Abstract
Meiosis requires that each chromosome finds its homologous partner and undergoes at least one crossover. X-Y chromosome segregation hinges on efficient crossing-over in a very small region of homology, the pseudoautosomal region (PAR). We find that mouse PAR DNA occupies unusually long chromosome axes, potentially as shorter chromatin loops, predicted to promote double-strand break (DSB) formation. Most PARs show delayed appearance of RAD51/DMC1 foci, which mark DSB ends, and all PARs undergo delayed DSB-mediated homologous pairing. Analysis of Spo11β isoform-specific transgenic mice revealed that late RAD51/DMC1 foci on the PAR are genetically distinct from early PAR foci and global foci, and that late PAR foci promote efficient X-Y pairing, recombination and male fertility. Our findings uncover specific mechanisms that surmount the unique challenges of X-Y recombination.
Meiotic recombination, initiated by programmed DSBs, promotes homologous chromosome (homolog) pairing during prophase I (1). A subset of DSBs matures into crossovers that physically connect homologs so they orient properly on the first meiotic spindle. Because sex chromosome recombination and pairing are restricted to the PAR (2), at least one DSB must form within this small region and the homologous PAR must be located and engaged in recombination leading to a crossover. Accordingly, the PAR in males exhibits high crossover frequency (2, 3) but sex chromosomes also mis-segregate more frequently than autosomes (4). Nevertheless, X-Y non-disjunction is rare, suggesting the existence of mechanisms that ensure successful X-Y recombination.
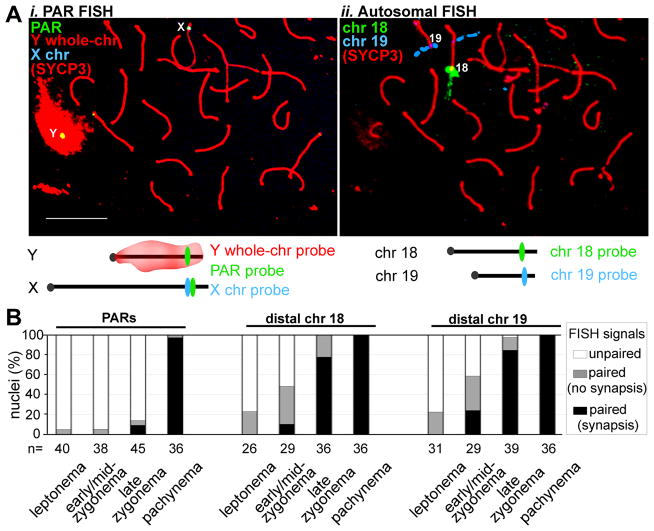
X-Y pairing is more challenging than autosomal pairing, as it cannot be mediated by multiple DNA interactions along the length of the chromosomes. We used fluorescence in situ hybridization (FISH) (5) to compare timing of meiotic X-Y and autosomal pairing in mice (Fig. 1). At leptonema when DSBs begin to form and only short chromosome axis segments are present, PAR and autosomal FISH probes were mostly unpaired. By early/mid-zygonema, when axes elongate and homologs become juxtaposed, distal ends of Chr 18 and 19 were paired in ~50% of nuclei; by late zygonema, these regions were paired in nearly all nuclei (Fig. 1B, fig. S1). In contrast, the X and Y PARs were rarely paired before pachynema (Fig. 1B), hence X-Y pairing is delayed compared to autosomes.
Figure 1.
Late PAR pairing during male meiosis. A. FISH assay for pairing. i–ii) Example of immunofluorescence (IF) and two sequential rounds of FISH on a late zygotene spermatocyte nucleus. Nuclei stained with an antibody against axis protein SYCP3 were subjected first to PAR FISH (i), then to distal-chr18 and distal-chr19 FISH (ii). Scale bar, 10 μm. B. Nuclei (%) with unpaired and paired (≤2 μm apart) FISH signals. Chromosome synapsis status was also recorded at sites of paired signals.
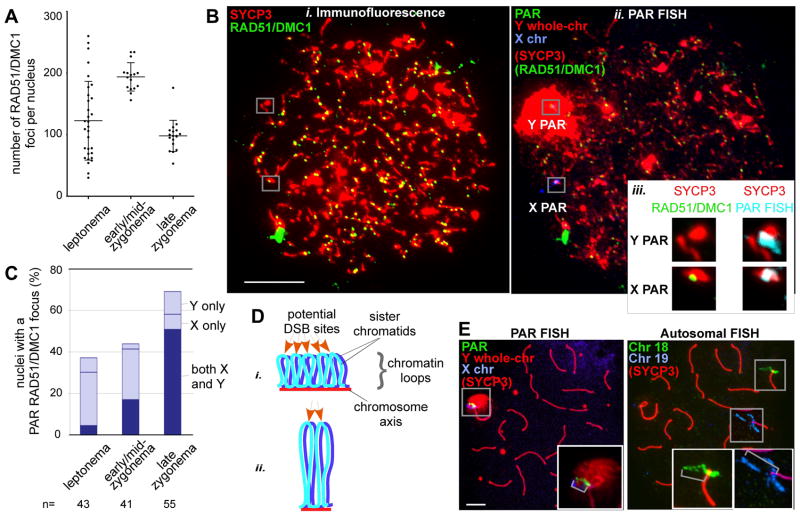
DSBs precede and are required for efficient homolog pairing in mouse meiosis (6, 7). Nucleus-wide (“global”) foci of DSB markers RAD51/DMC1 peak in number at early/mid-zygonema (Fig. 2A, (8, 9)). Because stable X-Y pairing occurs late, we asked whether PAR DSB kinetics are also delayed (Fig. 2B, fig. S2). More than half of cells had no RAD51/DMC1 focus in the PAR before late zygonema (Figs. 2B–C, fig. S2iii), distinct from global patterns. Only when global foci were already declining did the majority of cells (~70%) display PAR foci (Fig. 2C, fig. S2i). We interpret lack of PAR foci to indicate that DSBs have not yet formed. Thus we propose that PAR DSB formation and/or turnover are under distinct temporal control. We cannot exclude the alternative possibility that PAR DSBs have formed but are cytologically undetectable, for example because RAD51/DMC1 have not yet been loaded onto DSB ends, or because foci have already turned over (fig. S3). In either case, DSB dynamics/processing differs on the PAR.
Figure 2.
Distinct temporal and structural properties of the PAR. A. Nucleus-wide RAD51/DMC1 foci in spermatocytes (bars = mean±SD). B. Assay for PAR DSB formation. IF against RAD51/DMC1 and SYCP3 (i) and FISH (ii) with probes shown in Figure 1Ai on a leptotene spermatocyte nucleus. Scale bar, 10 μm. iii, Zoom-ins of Y and X PARs from i (29), and an overlay of the PAR FISH signal with SYCP3 (left), here with a RAD51/DMC1 focus only on the X PAR. C. Nuclei (%) with one or two PAR RAD51/DMC1 foci. D. Axis/loop segments as a determinant of DSB potential (after ref. 15). Only one homolog is shown. DNA organized on a longer axis into more and smaller loops (i) has more DSB potential than if the same DNA is organized on a shorter axis into fewer and larger loops (ii). E. Examples of chromatin extension (grey brackets in insets), see also Table 1. Scale bar, 5 μm.
Most sites marked by PAR RAD51/DMC1 foci appeared incapable of mediating stable pairing prior to early pachynema (~70% of late zygotene nuclei had foci, but <20% showed PAR pairing, Figs. 1B, 2C). The number of PAR foci per cell also increased over time. In leptonema and early/mid-zygonema, most cells with a PAR RAD51/DMC1 focus had only one (typically on X), whereas by late zygonema, two PAR foci were often present (both X and Y, Fig. 2C, fig. S2). Foci on both PARs could represent two independent DSBs. If so, then having more than one X-Y recombination interaction may stabilize pairing, similar to multiple interactions which stabilize pairing of autosomes (10). Alternatively, foci on both PARs could represent the two, separated ends of a single DSB (11, 12), with one focus marking the broken PAR and the second focus marking the other PAR (fig. S3A). In this “ends-apart” model, nuclei that have two PAR foci are those in which the X and Y PARs have successfully engaged each other. However, we found that most such nuclei showed no evidence of a preferential X-Y spatial relationship (fig. S3B), and most PAR pairing occurred abruptly at the zygonema-to-pachynema transition, i.e., after the stage when many cells displayed two PAR foci (compare Figs. 1B, 2C). Sex body formation (13) may facilitate this sudden completion of X-Y pairing by providing homology-independent X-Y proximity that simplifies the homology search.
The haploid mouse genome averages <1 DSB/10 Mb (Fig. 2A), whereas the <1 Mb PAR (14) undergoes 1–2 DSBs (Fig. 2C), 10–20-fold higher than genome average. We speculated that distinct higher order chromosome structure could render the PAR more conducive to DSB formation. Meiotic recombination is proposed to occur within DNA segments residing in chromatin loops that become transiently tethered to chromosome axes (15). Loop density per μm of axis is constant (16), producing an inverse relationship between loop size and axis length (17). DNA arranged into smaller loops may have higher DSB potential (Fig. 2D) (18); indeed, autosomal crossover frequency in male mice correlates with axis length (19). We found that PAR axes were disproportionately long relative to DNA length, incorporating ~1 Mb/μm of axis (Table 1A). At distal ends of Chr 18 and 19 (regions with relatively frequent crossing-over (19)), DNA content was 10–13 Mb/μm and correlated well with axis length, i.e., the distal ~10% of DNA occupied ~10% total axis length (Table 1A). The ≥10-fold difference between PAR and autosome axes is of the magnitude expected for a region that experiences >10-fold more DSBs. Axes of non-PAR portions of the X and Y had a more autosome-like DNA content (≥14 Mb/μm, fig. S4).
Table 1.
Chromosome axis lengths and chromatin extension in PARs and distal ends of Chr 18 and 19.
| A. DNA content vs. chromosome axis lengths (late zygonema) | |||||
|---|---|---|---|---|---|
| Chromosome | Total size (Mb) | Probe-distal region (Mb) | Total chromosome axis (μm)a | Probe-distal axis (μm)a | DNA content of probe- distal axis (Mb/μm) |
| Y | 95 | 0.7 | 4.2 ± 0.7 (17) | 0.7 ± 0.2 (20) | 1 |
| X | 167 | 0.7 | 12.7 ± 2.8 (13) | 0.8 ± 0.2 (23) | 1 |
| 18 | 91 | 8 | 6.1 ± 0.9 (11) | 0.6 ± 0.1 (12) | 13 |
| 19 | 61 | 6 | 5.1 ± 0.5 (11) | 0.6 ± 0.1 (10) | 10 |
| B. Length of chromatin extension from axes | |||||
|---|---|---|---|---|---|
| Locus | Probe size (kb)b | FISH signal extension, mean μm ± SD (number of observations)
|
|||
| Leptonema | Early/mid-zygonema | Late zygonema | Pachynema | ||
| Y PAR | 146 | 0.5 ± 0.2 (25) | 0.6 ± 0.3 (21) | 0.6 ± 0.3 (21) | 1.2 ± 0.5 (23)c |
| X PAR | 0.6 ± 0.4 (23) | 0.7 ± 0.5 (17) | 0.6 ± 0.5 (20) | ||
| Distal Chr 18 | 207 | 2.2 ± 0.8 (26) | 3.2 ± 1.5 (31)c | 4.5 ± 1.9 (35)c | 5.4 ± 2.2 (21)c |
| Distal Chr 19 | 182 | 2.3 ± 0.9 (18) | 3.6 ± 2.0 (33)c | 5.3 ± 3.0 (40)c | 5.9 ± 2.3 (23)c |
mean ± SD (number of observations)
size of BAC
some or all measurements are from paired FISH signals
Long PAR axes predict short chromatin loops. As a proxy for loop size we measured FISH signal extension from axes for probes in the PAR and autosomal subtelomeric regions (Fig. 2E, Table 1B). PAR FISH signals were substantially more compact at all stages (~3–7-fold less extended), consistent with smaller loops. Thus, chromosome structure could be one factor that facilitates high-frequency DSB formation in the PAR.
The distinct temporal and structural features outlined above raised the possibility that mechanisms ensuring efficient PAR recombination and pairing may be under different genetic control from autosomes. Characterization of a variant of SPO11, the evolutionarily conserved meiotic DSB catalyst (1), validated this hypothesis (Fig. 3). Two major mRNA splicing isoforms in mice and humans are Spo11α and Spo11β (7, 20–22), (Fig. 3Ai, fig. S5). Spo11β is expressed early in meiosis, when most DSBs are formed (Fig. 2A), whereas Spo11α is expressed later (7, 20, 23) (Fig. 3Aii, fig. S6A). Thus, SPO11β is likely responsible for most DSB formation.
Figure 3.
Genetic control of PAR recombination and pairing. A. Spo11 splice variants (see also fig. S4). i) Genomic organization and splicing. Spo11β includes exon 2, Spo11α excludes it. Y, catalytic tyrosine. ii–iii) Reverse transcriptase-PCR from flow-sorted meiocyte populations of adult mice. –RT, no reverse transcriptase; L/Z, leptonema/zygonema; P/D, pachynema/diplonema; S, spermatids. iv) SPO11 protein levels in adult testis extracts. Asterisk, a lower-mobility protein likely originating from the knock-out allele (fig. S5D). B. IF of SYCP1 and SYCP3 on pachytene nuclei (i) and of SYCP3 plus whole-chromosome FISH of early metaphase I spermatocyte nuclei (ii) from mice of the indicated genotypes. Inset in (i), schematic of X and Y chromosomes. Scale bars, 10 μm. (iii) Quantification of X-Y association; 57–65 nuclei scored/genotype. C. TUNEL-stained testis sections; apoptotic cells stain brown. Elongating spermatids (arrows) are rare in Spo11β-only mice. Inset shows a lagging chromosome (arrowhead) in a TUNEL-positive cell. D. RAD51/DMC1 focus counts in spermatocytes from control and Spo11β-only mice (bars = mean±SD). E. Nuclei (%) with PAR RAD51/DMC1 foci in mice of the indicated genotypes. 41–55 nuclei were scored per stage for each genotype. Asterisks indicate significant differences (P≤ 0.0002, two-tailed Mann-Whitney test). n.s., not significant, P=0.09.
We generated transgenic mice expressing Spo11βB cDNA (fig. S5) from a meiosis-specific promoter (24) (fig. S6B). Tg(Xmr-Spo11βB) transcript expression overlapped with Spo11β mRNA appearance in wild type (Fig. 3Aii, figs. S6A, S6C). In testis extracts of Spo11−/− Tg(Xmr-Spo11βB)+/+ (hereafter, “Spo11β-only”) mice, SPO11βB protein approximated the total level of SPO11 in wild type (Fig. 3Aiii). The transgene did not cause obvious meiotic phenotypes in mice heterozygous at the endogenous Spo11 locus (i.e., Spo11+/− Tg(Xmr-Spo11βB)+/+), and these mice were used as controls. The profound meiotic defects of Spo11−/− mice (no recombination, failure of homolog pairing and synapsis, infertility (6, 7, 25)) were mostly rescued by Tg(Xmr-Spo11βB) in both sexes: autosomal homologous pairing, synapsis and MLH1 focus formation (a crossover marker) appeared normal (Fig. 3Bi, fig. S7A). Moreover, ovaries of Spo11β-only mice contained abundant primordial follicles (Fig. S7B) and Spo11β-only females were fully fertile with normal litter sizes. Thus SPO11βB supports autosomal crossing-over, pairing and synapsis, and (in females) full meiotic progression and accurate chromosome segregation. Male meiosis was not fully rescued, however. Although sex bodies formed (fig. S7C), the X and Y failed to pair and synapse in ~70% of spermatocytes (Fig. 3B). Spo11β-only testis sections showed numerous apoptotic metaphase I cells (Fig. 3C), many with a lagging chromosome (Fig. 3C, inset), consistent with spindle checkpoint-induced apoptosis (9, 13, 26, 27) triggered by the failure of non-recombinant X and Y to orient properly on the metaphase I spindle. Few post-meiotic cells were formed and testis sizes were reduced (Fig. 3C, fig. S7D–E), and while some Spo11β-only males produced offspring, most were infertile.
Nucleus-wide numbers and timing of RAD51/DMC1 foci were indistinguishable between Spo11β-only and control males (Fig. 3D, fig. S7F) indicating that the X-Y pairing defect cannot be attributed to reduced global DSB levels (Fig. 3E). Similarly, the frequency of PAR RAD51/DMC1 foci in leptonema was not affected. In contrast, the percentage of late zygotene nuclei with a PAR focus was reduced in Spo11β-only males, consistent with a defect in a late-forming DSB population (PAR-specific, or possibly including a small subset of autosomal DSBs). ~70% of late zygotene nuclei lacked PAR foci (Fig. 3E), similar to the percentage of cells with X-Y pairing failure (Fig. 3Biii). Thus the few PAR foci that form early in both wild-type and Spo11β-only males seem to persist until late zygonema (fig. S4 discussion), at which time recombination-mediated X-Y pairing occurs. We propose that a lack of late PAR DSBs is the cause of infertility in Spo11β-only males. In females, two fully homologous X chromosomes make PAR recombination dispensable.
Spo11α is the only splice variant missing from Spo11β-only mice that is known to be developmentally regulated, and its expression in wild type correlates with the timing of late PAR DSBs as inferred from the appearance of RAD51/DMC1 foci. It is thus possible that SPO11α, by itself or in combination with SPO11β, is needed for DSB formation in late zygonema. In this scenario, late-forming PAR DSBs are genetically separable both from global DSBs and from early-forming PAR DSBs, and that the surge of late-forming PAR DSBs is crucial for efficient X-Y pairing and fertility. PAR recombination occasionally fails in humans, as evidenced by paternally inherited sex chromosome aneuploidies (e.g. Klinefelter or Turner syndromes (28)). Because Spo11 isoforms are conserved, we speculate that variation in Spo11 splicing patterns may be a human X-Y non-disjunction susceptibility trait.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 HD040916 (M.J., S.K); AIRC (MFAG grant 4765), MIUR, the Lalor Foundation, and the AICF (M.B.); and the Charles H. Revson Foundation (F.B.). We thank Margaret Leversha (MSKCC), Philippe Bois (Scripps Florida), and Katia Manova (MSKCC) for valuable advice and protocols. We are grateful to Keeney and Jasin lab members, especially Ignasi Roig, Esther de Boer and Francesca Cole, and to Neil Hunter (UC Davis) for insightful comments.
References and Notes
- 1.Cole F, Keeney S, Jasin M. Genes Dev. 2010;24:1201. doi: 10.1101/gad.1944710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouyer F, et al. Nature. 1986;319:291. doi: 10.1038/319291a0. [DOI] [PubMed] [Google Scholar]
- 3.Soriano P, et al. Proc Natl Acad Sci U S A. 1987;84:7218. doi: 10.1073/pnas.84.20.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Q, et al. Am J Med Genet. 2001;99:34. doi: 10.1002/1096-8628(20010215)99:1<34::aid-ajmg1106>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Materials and methods are available as supporting material on Science Online.
- 6.Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Mol Cell. 2000;6:989. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Romanienko PJ, Camerini-Otero RD. Mol Cell. 2000;6:975. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, et al. Dev Cell. 2007;12:863. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Barchi M, et al. PLoS Genet. 2008;4:e1000076. doi: 10.1371/journal.pgen.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner BM, Kleckner N. Cell. 1994;77:977. doi: 10.1016/0092-8674(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 11.Hunter N. In: Molecular Genetics of Recombination. Aguilera A, Rothstein R, editors. Vol. 17. Springer-Verlag; Heidelberg: 2007. pp. 381–442. [Google Scholar]
- 12.Storlazzi A, et al. Cell. 2010;141:94. doi: 10.1016/j.cell.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgoyne PS, Mahadevaiah SK, Turner JM. Nat Rev Genet. 2009;10:207. doi: 10.1038/nrg2505. [DOI] [PubMed] [Google Scholar]
- 14.Perry J, Palmer S, Gabriel A, Ashworth A. Genome Res. 2001;11:1826. doi: 10.1101/gr.203001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blat Y, Protacio RU, Hunter N, Kleckner N. Cell. 2002;111:791. doi: 10.1016/s0092-8674(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 16.Zickler D, Kleckner N. Annu Rev Genet. 1999;33:603. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- 17.Revenkova E, et al. Nat Cell Biol. 2004;6:555. doi: 10.1038/ncb1135. [DOI] [PubMed] [Google Scholar]
- 18.Kleckner N, Storlazzi A, Zickler D. Trends Genet. 2003;19:623. doi: 10.1016/j.tig.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Froenicke L, Anderson LK, Wienberg J, Ashley T. Am J Hum Genet. 2002;71:1353. doi: 10.1086/344714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanienko PJ, Camerini-Otero RD. Genomics. 1999;61:156. doi: 10.1006/geno.1999.5955. [DOI] [PubMed] [Google Scholar]
- 21.Keeney S, et al. Genomics. 1999;61:170. doi: 10.1006/geno.1999.5956. [DOI] [PubMed] [Google Scholar]
- 22.Bellani MA, Boateng KA, McLeod D, Camerini-Otero RD. Mol Cell Biol. 2010;30:4391. doi: 10.1128/MCB.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neale MJ, Pan J, Keeney S. Nature. 2005;436:1053. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romanienko PJ. Ph D dissertation. Cornell University; 1997. [Google Scholar]
- 25.Di Giacomo M, et al. Proc Natl Acad Sci U S A. 2005;102:737. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaker S, Cobb J, Pyle A, Handel MA. Dev Biol. 2002;249:85. doi: 10.1006/dbio.2002.0708. [DOI] [PubMed] [Google Scholar]
- 27.Odorisio T, Rodriguez TA, Evans EP, Clarke AR, Burgoyne PS. Nat Genet. 1998;18:257. doi: 10.1038/ng0398-257. [DOI] [PubMed] [Google Scholar]
- 28.Hall H, Hunt P, Hassold T. Curr Opin Genet Dev. 2006;16:323. doi: 10.1016/j.gde.2006.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.