Abstract
BACKGROUND
Depression and mortality have been studied separately in patients with coronary heart disease (CHD) and in populations healthy at study inception. This does not allow comparisons across risk-factor groups based on the cross-classification of depression and CHD status.
OBJECTIVE
To examine effects of depressive symptoms and coronary heart disease and their interactive associations on mortality in middle-aged adults followed over 5.6 years.
DESIGN AND SETTING
A prospective population-based cohort study of 5936 middle-aged men and women from the British Whitehall II study. We created 4 risk-factor-groups based on the cross classification of depressive symptoms and CHD status.
RESULTS
The age- and sex-adjusted hazard ratios for death from all causes were 1.67 (p<0.05) for participants with only CHD, 2.10 (p<0.001) for those with only depressive symptoms and 4.99 (p<0.001) for those with both CHD and depressive symptoms when compared to participants without either condition. The two latter risk-factor groups remained at increased risk after adjustments for relevant confounders. The relative excess risk due to the interaction between depressive symptoms and CHD for all-cause mortality was 3.58 (95% CI, −0.09–7.26), showing some evidence of an additive interaction. A similar pattern was also observed for cardiovascular death.
CONCLUSIONS
This study provides evidence that depressive symptoms are associated with an increased risk of all-cause and cardiovascular death and that this risk is particularly marked in depressive participants with co-morbid CHD.
Keywords: Coronary heart disease, depressive symptoms, survival
INTRODUCTION
The impact of depression on the development and prognosis of chronic diseases, coronary heart disease (CHD) in particular, has attracted significant research attention in recent years. In healthy populations, long-term prospective studies have found depression to be associated with the development of CHD, independently of other risk factors for CHD.[1, 2, 3] Successive meta-analyses show depression to increase the risk for the onset of CHD, with pooled relative risk between 1.6 and 1.8 in subjects with diagnosed depression or those reporting depressive symptoms.[1, 4, 5] Several other studies have shown that depression is also associated with increased risk of all-cause and cause-specific mortality in general populations. [3, 6, 7, 8] Furthermore, there is increasing evidence of the importance of depression as a prognostic factor as the outcomes of myocardial infarction (MI) have been found to be worse among depressive than non-depressive MI-patients after controlling for cardiac disease severity.[9, 10, 11] Findings from two recent systematic reviews have shown that in individuals with established CHD, depression is associated with an approximately 2-fold higher risk of death from all-cause and cardiac mortality.[12, 13]
However, these studies typically compared depressed and non depressed participants free of CHD or depressed and non depressed patients with CHD. This approach does not allow comparisons across risk-factor groups based on the cross-classification of depression and CHD status, and it remains uncertain whether the effect of depression on mortality in individuals with CHD is larger than that in healthy individuals. The present investigation was therefore conducted to examine effects of depressive symptoms and coronary heart disease and their interactive associations on mortality in middle-aged adults. We examined whether the association between depression and mortality varied among individuals with both CHD and depressive symptoms compared to those: 1) without both these conditions, 2) with depressive symptoms but not CHD, and 3) with CHD but no depressive symptoms.
MATERIAL & METHODS
Data are drawn from the Whitehall II study, established in 1985 as a longitudinal study to examine the socioeconomic gradient in health and disease among 10,308 civil servants (6,895 men and 3,413 women). All civil servants aged 35–55 years in 20 London based departments were invited to participate by letter, and 73% agreed. Baseline screening (Phase 1) took place during 1985–1988, and involved a clinical examination and a self-administered questionnaire. Subsequent phases of data collection have alternated between postal questionnaire alone [Phases 2 (1989–1990), 4 (1995–1996), 6 (2001) and 8 (2006)] and postal questionnaire accompanied by a clinical examination [Phases 3 (1991–1993), 5 (1997–1999) and 7 (2002–2004)]. Depressive symptoms were assessed by the Center for Epidemiologic Studies Depression Scale (CES-D) for the first time at phase 7 and this phase serves as the baseline for the present study. The University College London ethics committee approved the study.
Measures
Coronary heart disease (CHD)
CHD status at phase 7 was defined as non-fatal MI or ‘definite’ angina and was based on clinical examinations at phases 1, 3, 5, and 7 and records obtained from general practitioners and hospitals. Potential non-fatal myocardial infarction was ascertained by questionnaire items on chest pain (the World Health Organisation Rose questionnaire.[14]) and physician diagnosis of heart attack. Confirmation of myocardial infarction according to MONICA[15] criteria (Multinational Monitoring of Trends and Determinants in Cardiovascular Disease) was based on electrocardiograms, markers of myocardial necrosis, and chest pain history from medical records. Angina was assessed based on participant’s reports of symptoms with corroboration in medical records or abnormalities on a resting electrocardiogram, an exercise electrocardiogram, or a coronary angiogram.
Depressive symptoms
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D, Cronbach alpha = 0.83) at phase 7 of the Whitehall II study (2002–2004). The CES-D, a widely used and validated instrument, is a 20-item self-report questionnaire designed to measure depressive symptomatology in community studies. [16] A score ≥16 from a total possible score of 60 has been used extensively to distinguish depressed from nondepressed subjects [16].
Vital status
Mortality follow-up was available through the National Health Services Central Registry until 30th April 2009. Death certificates were coded using the 10th revision of the International Classification of Disease (ICD). All-cause mortality was the main outcome, in subsidiary analysis we also analysed death from cardiovascular diseases (CVD; ICD-10 codes I20–I25 and I60–I67).
Covariates
Sociodemographic measures
Sociodemographic measures included age, sex, ethnicity, and socioeconomic position (SEP) assessed by British civil service grade of employment taken from the phase 7 questionnaire.
Biobehavioural risk factors
Behavioural CVD risk factors were assessed using responses to the phase 7 questionnaire and categorised as follows: smoking status (never, ex, and current), physical activity (≥1.5 or <1.5 hours of moderate or vigorous exercise/week), alcohol consumption in the previous week was categorized as abstention, moderate (1–14 units for women/1–21 units for men), and high consumption (14+ units for women/21+ units for men). The following biological CVD risk factors were measured at phase 7 clinical examination: hypertension (systolic blood pressure ≥140 mm/Hg or diastolic blood pressure ≥90 mm/Hg), high total cholesterol (> 5 mmol/l), body mass index (BMI) (<20, 20–24.9, 25–29.9, or ≥30 kg/m2) and diabetes, assessed via glucose tolerance test at the medical screening or self-report of doctor diagnosis. Resting heart rate (RHR) was measured at the phase 7 screening clinic via electrocardiogram (ECG) from participants in the supine position following a standard protocol. The following classification was used to categorise RHR: RHR<80, and RHR>80 beats/minute (bpm).
Antidepressant medications
Data on antidepressant medications was drawn from the phase 7 questionnaire where participants were asked whether in the last 14 days they had taken antidepressants drugs prescribed by a doctor (yes/no).
Cardiovacular disease medications
CVD medications at phase 7 were also drawn from questions on whether in the last 14 days the participant had taken CVD drugs, including diuretics, beta blockers, ACE inhibitors, calcium channel blockers, other anti-hypertensive, prescribed by a doctor (yes/no).
Statistical analysis
We divided the study sample into 4 risk-factor groups based on the cross classification of depression (depression (CES-D ≥16) vs. no depression (CES-D <16)) and CHD (yes/no) at phase 7. Differences in these risk-factor groups as a function of the sample characteristics were assessed using a chi-square test
The risk of death was examined using Cox regression in 5 serially adjusted models, with participants without depressive symptoms and CHD constituting the reference group. Model 1 included adjustment for sex and age and Model 2 was additionally adjusted for ethnicity and SEP. Model 3 was as model 2 but additionally adjusted for smoking, BMI, alcohol consumption, physical activity, hypertension, total cholesterol, diabetes, and RHR. Model 4 was as model 2 plus adjustment for CVD medications and antidepressant medications. Finally, model 5 was the fully adjusted model with simultaneous adjustment for all covariates. In further analysis we changed the reference group and repeated these analyses in order to estimate the risk of death for participants with both depressive symptoms and CHD in comparison to those with depressive symptoms but without CHD and to those with CHD but without depressive symptoms. The time-dependent interaction terms between each predictor and logarithm of follow-up period were all non-significant confirming that the proportional hazards assumption was justified.
To examine whether the coexistence of depressive symptoms and CHD increased risk of death beyond that associated with each of them alone we tested for an interaction on the additive scale as defined by Rothman [17] for calculating the relative excess risk due to interaction (RERI) using the methods outlined by Andersson et al. [18]. In the absence of an interaction between depressive symptoms and CHD, the RERI is 0. RERI and the associated 95% CIs were computed by the delta method.[19]
RESULTS
Of the 10308 participants of the Whitehall II Study, the current analysis where the follow-up starts at Phase 7 of the study is based on a total of 5936 participants. Those not included in the analyses due to missing data were more likely to be: women (39.9% vs. 28.1%, p<0.001), non-white (15.5% vs. 7.7%, p <0.001), in the 70–74 age group (14.5% vs. 9.2%, p<0.001), and from lower SEP (34 % vs. 15 %, p<0.001). Of those included in the analysis, 170 died during the mean follow-up of 5.6 years (SD 0.7, range 0.03–6.6), of which, 47 from CVD. The mean age of participants at phase 7 was 61 years (SD 6.0). The prevalence of elevated depressive symptoms (CES-D score ≥16) was 14.9%. Participants with a history of CHD were more likely to have depressive symptoms (20% vs. 14%, p=0.001) than those without CHD.
Table 1 shows the baseline characteristics of the study participants. Participants with both depressive symptoms and CHD, as compared to those free of both conditions, were older and more likely to be from low socioeconomic position, non-White, antidepressant users, current smokers (along with those depressed participants without CHD), less physically active, obese, diabetic, treated by CVD medication and less likely to have high total blood cholesterol (all p≤0.009). As expected, results from additional analyses conducted separately for depression and CHD (data not shown in table), revealed that they both were associated with all cause mortality, the age-sex-adjusted hazards ratios (HRs) being 2.41 (p<0.001) and 2.02 (p<0.001) respectively. The corresponding HRs for CVD mortality were 2.83 (p=0.001) and 2.61 (p=0.004).
Table 1.
Baseline characteristics by presence or absence of CHD and depressive symptoms at phase 7
| Variables | N total | No CHD, not depressed | No CHD, Depressed | CHD, not depressed | CHD, depressed | P value/trend |
|---|---|---|---|---|---|---|
| Sex | 0.161 | |||||
| Male | 4268 | 3359 (72.9) | 491 (63.2) | 350 (79.0) | 68 (63.0) | |
| Female | 1668 | 1259 (27.1) | 286 (36.8) | 93 (21.0) | 40 (37.0) | |
| Age in years | < 0.001 | |||||
| < 54 | 1110 | 873 (18.9) | 203 (26.1) | 25 (5.6) | 9 (8.3) | |
| 54–59 | 1822 | 1453 (31.5) | 251 (32.3) | 97 (21.9) | 21 (19.4) | |
| 60–64 | 1259 | 998 (21.7) | 140 (18.0) | 98 (22.1) | 23 (21.3) | |
| 64–69 | 1196 | 896 (19.4) | 129 (16.6) | 137 (30.9) | 34 (31.5) | |
| 70–74 | 549 | 388 (8.4) | 54 (6.9) | 86 (19.4) | 21 (19.4) | |
| SEP | < 0.001 | |||||
| High | 2040 | 1661 (36.0) | 181 (23.3) | 178 (40.2) | 20 (18.5) | |
| Middle | 3032 | 2361 (51.2) | 415 (53.4) | 202 (45.6) | 54 (50.0) | |
| Low | 864 | 586 (12.7) | 181 (23.3) | 63 (14.2) | 34 (31.5) | |
| Ethnicity | < 0.001 | |||||
| White | 5485 | 4355 (94.3) | 669 (86.1) | 397 (89.6) | 74 (68.5) | |
| Other | 451 | 263 (5.7) | 108 (13.9) | 46 (10.4) | 34 (31.5) | |
| Antidepressant drugs | < 0.001 | |||||
| No | 5728 | 4505 (97.8) | 690 (88.8) | 437 (98.6) | 96 (88.9) | |
| Yes | 208 | 103 (2.2) | 87 (11.2) | 6 (1.4) | 12 (11.1) | |
| Smoking status | 0.001 | |||||
| Never smoker | 2678 | 2129 (46.2) | 334 (43.0) | 172 (38.8) | 43 (39.8) | |
| Ex smoker | 2282 | 1780 (38.6) | 255 (32.8) | 205 (46.3) | 42 (38.9) | |
| Current smoker | 680 | 490 (10.6) | 132 (17.0) | 45 (10.2) | 13 (12.0) | |
| Missing | 296 | 209 (4.5) | 56 (7.2) | 21 (4.7) | 10 (9.3) | |
| Alcohol consumption | 0.994 | |||||
| Abstainers | 973 | 662 (14.4) | 179 (23.0) | 91 (20.5) | 41 (38.0) | |
| Moderate | 3725 | 2983 (64.7) | 437 (56.2) | 258 (58.2) | 47 (43.5) | |
| High | 1158 | 920 (20.0) | 137 (17.6) | 85 (19.2) | 16 (14.8) | |
| Missing | 80 | 43 (0.9) | 24 (3.1) | 9 (2.0) | 4 (3.7) | |
| Exercise | < 0.001 | |||||
| ≥ 1.5 h/week | 5152 | 4074 (88.4) | 625 (80.4) | 379 (85.6) | 74 (68.5) | |
| < 1.5 h/week | 784 | 534 (11.6) | 152 (19.6) | 64 (14.4) | 34 (31.5) | |
| Hypertension | < 0.106 | |||||
| No | 4521 | 3590 (76.4) | 603 (77.6) | 324 (73.2) | 74 (68.5) | |
| Yes | 1410 | 1084 (23.5) | 174 (22.4) | 118 (26.6) | 34 (31.5) | |
| Missing | 5 | 4 (0.1) | 0 (0.0) | 1 (0.2) | 0 (0.0) | |
| BMI (kg/m2) | < 0.001 | |||||
| < 20 | 179 | 133 (2.9) | 37 (4.8) | 8 (1.8) | 1 (0.9) | |
| 20–24.9 | 2028 | 1638 (35.5) | 241 (31.0) | 127 (28.7) | 22 (20.4) | |
| 25–29.9 | 2606 | 2047 (44.4) | 320 (41.2) | 194 (43.8) | 45 (41.7) | |
| ≥ 30 | 1099 | 773 (16.8) | 176 (22.7) | 112 (25.3) | 38 (35.2) | |
| Missing | 24 | 17 (0.4) | 3 (0.4) | 2 (0.5) | 2 (1.9) | |
| Diabetes | < 0.001 | |||||
| No | 5656 | 4442 (96.4) | 727 (93.6) | 397 (89.6) | 89 (82.4) | |
| Yes | 281 | 166 (3.6) | 50 (6.4) | 46 (10.4) | 19 (17.6) | |
| High total cholesterol | < 0.001 | |||||
| No | 1335 | 912 (19.8) | 178 (22.9) | 197 (44.5) | 48 (44.4) | |
| Yes | 4491 | 3639 (79.0) | 583 (75.0) | 216 (48.7) | 53 (49.1) | |
| Missing | 110 | 57 (1.2) | 16 (2.1) | 30 (6.8) | 7 (6.5) | |
| Resting Heart Rate > 80 bpm | 0.803 | |||||
| No | 5051 | 3929 (85.3) | 648 (83.4) | 384 (86.7) | 90 (83.3) | |
| Yes | 885 | 679 (14.7) | 129 (16.6) | 59 (13.3) | 18 (16.7) | |
| CVD medications (Yes) | ||||||
| Diuretics | 494 | 348 (7.6) | 54 (6.9) | 70 (15.8) | 22 (20.4) | < 0.001 |
| Beta Blockers | 584 | 346 (7.5) | 60 (7.7) | 147 (33.2) | 31 (28.7) | < 0.001 |
| ACE Inhibitors | 649 | 392 (8.5) | 76 (9.8) | 144 (33.2) | 34 (31.5) | < 0.001 |
| Calcium Channel Blockers | 420 | 259 (5.6) | 43 (5.5) | 94 (21.2) | 24 (22.2) | < 0.001 |
| Other anti-hypertensive | 99 | 72 (1.6) | 8 (1.0) | 14 (3.2) | 5 (4.6) | 0.009 |
Table 2 presents the HRs for the associations with mortality, from all-causes and from CVD, in the groups resulting from the cross classification of CHD and depressive symptoms. Model 1 shows that compared with participants without CHD and depressive symptoms (the reference group) the age- and sex-adjusted hazard ratio for death from all-causes was higher in all three exposed groups: those with depressive participants but not CHD (1.67, 95% confidence interval (CI) 1.05–2.66), those with CHD but not depression (2.10, 95% CI 1.47– 3.13), and those with depression and CHD (4.99, 95% CI 2.89–8.62). After successive adjustments for sociodemographic characteristics (model 2), biobehavioural risk factors (model 3), antidepressant and CVD medications (model 4) and for all of these covariates (model 5), the magnitude of the associations was substantially reduced except in the “depressed but no CHD” group. Almost all attenuation of the association was related to controlling for biobehavioural risk factors. The fully-adjusted HR was highest in the group with both depression and CHD (HR=2.94, 95% CI 1.63–5.34) but the group with CHD but no depression was not robust to successive adjustment for covariates (HR=1.10, 95% CI 0.66– 1.82).
Table 2.
Hazard ratios of mortality from all causes and cardiovascular disease according to CHD and depressive symptoms.
| No CHD | CHD | ||||
|---|---|---|---|---|---|
| All-cause mortality | Depressive symptoms | ||||
| Model 1 | No | n events/n total** | 101/4608 | 22/443 | |
| HR (95% CI) | 1 (reference) | 1.67 (1.05–2.66)* | |||
| Yes | n events/n total** | 32/777 | 15/108 | ||
| HR (95% CI) | 2.10 (1.41–3.13)‡ | 4.99 (2.89–8.62)‡ | |||
| Depressive symptoms | |||||
| Model 2 | No | HR (95% CI) | 1 (reference) | 1.70 (1.06–2.71)* | |
| Yes | HR (95% CI) | 2.15 (1.44–3.23)‡ | 5.38 (3.07–9.42)‡ | ||
| Depressive symptoms | |||||
| Model 3 | No | HR (95% CI) | 1 (reference) | 1.25 (0.77–2.04) | |
| Yes | HR (95% CI) | 1.76 (1.17–2.64)† | 3.20 (1.79–5.75)‡ | ||
| Depressive symptoms | |||||
| Model 4 | No | HR (95% CI) | 1 (reference) | 1.37 (0.84–2.324) | |
| Yes | HR (95% CI) | 2.20 (1.46–3.32)‡ | 4.65 (2.62–8.24)‡ | ||
| Depressive symptoms | |||||
| Model 5 | No | HR (95% CI) | 1 (reference) | 1.10 (0.66–1.82) | |
| Yes | HR (95% CI) | 1.78 (1.17–2. 69)† | 2.94 (1.63–5.34)‡ | ||
| Cardiovascular mortality | Depressive symptoms | ||||
| Model 1 | No | n events/n total** | 25/4608 | 8/443 | |
| HR (95% CI) | 1 (reference) | 2.29 (1.02–5.14)* | |||
| Yes | n events/n total** | 9/777 | 5/108 | ||
| HR (95% CI) | 2.52 (1.17–5.43)* | 6.69 (2.54–17.54)‡ | |||
| Depressive symptoms | |||||
| Model 2 | No | HR (95% CI) | 1 (reference) | 2.34 (1.04–5.24)* | |
| Yes | HR (95% CI) | 2.46 (1.14–5.33)* | 6.73 (2.49–18.22)‡ | ||
| Depressive symptoms | |||||
| Model 3 | No | HR (95% CI) | 1 (reference) | 1.72 (0.74–4.01) | |
| Yes | HR (95% CI) | 2.22 (1.02–4.85)* | 4.45 (1.56–12.73)† | ||
| Depressive symptoms | |||||
| Model 4 | No | HR (95% CI) | 1 (reference) | 1.64 (0.70–3.84) | |
| Yes | HR (95% CI) | 2.57 (1.17–5.64)† | 4.95 (1.80–13.62)‡ | ||
| Depressive symptoms | |||||
| Model 5 | No | HR (95% CI) | 1 (reference) | 1.33 (0.54–3.28) | |
| Yes | HR (95% CI) | 2.37 (1.07–5.34)* | 3.91 (1.34–11.43)† | ||
p<0.05,
p<0.01,
p<0.001
Model 1: hazard ratios adjusted for sex and age.
Model 2: model 1 additionally adjusted for ethnicity and SEP
Model 3: model 2 additionally adjusted for BMI, alcohol consumption, smoking status, exercise, hypertension, total cholesterol, diabetes, and resting heart rate
Model 4: model 2 additionally adjusted for CVD medications (Diuretics, Beta Blockers, ACE Inhibitors, Calcium Channel Blockers, Other anti-hypertensives), and antidepressant medications
Model 5: all aforementioned
n events/n total is the same in all models.
The results for CVD mortality are essentially similar to those obtained for all-cause mortality. The age- and sex-adjusted HRs were 2.52 (95% CI, 1.17–5.43) for depressive participants without CHD, 2.29 (95% CI 1.02–5.14) for those with CHD but not depression and 6.69 (2.54–17.54) for those with both depression and CHD. The fully adjusted HRs attenuated by 49% in the group with both CHD and depression (HR= 3.91, 95% CI 1.34–11.43), and by 74% for participants with CHD but not depression (HR=1.33, 95% CI 0.54–3.28). For participants who were depressed but without CHD no substantial risk reduction was observed (HR= 2.37, 95% CI 1.07–5.34).
Figure 1 presents the risk of mortality, first all cause and then CVD mortality, among participants who were depressed and had CHD compared to those who were depressed but free from CHD. These results show 2.4-fold (p<0.006) and 2.7-fold higher (p=0.08) risk for all cause and CVD mortality respectively when adjustments were made for sex and age. Serial multivariable adjustment substantially attenuated these associations leading to non-significant associations for all-cause mortality (HR=1.66, p=0.13), and for CVD mortality (HR=1.65, p=0.40).
Figure 1.
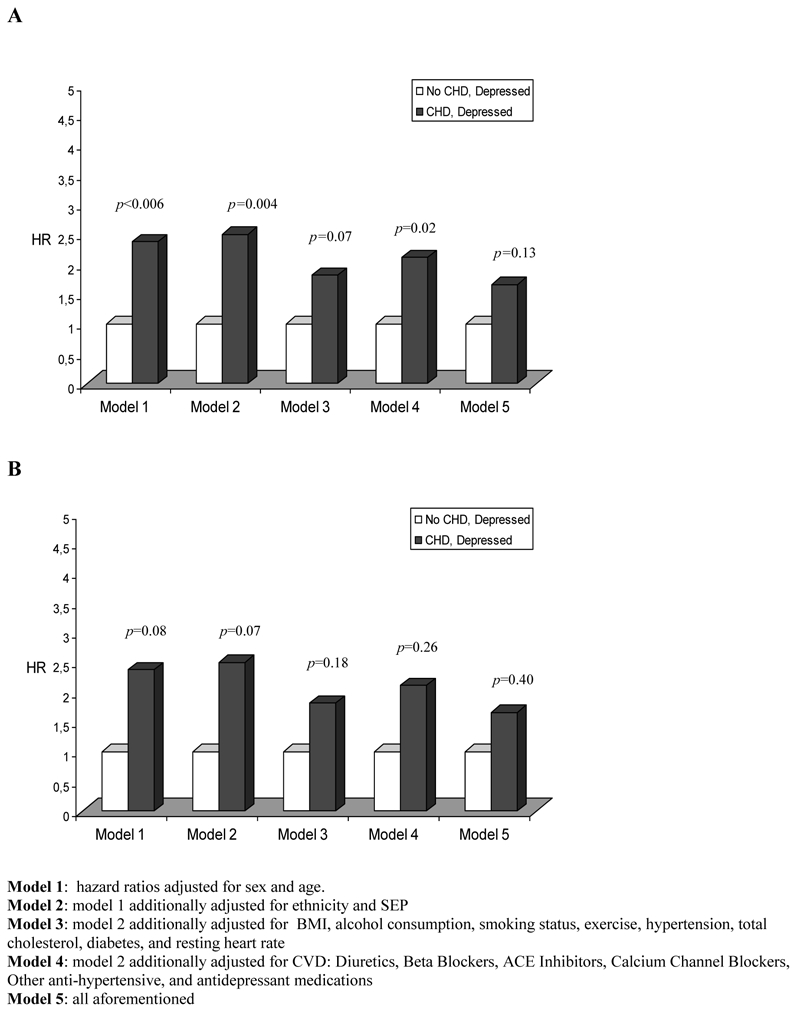
Hazard ratios of mortality for participants with both CHD and depressive symptoms comparatively to those with depressive symptoms but without CHD
A: All-cause mortality
B: Cardiovascular mortality
For results presented in Figure 2, the reference category is set to the group of individuals with CHD but no depressive symptoms. In model 1 (age and sex adjusted) the HRs associated with co-existing depression and CHD were 2.99 (p=0.001) and 2.92 (p=0.06) for all cause and CVD mortality, respectively. Serial multivariable adjustment showed no substantial attenuation for these associations.
Figure 2.
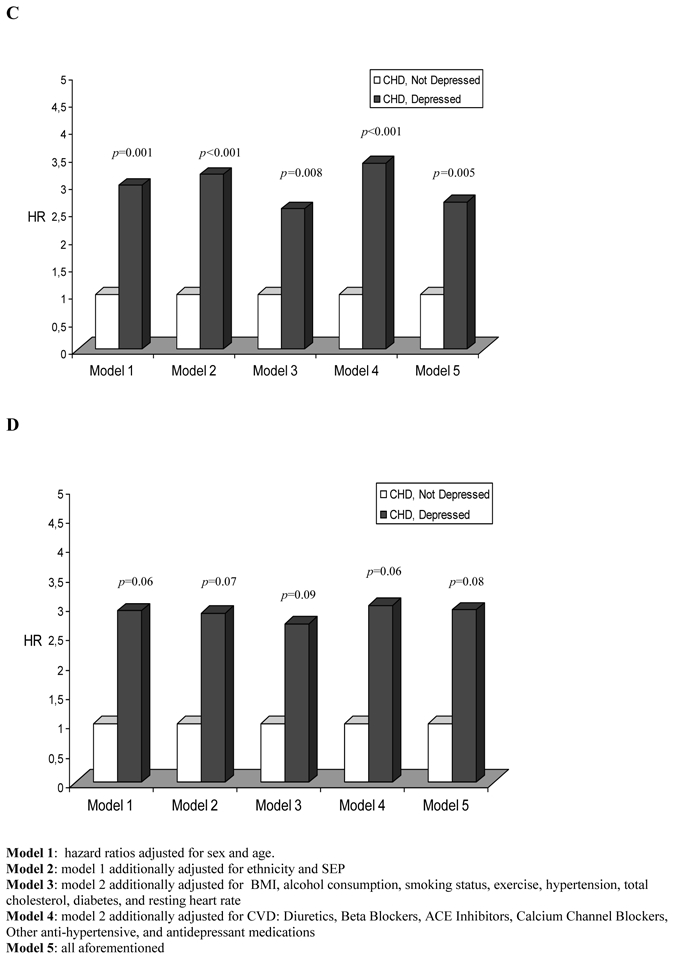
Hazard ratios of mortality for participants with both CHD and depressive symptoms comparatively to those with CHD but without depressive symptoms
C: All-cause mortality
D: Cardiovascular mortality
The RERI between depressive symptoms and CHD for all-cause mortality was 3.58 (95% CI, −0.09–7.26), providing some evidence for the presence of an additive interaction. The corresponding RERI for CVD mortality was 4.61 (95% CI, −4.04–13.26).
DISCUSSION
In this prospective study we sought to evaluate the differential effects of depressive symptoms on all-cause and CVD mortality in a large sample of middle-aged participants, with and without established CHD. We created 4 risk-factor groups based on the cross classification of depressive symptoms (yes/no) and CHD status (yes/no) at baseline. Our results show that compared to participants without depressive symptoms and CHD, the fully adjusted risks of mortality from all-cause and CVD were 2.9-fold and 3.9-fold higher for participants with both CHD and depressive symptoms, respectively. Further comparisons also indicated that the risks of death from all-causes and CVD were higher, but to a lesser extent, for these participants when specifically compared to those with depressive symptoms only or CHD only. There is some evidence of an additive interaction between depressive symptoms and CHD for mortality.
Findings in context of the literature
Several studies have examined the effect of depression on all-cause and CVD mortality in community and patients’ populations. Our results are in line with the findings reported in those studies. [3, 6, 9, 10, 11, 20] However, an important contribution of the present study to the current literature is that it is one of the first prospective cohort studies to compare the effects of depressive symptoms for mortality in individuals as a function of their CHD status. Our findings are based on a large well-characterized cohort with depressive symptoms assessed using a validated scale and biological risk factors assessed during a clinical examination. CHD events were ascertained using medical records and electrocardiograms data from clinical examinations. We were able to compare, within the same study, participants with both depressive symptoms and CHD to those free of one or both these conditions. We found fairly similar pattern of associations for both mortality outcomes examined, reinforcing therefore our confidence in the internal validity of our findings.
In the present study, the co-occurrence of depressive symptoms and CHD is associated with an increased risk of all-cause and CVD mortality beyond that due to depressive symptoms and CHD alone. The estimates for the additive interactions largely exceeded the value 0 (absence of interaction), even if the 95% confidence intervals for the RERIs were wide, particularly for CVD mortality. Indeed, we found that the RERI between depressive symptoms and CHD for all-cause mortality was 3.58 (4.61 for CVD death), suggesting that the relative risk of death from all-cause in depressive participants with CHD is 3.58 (4.61 for CVD death) more than if there were no interaction.
In order to test the robustness of our findings we repeated the main analysis by only considering participants with confirmed MI as CHD cases (see online appendix). Similar pattern of associations were observed as those presented in the main analysis in which CHD cases were participants with both confirmed MI and definite angina. This suggests that the combined effect of depressive symptoms and CHD on mortality outcomes is not sensitive to the definition of CHD events.
Further comparisons across risk-factor groups revealed (figures 1 and 2) that participants with both depressive symptoms and CHD were 2.4-fold (2.7-fold) and 3-fold (2.9-fold) higher age-sex-adjusted risk of all-cause (CVD) death when compared to those with only depression and only CHD, respectively. In combination, these observations illustrate the importance of the choice of the comparison group when estimating the effect size of depressive symptoms on health outcomes. The choice of the reference group seems also to be of great importance when testing the mediating role of third variables in the association between depression, morbidity and mortality. For instance the percentage of reduction in the age-sex adjusted hazards of death outcomes after multivariable adjustments for depressive participants with CHD varied from 49% to 51%, 53% to 62%, and 0% to 16% when compared to those without either depressive symptoms or CHD (table 2), those with only depressive symptoms (figure 1) and those with only CHD (figure 2), respectively.
Adjustment for sociodemographic characteristics, biobehavioural risk factors, antidepressant and CVD medications attenuated the increased risk of death in participants with only CHD towards the null, suggesting that this association was attributable to differences in baseline characteristics between the groups. However, this was not the case in those participants with only depressive symptoms and in those with both depressive symptoms and CHD, suggesting that the mechanisms through which depressive symptoms increase the risk of all-cause and CVD death remain to be understood. Further studies should explore other possible mechanisms that have not been addressed in the present study, including for example inflammatory processes [21], platelet activation [22], endothelial dysfunction [23], polyunsaturated fatty acids and plasma lipoproteins metabolism [24, 25] and the role of non adherence to medical regimens [26, 27]. Moreover, depressive symptoms may also be related to unfavorable trajectories of prognostic factors over time. Further longitudinal studies are needed to examine these mechanisms in detail.
Study limitations
In interpreting the present results, it is important to note some limitations. First, this cohort of civil servants did not include blue collar workers, unemployed or individuals with precarious jobs and younger adults; thus it is not representative of the general population, which may limit the generalisability of our findings. Second, we assessed depressive symptoms rather than clinical depression. However, it has been suggested that significant depressive symptomatology could be a risk factor for clinical depression [16]. Finally, as we had only 15 all-cause and 5 CVD deaths in the group of participants with both depressive symptoms and CHD, lack of statistical power is likely to be the main reason why the RERI values, though large, did not reach statistical significance.
Conclusions and implications
Despite these potential limitations, the present findings remain important by providing evidence that depressive symptoms are associated with an increased risk of all-cause and CVD death and that this risk is particularly marked in depressive participants with co-morbid CHD. A major implication of these results is the need for health care professionals to pay more attention to depression in their cardiac patients. Approximately 20% of participants with a history of CHD in our study had depressive symptoms, a figure consistent with those from previous studies estimating the prevalence of minor and major depression in patients with established CHD to be 27% and 20%, respectively [28, 29]. The findings stress the importance of developing interventions that are effective in reducing mortality due to depression. It should be noted that the mechanisms through which depressive symptoms increase mortality need to be better understood before evidence-based interventions can be developed.
Supplementary Material
Acknowledgments
We thank all participating civil service departments and their welfare personnel, and establishment officers; the Occupational Health and Safety Agency; the Council of Civil Service Unions; all participating civil servants in the Whitehall II study; all members of the Whitehall II study team. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
The Whitehall II study is supported by grants from the Medical Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (R01HL036310), US, NIH: National Institute on Aging (R01AG013196 and R01AG034454), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D and Catherine T MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. MK and JV are supported by the Academy of Finland (projects 124271, 124322, 129262 and 132944) and EU New OSH ERA Programme and MK is additionally supported by the BUPA Foundation, UK, the National Heart, Lung, and Blood Institute and the National Institute on Aging, USA. MJS is supported by a grant from the British Heart Foundation. AS-M is supported by a “European Young Investigator Award” from the European Science Foundation.
Footnotes
DECLARATION OF INTERESTS: None.
References
- 1.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–10. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 2.Anda R, Williamson D, Jones D, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–94. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–9. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 5.Rugulies R. Depression as a predictor for coronary heart disease. a review and metaanalysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 6.Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–8. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 7.Ryan J, Carriere I, Ritchie K, et al. Late-life depression and mortality: influence of gender and antidepressant use. Br J Psychiatry. 2008;192:12–8. doi: 10.1192/bjp.bp.107.039164. [DOI] [PubMed] [Google Scholar]
- 8.Mykletun A, Bjerkeset O, Dewey M, et al. Anxiety, depression, and cause-specific mortality: the HUNT study. Psychosom Med. 2007;69:323–31. doi: 10.1097/PSY.0b013e31803cb862. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. Jama. 1993;270:1819–25. [PubMed] [Google Scholar]
- 10.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–41. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 11.Dickens C, McGowan L, Percival C, et al. Depression is a risk factor for mortality after myocardial infarction: fact or artifact? J Am Coll Cardiol. 2007;49:1834–40. doi: 10.1016/j.jacc.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 12.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 13.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 14.Rose GA. Cardiovascular survey methods. Geneva: World Health Organization; Albany, N.Y: WHO Publications Centre USA [distributor]; 1982. [Google Scholar]
- 15.Gutzwiller F. Monitoring of cardiovascular disease and risk factor trends: experiences from the WHO/MONICA project. Annals of medicine. 1994;26:61–5. doi: 10.3109/07853899409147329. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385. [Google Scholar]
- 17.Rothman KJ. Epidemiology: an introduction. Oxford University Press; New York: 2002. [Google Scholar]
- 18.Andersson T, Alfredsson L, Kallberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–9. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–6. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Takeshita J, Masaki K, Ahmed I, et al. Are depressive symptoms a risk factor for mortality in elderly Japanese American men? : the Honolulu-Asia Aging Study. Am J Psychiatry. 2002;159:1127–32. doi: 10.1176/appi.ajp.159.7.1127. [DOI] [PubMed] [Google Scholar]
- 21.Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302–8. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Laghrissi-Thode F, Wagner WR, Pollock BG, et al. Elevated platelet factor 4 and betathromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry. 1997;42:290–5. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood A, Hinderliter AL, Watkins LL, et al. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–9. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–6. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Hamidifard S, Fakhari A, Mahboob S, et al. Plasma levels of lipoprotein (a) in patients with major depressive disorders. Psychiatry Res. 2009;169:253–6. doi: 10.1016/j.psychres.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Gehi A, Haas D, Pipkin S, et al. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508–13. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegelstein RC, Bush DE, Fauerbach JA. Depression, adherence behavior, and coronary disease outcomes. Arch Intern Med. 1998;158:808–9. doi: 10.1001/archinte.158.7.808. [DOI] [PubMed] [Google Scholar]
- 28.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. Jama. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 29.Carney RM, Rich MW, Tevelde A, et al. Major depressive disorder in coronary artery disease. Am J Cardiol. 1987;60:1273–5. doi: 10.1016/0002-9149(87)90607-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


