Abstract
Objectives
We examined trends and epidemiology of tuberculosis (TB) across racial/ethnic groups to better understand TB disparities in the United States, with particular focus on American Indians/Alaska Natives (AI/ANs) and Native Hawaiians/other Pacific Islanders (NH/PIs).
Methods
We analyzed cases in the U.S. National Tuberculosis Surveillance System and calculated TB case rates among all racial/ethnic groups from 2003 to 2008. Socioeconomic and health indicators for counties in which TB cases were reported came from the Health Resources and Services Administration Area Resource File.
Results
Among the 82,836 TB cases, 914 (1.1%) were in AI/ANs and 362 (0.4%) were in NH/PIs. In 2008, TB case rates for AI/ANs and NH/PIs were 5.9 and 14.7 per 100,000 population, respectively, rates that were more than five and 13 times greater than for non-Hispanic white people (1.1 per 100,000 population). From 2003 to 2008, AI/ANs had the largest percentage decline in TB case rates (−27.4%) for any racial/ethnic group, but NH/PIs had the smallest percentage decline (−3.5%). AI/ANs were more likely than other racial/ethnic groups to be homeless, excessively use alcohol, receive totally directly observed therapy, and come from counties with a greater proportion of people living in poverty and without health insurance. A greater proportion of NH/PIs had extrapulmonary disease and came from counties with a higher proportion of people with a high school diploma.
Conclusions
There is a need to develop flexible TB-control strategies that address the social determinants of health and that are tailored to the specific needs of AI/ANs and NH/PIs in the U.S.
Among the 9.2 million new cases of tuberculosis (TB) and 1.7 million deaths due to TB globally each year,1 the absolute magnitude of TB among the estimated 370 million indigenous people worldwide2 is not known because few disaggregated surveillance data exist. However, evidence from nations where data are available, such as the U.S., Canada, Australia, and New Zealand, suggests that TB rates are higher among indigenous than nonindigenous people.3–7
Disparities in health, including differences in TB risk and burden, between indigenous and nonindigenous people are the result of the complex interplay among the individual, the community, and the social determinants of health.8,9 Determinants of health extend beyond individual-level traits and behaviors and involve social and economic factors that operate at the community or population level.10,11 It is critical to understand the factors that underlie poorer health status and outcomes among indigenous people to effectively address and narrow the health gap.
In November 2008, indigenous leaders and TB experts from around the world met in Canada to develop a strategy for reducing the burden of TB among indigenous people globally.12 Poverty and other social determinants of health were acknowledged as factors influencing indigenous people's vulnerability to TB.13 Furthermore, participants highlighted the need for documenting and monitoring the burden of TB in indigenous people and better understanding the social realities of these groups to design more effective TB interventions.
The term “indigenous” has many different usages. The United Nations approach uses various concepts, including self-identification and recognition by one's community as indigenous and constituting non-dominant groups of society.14 Two broad categories of indigenous groups in the U.S. are the American Indians/Alaska Natives (AI/ANs) and the Native Hawaii-ans/other Pacific Islanders (NH/PIs). These groups encompass many people, each with distinct historical identities. In 2008, an estimated 2.3 million and 435,000 U.S. residents identified themselves as solely AI/AN and NH/PI, respectively.15
TB is a public health concern for AI/ANs and NH/PIs in the U.S. For example, from 1993 to 2002, AI/ANs experienced the smallest decrease in TB case rates of any U.S.-born racial/ethnic group, and the rate was almost six times greater than for non-Hispanic white people in 2002.16 More recently, several large TB outbreaks among NH/PIs have occurred.17 However, to date, few studies have examined individual and community similarities and differences among TB cases in indigenous and nonindigenous groups at the national level.
To update our understanding and further examine the magnitude of TB in indigenous people, and to inform public health approaches to address TB disparities in the U.S., we described the TB trends and epidemiology of AI/ANs and NH/PIs relative to other racial/ethnic groups during 2003–2008. We also examined county-level socioeconomic and health indicators from counties in which AI/AN and NH/PI TB cases were reported.
METHODS
Data systems
We analyzed data from two systems: the National TB Surveillance System (NTSS) and the 2007 Health Resources and Services Administration (HRSA) Area Resource File (ARF). NTSS is the Centers for Disease Control and Prevention (CDC)-based national surveillance system in which health departments in the 50 states and the District of Columbia (DC) electronically report verified TB cases to CDC using standardized definitions.18 The NTSS is used to monitor the frequency and distribution of TB and guide TB-control efforts in the U.S. The HRSA ARF is a national county-level health resource information database containing more than 6,000 variables from more than 50 sources.19 It contains information on health facilities, training programs and professions, measures of resource scarcity, health status, economic activity, and socioeconomic and environmental characteristics.
Study population
We analyzed reports of all TB cases submitted to NTSS from all 50 states and DC between January 1, 2003, and December 31, 2008. Reports included demographic, clinical, case management, and laboratory data.20
Definitions
In this study, race/ethnicity was based on self-report. The U.S. Census Bureau, which uses self-reported data, defines AI/ANs as “people having origins in any of the original peoples of North and South America (including Central America), and who maintain tribal affiliation or community attachment.” NH/PI is defined as “people having origins in any of the original peoples of Hawaii, Guam, Samoa, or other Pacific Islands.”21
The race/ethnicity variable in NTSS is based on each patient's self-identity. For ethnicity, patients can choose either “Hispanic or Latino” or “not Hispanic or Latino.” For race, TB patients select one or more of the following: AI/AN, NH/PI, Asian, black or African American, or white. For this analysis, we categorized race/ethnicity into Hispanic or Latino or, among non-Hispanic people, AI/AN, NH/PI, Asian, black or African American, or white. Few patients selected no race or ethnicity or multiple races in NTSS (n=464), and among non-Hispanic people, only cases reported as single race were included in the analysis.
We defined a U.S.-born person as someone born in the U.S. or its associated jurisdictions, or someone born in a foreign country but having at least one parent who was a U.S. citizen. Others were classified as foreign-born. For the groups of AI/ANs and NH/PIs to be consistent with the aforementioned definitions and to minimize the level of misclassification, we excluded TB cases that were AI/AN and NH/PI documented as originating from countries not considered part of our definitions (n=12 for AI/AN and n=35 for NH/PI).
Design and data analysis
Taking Schneider's article examining TB in AI/ANs between 1993 and 200216 as a model for this analysis, we used NTSS data to describe patient sociodemographic, geographic, clinical, and bacteriological characteristics and treatment outcomes by racial/ethnic group between 2003 and 2008. Missing or unknown data were removed from the analyses if they comprised less than 5% of the total.
To examine the bivariate relationships between racial/ethnic groups and patient characteristics, we calculated unadjusted odds ratios (ORs) and 95% confidence intervals (CIs). For each comparison with AI/AN and NH/PI, the other racial/ethnic categories were used as the reference group. We used the Chi-square test for categorical variables. We conducted additional Chi-square tests to examine differences in characteristics between AI/ANs and NH/PIs and each of the other racial/ethnic groups during the study period. We defined statistical significance as p<0.05. We used the U.S. Census annual population estimates15 to calculate national case rates by racial/ethnic group. We examined trends in TB case rates during the study period for AI/ANs, NH/PIs, and other racial/ethnic groups.
We also compared characteristics of counties in which TB cases that were AI/AN and NH/PI were reported with characteristics of counties in which TB cases of other racial/ethnic groups originated. We linked each TB case from NTSS to county-level socioeconomic and health-care data from the county in which the case was reported. At least one TB case was needed for inclusion of a county.
We obtained county-level data from the 2007 HRSA ARF and linked them to TB cases using federal information processing standards codes.22 Because indicators within the ARF come from different sources (e.g., the Census Bureau, National Center for Health Statistics, Centers for Medicare and Medicaid Services, and Bureau of Labor Statistics), which collect data at different intervals, and not always annually, county-level ARF indicators were selected for this analysis only if they overlapped with the study period and, of these, ARF indictors collected in the middle of 2003 and 2008 were selected if available. For each of the county-level indicators, we examined differences in means and average proportions by racial/ethnic groups using analysis of variance testing. We conducted the Bonferroni test to compare pairs of group means, specifically examining differences in county characteristics between AI/AN, NH/PI, and other racial/ethnic groups.
Ethical considerations
The TB data for this study were part of a surveillance system without personal identifiers and, therefore, did not require human subjects research approval. However, the study was reviewed and approved by CDC's Division of TB Elimination Analytic Steering Committee.
RESULTS
Description of TB cases
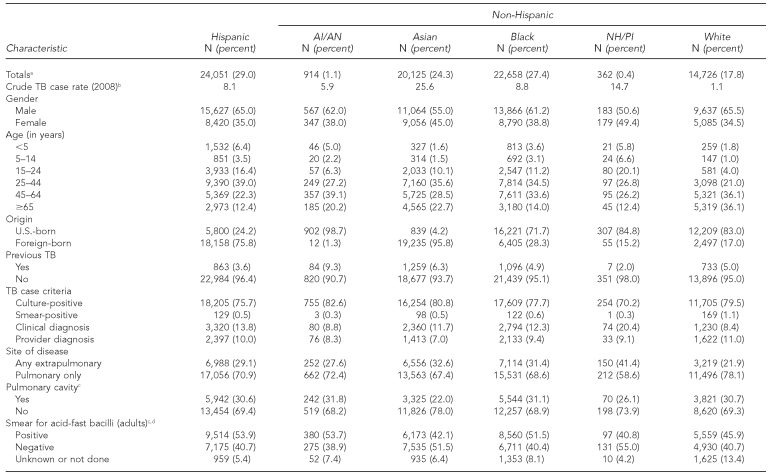
Of the 82,836 TB cases reported between 2003 and 2008, 914 (1.1%) were among AI/ANs and 362 (0.4%) were among NH/PIs, with NH/PIs having the smallest absolute number of cases reported compared with all other racial/ethnic groups (Table 1). In comparison with all other groups, the proportion of patients who were female was highest among NH/PI patients (49.4%) and, on average, NH/PI patient were significantly younger than all other groups except for Hispanic patients (p<0.05, all comparisons). The four states reporting the largest number of TB cases among NH/PIs were Hawaii (n=99), California (n=81), Washington (n=42), and Arkansas (n=42); the majority of TB cases among AI/ANs were reported from Alaska (n=209), Oklahoma (n=164), Arizona (n=94), and New Mexico (n=73) (data not shown).
Table 1.
Demographic and clinical characteristics of TB cases, by racial/ethnic group: U.S., 2003–2008 (n=82,836)
aCell counts may not always add up to totals because of missing values.
bTB case rate includes only TB cases counted in 2008; TB case rate = TB cases per 100,000 population.
cIncludes only people with any pulmonary TB
dAdult is defined as a person aged 15 years and older.
eHIV status does not include data from Vermont or California; unknown or missing = indeterminate, refused, not offered, test done but unknown, or unknown.
TB = tuberculosis
AI/AN = American Indian/Alaska Native
NH/PI = Native Hawaiian/other Pacific Islander
HIV = human immunodeficiency virus
TB rates
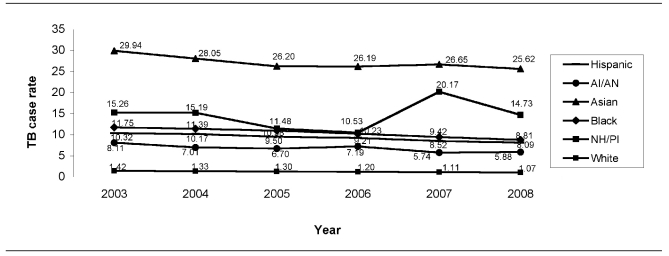
In 2008, TB case rates for AI/ANs and NH/PIs were 5.9 and 14.7 per 100,000 population, respectively, rates that were more than five and 13 times greater than for non-Hispanic white people (1.1 per 100,000 population) (Table 1, Figure). Among all racial/ethnic groups, AI/ANs had the second lowest case rate overall in 2008. From 2003 to 2008, AI/ANs experienced the largest percentage decline in TB case rate (−27.4%) for any racial/ethnic group, whereas NH/PIs experienced the smallest percentage decline overall (−3.5%). The number of TB cases among NH/PIs almost doubled between 2006 and 2007, which can be partly explained by increases in the number of patients born in the Federated States of Micronesia and the Marshall Islands who were residing and reported in the U.S. during that time. However, between 2007 and 2008, there was a steep decline in TB case rates among NH/PIs (−27.0%).
Figure.
TB case rates, by race/ethnicity: U.S., 2003–2008 (n=82,836)
AI/AN = American Indian/Alaska Native
NH/PI = Native Hawaiian/other Pacific Islander
TB = tuberculosis
Clinical characteristics
Compared with all other groups, TB patients who were AI/AN had a greater proportion of previous TB (9.3%, p<0.001, all comparisons) and were more frequently diagnosed with a positive culture (82.6%, p<0.001, all comparisons) (Table 1).
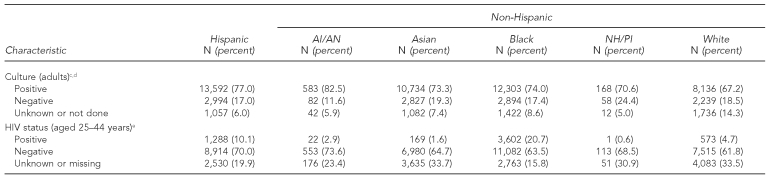
As shown in Table 1, 53.7% of AI/ANs had positive sputum-smear microscopy results, while 31.8% had pulmonary cavities, a marker for disease severity. Compared with other groups, NH/PIs more commonly had extrapulmonary disease (41.4%, p<0.001, all comparisons) and were diagnosed clinically (i.e., without microbiology confirmation) (20.4%, p<0.0001, all comparisons). A smaller fraction of NH/PIs had pulmonary cavities (26.1%) and positive acid-fast bacilli results (40.8%). Both groups had a low prevalence rate of human immunodeficiency virus (HIV) infection (<3.0%) compared with white (4.7%), Hispanic (10.1%), and black (20.7%) patients; 23.4% and 30.9% of AI/ANs and NH/PIs, respectively, did not know their HIV status. AI/AN was the only racial/ethnic group to have no multidrug-resistant (i.e., resistant to at least isoniazid and rifampin) cases reported during this study period. The proportion of cases with multidrug resistance for all other groups ranged from 2.0% (Asian patients) to 0.7% (white patients) (data not shown).
Social and behavioral characteristics
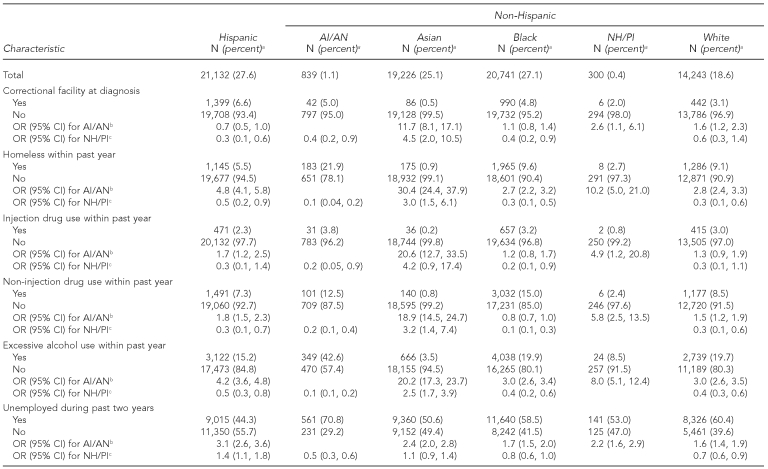
Among patients aged 18 years and older, a significantly greater proportion of AI/ANs than other groups were unemployed during the past two years (70.8%, p<0.0001, all comparisons), reported excessive alcohol use within the past year (42.6%, p<0.0001, all comparisons), and were homeless within the past year (21.9%, p<0.0001, all comparisons) (Table 2). For example, AI/ANs were three times more likely than non-Hispanic white patients (OR=2.8, 95% CI 2.4, 3.3) to be homeless. A greater proportion of AI/AN patients (5.0%) were in a correctional facility at the time of diagnosis than any other group, except Hispanic patients (6.6%); this difference was statistically significant (p<0.05, all comparisons, except for black patients).
Table 2.
Social and behavioral characteristics among patients with TB aged ≥18 years, by racial/ethnic group: U.S., 2003–2008 (n=76,481)
aCell counts may not always add up to totals because of missing values.
bComparison of AI/AN vs. other racial/ethnic groups. The reference group, the racial/ethnic group being compared with AI/AN, changes for each column.
cComparison of NH/PI vs. other racial/ethnic groups. The reference group, the racial/ethnic group being compared with NH/PI, changes for each column.
TB = tuberculosis
AI/AN = American Indian/Alaska Native
NH/PI = Native Hawaiian/other Pacific Islander
OR = odds ratio
CI = confidence interval
In contrast, patients who were NH/PI were generally less likely than those of other racial/ethnic groups, except Asians, to report homelessness or the use of non-injection drugs or excessive alcohol in the past year. For example, NH/PIs were significantly less likely than all other racial/ethnic groups to be homeless (p<0.05, all comparisons except for Asians), use non-injection drugs (p<0.05, all comparisons except for Asians), and report excessive alcohol use (p<0.05, all comparisons except for Asians). However, patients who were NH/PI were approximately three times more likely than Asian patients to report these characteristics. NH/PI TB cases were significantly more likely than Hispanic TB cases to be unemployed during the past two years (OR=1.4, 95% CI 1.1, 1.8).
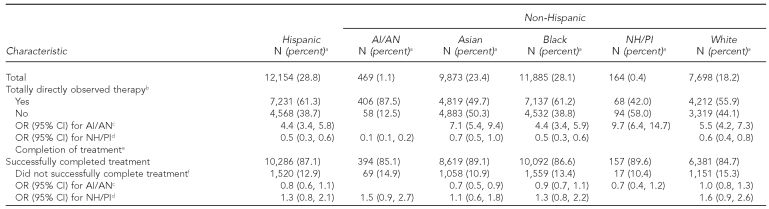
Treatment characteristics
Final treatment outcomes were available through 2005. AI/ANs had the largest proportion of cases receiving directly observed therapy (DOT) during the total course of treatment (87.5%, p<0.0001, all comparisons), making them four to 10 times more likely than those in other racial/ethnic groups to receive total DOT (Table 3). AI/ANs were only slightly less likely to complete treatment than Asian or NH/PI TB patients (p<0.01), but had similar treatment completion rates as other racial/ethnic groups (p≥0.05, all comparisons). The proportion of NH/PIs who successfully completed treatment was 89.6%. Compared with patients in other groups, NH/PIs were the least likely to have received DOT during the total course of therapy (42.0%), and instead had a greater proportion of people receiving self-administered treatment or partial provision by DOT.
Table 3.
Treatment characteristics among patients with TB, by racial/ethnic group: U.S., 2003–2005 (n=42,243)
aCell counts may not always add up to totals because of missing values.
bCompletely throughout the course of treatment
cComparison of AI/AN vs. other racial/ethnic groups. The reference group, the racial/ethnic group being compared with AI/AN, changes for each column.
dComparison of NH/PI vs. other racial/ethnic groups. The reference group, the racial/ethnic group being compared with NH/PI, changes for each column.
en=41,310, excludes unknown or missing data
fIncludes the following outcomes: moved, lost, uncooperative or refused, died, and other
TB = tuberculosis
AI/AN = American Indian/Alaska Native
NH/PI = Native Hawaiian/other Pacific Islander
OR = odds ratio
CI = confidence interval
Compared with other racial/ethnic groups, smaller proportions of AI/ANs (49.9%) and NH/PIs (40.9%) received care for TB exclusively from the health department, with Hispanic patients most commonly using the health department only (63.0%). Overall, Asians had the largest proportion of cases managed exclusively by private providers (25.0%), compared with 19.0% of cases among both AI/ANs and NH/PIs (data not shown).
County characteristics
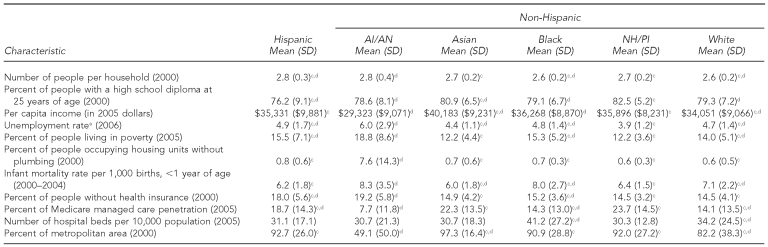
Table 4 presents averages of county-level demographic, economic, and health characteristics for counties in which TB cases were reported. Compared with all other racial/ethnic groups, TB patients who were AI/AN came from counties that, on average, had larger household sizes, higher unemployment rates, a greater percentage of people living in poverty, a greater proportion of housing units without plumbing, higher infant mortality rates, a higher proportion of people living without health insurance, a smaller percentage of Medicare penetration, and a higher proportion of people living in a nonmetropolitan area. Overall, TB patients who were AI/AN originated from counties that had fewer resources than those from which NH/PIs were reported.
Table 4.
Characteristics of counties from which patients with TB originated, by racial/ethnic group: U.S., 2003–2008 (n=82,836)a,b
The numbers reported are means or average proportions and SDs at the county-level for counties in which the TB cases originated.
All relationships are significant at p<0.0001 for overall association between race/ethnicity and county-level characteristic.
cIndicates significant difference vs. AI/AN (p<0.05)
dIndicates significant difference vs. NH/PI (p<0.05)
eThe number of unemployed people aged ≥16 years as a percentage of the civilian labor force
TB = tuberculosis
AI/AN = American Indian/Alaska Native
NH/PI = Native Hawaiian/other Pacific Islander
SD = standard deviation
NH/PIs came from counties that, on average, had a significantly higher proportion of people with at least a high school diploma by 25 years of age, with an average of 82.5% of people graduating from high school compared with 78.6% for counties in which TB patients who were AI/AN originated.
DISCUSSION
In this study, we documented the epidemiology of TB in AI/ANs and NH/PIs in the U.S. during 2003–2008. We found important similarities and differences between these two groups as well as with other racial/ethnic groups in the U.S. Although the absolute numbers of cases among AI/ANs and NH/PIs were smaller than among the other racial/ethnic groups, their case rates exceeded those of non-Hispanic white people. During the five-year study period, the greatest percentage decline in rates was observed for AI/ANs. This finding may be related, in part, to the smaller proportion of patients in this group who were foreign-born compared with other groups; in recent years, TB case rates among the U.S.-born population have been decreasing at a faster pace than those for foreign-born people.18
Conversely, NH/PIs experienced the largest overall percentage increase in rates from 2003 to 2008, due in large part to an increase in cases in 2007. It is important to interpret these changes with caution, however, because of the potential for unstable estimates due to the small number of TB cases in this group. A small change in the number of cases reported in NH/PIs (e.g., due to an individual clinician's diagnostic and practicing patterns) could affect the case rates and trends. Overall, case rates for NH/PIs and AI/ANs are higher than the CDC's 2000 national interim target of 3.5 per 100,000 population.23 These differences in rates of TB constitute important health disparities. Eliminating racial/ethnic health disparities is a goal of several federal initiatives, including Healthy People 2020.24
We found that the proportion of AI/AN TB patients who were homeless, unemployed, or used alcohol excessively was higher than other groups, similar to the findings for 1993–2002.16 Almost one-quarter of AI/AN TB patients were homeless within the past year, which is significantly greater than the proportion found for other racial/ethnic groups. TB already has been recognized as an important health threat for homeless AI/ANs. Almost half of the patients in a TB outbreak among homeless people in Washington State from 2002 to 2003 were AI/AN.25 Early detection and treatment of TB disease among homeless people is a priority for TB prevention and control activities in the U.S.26 Furthermore, we found that a large proportion of TB patients (43%) who were AI/AN included reports of excessive alcohol use within the past year, almost three times that reported for all TB patients ≥15 years of age in the U.S. between 1997 and 2006 (15.1%).27 These findings highlight the importance of TB-control programs to work together with poverty reduction and alcohol abuse programs to simultaneously manage the diseases of addiction and TB and address the underlying causes of disease in AI/ANs.
An important finding in this study was that TB cases among AI/ANs were reported from communities that tended to have fewer resources. Approximately half of the TB cases in AI/ANs were reported from rural communities, which may add to the challenges of TB case detection, management, and contact investigations in AI/ANs due to the distances health-care personnel and patients must travel.16,28 Compared with the counties for other racial/ethnic groups, counties in which AI/ANs resided had the greatest proportion of the population who were living in poverty, unemployed, and without health insurance. Previous studies have found community- and population-level factors (e.g., level of education and socioeconomic status in a community) to be associated with TB risk,29,30 and improvements in population health and health services are related to improvements in TB outcomes.31 Poverty is an important social determinant of disease and a key obstacle to health and health care.32 Promotion of equity and pro-poor policies in TB prevention and control activities, including working to improve the conditions of daily life among AI/ANs in the U.S., is needed.8,29
We found that AI/ANs more commonly received DOT throughout TB treatment than other racial/ethnic groups, despite the barriers to successful DOT present in many AI/AN communities. Similar to a study comparing indigenous groups with the general population in Canada,5 we found AI/AN TB patients had comparable treatment completion rates as other racial/ethnic groups. The DOT patient-centered approach to treatment support is recommended as a core element of TB care and control efforts,33,34 and DOT has been associated with improved TB treatment outcomes.35,36 Access to guaranteed health care and, hence, total DOT through Indian Health Service (IHS), tribal, and urban Indian health-care facilities, may have contributed to the recent declines in case rates among AI/ANs over time. In addition to the ongoing decrease in TB case rates in AI/ANs found in this and previous studies,16 these findings highlight the success of recent TB-control efforts focusing on AI/ANs in the U.S.
We found that NH/PIs were more likely to be female, younger, and diagnosed with extrapulmonary disease. These findings were similar to a previous study using NTSS data comparing Asian/Pacific Islanders with non-Hispanic white people in the U.S. between 1993 and 2006.37 Other studies in the U.S. have found associations between extrapulmonary disease and nonwhite race and/or ethnicity,38,39 region of birth,40 and genotypic lineage.41,42 We also found that, compared with other groups, NH/PIs were more frequently diagnosed clinically and less commonly had HIV infection, a known risk factor for extrapulmonary disease.43 These findings are important for informing TB-control strategies among NH/PI TB patients who lack common risk factors for extrapulmonary disease to ensure disease detection and provision of appropriate TB treatment.
We found that TB cases among NH/PIs are reported from communities in which, on average, there was a greater proportion of people with at least a high school diploma and the lowest unemployment rate. Although TB cases in NH/PIs were reported from communities that tended to have more resources, they may have difficulties in accessing those resources. In a study involving 50 NH/PI TB patients from the Marshall Islands living in Arkansas from 2000 to 2005, 65% of symptomatic TB patients had delayed diagnosis (>60 days from symptom onset), largely owing to patients not seeking medical care, difficulties navigating the health-care system, and language and transportation barriers.44 Furthermore, previous studies have found that individual-level TB risk factors, such as diabetes, are prevalent in NH/PIs45,46 and, thus, may have contributed to the high rates of TB in this population, regardless of the level of community resources. However, because more extensive individual-level data were not available, we were unable to assess this possibility in our study. To reduce health disparities, the recent increases in the TB case rate among NH/PIs found in this study highlight the growing need to better understand the obstacles to and best strategies for TB prevention and control in this group.
For this study, we used self-reported race/ethnicity data based on categories used by the Census Bureau. We recognize that the racial/ethnic minority groups are, in reality, heterogeneous and constitute unique individuals and groups within each broad classification. However, to monitor progress or setbacks in inequalities in health,47 researchers and advocacy groups have been promoting more disaggregated health data to quantify health issues; teach others about disparities; and improve planning, funding, and health-care delivery in underserved communities.48 In this study, the differences found between NH/PIs and AI/ANs highlight the need to develop flexible TB-control strategies tailored to the specific needs of the unique racial/ethnic groups in the U.S. More comprehensive studies can help to more thoroughly understand the epidemiology of TB within the NH/PI and AI/AN groups.
Limitations
Our study had several limitations. First, there was the potential for misclassification for race/ethnicity. For example, in a study of HIV/acquired immunodeficiency syndrome reporting systems, the authors found racial misclassification of AI/AN was associated with degree of ancestry of AI/ANs and living in an urban setting.49 In this study, race/ethnicity was self-reported, and we restricted our analyses to only those who reported a single race/ethnicity. However, it is unlikely that misclassification introduced a systematic bias, as there is no reason to expect the accuracy of self-reporting of a single race/ethnicity to be different among groups. Any misclassification would most likely have resulted in under-estimating case counts among AI/ANs or NH/PIs. We attempted to further limit the level of misclassification of AI/ANs and NH/PIs by excluding cases originating from countries or territories that did not fit the race/ethnicity definitions.
Second, we only included cases in the NTSS, so our analysis missed any TB cases that were not reported or not registered in the system because of counting criteria. The IHS, which provides health care for approximately 60% of AI/ANs, has an independent system for recording cases of TB and other diseases among inpatients and outpatients. While there are guidelines in place for the IHS to report TB cases to local and state authorities, the extent to which this occurs is not well understood; an evaluation is planned to compare TB cases reported in the NTSS with data on TB cases identified in the IHS system at selected sites.
Finally, we recognize the potential for ecological fallacy when applying the average characteristics at a county level to individuals within that community. It was not possible to tease out the indirect influence of community-level factors in this study, given the complex matrix of other factors such as biology and the population's historical experience with the epidemic that are also in play. While our data were not structured to specifically use statistical methods that allow for the consideration of the hierarchy of factors, this analysis was intended to help inform future studies on community-level factors that may be of importance; these studies should gather data from various sources that will allow for the incorporation of these methods.
CONCLUSIONS
This study updates our current understanding of the TB epidemiology among indigenous people, AI/ANs and NH/PIs, relative to other racial/ethnic groups in the U.S. by describing individual and community characteristics that may be of importance for TB cases and rates, and how those characteristics vary across different racial/ethnic groups. By linking community-level and patient-level data, we were able to examine contextual information about the general status of health and wealth in the communities in which TB cases originated. These findings help to improve our understanding and highlight the importance of addressing both individual- and community-level social determinants of TB, especially among indigenous groups in the U.S. TB programs and initiatives that focus interventions among AI/AN and NH/PI groups should also address the social determinants of health.
Additional studies are necessary to further understand the social, cultural, and environmental factors that underlie health disparities in indigenous populations. Hierarchical theory and multilevel modeling that includes individual and community characteristics, and the complex interrelationship of the two, can help further delineate modifiable factors that may lessen the risk of TB and other diseases among these groups.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Indian Health Service.
REFERENCES
- 1.World Health Organization. Global tuberculosis control 2009—- epidemiology, strategy, financing. Geneva: WHO; 2009. [Google Scholar]
- 2.UN Permanent Forum on Indigenous Issues. State of the world's indigenous peoples. New York: United Nations; 2009. [cited 2011 Mar 29]. Also available from: URL: http://www.un.org/esa/socdev/unpfii/documents/SOWIP_web.pdf. [Google Scholar]
- 3.Das D, Baker M, Calder L. Tuberculosis epidemiology in New Zealand: 1995–2004. N Z Med J. 2006;119:U2249. [PubMed] [Google Scholar]
- 4.Fanning A. Tuberculosis: 1. Introduction. CMAJ. 1999;160:837–9. [PMC free article] [PubMed] [Google Scholar]
- 5.FitzGerald JM, Wang L, Elwood RK. Tuberculosis: 13. Control of the disease among aboriginal people in Canada. CMAJ. 2000;162:351–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Trends in tuberculosis—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(10):249–53. [PubMed] [Google Scholar]
- 7.Barry C, Konstantinos A, National Tuberculosis Advisory Committee. Tuberculosis notifications in Australia, 2007. Commun Dis Intell. 2009;33:304–15. [PubMed] [Google Scholar]
- 8.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–6. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 9.Basta PC, Coimbra CE, Jr, Escobar AL, Santos RV, Alves LC, Fonseca Lde S. Survey for tuberculosis in an indigenous population of Amazonia: the Suruí of Rondônia, Brazil. Trans R Soc Trop Med Hyg. 2006;100:579–85. doi: 10.1016/j.trstmh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization, Commission on Social Determinants of Health. Geneva: WHO; 2008. [cited 2010 Apr 7]. Closing the gap in a generation: health equity through action on the social determinants of health. Also available from: URL: http://www.who.int/social_determinants/thecommission/finalreport/en/index.html. [DOI] [PubMed] [Google Scholar]
- 11.King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. 2009;374:76–85. doi: 10.1016/S0140-6736(09)60827-8. [DOI] [PubMed] [Google Scholar]
- 12.Assembly of First Nations and Inuit Tapiriit Kanatami. A strategic framework for action on tuberculosis control in indigenous communities: a global indigenous peoples' initiative to stop TB. 2009. [cited 2010 Apr 7]. Available from: URL: http://fnpublichealth.ca/wp-content/uploads/PDF/GI-STOP-TB-StratActPlan.pdf.
- 13.Assembly of First Nations and Inuit Tapiriit Kanatami. A global indigenous peoples' initiative to stop TB: our children, our future: executive summary. Presented at the STOP-TB Experts Meeting; 2008 Nov 12–14; Toronto. [cited 2010 Apr 7]. Also available from: URL: http://fnpublichealth.ca/wp-content/uploads/PDF/GI-STOP-TB-ExecSum.pdf.
- 14.United Nations Permanent Forum on Indigenous Issues. Fifth session of the UNPFII: fact sheet 1: indigenous peoples and identity. [cited 2010 Apr 7]. Available from: URL: http://www.un.org/esa/socdev/unpfii/documents/5session_factsheet1.pdf.
- 15.Census Bureau (US). Annual estimates of the resident population by sex, race, and Hispanic origin for the United States: April 1, 2000, to July 1, 2008 (NC-EST2008-03) [cited 2010 Apr 7]. Available from: URL: http://www.census.gov/popest/national/asrh/NC-EST2008-srh.html.
- 16.Schneider E. Tuberculosis among American Indians and Alaska Natives in the United States, 1993–2002. Am J Public Health. 2005;95:873–80. doi: 10.2105/AJPH.2004.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Two simultaneous outbreaks of multidrug-resistant tuberculosis—Federated States of Micronesia, 2007–2009. MMWR Morb Mortal Wkly Rep. 2009;58(10):253–6. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC). Reported tuberculosis in the United States, 2008. Atlanta: Department of Health and Human Services (US); 2009. [cited 2010 Apr 7]. Also available from: URL: http://www.cdc.gov/tb/statistics/reports/2008/default.htm. [Google Scholar]
- 19.Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Professions (US). Area resource file, 2007. Rockville (MD): HHS; 2009. [Google Scholar]
- 20.Centers for Disease Control and Prevention (US). Report of verified case of tuberculosis (RVCT) in the United States. OMB No. 0920-0020. Atlanta: CDC; 2003. [Google Scholar]
- 21.Census Bureau (US). Census 2000 brief: overview of race and Hispanic origin: 2000. March 2001. [cited 2010 Apr 7]. Available from: URL: http://www.census.gov/prod/2001pubs/cenbr01-1.pdf.
- 22.National Institute of Standards and Technology (US). Index of codes for FIPS 6-4. [cited 2011 Apr 7]. Available from: URL: http://www.itl.nist.gov/fipspubs/co-codes/states.htm#US_TERR_SEC.
- 23.A strategic plan for the elimination of tuberculosis in the United States. MMWR Morb Mortal Wkly Rep. 1989;38(S-3):1–25. [PubMed] [Google Scholar]
- 24.Department of Health and Human Services (US). About Healthy People. [cited 2011 Feb 11]]. Available from: URL: http://healthypeople.gov/2020/about/default.aspx.
- 25.Public health dispatch: tuberculosis outbreak among homeless persons—King County, Washington, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52(49):1209–10. [PubMed] [Google Scholar]
- 26.Prevention and control of tuberculosis among homeless persons. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Morb Mortal Wkly Rep. 1992;41(RR-5):13–23. [PubMed] [Google Scholar]
- 27.Oeltmann JE, Kammerer JS, Pevzner ES, Moonan PK. Tuberculosis and substance abuse in the United States, 1997–2006. Arch Intern Med. 2009;169:189–97. doi: 10.1001/archinternmed.2008.535. [DOI] [PubMed] [Google Scholar]
- 28.Progressing toward tuberculosis elimination in low-incidence areas of the United States. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Morb Mortal Wkly Rep. 2002;51(RR-5):1–14. [PubMed] [Google Scholar]
- 29.de Alencar Ximenes RA, de Fátima Pessoa Militão de Albuquerque M, Souza WV, Montarroyos UR, Diniz GT, Luna CF, et al. Is it better to be rich in a poor area or poor in a rich area? A multilevel analysis of a case-control study of social determinants of tuberculosis. Int J Epidemiol. 2009;38:1285–96. doi: 10.1093/ije/dyp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1016–20. doi: 10.1164/ajrccm.157.4.9704036. [DOI] [PubMed] [Google Scholar]
- 31.Oxlade O, Schwartzman K, Behr MA, Benedetti A, Pai M, Heymann J, et al. Global tuberculosis trends: a reflection of changes in tuberculosis control or in population health? Int J Tuberc Lung Dis. 2009;13:1238–46. [PubMed] [Google Scholar]
- 32.World Health Organization. Addressing poverty in TB control. Options for national TB control programmes. Geneva: WHO; 2005. [cited 2010 Apr 7]. Also available from: URL: http://www.who.int/tb/challenges/poverty/en/index.html. [Google Scholar]
- 33.World Health Organization. The stop TB strategy: building on and enhancing DOTs to meet the TB related millennium development goals. Geneva: WHO; 2006. [Google Scholar]
- 34.Treatment of tuberculosis [published erratum appears in MMWR Recomm Rep 2005;53(51&52):1203] MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 35.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines Panel [published erratum appears in JAMA 1998;280:134] JAMA. 1998;279:943–8. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 36.Anuwatnonthakate A, Limsomboon P, Nateniyom S, Wattanaamornkiat W, Komsakorn S, Moolphate S, et al. Directly observed therapy and improved tuberculosis treatment outcomes in Thailand. PLoS One. 2008;3:e3089. doi: 10.1371/journal.pone.0003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manangan L, Elmore K, Lewis B, Pratt R, Armstrong L, Davison J, et al. Disparities in tuberculosis between Asian/Pacific Islanders and non-Hispanic whites, 1993–2006. Int J Tuberc Lung Dis. 2009;9:1077–85. [PubMed] [Google Scholar]
- 38.Rieder HL, Snider DE, Jr, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141:347–51. doi: 10.1164/ajrccm/141.2.347. [DOI] [PubMed] [Google Scholar]
- 39.Asghar RJ, Pratt RH, Kammerer JS, Navin TR. Tuberculosis in South Asians living in the United States, 1993–2004. Arch Intern Med. 2008;168:936–42. doi: 10.1001/archinte.168.9.936. [DOI] [PubMed] [Google Scholar]
- 40.Wilberschied LA, Kaye K, Fujiwara PI, Frieden TR. Extrapulmonary tuberculosis among foreign-born patients, New York City, 1995 to 1996. J Immigr Health. 1999;1:65–75. doi: 10.1023/A:1021828321167. [DOI] [PubMed] [Google Scholar]
- 41.Kong Y, Cave MD, Zhang L, Foxman B, Marrs CF, Bates JH, et al. Association between Mycobacterium tuberculosis Beijing/W lineage strain infection and extrathoracic tuberculosis: insights from epidemiologic and clinical characterization of the three principal genetic groups of M. tuberculosis clinical isolates. J Clin Microbiol. 2007;45:409–14. doi: 10.1128/JCM.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Sadr WM, Tsiouris SJ. HIV-associated tuberculosis: diagnostic and treatment challenges. Semin Respir Crit Care Med. 2008;29:525–31. doi: 10.1055/s-0028-1085703. [DOI] [PubMed] [Google Scholar]
- 44.Chideya S, Harrington T, Mukasa L, Bahktawar I. Disproportionate burden of tuberculosis among Marshall Islanders living in Arkansas, 2000–2005. 11th Annual Conference, International Union of Tuberculosis and Lung Disease—North American Region; 2007; Feb 22–24; Vancouver. [Google Scholar]
- 45.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27:66–9. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 46.Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis. 2009;19:49–55. [PMC free article] [PubMed] [Google Scholar]
- 47.Krieger N. Counting accountably: implications of the new approaches to classifying race/ethnicity in the 2000 census. Am J Public Health. 2000;90:1687–9. doi: 10.2105/ajph.90.11.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stafford S. Caught between “the rock” and a hard place: the native Hawaiian and Pacific Islander struggle for identity in public health. Am J Public Health. 2010;100:784–9. doi: 10.2105/AJPH.2009.191064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertolli J, Lee LM, Sullivan PS, AI/AN Race/Ethnicity Data Validation Workgroup. Racial misidentification of American Indians/Alaska Natives in the HIV/AIDS reporting systems of five states and one urban health jurisdiction, U.S., 1984–2002. Public Health Rep. 2007;122:382–92. doi: 10.1177/003335490712200312. [DOI] [PMC free article] [PubMed] [Google Scholar]