Abstract
Objectives
We assessed the extent to which Centers for Disease Control and Prevention (CDC) recommendations have influenced routine HIV testing among Massachusetts community health center (CHC) personnel, and identified specific barriers and facilitators to routine testing.
Methods
Thirty-one CHCs were enrolled in the study. We compared those that did and did not receive funding support from the federal Ryan White HIV/AIDS Program. An anonymous survey was administered to a maximum five personnel from each CHC, including a senior administrator, the medical director, and three medical providers. Overall, 137 participants completed the survey.
Results
Among all CHCs, 53% of administrators reported having implemented routine HIV testing at their CHCs; however, only 33% of medical directors/providers reported having implemented routine HIV testing in their practices (p<0.05). Among administrators, 60% of those from Ryan White-supported CHCs indicated that both they and their CHCs were aware of CDC's recommendations, compared with 27% of administrators from non-Ryan White-supported CHCs. The five most frequently reported barriers to the implementation of routine HIV testing were (1) constraints on providers' time (68%), (2) time required to administer counseling (65%), (3) time required to administer informed consent (52%), (4) lack of funding (35%), and (5) need for additional training (34%). In a multivariable logistic regression model, the provision of on-site HIV testing by nonmedical staff resulted in increased odds of conducting routine HIV testing (odds ratio [OR] = 9.84, 95% confidence interval [CI] 1.77, 54.70). However, the amount of time needed to administer informed consent was associated with decreased odds of providing routine testing (OR=0.21, 95% CI 0.05, 0.92).
Conclusions
Routine HIV testing is not currently being implemented uniformly among Massachusetts CHCs. Future efforts to increase implementation should address personnel concerns regarding time and staff availability.
In the United States, more than one million people are estimated to be living with human immunodeficiency virus (HIV); 21% are undiagnosed and/or remain unaware of their HIV infection.1–3 Almost 40% are diagnosed late in the course of infection and receive an acquired immunodeficiency syndrome (AIDS) diagnosis within one year of their first positive HIV test result.3 To address the large number of undiagnosed HIV cases and high proportion of individuals presenting late to care, the Centers for Disease Control and Prevention (CDC) published revised recommendations in September 2006 that sought to establish HIV testing as a routine component of medical care similar to other screening procedures. Specifically, the CDC guidelines recommend that providers in all health-care settings, including hospital emergency departments, primary care practices, and community clinics, offer voluntary HIV testing to all patients aged 13–64 years and all pregnant women as an opt-out procedure, meaning that patients are to be notified that an HIV test will be conducted unless the patient declines.4 Separate written consent and prevention counseling as prerequisites for testing are no longer recommended.
Release of the revised recommendations has sparked a national debate, and responses among the medical community have been mixed, with a majority of U.S. health professional organizations endorsing all or parts of the CDC recommendations.5–7 While potentially increasing rates of HIV testing by streamlining consent8 and reducing associated stigma through normalization as a routine clinical procedure,9,10 the elimination of a separate consent process and mandatory prevention counseling remains incompatible with several state laws or regulations and has been met with some concern.5,11–14 Physician barriers to HIV testing include insufficient time, burdensome consent process, lack of knowledge/training about HIV testing and the CDC revised recommendations, difficulty locating HIV testing consent forms, lack of patient acceptance, competing priorities, and inadequate reimbursement.15,16
A growing body of research has examined efforts to improve HIV testing rates in a variety of health-care settings, including a public, urban medical care system,17 U.S. Department of Veterans Affairs health-care facilities,18,19 hospital emergency departments,20 a sexually transmitted disease (STD) clinic,21 and community health centers (CHCs).22–24 However, while studies of CHCs have described programs to implement routine testing and largely reported patient-level data, little research to date has examined barriers to implementation among CHC personnel. CHCs represent an important source of primary care for people who are low-income, from racial/ethnic and sexual minority groups, immigrants, and those seeking mental health and substance abuse treatment services.25 These populations are also disproportionately affected by HIV/AIDS, suggesting that CHCs can and do serve as an important resource for HIV/AIDS prevention and treatment.26 In fact, from 1999 to 2004, CHCs conducted 7% of the total HIV tests supported by CDC yet identified 12% of the total HIV-positive results.27
In Massachusetts, providers face unique barriers to implementing routine testing. Despite the issuance of a June 2009 clinical advisory by the state health department supporting routine HIV testing in primary and urgent care settings, state law requires specific written informed consent before testing a patient for HIV, which is inconsistent with CDC's recommendation to no longer require separate informed consent.28–31 Consequently, written informed consent may be perceived as a barrier for providers to offer routine testing to patients, as this process typically requires a detailed conversation and providers are often working under already limited time constraints.8,16 Understanding the facilitators and barriers to the implementation of routine HIV testing among CHCs in Massachusetts may have relevance to other U.S. states, where laws remain inconsistent with CDC HIV testing guidelines.30,31
We sought to gain a better understanding of HIV testing efforts among Massachusetts CHC personnel, including awareness of the CDC revised recommendations and any efforts to implement and support routine HIV testing in primary care settings. Analyses were stratified by respondent type (i.e., medical provider, administrator, and director) and funding mechanism, comparing health centers that did and did not receive support from the Ryan White HIV/AIDS Program, the federal program primarily responsible for HIV-related health services.32 Understanding the barriers and facilitators to implementing CDC's revised recommendations may prove useful for designing educational materials and structural or individual-level interventions that will aid in conducting testing procedures in a more effective and efficient way.
METHODS
Participants and procedures
There are 52 CHCs in Massachusetts; 42% receive funding support from at least one part of the Ryan White HIV/AIDS Program.33,34 Seeking statewide representation, health centers participating in the study were selected based on community rates of HIV/AIDS, then geographic location, and factors associated with health disparities, including racial/ethnic composition and poverty levels. From April to December 2008, 31 CHCs representing 60% of the state's total number of CHCs were enrolled in the study: 15 health centers that received Ryan White funding support were compared with 16 health centers that did not directly receive Ryan White funding, matched on location, community composition, and rates of HIV/AIDS. Twenty-three CHCs were located within the Greater Boston metropolitan area, four health centers were from the southeast and Cape Cod, and four were from the state's central and western districts.
Recruitment
Following the identification of potential health centers, the study team contacted a senior administrator from each CHC to explain the study and invite the organization to participate. A staff person at each agency was next identified to serve as a study liaison, subsequently explaining the study to the appropriate personnel, distributing study materials, and coordinating receipt of compensation. Only three health centers that were asked to participate declined and were replaced with comparable health centers.
Survey implementation
An anonymous survey was administered to a maximum five personnel from each CHC, including (1) a senior-level administrator of the agency (e.g., the executive director or another high-level administrator knowledgeable of the agency's HIV/AIDS services), (2) the medical director of the agency, and (3) three medical providers (e.g., medical doctors, nurse practitioners, and physician's assistants). Participants completed the survey either through a secure website or on paper, returning paper surveys to the study team via self-addressed stamped envelopes. Compensation for completion of each survey was $500, which was paid directly to the health center. Study participation was completely voluntary for both individual respondents and their respective health centers, and all study procedures received appropriate institutional review board approvals, including a waiver of informed consent for participants completing the anonymous survey.
Measures
Survey questions were adapted from a national survey of CHCs regarding HIV testing, prevention, care, and treatment practices, and a national survey to assess health department efforts to implement and support routine HIV testing programs.35,36 The anonymous survey asked questions regarding the provision of HIV services, including the gender, age, and race/ethnicity of HIV-infected patients; and perceptions, understanding, and experiences with HIV testing, including routine HIV testing. Administrators were also asked questions about relevant agency operations, such as funding for HIV services, their understanding of the CDC revised recommendations, and the regulatory and legal environment regarding routine HIV testing. Medical directors and other medical providers were instructed to respond from the perspective of their individual clinical practice; administrators were asked to respond from the perspective of their respective health centers. For this study, routine HIV testing was defined as “voluntary HIV testing performed for all patients in a setting unless the patient specifically declines HIV testing (i.e., ‘opt-out’ testing).”36
To the question, “What are the most significant barriers to implementing routine HIV testing in your health center and related divisions, settings, or programs?”, respondents were asked to indicate all that applied among a list of 27 possible barriers. Additional health center factors measured in the survey were the personnel, technologies, and settings used to conduct HIV testing, and the presence of written procedures, including those that require providers to offer HIV testing, provide risk assessments and pre/posttest counseling, and discuss sexual behaviors and substance use with patients. The survey also asked about intentions to address routine HIV testing in the future.
Data analysis
We used SAS® version 9.137 to perform analyses and determined statistical significance at p<0.05.
As medical directors and other medical providers were instructed to respond from their individual clinical practice, these responses were combined for descriptive statistics, which were conducted to compare responses between health center administrators and medical directors/providers and to compare responses between health centers that did and did not receive direct Ryan White funding support at the time of the study. We used Chi-square global tests of independence and Fisher's exact tests to examine independent associations between variables. Due to the non-symmetric distributions of the number of HIV-infected patients served in the past 12 months, the annual number of HIV tests performed, and annual funding for HIV testing, median and interquartile range (IQR) values were calculated to capture the general tendency of the data. We used the Wilcoxon rank-sum test, a nonparametric equivalent to the two-sample t-test, to test these distributions with respect to the median.
Bivariate and multivariable logistic regression models
Dependent variable.
For the purpose of this analysis, the primary outcome was a dichotomous measure of whether or not respondents indicated that they, or in the case of administrators, their respective health centers, had implemented routine HIV testing. Specifically, medical directors and providers were asked, “Have you implemented routine HIV testing in your practice?”; administrators were asked, “Has your health center implemented routine HIV testing in any care setting?”
Independent variables.
Independent variables for this analysis included demographic profiles of each health center, including the number, gender, age, and racial/ethnic composition of HIV-infected patients; the type of respondent (administrator, medical director, or medical provider); the 10 most frequently reported perceived barriers to conducting routine HIV testing; and other health center factors, such as personnel and technologies used for testing, and the presence of required written procedures.
We used bivariate logistic regression procedures to examine associations between our outcome of interest and each independent variable. To fit the most parsimonious multivariable model, we used the backward elimination process, in which we started with a model that contained all the statistically significant bivariate predictors and used SAS procedures to systematically remove the largest nonsignificant p-value terms until we identified a subset model that consisted of entirely statistically significant terms.
RESULTS
Health center characteristics and HIV testing
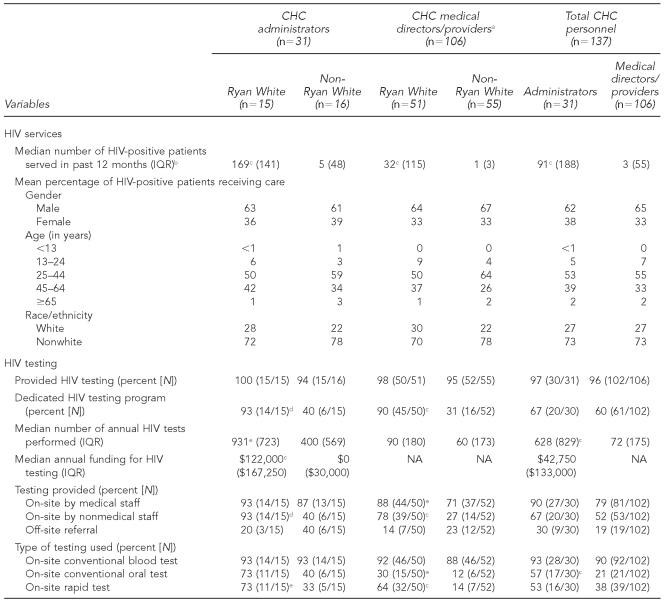
A total of 137 health center personnel completed surveys, including 31 senior administrators, 29 medical directors, and 77 medical providers. Table 1 presents the frequencies of HIV services, including routine HIV testing, as reported by Ryan White- vs. non-Ryan White-supported health center personnel.
Table 1.
HIV services provision among personnel of 15 Ryan White-supported and 16 non-Ryan White-supported CHCs in Massachusetts, April-–December 2008 (n=137)
aAdministrator responses reflect overall agency operations. Medical director and medical provider responses reflect individual practices within their respective health centers; therefore, these responses have been combined.
bRyan White health centers served an estimated median 17,500 (IQR=21,750) individual patients in the past 12 months, compared with a median 8,000 (IQR=6,750) individual patients at non-Ryan White health centers (not significant).
cp<0.001
dp<0.01
ep<0.05
CHC = community health center
HIV = human immunodeficiency virus
IQR = interquartile range
NA = not available
CDC = Centers for Disease Control and Prevention
STD = sexually transmitted disease
Health center characteristics.
While there were no significant differences regarding the gender, age, or race/ethnicity of HIV-infected patients at either Ryan White- or non-Ryan White-supported health centers, the Ryan White-supported health centers served a significantly higher number of HIV-infected patients. Per administrators, whose responses reflect overall agency operations, Ryan White health centers served a median of 169 (IQR=141) HIV-infected patients in the past 12 months, compared with five (IQR=48) HIV-infected patients at non-Ryan White-supported health centers (p<0.001). Similarly, Ryan White medical providers served an annual median of 32 (IQR=115) HIV-infected patients, compared with a median of one (IQR=3) HIV-infected patient seen by non-Ryan White-supported CHCs (p<0.001). Overall, Ryan White health centers served an estimated median of 17,500 (IQR=21,750) individual patients in the past 12 months, compared with a median of 8,000 (IQR=6,750) individual patients at non-Ryan White-supported health centers (not significant, data not shown).
HIV testing.
Per administrators, 100% of Ryan White-supported and 94% of non-Ryan White-supported CHCs provided HIV testing, with a higher proportion of Ryan White-supported health centers offering a dedicated HIV testing program (93% vs. 40%, p<0.01) and performing a higher median annual number of HIV tests (n=931 [IQR=723] vs. n=400 [IQR=569], p<0.05). A higher proportion of administrators and medical directors/providers at Ryan White-supported health centers reported that HIV testing was conducted on-site by both medical staff (88% Ryan White CHCs vs. 71% non-Ryan White CHCs, p<0.05) and nonmedical staff (78% Ryan White CHCs vs. 27% non-Ryan White CHCs, p<0.001), with a higher proportion of non-Ryan White-supported health centers providing off-site referrals (Table 1).
While personnel from both types of health centers were similar in the proportion reporting the use of on-site conventional blood tests to conduct HIV testing, Ryan White-supported health center personnel reported higher frequencies in the use of on-site conventional oral testing (30% Ryan White providers vs. 12% non-Ryan White providers, p<0.05) and on-site rapid testing (64% Ryan White providers vs. 14% non-Ryan White providers, p<0.001) (Table 1). Ninety-seven percent of Ryan White-supported personnel and 100% of non-Ryan White-supported personnel indicated that patients were required to sign written consent for HIV tests provided by their health centers (data not shown).
Routine HIV testing.
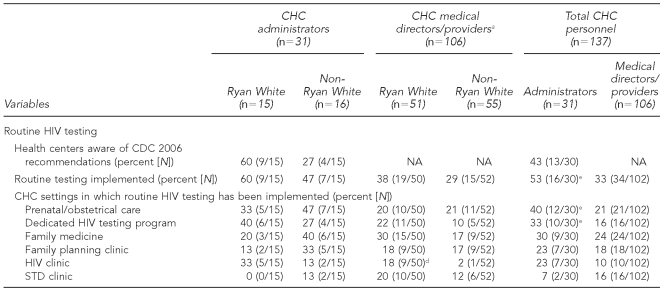
Overall, 38% of survey respondents identified that routine testing had been implemented in their health centers. Among these respondents, routine testing had been primarily implemented in the divisions of prenatal/obstetrical care (66%), family medicine (66%), dedicated HIV testing program (52%), family planning (50%), STD clinic (36%), and HIV clinic (34%).
Sixty percent of senior administrators from Ryan White-supported CHCs indicated that both they and their health centers were aware of CDC's 2006 revised recommendations, compared with 27% of -administrators from non-Ryan White-supported CHCs. Among all CHCs, 53% of administrators reported having implemented routine HIV testing at their health centers; however, among medical directors/providers, only 33% reported having implemented routine HIV testing in their practices (p<0.05).
Perceived barriers to implementing routine HIV testing
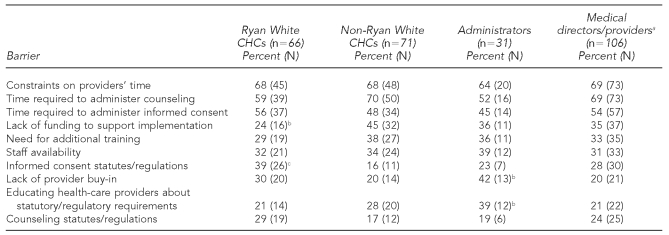
As shown in Table 2, the 10 most frequently reported barriers to implementing routine HIV testing among all respondents were (1) constraints on providers' time (68%), (2) time required to administer counseling (65%), (3) time required to administer informed consent (52%), (4) lack of funding to support implementation (35%), (5) need for additional training (34%), (6) staff availability (33%), (7) informed consent statutes/regulations (27%), (8) lack of provider buy-in (25%), (9) educating health-care providers about statutory/regulatory requirements (25%), and (10) counseling statutes/regulations (23%). Two significantly different barriers among administrators compared with medical directors/providers were lack of provider buy-in (42% vs. 20%, p<0.05) and the need to -educate health-care providers about statutory/regulatory requirements (39% vs. 21%, p<0.05). Among Ryan White-supported health centers compared with non-Ryan White-supported health centers, two significantly different barriers were a lack of funding to support implementation (24% vs. 45%, p<0.05) and informed consent statutes/regulations (39% vs. 16%, p<0.01).
Table 2.
10 most frequently reported barriers to routine HIV testing implementation by Ryan White status and CHC personnel in Massachusetts, April–December 2008 (n=137)
aAdministrator responses reflect overall agency operations. Medical director and medical provider responses reflect individual practices within their respective health centers; therefore, these responses have been combined.
bp<0.05
cp<0.01
HIV = human immunodeficiency virus
CHC = community health center
Bivariate associations of provider characteristics, perceived barriers to routine HIV testing, and other CHC factors to providing routine HIV testing
As shown in Table 3, variables significantly associated with providing routine HIV testing according to CDC guidelines included:
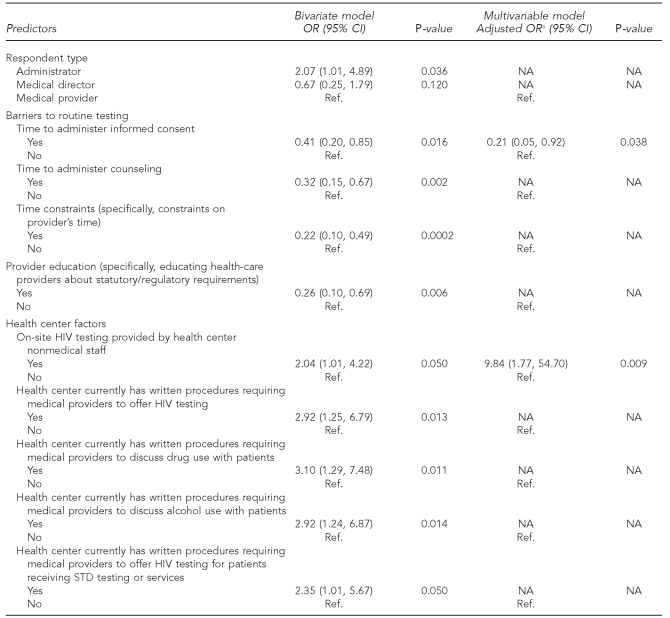
The type of respondent was differentially associated with routine HIV testing, such that administrators were more likely than medical providers to report that their health center conducted routine HIV testing (odds ratio [OR] = 2.07, 95% confidence interval [CI] 1.01, 4.89).
Several perceived barriers to conducting routine HIV testing were significantly associated with lower odds of providing routine HIV testing according to the CDC guidelines. These included time to administer informed consent (OR=0.41, 95% CI 0.20, 0.85), time to administer pre- and posttest HIV counseling (OR=0.32, 95% CI 0.15, 0.67), provider time constraints (OR=0.22, 95% CI 0.10, 0.49), and educating health-care providers about statutory/regulatory requirements (OR=0.26, 95% CI 0.10, 0.69).
Several health center-specific factors were associated with increased odds of providing routine HIV testing according to CDC guidelines, including: if on-site HIV testing was provided by nonmedical staff (OR=2.04, 95% CI 1.01, 4.22), if the health center had written procedures requiring medical providers to offer HIV testing (OR=2.92, 95% CI 1.25, 6.79), if the health -center had written procedures requiring medical providers to discuss drug use (OR=3.10, 95% CI 1.29, 7.48) or alcohol use (OR=2.92, 95% CI 1.24, 6.87) with patients, and if the health center had written procedures requiring medical providers to offer HIV testing for patients receiving STD testing or services (OR=2.35, 95% CI 1.01, 5.67).
Table 3.
Bivariate and multivariable predictors of routine HIV testing among 31 Massachusetts community health centers, April–December 2008 (n=137)
aBackward elimination was used to construct the final multivariable model. All variables presented in the bivariate analyses were used in the backward elimination process, which fit three variables represented above.
HIV = human immunodeficiency virus
OR = odds ratio
CI = confidence interval
NA = not available
Ref. = reference group
STD = sexually transmitted disease
Backward elimination logistic regression predicting routine HIV testing
The bivariate and multivariable predictors of routine HIV testing among the sample are presented in Table 3. In a final multivariable model, significant unique predictors of conducting routine HIV testing included:
The perceived barrier of the amount of time necessary to administer informed consent was associated with decreased odds of providing routine HIV testing (adjusted OR=0.21, 95% CI 0.05, 0.92).
The provision of on-site HIV testing by nonmedical staff resulted in increased odds of a center providing routine testing (adjusted OR=9.84, 95% CI 1.77, 54.70).
DISCUSSION
Findings from this study suggest that during the two years following the release of the 2006 revised CDC recommendations, routine HIV testing was still not being implemented uniformly among Massachusetts CHCs, despite the fact that between 2005 and 2007, 31% of all individuals newly diagnosed with HIV in the state progressed to an AIDS diagnosis within two months.38 Overall, only 38% of survey respondents identified that routine HIV testing had been implemented in their health centers; 53% of administrators reported having implemented routine HIV testing at their health centers compared with only 33% of medical directors and providers (p<0.05), suggesting that few health center personnel have actually implemented routine HIV testing.
Additionally, while health centers differed in the provision of HIV care and testing based on whether or not they received Ryan White funding, there were few significant differences found among CHCs regarding the implementation of routine HIV testing. For example, compared with health centers not receiving any Ryan White support, Ryan White-supported health centers served a higher annual number of HIV-infected patients (median: 169 vs. 5, p<0.001) and individual patients (median: 17,500 vs. 8,000, not significant), with a higher proportion of Ryan White-supported health centers offering a dedicated HIV testing -program (93% vs. 40%, p<0.01). However, there were no significant differences in the proportion of health center personnel reporting the implementation of routine testing based on Ryan White status, and the estimated annual number of HIV tests at both Ryan White-supported and non-Ryan White-supported health centers (median: 931 vs. 400, p<0.05) suggests that only 5% of patients are being screened among this sample of Massachusetts CHCs.
The most salient barriers to conducting routine HIV testing included concerns regarding informed consent and counseling statutes, time constraints, lack of provider acceptance, and the need for additional training and education. Furthermore, in a multivariable model, the perceived time required to administer informed consent was associated with decreased odds of providing routine HIV testing, whereas the provision of on-site HIV testing by nonmedical staff resulted in increased odds of conducting routine HIV testing. This finding may provide useful evidence for designing structural interventions with CHCs. For example, efforts to increase HIV testing rates in clinical settings have included the elimination of separate written informed consent and laboratory requisition forms; instead, HIV testing is simply incorporated into standard requisition forms and documenting consent in patient medical charts.17 These changes have led to increased testing rates, including among those considered at highest risk for HIV.8,17 While Massachusetts state law continues to require separate written consent for HIV testing, a state health department clinical advisory in June 2009 supported the inclusion of this written consent on a patient's consent form for general medical care. Additionally, the advisory clarifies language regarding the provision of counseling and laboratory requisition forms, addressing several of the barriers identified by this study.28
Additional strategies have sought to streamline the testing process through the use of attending nurses (rather than physicians or counselors) to initiate testing, rapid testing technologies, electronic clinical reminders to encourage providers to offer HIV testing, and provider and patient education and marketing.18,19,21,22,39 Future efforts to increase rates of routine HIV testing among Massachusetts CHCs should consider strategies regarding time; funding; and staff education, training, and availability.
Limitations
This study was subject to several limitations. Although this study is the most comprehensive assessment of the barriers and facilitators to routine HIV testing among U.S. CHCs, it was based upon a convenience sample and did not evaluate every provider and/or health center in Massachusetts. As a cross-sectional survey, it was not possible to assess any temporal developments that may have occurred during the course of the study, such as stakeholder discussions or state-level policy changes. Given that a bill to revise the state informed consent law had been introduced but not enacted,5 and that the Massachusetts Department of Public Health had released a June 2008 memorandum clarifying the state's opt-in policy,40 it is unlikely that widespread policy changes had recently occurred that would be sufficient to shift provider attitudes to any significant degree. Also, as one of only nine states with laws or regulations that required separate written consent for HIV testing of nonpregnant adults at the time of this study,5 it is important to note that generalizability of study findings to health centers in states without these requirements may be limited.
Another potential limitation to the interpretation of findings was the study definition of routine HIV testing as an opt-out procedure,36 which would suggest that respondents affirming implementation of routine testing were in violation of Massachusetts law. However, this limitation was unlikely given that 99% of survey respondents indicated that patients were required to sign written consent for HIV testing. Rather, as this study demonstrates, there is a need for improved education and training regarding the CDC recommendations.
CONCLUSIONS
As the U.S. HIV epidemic continues unabated, novel approaches to prevention and treatment are urgently needed. The 2006 CDC revisions to longstanding testing guidelines seek to increase the number of people aware of their HIV status through the normalization of testing as a routine clinical procedure. CHCs play a crucial role in the delivery of primary care to many of society's most vulnerable populations, including low-income individuals, people in racial/ethnic and sexual minority groups, immigrants, and those seeking mental health and substance abuse treatment services. Because these populations continue to be disproportionately affected by HIV/AIDS, it is appropriate that researchers and policy makers examine conditions and devise strategies to increase rates of HIV testing in these settings.
Given this study's findings that routine HIV testing is not being implemented consistently among Massachusetts CHCs, future efforts to increase implementation should consider addressing concerns regarding time, by streamlining ways to obtain informed consent and provide counseling, improving provider buy-in, and increasing staff availability. Development of revised testing protocols addressing these concerns and tailored to the expressed needs of individual health centers is warranted.
Footnotes
This project was supported by a Testing and Linkage to Care grant (#2691) from Gilead Sciences, Inc. awarded to Drs. Mayer and Mimiaga, who exercised full control over the project. The content is solely the responsibility of the authors and does not necessarily represent the official view of Gilead Science, Inc.
REFERENCES
- 1.Persons tested for HIV—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(31):845–9. [PubMed] [Google Scholar]
- 2.HIV prevalence estimates—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–6. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (US) HIV/AIDS surveillance report, 2007. Vol. 19. Atlanta: CDC; 2009. [Google Scholar]
- 4.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR14):1–17. [PubMed] [Google Scholar]
- 5.Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, Mayer KH. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. JAMA. 2008;300:945–51. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Huffman L. Preventive Services Task Force. Rockville (MD): Department of Health and Human Services (US), Agency for Healthcare Research and Quality; 2007. Screening for human immunodeficiency virus: focused update of a 2005 systematic evidence review for the U.S. AHRQ Pub. No. 07-0597-EF-1. [PubMed] [Google Scholar]
- 7.US. Preventive Services Task Force. Screening for HIV: recommendation statement. Ann Intern Med. 2005;143:32–7. doi: 10.7326/0003-4819-143-1-200507050-00008. [DOI] [PubMed] [Google Scholar]
- 8.Zetola NM, Grijalva CG, Gertler S, Hare CB, Kaplan B, Dowling T, et al. Simplifying consent for HIV testing is associated with an increase in HIV testing and case detection in highest risk groups, San Francisco January 2003–June 2007. PLoS One. 2008;3:e2591. doi: 10.1371/journal.pone.0002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton KA. Changing epidemiology of HIV/AIDS in the United States: implications for enhancing and promoting HIV testing strategies. Clin Infect Dis. 2007;45(Suppl 4):S213–20. doi: 10.1086/522615. [DOI] [PubMed] [Google Scholar]
- 10.Smith R, Zetola NM, Klausner JD. Beyond the end of exceptionalism: integrating HIV testing into routine medical care and HIV prevention. Expert Rev Anti Infect Ther. 2007;5:581–9. doi: 10.1586/14787210.5.4.581. [DOI] [PubMed] [Google Scholar]
- 11.Burrage JW, Zimet GD, Cox DS, Cox AD, Mays RM, Fife RS, et al. The Centers for Disease Control and Prevention revised recommendations for HIV testing: reactions of women attending community health clinics. J Assoc Nurses AIDS Care. 2008;19:66–74. doi: 10.1016/j.jana.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanssens C. Legal and ethical implications of opt-out HIV testing. Clin Infect Dis. 2007;45(Suppl 4):S232–9. doi: 10.1086/522543. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, del Rio C. Understanding the patient's perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev. 2004;16:101–14. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- 14.Henry J Kaiser Family Foundation. CDC recommends routine HIV testing for U.S. residents ages 13 to 64, dropping written consent, pretest counseling requirements [press release] 2006. Sep 22, [cited 2009 Apr 28]. Available from: URL: http://www.thebody.com/content/art38162.html.
- 15.Burke RC, Sepkowitz KA, Bernstein KT, Karpati AM, Myers JE, Tsoi BW, et al. Why don't physicians test for HIV? A review of the US literature. AIDS. 2007;21:1617–24. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 16.Jain CL, Wyatt CM, Burke R, Sepkowitz K, Begier EM. Knowledge of the Centers for Disease Control and Prevention's 2006 routine HIV testing recommendations among New York City internal medicine residents. AIDS Patient Care STDS. 2009;23:167–76. doi: 10.1089/apc.2008.0130. [DOI] [PubMed] [Google Scholar]
- 17.Zetola NM, Klausner JD, Haller B, Nassos P, Katz MH. Association between rates of HIV testing and elimination of written consents in San Francisco. JAMA. 2007;297:1061–2. doi: 10.1001/jama.297.10.1061. [DOI] [PubMed] [Google Scholar]
- 18.Anaya HD, Hoang T, Golden JF, Goetz MB, Gifford A, Bowman C, et al. Improving HIV screening and receipt of results by nurse-initiated streamlined counseling and rapid testing. J Gen Intern Med. 2008;23:800–7. doi: 10.1007/s11606-008-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz MB, Hoang T, Bowman C, Knapp H, Rossman B, Smith R, et al. A system-wide intervention to improve HIV testing in the Veterans Health Administration. J Gen Intern Med. 2008;23:1200–7. doi: 10.1007/s11606-008-0637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons MS, Lindsell CJ, Haukoos JS, Almond G, Brown J, Calderon Y, et al. Nomenclature and definitions for emergency department human immunodeficiency virus (HIV) testing: report from the 2007 conference of the National Emergency Department HIV Testing Consortium. Acad Emerg Med. 2009;16:168–77. doi: 10.1111/j.1553-2712.2008.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks L, Rietmeijer CA, McEwen D, Subiadur JA, Mettenbrink CJ. Normalizing HIV testing in a busy urban sexually transmitted infections clinic. Sex Transm Dis. 2009;36:127–8. doi: 10.1097/OLQ.0b013e318191701c. [DOI] [PubMed] [Google Scholar]
- 22.Weis KE, Liese AD, Hussey J, Coleman J, Powell P, Gibson JJ, et al. A routine HIV screening program in a South Carolina community health center in an area of low HIV prevalence. AIDS Patient Care STDS. 2009;23:251–8. doi: 10.1089/apc.2008.0167. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham CO, Doran B, DeLuca J, Dyksterhouse R, Asgary R, Sacajiu G. Routine opt-out HIV testing in an urban community health center. AIDS Patient Care STDS. 2009;23:619–23. doi: 10.1089/apc.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers JJ, Modica C, Dufour MS, Bernstein C, McNamara K. Routine rapid HIV screening in six community health centers serving populations at risk. J Gen Intern Med. 2009;24:1269–74. doi: 10.1007/s11606-009-1070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Association of Community Health Centers. America's health centers. [cited 2009 Jun 18]. Available from: URL: http://www.nachc.com/client/documents/America's_Health_Centers_updated_3.09.pdf.
- 26.National Association of Community Health Centers. Fact sheet: health centers on the front lines of HIV/AIDS prevention and treatment. 2007. Jun, [cited 2009 Jun 18]. Available from: URL: http://www.nachc.com/client/documents/issues-advocacy/policy-library/research-data/fact-sheets/HIVAIDS_Fact_Sheet2.pdf.
- 27.Centers for Disease Control and Prevention (US) HIV counseling and testing at CDC-supported sites—United States, 1999–2004. Atlanta: CDC; 2006. [Google Scholar]
- 28.Massachusetts Department of Public Health. Clinical advisory: routine HIV screening in primary and urgent care settings in Massachusetts. Boston: The Commonwealth of Massachusetts Executive Office of Health and Human Services, Department of Public Health; 2009. [Google Scholar]
- 29. General Laws of Massachusetts. Title XVI, Ch. 111, §70F.
- 30.Mahajan AP, Stemple L, Shapiro MF, King JB, Cunningham WE. Consistency of state statutes with the Centers for Disease Control and Prevention HIV testing recommendations for health care settings. Ann Intern Med. 2009;150:263–9. doi: 10.7326/0003-4819-150-4-200902170-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf LE, Donoghoe A, Lane T. Implementing routine HIV testing: the role of state law. PLoS One. 2007;2:e1005. doi: 10.1371/journal.pone.0001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Department of Health and Human Services (US), Health Resources and Services Administration. The HIV/AIDS program: Ryan White Parts A-F. [cited 2011 Jun 24]. Available from: URL: http://hab.hrsa.gov.
- 33.Massachusetts League of Community Health Centers. About us. [cited 2008 Mar 25]. Available from: URL: http://www.massleague.org/About/MissionAndRole.php.
- 34.Boston AIDS Consortium. Persevering in the struggle against AIDS: the Boston EMA HIV Health Services Planning Council comprehensive plan 2005–2008. Boston: Boston AIDS Consortium; 2005. [Google Scholar]
- 35.National Association of Community Health Centers. National assessment of health center HIV testing, prevention, care and treatment practices. Bethesda (MD): NACHC; 2005. [Google Scholar]
- 36.National Alliance of State and Territorial AIDS Directors. Report on findings from an assessment of health department efforts to implement HIV screening in health care settings. 2007. [cited 2007 Dec 12]. Available from: URL: http://www.nastad.org/Docs/highlight/2007626_NASTAD_Screening_Assessment_Report_062607.pdf.
- 37.SAS Institute, Inc. SAS®: Version 9.1. Cary (NC): SAS Institute, Inc.; 2003. [Google Scholar]
- 38.Massachusetts Department of Public Health, HIV/AIDS Bureau. Massachusetts HIV/AIDS data fact sheet: the Massachusetts HIV/AIDS epidemic at a glance. Boston: Massachusetts Department of Public Health, HIV/AIDS Bureau; 2008. [cited 2009 Jul 13]. Also available from: URL: http://www.mass.gov/Eeohhs2/docs/dph/aids/2008_profiles/epidemic_glance.pdf. [Google Scholar]
- 39.Cohan D, Sarnquist C, Gomez E, Feakins C, Maldonado Y, Zetola N. Increased uptake of HIV testing with the integration of nurse-initiated HIV testing into routine prenatal care. J Acquir Immune Defic Syndr. 2008;49:571–3. doi: 10.1097/QAI.0b013e31818d5e11. [DOI] [PubMed] [Google Scholar]
- 40.Massachusetts Department of Public Health. Routine HIV counseling and testing of pregnant women clinical advisory update. Boston: The Commonwealth of Massachusetts Executive Office of Health and Human Services, Massachusetts Department of Public Health; 2008. [Google Scholar]