Abstract
Objectives
We described the outbreak investigation and control measures after the Minnesota Department of Health identified a cluster of tuberculosis (TB) cases among Guatemalan immigrants within three rural Minnesota counties in August 2008.
Methods
TB cases were diagnosed by tuberculin skin test followed by chest radiography and sputum testing for Mycobacterium tuberculosis (M. tuberculosis). We reviewed medical records, interviewed patients, and completed a contact investigation for each infectious case. We used isolate genotyping to confirm epidemiologic links between cases.
Results
The index case was a six-month-old U.S.-born male with Guatemalan parents. Although he experienced four months of cough and fever, TB was not considered at two medical visits but was diagnosed upon hospitalization in May 2008. The presumed source of infection was a Guatemalan male aged 25 years who sang in a band that practiced in the infant’s house and whose pulmonary TB was diagnosed at hospitalization in June 2008, despite his having sought medical attention for symptoms seven months earlier. Among the 16 identified TB cases, 14 were outbreak-related. Three genetically distinct M. tuberculosis strains circulated. Of 150 contacts of the singer, 62 (41%) had latent TB infection and 13 (9%), including 10 children, had TB disease.
Conclusions
In this outbreak, delayed diagnoses contributed to M. tuberculosis transmission. Isolate genotyping corroborated the social links between outbreak-related patients. More timely diagnosis of TB among immigrants and their children can prevent TB transmission among communities in rural, low-incidence areas that might have limited resources for contact investigations.
During 2008, approximately nine million cases and two million deaths from tuberculosis (TB) occurred worldwide;1 59% of U.S. TB cases occurred among foreign-born people.2 In 2008, the incidence of TB in Minnesota (4.0 per 100,000 population) was lower than the overall incidence in the United States (4.2 per 100,000 population). Similar to national trends, the majority of TB cases in Minnesota occurred in urban rather than rural areas.3 Also similar to national trends, the majority of TB cases in Minnesota were among foreign-born people (82% during 2004–2008).3
Refugees and immigrants who apply for legal residence in the U.S. are required to undergo TB screening.4 People entering the U.S. on nonimmigrant visas (e.g., temporary workers) are not screened for TB; however, studies have detected TB among nonimmigrant visitors to the U.S.5,6 In Minnesota, the majority of TB-control infrastructure is in urban areas, although rural areas have experienced increased racial/ethnic diversification during the past decades because of immigration, including economic migrants,7 thereby potentially creating new challenges for rural TB control. Specifically, one challenge in the context of declining overall U.S. TB prevalence is that it might not be suspected when TB is rare in a community and when TB is an uncommon diagnosis in providers' experiences.
In August 2008, the Minnesota Department of Health (MDH) notified the Centers for Disease Control and Prevention (CDC) of a possible outbreak in three rural adjacent southwestern Minnesota counties that included four confirmed and nine suspected TB cases, some associated with possible social links to TB patients in neighboring states. All except one patient were Guatemalan or a U.S.-born child with Guatemalan parents. Among these U.S.-born children was a male aged six months with TB diagnosed in May 2008 and with no known source of infection. Reports conflicted regarding the social links between this infant with newly diagnosed illness and cases previously reported in the county. We describe the outbreak investigation initiated on the basis of this index patient.
METHODS
Population and setting
The investigation occurred in three adjacent rural counties in Minnesota, which, according to the Minnesota 2000 Census, had 42,429 people, 10% of whom were of Hispanic or Latino ethnicity, and 7% of whom were foreign-born.8 Agriculture and manufacturing are common sources of employment within the counties. Two main private health-care providers are available for the counties, but patients are referred out of county for specialty medical care. As in many rural parts of the U.S., these three counties had no full-time TB-control staff. Instead, public health nurses who perform multiple activities also manage TB contact investigations and provide directly observed therapy (DOT) for TB patients free of charge.
Outbreak investigation
On August 4, 2008, a team from MDH and CDC arrived to assist local health partners in this investigation. Medical records were reviewed and patients were interviewed about their history of symptoms, which were used to estimate their infectious period.9 An outbreak case was defined as either being confirmed by a positive culture of Mycobacterium tuberculosis (M. tuberculosis) with a genotype matching the outbreak strain or, when laboratory confirmation was unavailable, a clinical diagnosis of TB in a contact of an outbreak-associated patient in this rural three-county area of Minnesota during 2008. Excluded cases were those determined not to be part of the outbreak because they were infected with M. tuberculosis genotypes that did not match the outbreak strain. The National TB Genotyping Service provided genotyping of M. tuberculosis isolates.10
A contact investigation was completed for each infectious TB patient.9 Contacts were prioritized for a tuberculin skin test (TST) on the basis of degree of exposure and risk for severe TB disease. Interferon gamma release assay, an alternative diagnostic test for detecting TB infection, was not available in this area of the state at the time of the outbreak.11 In multiple settings, additional contacts were identified from named contacts. For example, a supervisor who was a named contact encouraged other coworkers, who were not necessarily named contacts, to undergo TB testing by the health department. Church contacts were identified through a church pastor, and TSTs were administered at the church. Information on legal residency or visa status was not sought because of concerns that doing so might prevent contacts from being named or from seeking a TST.
Contacts with a positive TST result (≥5 millimeters [mm]) had subsequent chest radiography and, if abnormal or if signs and symptoms of TB were present, sputum testing for M. tuberculosis at either MDH or the South Dakota Department of Health public health laboratories. Two polymerase chain reaction (PCR)-based genotyping methods, spoligotype and 12-locus mycobacterial interspersed repetitive unit (MIRU) analysis, were used to examine the genetic relatedness of strains.10 To compare groups with multiple types of contact, we calculated prevalence ratios (PRs) and 95% confidence intervals (CIs) to evaluate TB exposure risk for each mutually exclusive group.
RESULTS
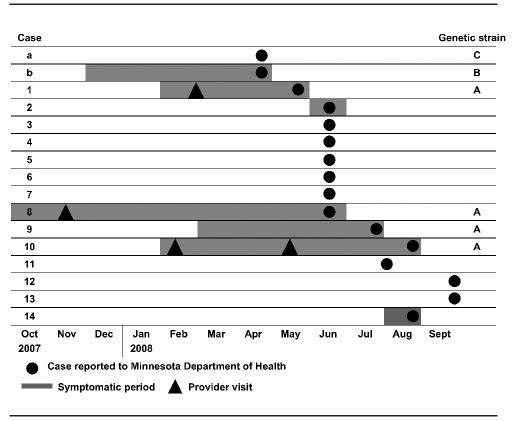
During January–September 2008, a total of 16 TB cases were reported from three adjacent counties, including 14 cases linked to the outbreak (Figure 1). Two people with TB (cases a and b) were reported in April, before the outbreak index patient (case 1) was reported. One of these (case a) was in a 5-year-old U.S-born female with Guatemalan parents who was identified during a contact investigation of a Guatemalan adult. Both case a and case b—a U.S.-born white male aged 40 years—were subsequently determined not to be part of the outbreak because they were infected with M. tuberculosis genotypes that did not match the outbreak strain.
Figure 1.
Timing of TB cases in a Minnesota TB outbreak investigation, 2007–2008
The index patient (case 1) in the outbreak was a six-month-old U.S.-born male with Guatemalan parents whose family had arrived in the U.S. in 1997. He experienced four months of cough and fever that had begun in February 2008. TB was not considered at a medical visit in February in which the infant was treated with antibiotics (type unknown). He remained symptomatic and in May 2008, he was taken to the emergency department with coughing and night sweats, administered albuterol and antibiotics (type unknown), and discharged. Two days later he was hospitalized with pneumonia, had extensive consolidation of the right lung and limited pleural effusion on chest radiography, and a 10-mm-induration TST result; TB was diagnosed. Bronchoalveolar lavage fluid was culture-positive for M. tuberculosis susceptible to isoniazid, rifampin, ethambutol, and pyrazinamide.
The June 2008 investigation involving case 1 did not identify a source case, but revealed that both mother and father had evidence of TB infection (TST results for the mother and father were 20- and 22-mm induration, respectively). In addition, in the same household, all four siblings and two young cousins (aged 2–13 years) had active TB disease (cases 2–7) (Figure 1). Five of the children did not have clinical symptoms but had positive TST results and evidence of TB on chest radiography or computerized tomography. One child (aged 2 years) had a negative TST result but had respiratory symptoms (cough and cold) and radiologic evidence of TB. All pediatric patients, including the infant index patient, began four-drug (isoniazid, rifampin, ethambutol, and pyrazinamide) DOT, and the parents began treatment for latent TB infection.
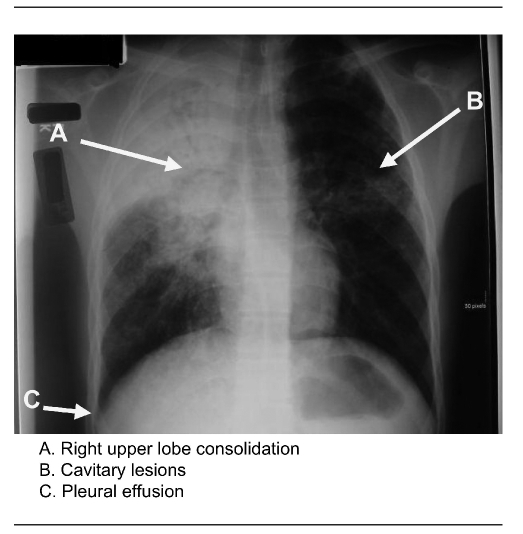
The same month, the presumed source for the children (cases 1–7) was identified when a 25-year-old Guatemalan-born male (case 8) was hospitalized with active pulmonary TB. His symptoms had begun in October 2007, and he had sought care at an urgent care facility in November 2007 for a fever and productive cough. Bronchitis was diagnosed, and he was prescribed an oral cephalosporin antibiotic, cough suppressant, and antipyretics. (Diagnostic evaluation had not included a TST or chest radiograph.) He remained symptomatic but did not seek further care until his condition deteriorated in June 2008, when he returned to the emergency department with a productive cough, fever, night sweats, and a 25-pound weight loss since January. He was medically transported from the local hospital to a regional hospital in which an airborne infection isolation room and infectious disease consultation were available. He had a negative TST result, but a chest radiograph revealed pulmonary infiltrates and a pleural effusion (Figure 2). Sputum samples were acid-fast bacillus smear-positive and culture-positive for drug-susceptible M. tuberculosis. He began a four-drug treatment for TB and was discharged to continue DOT in July 2008.
Figure 2.
Chest radiography of TB outbreak source case in a Minnesota TB outbreak, 2008
TB = tuberculosis
The contact investigation associated with case 8 identified multiple household, workplace, and social contacts. Of note, this patient was a singer in the music group managed by the father of the index patient (case 1). He had sung in the group during his entire infectious period (October 2007–June 2008). During this time, the group had played regularly at band members' homes (<20 people), the house of the index patient (<15 people), community centers (60–100 people), church services (maximum audience = 100 people), and other functions. Contacts of the case 8 patient were also identified in two households (seven people), at the patient's workplace (30 people), and other social settings (e.g., a sports team).
In July 2008, while the contact investigation associated with case 8 was ongoing, a 19-year-old Guatemalan-born male received a TB diagnosis (case 9) after he presented at a hospital with symptoms of a predominantly dry cough with intermittent hemoptysis, fever, 15-pound weight loss, and fatigue that began in March 2008. Although patients 8 and 9 did not name each other as contacts, they worked together, occasionally carpooled, and named common contacts. Patient 9 had not played in the band, visited the index patient's household, or participated in church community events. He reported not knowing anyone with TB or illness similar to his own.
Other cases were identified through contact investigations during July–September 2008. A 26-year-old Guatemalan-born male (case 10) who was a frequent visitor to the household of case 8 was also named as a workplace contact of case 9. The case 10 patient had a history of a medical visit for chest pain in February 2008 when a pleural effusion was documented. He returned in May 2008 with continued chest pain, but no cough or fever; he was referred to a pulmonologist but did not follow up. During these two medical visits, TB was not suspected. Then in August 2008, after the local health department recommended he have a TST as part of the ongoing TB contact investigations, and after experiencing epistaxis, dry cough, weight loss, and chest pain, he returned to the same facility that provided his initial medical care. TB was subsequently diagnosed on the basis of a positive TST result (15-mm induration), a chest radiograph that revealed bilateral perihilar infiltrates, and a sputum sample that was culture-positive for M. tuberculosis.
Four additional people were socially linked to the case 8 patient: an asymptomatic household contact aged 4 years (case 11) with a positive TST result (10-mm induration) and hilar adenopathy; two asymptomatic contacts aged 4 years (TST results 10- and 14-mm induration and infiltrates on chest radiographs) with multiple exposures to the band (cases 12 and 13); and a 37-year-old Guatemalan woman (TST result 10-mm induration and nodular infiltrates on chest radiograph) who occasionally visited the church (case 14).
Results of genotyping confirmed that the M. tuberculosis isolates from the singer (case 8) and two of his contacts (case 9 and case 10) matched the outbreak genotype (case 1) (i.e., PCR08788, or spoligotype 676177607760771 and MIRU 22432615332 [strain A]). Genotyping results of cases a and b demonstrated that they both differed substantially from the outbreak and each other (case a: PCR00226, or spoligotype 777777607760771 and MIRU 124326153324 [strain C]; case b: PCR00082, or spoligotype 777777777760771 and MIRU 223325154322 [strain B]).
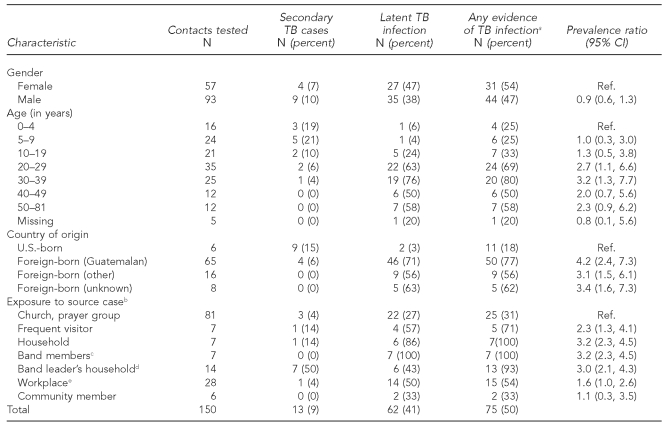
In total, 150 contacts were identified who could be linked to the case 8 patient; 13 had secondary cases of TB disease and 62 had latent TB infection. The majority of cases were reported during June (Figure 1). The prevalence of TB infection (either TB disease or latent TB infection) was greatest among foreign-born contacts compared with U.S.-born contacts, and greatest among household contacts, frequent visitors, and band members (Table). Foreign-born contacts were from Guatemala (n=65), Mexico (n=13), Honduras (n=2), and El Salvador (n=1); eight contacts did not report a country of origin. The number of years living in the U.S. was unrelated to TB infection for the 64 non-U.S.-born contacts who reported this information (median = 6 years; range: 1–24 years). Twenty contacts reported having a TST before this outbreak investigation. Although we did not collect information on visa status, 15 people in the investigation (contacts and patients) provided at least one pseudonym, creating a challenge for contact tracing by name. TB cases were more common among children, whereas the proportion with latent TB infection increased at older ages (Table).
Table.
Characteristics of contacts of TB patients investigated during an outbreak in Minnesota, 2008
aCombines latent TB infection and TB disease
bIncludes only contacts with relationship to source case
cAll groups are mutually exclusive; two band members were classified as household contacts.
dIncludes index case and contacts from source case investigation
eIncludes workplace contacts of source case and secondary case
TB = tuberculosis
CI = confidence interval
Ref. = reference group
All infected people received free treatment through county public health services. TB treatment was DOT and, as with contact investigations, required bilingual outreach workers.
DISCUSSION
In this low-incidence rural area (pre-outbreak range: 0–4 cases per year throughout the three counties during 1997–2007), this outbreak resulted in a substantial increase in the need for TB investigation and case management. The challenges encountered in this outbreak have been noted in other rural settings with limited TB resources.12 During the outbreak investigation, local public health resources became overwhelmed by case reporting, contact investigations, and DOT implementation. Case management and investigations also required language interpretation services. Despite these barriers, detection of the first cases in this outbreak led to the identification of additional patients who benefited from early intervention and treatment, preventing additional morbidity and transmission. As of July 2010, no additional outbreak-associated cases were identified after October 2008.
This outbreak might have been prevented had delays not occurred both in patients seeking care and clinicians correctly diagnosing TB. The diagnosis of TB was delayed for the index patient, the source patient, and an additional patient who had had multiple medical visits but only had TB suspected as a result of the health department's TB contact investigations. The singer, likely contagious for almost a year, appears to have been the source of exposure for all identified -secondary cases on the basis of both the social links and the matching genotypes. Spread of M. tuberculosis through singing has been documented elsewhere.13,14 In the role of the singer in a band that played in community settings, repeated opportunities existed for transmission of aerosolized M. tuberculosis from the source patient to contacts. Groups with the highest likelihood of infection were the most exposed household contacts and band members.
The results of this outbreak investigation highlight the importance of suspecting and diagnosing TB among foreign-born populations and their children in the U.S. In 2007, reported TB incidence in Guatemala was 63 per 100,000 population compared with approximately four per 100,000 population in the U.S.1 All outbreak cases occurred among Guatemalan-born adults or U.S.-born children of Guatemalan parents. Older Guatemalan contacts might have become infected in Guatemala. During this outbreak, latent TB infection was increasingly common among older and foreign-born contacts, compared with children and U.S.-born contacts. Regardless of the source of their infection, whether imported or acquired locally, identifying and treating latent TB infection among immigrant communities at high risk will help prevent future outbreaks. CDC provides guidance for overseas and domestic TB screening for arriving immigrants suspected of having TB.15 However, few people in this investigation reported prior TB screening, and this guidance does not apply to the U.S.-born children of foreign-born people.
In this outbreak, a number of people were known by multiple names, which both complicated name-based contact tracing and possibly indicated undocumented immigrant status. In other settings, undocumented foreign-born patients have had a longer symptomatic period before TB diagnosis than documented foreign-born patients.16 Such barriers as concerns regarding immigration status might have influenced people's willingness to share contact information or seek TB testing; it might also have caused them to delay seeking health care.17
We did not ask contacts about their residency status because it did not affect access to TB evaluation by the health department. Contacts often provided multiple aliases, possibly indicating undocumented residency status; however, provision of contact information also demonstrates that local public health staff had established a trusting relationship with this immigrant community. Success in identifying and locating contacts among immigrant workers was likely influenced by the health department's ability to establish a level of trust with the community, specifically by the support of the community church leaders and their facilitation of TB screening.
Genotyping confirmed the social links between patients and illustrated molecular epidemiology's usefulness for TB control and surveillance. Genotyping M. tuberculosis isolates has been used elsewhere to detect clusters for further investigation in rural settings18–20 and among immigrant communities.17 The matching genotype shared by the source patient, the U.S.-born index patient, and additional contacts was further evidence of a common strain of TB that was shared among community members. The genotypic evidence that the two cases preceding the index patient's diagnosis were not part of the outbreak reassured public health investigators that previous contact investigations had been adequate, preventing unnecessarily expansive investigations and further use of limited resources.
TB cases in this outbreak often occurred among children for whom TB diagnosis may be challenging, risk for progression to TB disease is greater, and the sequelae of TB disease are more severe.21 Undiagnosed TB among infants, as observed in regard to this index patient, is particularly concerning because of the risk for severe outcomes.21 Diagnosing TB among young children can be difficult, and gastric aspiration might be necessary to obtain samples for culture.10 Although this medical procedure was able to be provided for the children involved in the outbreak investigation, identifying clinicians familiar with this procedure was an additional challenge for this rural community.
CONCLUSIONS
This outbreak highlights key aspects of TB control in the United States, including timely case identification, accurate diagnosis, completeness of contact investigations, and usefulness of genotyping by the National TB Genotyping Service. In this outbreak, delayed diagnoses contributed to M. tuberculosis transmission among Guatemalan immigrants and their children. Early TB recognition and control can be facilitated by an understanding of local TB epidemiology by clinicians and collaboration with public health agencies. More timely diagnosis of TB among immigrants and their children can prevent TB transmission among communities in rural, low-incidence areas that might have limited resources for contact investigations.
Footnotes
The investigation was conducted with the input, advice, support, and collaboration of Nobles-Rock Community Health Services, the Watonwan County Public Health Department, the Minnesota Department of Health, and the Centers for Disease Control and Prevention (CDC).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC.
REFERENCES
- 1.World Health Organization. Global tuberculosis control—epidemiology, strategy, financing. Geneva: WHO; 2009. [Google Scholar]
- 2.Trends in tuberculosis—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(10):249–53. [PubMed] [Google Scholar]
- 3.Minnesota Department of Health. Annual summary of communicable diseases reported to the Minnesota Department of Health, 2008: tuberculosis. St. Paul (MN): Minnesota Department of Health; 2009. [Google Scholar]
- 4.Reports of Council on Scientific Affairs. Screening nonimmigrant visitors to the United States for tuberculosis (Resolution 421,l-98) Chicago: American Medical Association; 1999. [DOI] [PubMed] [Google Scholar]
- 5.Weis SE, Moonan PK, Pogoda JM, Turk L, King B, Freeman-Thompson S, et al. Tuberculosis in the foreign-born population of Tarrant County, Texas, by immigration status. Am J Respir Crit Care Med. 2001;164:953–7. doi: 10.1164/ajrccm.164.6.2102132. [DOI] [PubMed] [Google Scholar]
- 6.LoBue PA, Moser KS. Screening of immigrants and refugees for pulmonary tuberculosis in San Diego County, California. Chest. 2004;126:1777–82. doi: 10.1378/chest.126.6.1777. [DOI] [PubMed] [Google Scholar]
- 7.Fennelly K. Latinos, Africans, and Asians in the North Star state: immigrant communities in Minnesota. In: Gozdziak EM, Martin SF, editors. Beyond the gateway: immigrants in a changing America. Lanham (MD): Lexington Books, Rowman – Littlefield Publishers, Inc.; 2005. pp. 111–51. [Google Scholar]
- 8.Minnesota State Data Center. Census of population and housing. 2000 Census datanet. [cited 2010 Jan 20]. Available from: URL: http://www.lmic.state.mn.us/datanetweb/php/census2000/c2000.html.
- 9.National Tuberculosis Controllers Association. Guidelines for the investigation of contacts of persons with infectious tuberculosis. MMWR Recomm Rep. 2005;54(RR-15):1–47. [PubMed] [Google Scholar]
- 10.National TB Controllers Association/Centers for Disease Control and Prevention (US) Advisory Group on Tuberculosis Genotyping. Guide to the application of genotyping to tuberculosis prevention and control: handbook for TB controllers, epidemiologists, laboratorians, and other program staff. Atlanta: CDC; 2004. [Google Scholar]
- 11.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 12.Phillips L, Carlile J, Smith D. Epidemiology of a tuberculosis outbreak in a rural Missouri high school. Pediatrics. 2004;113:e514–9. doi: 10.1542/peds.113.6.e514. [DOI] [PubMed] [Google Scholar]
- 13.Loudon RG, Roberts RM. Singing and the dissemination of tuberculosis. Am Rev Respir Dis. 1968;98:297–300. doi: 10.1164/arrd.1968.98.2.297. [DOI] [PubMed] [Google Scholar]
- 14.Mangura BT, Napolitano EC, Passannante MR, McDonald RJ, Reichman LB. Mycobacterium tuberculosis miniepidemic in a church gospel choir. Chest. 1998;113:234–7. doi: 10.1378/chest.113.1.234. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (US). CDC immigrant requirements: technical instructions for tuberculosis screening and treatment—using cultures and directly observed therapy. 2009. [cited 2010 Jul 1]. Available from: URL: http://www.cdc.gov/immigrantrefugeehealth/pdf/tuberculosis-ti-2009.pdf.
- 16.Achkar JM, Sherpa T, Cohen HW, Holzman RS. Differences in clinical presentation among persons with pulmonary tuberculosis: a comparison of documented and undocumented foreign-born versus US-born persons. Clin Infect Dis. 2008;47:1277–83. doi: 10.1086/592572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tardin A, Dominicé Dao M, Ninet B, Janssens JP. Tuberculosis cluster in an immigrant community: case identification issues and a transcultural perspective. Trop Med Int Health. 2009;14:995–1002. doi: 10.1111/j.1365-3156.2009.02325.x. [DOI] [PubMed] [Google Scholar]
- 18.Dillaha JA, Yang Z, Ijaz K, Eisenach KD, Cave MD, Wilson FJ, et al. Transmission of Mycobacterium tuberculosis in a rural community, Arkansas, 1945–2000. Emerg Infect Dis. 2002;8:1246–8. doi: 10.3201/eid0811.020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobbs KG, Lok KH, Bruce F, Mulcahy D, Benjamin WH, Dunlap NE. Value of Mycobacterium tuberculosis fingerprinting as a tool in a rural state surveillance program. Chest. 2001;120:1877–82. doi: 10.1378/chest.120.6.1877. [DOI] [PubMed] [Google Scholar]
- 20.Haddad MB, Diem LA, Cowan LS, Cave MD, Bettridge J, Yun L, et al. Tuberculosis genotyping in six low-incidence states, 2000–2003. Am J Prev Med. 2007;32:239–43. doi: 10.1016/j.amepre.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]