previously described.47 Cells were washed twice with free F12 medium and then incubated for 4 h in 1 mL of the same media containing 12 μCi [3H]adenine. The cells were then incubated at 37 °C for 40 min in the presence of 50 μM forskolin, peptidase inhibitors (10 μM bestatin, 30 μM captopril, and 50 μM L-leucyl-L-leucine) and varying concentrations of the peptide ligand (0.1–10,000 nM in 10-fold dilutions). Incubations were terminated by the addition of 30 μL of stop solution (2% sodium dodecyl sulfate and 1.3 mM cyclic adenosine monophosphate (cAMP) in water), followed by the addition of 100 μL of conc perchloric acid and 750 μL water. [14C]cAMP (500 cpm in 50 μL) was added to each well to correct for recovery. After transferring the contents of the wells to 1.5 mL centrifuge tubes, 12 M KOH was added to neutralize the samples. The resulting precipitates were pelleted by centrifugation at 10,000g for 10 minutes. cAMP in the supernatants was isolated by sequential chromatography over BioRad AG-50W-X4 cation exchange resin and neutral alumina. The concentrations of [3H]cAMP and [14C]cAMP in the eluants were determined simultaneously by scintillation counting. Counts were corrected for crossover and recovery. The efficacies of the analogs are expressed relative to the reference compound Dyn A-(1-13)NH2. The results presented are the mean ± SEM from at least three separate assays.
Supplementary Material
Acknowledgments
We thank Dr. David VanderVelde from the University of Kansas NMR Laboratory for assistance with configurational assignment based on the NMR data. Funding was provided by National Institute of Drug Abuse grant R01 DA18832.
Footnotes
Abbreviations: Abbreviations used for amino acids follow the rules of the IUPAC-IUB Joint Commission of Biochemical Nomenclature (Eur. J. Biochem. 1984, 138, 9–37). Amino acids are the L-configuration except where indicated otherwise. AC, adenylyl cyclase; AllGly, allylglycine; cAMP: cyclic adenosine monophosphate; DAMGO, ([D-Ala2,MePhe4,glyol]enkephalin; Dap, 2,3-diaminopropionic acid; DCM, dichloromethane; DIEA, N,N-diisopropylethylamine; DMF, N,N-dimethylformamide; DMSO, dimethyl sulfoxide; DPDPE, cyclo[D-Pen2,D-Pen5]enkephalin; Dyn, dynorphin; ESI-MS, electrospray ionization-mass spectrometry; Fmoc, fluorenylmethoxycarbonyl; HOBt, hydroxybenzotriazole; HPLC, high-performance liquid chromatography; PAL-PEG-PS, Peptide Amide Linker-poly(ethylene glycol)-polystyrene); PyBOP, benzotriazole-1-yloxytripyrrolidinophosphonium hexafluorophosphate; RCM, ring-closing metathesis; SAR, structure-activity relationships; SPPS, solid phase peptide synthesis; TFA, trifluoroacetic acid; TIPS, triisopropylsilane.
Supporting Information Available. The 1H-NMR data of these cyclic Dyn A analogs. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Aldrich JV, Vigil-Cruz SC. Narcotic Analgesics. In: Abraham DJ, editor. Burger’s Medicinal Chemistry & Drug Discovery. Vol. 6. John Wiley & Sons, Inc; New York: 2003. pp. 329–481. [Google Scholar]
- 2.Millan MJ. κ-Opioid Receptors and Analgesia. Trends Pharmacol Sci. 1990;11:70–76. doi: 10.1016/0165-6147(90)90321-x. [DOI] [PubMed] [Google Scholar]
- 3.Riviere PJM, Junien JL. Opioid Receptors, Targets for New Gastrointestinal Drug Development. In: Gaginella TS, Guglietta A, editors. Drug Development, Molecular Targets for GI Diseases. Humana Press; Totowa, NJ: 2000. pp. 203–238. [Google Scholar]
- 4.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the Pathophysiology of Drug Addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortella FC, Decoster MA. Kappa Opioids: Therapeutic Considerations in Epilepsy and CNS Injury. Clin Neuropharmacol. 1994;17:403–416. [PubMed] [Google Scholar]
- 6.Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM, Peterson PK. κ Opioid Receptors in Human Microglia Downregulate Human Immunodeficiency Virus 1 Expression. Proc Natl Acad Sci US A. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson PK, Gekker G, Lokensgard JR, Bidlack JM, Chang AC, Fang X, Portoghese PS. κ-Opioid Receptor Agonist Suppression of HIV-1 Expression in CD4+ Lymphocytes. Biochem Pharmacol. 2001;61:1145–1151. doi: 10.1016/s0006-2952(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine Pituitary Dynorphin: Complete Amino Acid Sequence of the Biologically Active Heptadecapeptide. Proc Natl Acad Sci U.S.A. 1981;78:7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caudle RM, Mannes AJ. Dynorphin: Friend or Foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 10.Chavkin C, Goldstein A. Specific Receptor for the Opioid Peptide Dynorphin: Structure-Activity Relationships. Proc Natl Acad Sci US A. 1981;78:6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwyzer R. Estimated Conformation, Orientation, and Accumulation of Dynorphin A-(1-13)- Tridecapeptide on the Surface of Neutral Lipid Membranes. Biochemistry. 1986;25:4281–4286. doi: 10.1021/bi00363a016. [DOI] [PubMed] [Google Scholar]
- 12.Schwyzer R. Molecular Mechanism of Opioid Receptor Selection. Biochemistry. 1986;25:6335–6342. doi: 10.1021/bi00368a075. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JW, Osapay G. Determining the Functional Conformations of Biologically Active Peptides. Acc Chem Res. 1990;23:338–344. [Google Scholar]
- 14.Renugopalakrishnan V, Paraka RS, Bhargava HN. Conformational Features of Opioid Peptides: Ligand Receptor Interactions. In: Szekely JI, Ramabadran K, editors. Opioid Peptides, Biochemistry and Applied Physiology. IV. CRC Press; 1990. pp. 53–114. [Google Scholar]
- 15.Lancaster CRD, Mishra PK, Hughes DW, St-Pierre SA, Bothner-By AA, Epand RM. Mimicking the Membrane-Mediated Conformation of Dynorphin A-(1-13)-Peptide: Circular Dichroism and Nuclear Magnetic Resonance Studies in Methanolic Solution. Biochemistry. 1991;30:4715–4726. doi: 10.1021/bi00233a012. [DOI] [PubMed] [Google Scholar]
- 16.Kallick DA. Conformation of Dynorphin A(1-17) Bound to Dodecylphosphocholine Micelles. J Am Chem Soc. 1993;115:9317–9318. [Google Scholar]
- 17.Deber CM, Madison V, Blout ER. Why Cyclic Peptides? Complementary Approaches to Conformations. Acc Chem Res. 1976;9:106–113. [Google Scholar]
- 18.Freidinger RM, Veber DF, Perlow DS. Bioactive Conformation of Luteinizing Hormone-Releasing Hormone: Evidence from a Conformationaily Constrained Analog. Science. 1980;210:656–658. doi: 10.1126/science.7001627. [DOI] [PubMed] [Google Scholar]
- 19.Hruby VJ, Agnes RS. Conformation-Activity Relationships of Opioid Peptides with Selective Activities at Opioid Receptors. Biopolymers Pept Sci. 1999;51:391–410. doi: 10.1002/(SICI)1097-0282(1999)51:6<391::AID-BIP3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Hruby VJ. Design in Topographical Space of Peptide and Peptidomimetic Ligands That Affect Behavior. A Chemist’s Glimpse at the Mind-Body Problem. Acc Chem Res. 2001;34:389–397. doi: 10.1021/ar990063q. [DOI] [PubMed] [Google Scholar]
- 21.Chappa AK. Ph D Thesis. Department of Pharmaceutical Chemistry, The University of Kansas; Lawrence: 2007. Biopharmaceutical Aspects of the Development of Peptides as CNS Drug Delivery Vectors and Therapeutic Agents: Studies with Substance P and Dynorphin A Analogs. [Google Scholar]
- 22.Sako Y, Goto Y, Murakami H, Suga H. Ribosomal Synthesis of Peptidase-Resistant Peptides Closed by a Nonreducible Inter-Side-Chain Bond. ACS Chem Biol. 2008;3:241–249. doi: 10.1021/cb800010p. [DOI] [PubMed] [Google Scholar]
- 23.Miranda LP, Winters KA, Gegg CV, Patel A, Aral J, Long J, Zhang J, Diamond S, Guido M, Stanislaus S, Ma M, Li H, Rose MJ, Poppe L, Veniant MM. Design and Synthesis of Conformationally Constrained Glucagon-Like Peptide-1 Derivatives with Increased Plasma Stability and Prolonged in Vivo Activity. J Med Chem. 2008;51:2758–2765. doi: 10.1021/jm701522b. [DOI] [PubMed] [Google Scholar]
- 24.Schiller PW, Nguyen TMD, Lemieux C. Synthesis and Opioid Activity Profiles of Cyclic Dynorphin Analogs. Tetrahedron. 1988;44:733–743. [Google Scholar]
- 25.Kawasaki AM, Knapp RJ, Kramer TH, Wire WS, Vasquez OS, Yamamura HI, Burks TF, Hruby VJ. Design and Synthesis of Highly Potent and Selective Cyclic Dynorphin A Analogs. J Med Chem. 1990;33:1874–1879. doi: 10.1021/jm00169a007. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki AM, Knapp RJ, Kramer TH, Walton A, Wire WS, Hashimoto S, Yamamura HI, Porreca F, Burks TF, Hruby VJ. Design and Synthesis of Highly Potent and Selective Cyclic Dynorphin A Analogs. 2. New Analogs. J Med Chem. 1993;36:750–757. doi: 10.1021/jm00058a012. [DOI] [PubMed] [Google Scholar]
- 27.Collins N, Hruby VJ. Prediction of the Conformational Requirements for Binding to the κ-Opioid Receptor and Its Subtypes. I. Novel α-Helical Cyclic Peptides and Their Role in Receptor Selectivity. Biopolymers. 1994;34:1231–1241. doi: 10.1002/bip.360340911. [DOI] [PubMed] [Google Scholar]
- 28.Meyer JP, Collins N, Lung FD, Davis P, Zalewska T, Porreca F, Yamamura HI, Hruby VJ. Design, Synthesis, and Biological Properties of highly Potent Cyclic Dynorphin A Analogs. Analogs Cyclized between Positions 5 and 11. J Med Chem. 1994;37:3910–3917. doi: 10.1021/jm00049a010. [DOI] [PubMed] [Google Scholar]
- 29.Arttamangkul S, Murray TF, DeLander GE, Aldrich JV. Synthesis and Opioid Activity of Conformationally Constrained Dynorphin A Analogs. 1. Conformational Constraint in the “Message” Sequence. J Med Chem. 1995;38:2410–2417. doi: 10.1021/jm00013a016. [DOI] [PubMed] [Google Scholar]
- 30.Lung FDT, Collins N, Stropova D, Davis P, Yamamura HI, Porreca F, Hruby VJ. Design, Synthesis, and Biological Activities of Cyclic Lactam Peptide Analogues of Dynorphin A(1-11)-NH2. J Med Chem. 1996;39:1136–1141. doi: 10.1021/jm950369c. [DOI] [PubMed] [Google Scholar]
- 31.Arttamangkul S, Ishmael JE, Murray TF, Grandy DK, DeLander GE, Kieffer BL, Aldrich JV. Synthesis and Opioid Activity of Conformationally Constrained Dynorphin A Analogues. 2.1 Conformational Constraint in the “Address” Sequence. J Med Chem. 1997;40:1211–1218. doi: 10.1021/jm960753p. [DOI] [PubMed] [Google Scholar]
- 32.Fu GC, Grubbs RH. The Application of Catalytic Ring-Closing Olefin Metathesis to the Synthesis of Unsaturated Oxygen Heterocycles. J Am Chem Soc. 1992;114:5426–5427. [Google Scholar]
- 33.Miller SJ, Blackwell HE, Grubbs RH. Application of Ring-Closing Metathesis to the Synthesis of Rigidified Amino Acids and Peptides. J Am Chem Soc. 1996;118:9606–9614. [Google Scholar]
- 34.Stymiest JL, Mitchell BF, Wong S, Vederas JC. Synthesis of Oxytocin Analogues with Replacement of Sulfur by Carbon Gives Potent Antagonists with Increased Stability. J Org Chem. 2005;70:7799–7809. doi: 10.1021/jo050539l. [DOI] [PubMed] [Google Scholar]
- 35.Reichwein JF, Versluis C, Liskamp RMJ. Synthesis of Cyclic Peptides by Ring-Closing Metathesis. J Org Chem. 2000;65:6187–6195. doi: 10.1021/jo000759t. [DOI] [PubMed] [Google Scholar]
- 36.Nutt RF, Veber DF, Saperstein R. Synthesis of Nonreducible Bicyclic Analogs of Somatostatin. J Am Chem Soc. 1980;102:6539–6545. [Google Scholar]
- 37.Berezowska I, Chung NN, Lemieux C, Wilkes BC, Schiller PW. Cyclic Dermorphin Tetrapeptide Analogues Obtained via Ring-Closing Metathesis. Acta Biochim Pol. 2006;53:73–76. [PubMed] [Google Scholar]
- 38.Berezowska I, Chung NN, Lemieux C, Wilkes BC, Schiller PW. Dicarba Analogues of the Cyclic Enkephalin Peptides H-Tyr-c[D-Cys-Gly-Phe-D(or L)-Cys]NH2 Retain High Opioid Activity. J Med Chem. 2007;50:1414–1417. doi: 10.1021/jm061294n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mollica A, Guardiani G, Davis P, Ma SW, Porreca F, Lai J, Mannina L, Sobolev AP, Hruby VJ. Synthesis of Stable and Potent δ/μ; Opioid Peptides: Analogues of H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH by Ring-Closing Metathesis. J Med Chem. 2007;50:3138–3142. doi: 10.1021/jm061048b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Story SC, Murray TF, Delander GE, Aldrich JV. Synthesis and Opioid Activity of 2- Substituted Dynorphin A-(1-13) Amide Analogs. Int J Pept Prot Res. 1992;40:89–96. doi: 10.1111/j.1399-3011.1992.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 41.Turcotte A, Lalonde J-M, St-Pierre S, Lemaire S. Dynorphin-(1-13). I. Structure-Function Relationships of Ala-Containing Analogs. Int J Pept Protein Res. 1984;23:361–367. [PubMed] [Google Scholar]
- 42.Bovey FA, Mirau PA, Gutowsky HS. Nuclear Magnetic Resonance Spectroscopy. 2. Academic Press; San Diego: 1988. [Google Scholar]
- 43.Braun S, Kalinowski HO, Berger S. 150 and More Basic NMR Experiments. 2. Wiley-VCH; Weinheim: 1998. [Google Scholar]
- 44.Silverstein RM, Webster FX. Spectrometric Identification of Organic Compounds. John Wiley & Sons, Inc; New York: 1997. [Google Scholar]
- 45.Patkar KA, Yan X, Murray TF, Aldrich JV. [Nα-BenzylTyr1,cyclo(D-Asp5,Dap8)]-dynorphin A-(1-11)NH2 Cyclized in the “Address” Domain Is a Novel κ-Opioid Receptor Antagonist. J Med Chem. 2005;48:4500–4503. doi: 10.1021/jm050105i. [DOI] [PubMed] [Google Scholar]
- 46.Vig BS, Murray TF, Aldrich JV. Synthesis and Opioid Activity of Side-Chain-to-Side-Chain Cyclic Dynorphin A-(1-11) Amide Analogues Cyclized between Positions 2 and 5. 1. Substitutions in Position 3. J Med Chem. 2004;47:446–455. doi: 10.1021/jm030298e. [DOI] [PubMed] [Google Scholar]
- 47.Soderstrom K, Choi H, Berman FW, Aldrich JV, Murray TF. N-Alkylated Derivatives of [D-Pro10]Dynorphin A-(1-11) Are High Affinity Partial Agonists at the Cloned Rat κ-Opioid Receptor. Eur J Pharmacol. 1997;338:191–197. doi: 10.1016/s0014-2999(97)81948-6. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color Test for Detection of Free Terminal Amino Groups in the Solid-Phase Synthesis of Peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 49.Sole NA, Barany G. Optimization of Solid-Phase Synthesis of [Ala 8]-Dynorphin A. J Org Chem. 1992;57:5399–5403. [Google Scholar]
- 50.Bennett MA, Murray TF, Aldrich JV. Structure-Activity Relationships of Arodyn, a Novel Acetylated Kappa Opioid Receptor Antagonist. J Pept Res. 2005;65:322–332. doi: 10.1111/j.1399-3011.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 51.Cheng YC, Prusoff WH. Relationship Between the Inhibition Constant (Ki) and the Concentration of Inhibitor Which Causes 50 Percent Inhibition (IC50) of an Enzymatic Reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.