Abstract
Although Cytomegalovirus (CMV) infection is largely benign in immunocompetent people, the specific T cell responses associated with control of this persistent virus are enormous and must be maintained for life. These responses may increase with advanced age and have been linked to an “Immune Risk Profile” (IRP) that is associated with poor immune responsiveness and increased mortality in aged individuals. Based on this association, it has been suggested that CMV-specific T cell responses might become dysfunctional with age, and thereby contribute to the development of immune senescence by homeostatic disruption of other T cell populations, diminished control of CMV replication, and/or excess chronic inflammation. Here, we use the rhesus macaque (RM) model of aging to ask whether the quantity and quality of CMV-specific T cell responses differ between healthy adult RM and elderly RM that manifest hallmarks of immune aging. We demonstrate that the size of the CD4+ and CD8+ CMV-specific T cell pools are similar in adult vs. old RM, and show essentially identical phenotypic and functional characteristics, including a dominant effector memory (EM) phenotype, identical patterns of IFN-γ, TNF-α and IL-2 production and cytotoxic degranulation, and comparable functional avidities of optimal epitope-specific CD8+ T cells. Most importantly, the response to and protection against an in vivo CMV challenge were identical in adult and aged RM. These data indicate that CMV-specific T cell immunity is well maintained in old RM, and argue against a primary role for progressive dysfunction of these responses in the development of immune senescence.
INTRODUCTION
Aging may be accompanied with a decline in immune function characterized by poor responses to vaccination and increased morbidity and mortality from infectious diseases (1–4). This functional decline is associated with complex, but characteristic, changes in both the innate and adaptive immune system, collectively referred to as “immune senescence” (5, 6). Among the most consistent and dramatic age-related changes are those that occur in T cell homeostasis and function, manifesting in blood as 1) decreased CD4:CD8 T cell ratios, 2) loss of naïve cells and relative expansion of differentiated EM cells, 3) oligoclonality/clonal expansions, 4) poor proliferative responses, and 5) changes in cytokine secretion patterns (5, 7–12). These immunologic changes, which typically occur coordinately, have also been strongly correlated with evidence of persistent infection with the ubiquitous β-herpesvirus CMV, and together, these features constitute an IRP that in some studies has been predictive of increased mortality in aged individuals (7, 10–16).
These associations have led to the hypothesis that immune senescence may be infectious -- a consequence of long-term exposure to, and immunologic control of, persistent infections, in particular, CMV (7, 13, 15, 17). CMV is among the most immunogenic of known viruses, eliciting stable frequencies of specific T cells in CMV+ adults that average 10% of both the CD4+ and CD8+ memory compartments in blood (18). Frequencies of CMV-specific T cells can be even higher in aged individuals (19–21), and given that CMV-specific T cell responses are characterized by 1) a dominant EM (CD28−, CD27−, CCR7−) phenotype, 2) functional characteristics commensurate with this phenotype (high effector cytokine production, relatively low IL-2 production, expression of cytotoxic apparatus, poor proliferation), and 3) highly hierarchical clonotypic repertoires (with the top clonotypes manifesting frequencies >1%), these responses clearly underlie many of the features of the IRP (7, 13, 15, 17, 22–28). Indeed, it has been postulated that an increasingly dysfunctional, pro-inflammatory, CMV-specific T cell response expands with advanced age, driving out other T cell populations and causing both the IRP and a significant component of age-associated immune deficiency (7, 13, 17, 29). On the other hand, overt CMV disease is rare in the elderly (30, 31), healthy aged individuals may also be CMV+ (32, 33), and other age-related mechanisms such as thymic involution and cessation of new T cell production clearly play a major role in naïve cell deficiency (17, 34, 35) and likely, the poor responsiveness of the elderly to new Ags. Thus, the large CMV-specific effector memory response may simply be better preserved than other T cell populations, persisting while other populations decline (36).
The issue of whether CMV infection plays a causal role in immune senescence, and if so, the understanding of the mechanism(s) by which this virus manifests these changes, has critical implications for the clinical approach to elderly individuals. Obviously, if CMV infection and/or the CMV-specific T cell response are culpable in the development of immune senescence, therapy for immune senescence should be directed at preventing or treating CMV infection or interfering with the mechanism by which CMV and/or CMV-specific T cell responses cause the deleterious changes. Alternatively, if CMV and the CMV-specific T cell response are simply bystanders to the pathogenesis of functional immune senescence, therapy should be directed towards these other non-CMV-related causal mechanisms. When manifest in middle-aged adults, the IRP does not portend a poor prognosis (37), suggesting a progressive process that develops in late middle age to advanced age. One possibility is that the CMV-specific T cell response deteriorates during this timeframe, undergoing slow, but progressive, dysfunction, allowing more frequent and/or higher magnitude episodes of viral replication. This antigenic stimulation might expand the CMV-specific response, and elicit an ever-increasing level of dysfunction by exhausting any remaining non-senescent T cell populations and/or stimulating production of dysfunctional progeny. Such a reinforcing cycle of viral replication and dysfunction might then cause generalized immune dysfunction by displacing naïve and/or central memory T cell populations (13), and/or by creating a chronic inflammatory response that negatively affects the homeostasis of these other crucial T cell populations (38).
To address the question of whether CMV-specific T cell responses deteriorate with age and lose protective capacity, we turned to the rhesus macaque (RM) model. This model manifests the major features of immune senescence found in humans (35, 39–42), as well as a highly homologous rhesus CMV (RhCMV) that manifests similar immunobiology as human CMV, including the same high frequency EM-biased CD4+ and CD8+ T cell responses observed in CMV+ people (41, 43–46). Indeed, if chronic viral Ag exposure underlies deterioration of the CMV-specific T cell responses and ultimately the overall T cell compartment, the RM model might be expected to highlight this pathogenic mechanism as all colony-raised RM become CMV+ in the first year of life, and RhCMV, unlike HCMV [which is shed intermittently in the chronic phase of infection; (47–49)], is continually shed in the urine and saliva of the vast majority, if not all, animals (44, 46, 50). Here, we compare in detail the CMV-specific CD4+ and CD8+ T cell responses in adult and aged RM, and demonstrate that both the ex vivo and in vivo function of these responses are unchanged with advanced age, even when other features of immune senescence are present. These results contradict the occurrence of a general deterioration of CMV-specific EM T cell function with advanced age, and argue against the hypothesis that such age-associated deterioration is a requirement for development of immune senescence.
MATERIALS AND METHODS
Animals
Colony-bred male and female RM of Indian origin were maintained according to the federal, state and local guidelines. All experiments were approved by the Institutional Animal Care and Use Committee at the Oregon National Primate Research Center (ONPRC). RM with tumors, amyloidosis, or signs of significant clinical disease at the time of initial assignment were excluded from the study. The overall cohort consisted of 21 young adult (average = 7.8 years, range 7–10 years; 12 males, 9 females) and 36 old (average = 22.8 years, range 19–26 years, 15 males, 21 females) RM, which naturally acquired RhCMV in their first year of life. The in vivo challenge assay was performed on a subset of this overall cohort consisting of 11 young adult (average = 8.5 years, range 7–10 years; 6 males, 5 females) and 9 old (average =23.2 years, range 20–27 years; 4 males, 5 females) RM. Pooled data from multiple independent experiments is shown throughout. The number of RM used in any particular analysis was a variable subset of the overall cohort, dependent on the availability of RM at the time of analysis, or (where indicated) on defined inclusion criteria applied to the entire RM cohort. Bronchoalveolar lavage lymphocyte (BAL) collection and sampling was performed as previously described (41). In the RhCMV super-infection experiments, RM were challenged by subcutaneous injection of 107 plaque forming units of RhCMV strain 68.1, as previously described (44).
Ags and Abs
RhCMV-specific CD4+ T cell responses were identified by the response to RhCMV (Cercopithecine herpesvirus 8) Ag preparations containing lysates of RhCMV (strain 68.1; American Type Culture Collection VR-677)-infected monolayers of telomerized RM fibroblasts (44) combined with cell-free viral proteins concentrated from the culture medium of these infected cells (2:1 ratio of lysate to supernatant concentrate). Proteins were concentrated from infected cell supernatants by precipitation with 90% saturated ammonium sulfate, centrifugation (10,000 × g, 30 min, 4°C), resuspension in PBS, and finally, extensive dialysis against PBS (10,000d MW exclusion). RhCMV-specific CD8+ T cell responses were identified by the response to consecutive, overlapping 15-mer peptide pools (11 amino acid overlap) spanning the entire sequences of immediate-early (IE)-1 and IE-2 RhCMV genes (Intavis, Reutlingen, Germany). The final concentration of each peptide in the pool was 2μg/test. The mAbs L200 (CD4; AmCyan, PerCP-Cy5.5), SP34-2 (CD3; Pacific Blue, Alexa-700), SK-1 (CD8α; PerCP-Cy5.5, PE, unconjugated), CD28.2 (CD28; PE-Texas Red, PerCP-Cy5.5, unconjugated), DX2 (CD95; PE-Cy7, APC, PE), 15053 (CCR7; FITC), B56 (Ki67; FITC, PE), FN50 (CD69; PE, PE-Texas Red), 9F10 (CD49d; unconjugated), MAB11 (TNFα; FITC, PE-Cy7), H3A3 (CD107a; FITC), H4B4 (CD107b; FITC), B27 (IFN γ; APC), and MQ1-17H12 (IL-2; PE) were obtained from BD Biosciences. MAb FN18 (CD3) was produced in house and conjugated to Pacific Blue or Alexa700 using a conjugation kit from Life Technologies.
Cell Preparation and Stimulation
Citrated venous blood was centrifuged on a Ficoll-Hypaque density gradient (Sigma-Aldrich) to separate PBMCs. A small aliquot (100 μl) of whole blood was used at each bleed to define absolute lymphocyte counts in an Ac•T 5diff™ hematologic analyzer (Beckman-Coulter). PBMC and BAL were washed in HBSS and resuspended in RPMI-1640 medium, supplemented with 10% heat-inactivated FCS (HyClone), 2mM L-Glutamine (Sigma-Aldrich), 1mM Na-pyruvate (Sigma-Aldrich), and 50μM β-Mercaptoethanol (Sigma-Aldrich) (R10). To evaluate RhCMV-specific CD4+ T cell responses, 106 cells in 1 ml of R10 were placed in 17×100mm polystyrene tubes pre-coated with goat Fab fragments (2.5μg/ml) against mouse IgG (H+L), and then coated with costimulatory CD28 and CD49d antibodies (2.5μg/ml of each). Precoating and coating were done on stimulation day, in PBS, at 37°C for 1h, with PBS washes between procedures and following coating. Six μl of RhCMV lysate was added for 6h total, in the presence of Brefeldin A (10μg/ml) for the last 5h. Stimulation with 15-mer peptide pools was performed in the same volume format, but in polypropylene tubes. Co-stimulatory antibodies were added directly in the tube (at 0.5μg/ml each). To evaluate dynamic responses upon in vivo challenge, these assays were adapted to a 96-well plate format, where 5×105 cells in 200μl R10 were stimulated with lysates or 15-mer peptide pools for 9h (last 8h in presence of Brefeldin A), and co-stimulation as above. In all cases, a negative control tube that contained media and co-stimulatory antibodies was set up in parallel.
Immunofluorescence Staining and Flow Cytometric Analysis
Flow cytometric analysis was performed on an LSR-II apparatus (BD Biosciences) with Pacific Blue, AmCyan, FITC, PE, PE-Texas Red, PerCP-Cy5.5, PE-Cy7, APC, Alexa 700 and APC-Cy7 as the available fluorescent channels. Analysis was done with FlowJo software (Treestar, Ashland, OR). In all cases, gating on the lymphocyte population was followed by the separation of the CD3+ T cell subset, and progressive gating on CD4+ and CD8+ T cellsubsets. Naïve cells were defined by CD28 vs. CD95 expression patterns, as previously described (41). Among memory T cells, quadrant analysis of the CD28 and CCR7 parameters defined the central, transitional effector, and effector and memory subsets, as described (51). For the detection of Ag-responding subsets we gated the CD4+ or CD8+ subsets on intracellular expression of CD69 and any of the IFN-γ, TNF-α or IL-2 cytokines (46). Boolean gates of any of these combinations were generated, and in select experiments, cells responding by production of any of the cytokines were phenotyped with respect to CD28 and CCR7 expression, as described (46). For the detection of degranulation responses to antigen stimulation, CD107a and CD107b antibodies were added to the cells during the stimulation assay, so as to detect externalization of cytotoxic granule membrane proteins upon granule fusion with the cell membrane (25).
Functional Avidity Assessment
Immunodominant peptides indicated in Table 1 were used at graded concentrations from 100μg/ml to 1pg/ml to stimulate PBMCs for 6h (last 5h in presence of Brefeldin A). IFN-γ, TNF-α or CD107a/b responses were measured as detailed above, and normalized to maximum response, to generate dose-response curves and calculate peptide concentrations corresponding to 50% of maximum response.
Table 1. CD8+ T cell response to optimal IE peptides (% responding in the blood memory population).
| Peptide | ADULT mean±SD (n) | OLD mean±SD (n) | Kruskal-Wallis test (p) |
|---|---|---|---|
| VTTLGMALY | 0.92 ± 0.98 (4) | 5.19 ± 4.53 (8) | >0.05 |
| SGVLPENVP | 3.38 ± 2.41 (4) | 7.63 ± 10.7 (5) | >0.05 |
| SEDLQMTVI | 6.57 ± 9.23 (2) | 6.43 ± 8.59 (2) | n/a |
n/a = not applicable, given n=2 for adult and aged groups
RhCMV Quantification Using Real-Time PCR (qPCR)
RhCMV DNA in blood or BAL samples were quantified by qPCR amplification of a segment of the RhCMV IE-2 gene, as previously described (44). Nucleic acid was purified from samples using a Magnapure Compact instrument (Roche Diagnostics) according to the manufacturer’s instructions. Total nucleic acid in the samples was quantified by absorbance at 260nm using a Nanodrop ND-1000 spectrophotometer. Duplicate 10μl samples of purified nucleic acid were analyzed and the resulting copies per sample RhCMV IE-2 gene were divided by the μg total nucleic acid in the sample.
TCRB Spectratyping
TCRB transcripts were analyzed for CDR3 region length polymorphism by PCR spectratyping, as previously described (40). We monitored spectratypes in PBMC over 3 years, with 4 time points roughly 1 year apart. All RM were analyzed at least twice. TCRB spectratype profiles were independently evaluated for morphology by 3 independent researchers; profiles were defined as “Gaussian” or “clonal” when at least two assessments categorized a Vβ as such.
Statistical Analysis
Statistical analysis was performed with methods indicated in the text, and using SAS software, version 9.2 (SAS), or Prism software (Graphpad). Assessments were considered non-significant (ns) if the p-value >0.05.
RESULTS
Loss of naïve cells and T cell diversity in old monkeys
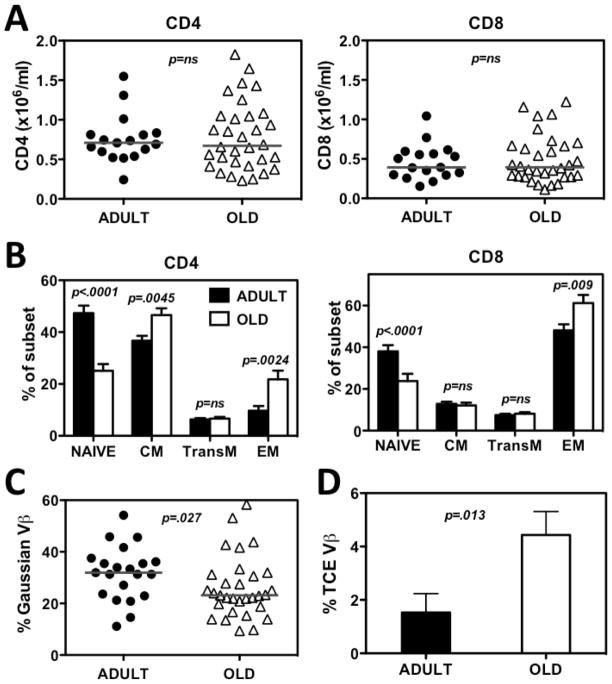
The overall goal of this project was to characterize changes in the quantity and/or quality of RhCMV-specific T cell responses associated with old age. To accomplish this goal, we assembled a cohort of randomly selected adult (7–10 years of age) and old (19–26 years of age) RM (roughly corresponding to 20–30 and 60–80 years of age, respectively, in the human lifespan). Our use of adult rather than juvenile RM as the baseline population allowed us to characterize changes associated with aging, and not with the maturation of the immune system. We first assessed the impact of aging on the size and composition of the overall T cell compartment to provide context for changes in the RhCMV-specific T cell populations. As shown in Fig. 1A, the absolute counts of total CD4+ and CD8+ T cells were comparable among the adult and old RM cohorts, but the relative composition of these subsets was significantly different. The proportion of naïve cells within both the CD4+ and CD8+ T cell lineages, defined as CD28intCD95lo (41), was significantly lower in old monkeys (Fig. 1B), consistent with previous results (40, 41). Memory cells were sub-classified by the expression of CD28 and CCR7 into central memory (CM) subsets (CD28+CCR7+), transitional effector memory (TransEM) cells (CD28+CCR7−) and effector memory (EM) cells (CD28−CCR7−) (51). With regard to these subsets, the old RM manifested a significant increase in relative size of the EM population in both lineages, as well as a modest CD4+ T cell-specific increase in the proportion of CM cells (Fig. 1B). Notably, the proportion of TransEM cells, which include the highest fraction of proliferating cells and is a general reflection of ongoing EM cell production (51), was not different between the adult and aged RM.
Figure 1. Comparison of CD4 and CD8 T cell subset distribution and TCRB-defined clonality in adult vs. old RM.
(A) Absolute lymphocyte counts were multiplied by the fraction of CD3+/CD4+ and CD3+/CD8+ small lymphocytes determined by flow cytometry to determine absolute CD4+ and CD8+ T cell counts in blood. Symbols show values obtained in individual adult or old monkeys, horizontal bars denote medians. P value was obtained by a two-tailed Mann-Whitney test. (B) PBMC were further analyzed for CD28, CCR7, and CD95 expression to determine frequencies of naïve, CM, TransEM, and EM subsets within the overall CD4+ and CD8+ T cell populations (see text). Histograms show mean frequencies of the indicated subsets in the adult and old RM cohorts, error bars show SEM. Indicated p values were obtained by mixed model ANOVA followed by step-down correction. (C) PBMC RNA was PCR analyzed with 24 primers specific for each TCRVB family followed by PAGE and densitometry to identify Vβ families showing a Gaussian distribution of PCR product densities over their length. Symbols indicate frequencies of TCRVB chains with Gaussian PCR products in individual monkeys over three years. Horizontal lines are medians; p value was obtained by a two-tailed Mann-Whitney test. (D) The same analysis as in C was performed to identify TCRVB families carrying a single PCR band over at least 2 consecutive years. Average frequencies of their occurrence in the adult and old monkey cohort are indicated. Error bars show SEM; p value was obtained by a two-tailed Mann-Whitney test.
A key characteristic of an aging immune system is a general loss of T cell repertoire diversity and the appearance of prominent clonal T cell expansions, reflecting both the decline of the highly diverse naïve population, and the expansion or relative preservation of dominant memory clonotypes (9, 15, 52). To establish whether our adult and aged RM cohorts differed with respect to repertoire diversity and frequency of clonal expansions, we monitored CDR3 region length-polymorphism of TCRB transcripts by PCR spectratyping (53). Cell populations exhibiting high TCRB diversity exhibit a bell-shaped (Gaussian) distribution of CDR3 region lengths, whereas populations with narrow repertoire show distorted profiles, and in extreme cases of repertoire reduction can show a single PCR product band. We analyzed all 24 TCRBV chains in these adult and old RM over 3 consecutive years, and quantified their relative percentage of profiles with Gaussian CDR3 size distributions and with highly skewed (single peak) distributions. As TCRBV populations with single peaks can represent reversible expansions of T cells that recently encountered an antigen (e.g., cells in the TransEM subset), or stable age-associated (potentially pathogenic) T cell clonal expansions (TCE; e.g., cells in the EM subset) (52, 54), we determined the frequencies of TCRBV families exhibiting the same TCE profile in at least two consecutive time points spaced at least a year apart. Notably, these analyses revealed that old RM manifested significantly lower frequencies of Gaussian TCRB profiles (Fig. 1C), and a significantly higher frequency of TCRB profiles showing a stable, single peak (Fig. 1D). Overall, 58.8% of old RM had at least one stable TCE, vs. only 23.8% of young adult RM, which was a significant difference (p=0.014; two-tailed Fisher’s exact test). Taken together, these data argue for an age-associated skewing of the TCRB repertoire in the RM of our old cohort.
In separate studies, we have confirmed functional differences in T cell responses in our adult vs. young RM cohorts, showing that the old cohort exhibited significantly weaker antibody and CD8+ T cell responses to an immunization with a live modified vaccinia strain Ankara (35). Thus, the old RM cohort studied here exhibited significant changes in the composition and function of the T cell compartment, consistent with immune senescence, and reminiscent of the IRP defined in aged human subjects.
RhCMV-specific CD4+ T cell responses in adult vs. old monkeys
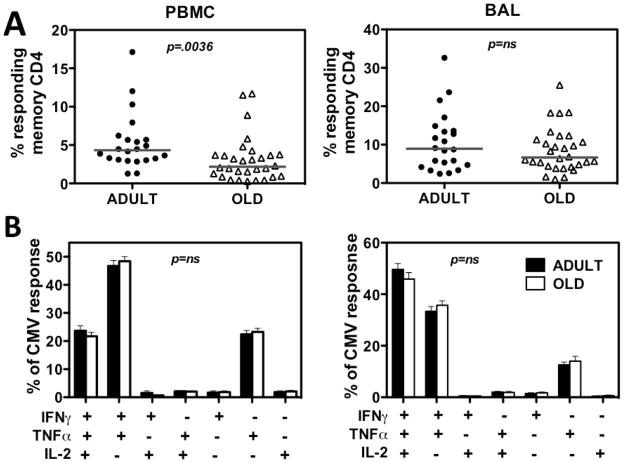
RhCMV-specific CD4+ T cell responses were quantified in both PBMC and BAL, the latter representing an accessible mucosal effector site (41), by in vitro stimulation with whole viral Ag preparations derived from RhCMV-infected fibroblasts, and were assessed for CD69 upregulation and IFN-γ, TNF-α and IL-2 production by CD4+ memory T cells. The median frequency of T cells within the CD4+ memory compartment in blood (PBMC) specifically responding to RhCMV Ag with a cytokine response (any of the 3 cytokines analyzed, in any combination) was significantly higher in the adult RM than in the old RM (Fig. 2A – left), but there was no difference in these frequencies in the BAL compartment [Fig. 2A – right; note: BAL T cells are essentially 100% memory in phenotype (41)]. To compare the profile of cytokine responses in adult vs. old RM, we analyzed the frequency of RhCMV-responding cells exhibiting any combination of the 3 cytokines, and defined the contribution of each cytokine profile to the total response. Aging-related changes in these cytokine synthesis profiles were not observed in either PBMC or BAL responses (Fig. 2B). In summary, our data indicate that aging is associated with a modest decline in the frequency of CMV-specific CD4+ T cells in the blood memory compartment, but not in the BAL memory compartment, and no qualitative changes in cytokine profiles were observed in either site.
Figure 2. Comparison of RhCMV-specific CD4+ T cell responses in adult vs. old RM.
(A) PBMC and BAL cells were assessed for CD4+ T cell responses to RhCMV Ag preparations with cytokine flow cytometry as described in the Methods. The frequency of CD4+ T cells expressing CD69 and any combination of IFN-γ, TNF-α and IL-2 expression after incubation with and without RhCMV Ag preparation was determined, with the difference between these frequencies reported as the RhCMV-specific CD4+ T cell response frequency. These values were then normalized to the memory population size in each RM. Symbols show individual monkeys, horizontal lines denote medians. P value was obtained by a two-tailed Mann-Whitney test. (B) To determine if the pattern of IFN-γ, TNF-α and IL-2 production by RhCMV Ag-stimulated CD4+ T cells differed in adult vs. old RM, the contribution of each possible combination of these cytokines to the overall response was determined by Boolean gating, and statistically analyzed by a mixed effects regression model analysis. Histograms indicate average frequencies of each of the indicated patterns in adult vs. old responder cells; error bars are SEM.
RhCMV-specific CD8+ T cell responses in adult vs. old monkeys
We next characterized RhCMV-specific responses in CD8+ T cells in PBMC and BAL. As Ag preparations composed of lysates and/or supernatants from RhCMV-infected cells are suboptimal antigens for the induction of MHC class I-restricted (CD8+) T cell responses, we used a pool of overlapping, consecutive 15-mer peptides spanning two immunodominant RhCMV genes (IE-1 and IE2-) to evaluate RhCMV-specific CD8+ T cell responses in our cohorts. The rationale for selection of IE-1 and IE-2 for evaluation of RhCMV-specific CD8+ T cells was two-fold. First, pan-proteome CD8+ T cell response analysis of HCMV-specific responses in HCMV+ humans has demonstrated that IE-1 and IE-2 are among the most frequently recognized HCMV proteins, and manifest the highest response frequencies of all HCMV proteins (18). In addition, IE-1-specific CD8+ responses have been closely correlated with clinical outcome after transplantation (55, 56). Second, pilot studies in RM demonstrated that IE-1 and IE-2 were targeted by CD8+ T cells in the vast majority (albeit not all) of monkeys with robust response frequencies (far greater in both frequency and response size than responses to 2 RhCMV pp65 homologues; data not shown).
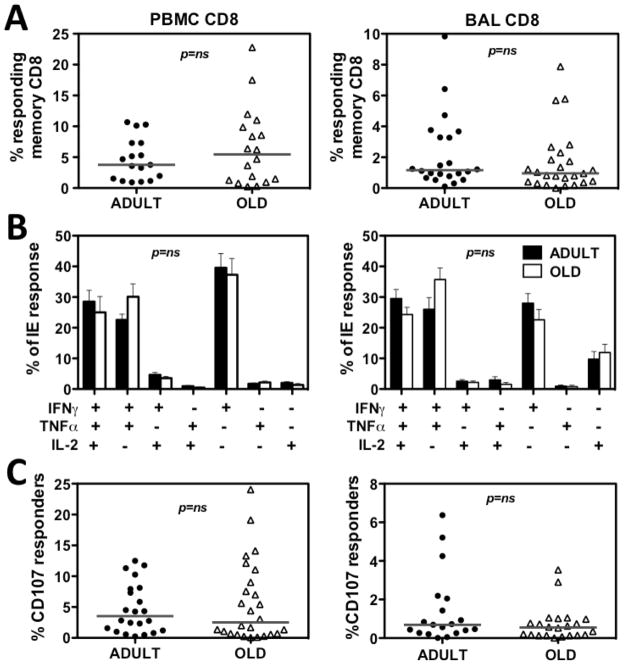
As shown in Fig. 3A and B, neither the distribution of overall response frequencies, nor the cytokine profiles of RhCMV(IE)-specific CD8+ T cell responses in PBMC or BAL were significantly different between the adult and old RM cohorts. Moreover, the frequency of cells undergoing Ag-triggered externalization of cytotoxic granules after IE peptide exposure was not significantly different between adult and old RM in both PBMC and BAL. Four old animals showed low (<0.2%) responses to the IE-1 + IE-2 peptide pools in PBMC and in BAL by all measures. As not all RhCMV+ RM recognize epitopes within the IE proteins (data not shown), it is probable that these RM were not deficient in RhCMV responsiveness, but rather simply fell into this non-recognition group. In any case, the exclusion of these 4 RM from the analysis only decreased the difference between adult and old cohorts (data not shown), and therefore this consideration did not alter the conclusion that CD8 responses to CMV antigen did not significantly change with age by these criteria.
Figure 3. Comparison of RhCMV/IE-specific CD8+ T cell responses in adult vs. old RM.
(A) PBMC and BAL cells were assessed for CD8+ T cell responses to mixes of consecutive, overlapping (11 amino acid overlap) 15-mer peptides comprising the RhCMV IE-1 and IE-2 proteins with cytokine flow cytometry as described in the Methods. The frequency of CD8+ T cells expressing CD69 and any combination of IFN-γ, TNF-α and IL-2 expression after incubation with and without RhCMV IE peptides was determined, with the difference between these frequencies reported as the RhCMV-specific CD8+ T cell response frequency. These values were then normalized to the memory population size in each RM. Symbols show individual monkeys, horizontal lines denote medians. P value was obtained by a two-tailed Mann-Whitney test. (B) To determine if the pattern of IFN-γ, TNF-α and IL-2 production by RhCMV/IE peptide-stimulated CD8+ T cells differed in adult vs. old RM, the contribution of each possible combination of these cytokines to the overall response was determined by Boolean gating, and statistically analyzed by a mixed effects regression model analysis. Histograms indicate average frequencies of each of the indicated patterns in adult vs. old responder cells; error bars are SEM. Significance was assessed by mixed-effects regression model analysis. (C) The frequency of CD8+ T cells manifesting externalization of cytotoxic granules in response to RhCMV/IE peptide vs. control stimulation in PBMC (left) and BAL (right) was determined by CD107a and CD107b staining. These values were then normalized to the memory population size in each RM. Symbols show individual monkeys, horizontal lines denote medians. P value was obtained by two-tailed Mann-Whitney test.
Absolute CD4+ and CD8+ T cell response in old monkeys
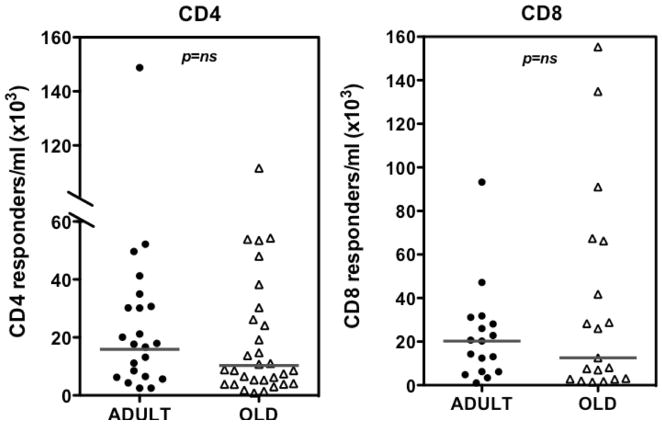
To further compare the size of RhCMV-specific CD4+ and CD8+ T cell populations, we determined the absolute count of these cells in the blood in the adult and old RM, using the same intracellular cytokine assays described in Figs. 2 and 3. Importantly, we found no significant difference in the absolute counts of RhCMV-specific CD4+ or CD8+ T cells between adult and old monkeys (Fig. 4). Note that while the frequency of CD4+ T cell responders in the CD4+ memory T cell pool was somewhat decreased in old RM (Fig. 2A), the increased number of total circulating memory cells in the old cohort compensated for this reduction (Fig. 4).
Figure 4. Comparison of absolute counts of RhCMV-specific CD4+ and CD8+ T cells in adult vs. old RM.
PBMC cells were stimulated to elicit cytokine responses in CD4+ or CD8+ T cells as shown in Figs. 2 and 3, respectively. Blood lymphocytes were counted in parallel, and this value was multiplied by the fraction of CD4+ or CD8+ RhCMV-specific lymphocytes to define the absolute count of RhCMV-specific CD4+ and CD8+ T cells. Symbols show individual monkeys, horizontal lines denote medians. P value was obtained by a two-tailed Mann-Whitney test.
Phenotypes of RhCMV-specific T cells in adult and old monkeys
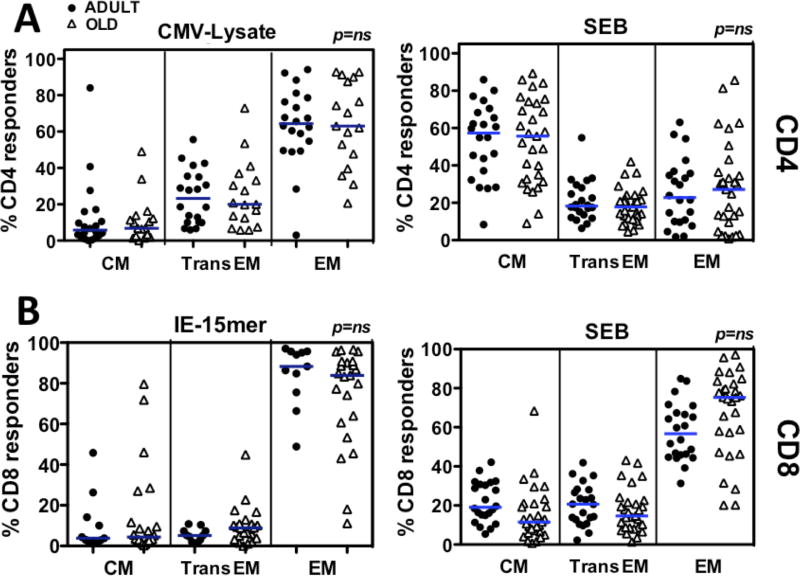
Both human CMV- and RhCMV-specific T cells in blood manifest a predominantly EM phenotype (23, 24, 41, 46). To determine if aging accentuates this phenotypic bias, PBMC were stimulated with RhCMV antigen (whole Ag preparations and IE peptides, as in Figs. 2A and 3A) and cytokine-responding cells were categorized by CD28 and CCR7 surface expression into CM, TransEM or EM subsets of memory cells, as described in Fig. 1. In parallel, PBMC were stimulated by the superAg Staphylococcus enterotoxin B (SEB) and similarly phenotyped to demonstrate the distribution of a generic functional response among the CM, TransEM, or EM subsets. As shown in Fig. 5A, RhCMV-specific CD4+ T cells manifested a similar EM bias in both adult and old RM, in contrrast to the predominant CM phenotype of SEB-responsive cells. As shown in Fig. 1, the overall CD8+ memory compartment is much more EM-biased than the CD4+ memory compartment, and this overall bias increases with age. In keeping with this, the SEB-responsive CD8+ T cell subset was more EM-biased in old RM than adult RM (Fig. 5B). Importantly, however, the RhCMV(IE)-specific CD8+ T cell responses were even more EM-biased than the overall or SEB-responsive population, and this marked bias was similar in the adult and old RM cohorts. In conclusion, RhCMV-specific T cells are highly EM-biased in both adult and old RM, and aging did not increase the extent of this bias.
Figure 5. Comparison of the phenotypically-defined differentiation state of RhCMV-specific T cell responses in adult vs. old RM.
PBMC were stimulated with SEB (200ng/ml), whole RhCMV Ag preparations, RhCMV(IE) peptide mixes or no Ag, as described in Figs. 2 and 3. Cells were analyzed for the expression of CD3, CD4, CD8, CD69, IFN-γ, TNF-α and IL-2, as described in Figs. 2 and 3, and in addition, the surface markers CD28 and CCR7, with the CD28 vs. CCR7 phenotype (CM, TransEM, and EM designations as in Fig. 1) determined for all responding cells (CD69+ plus any combination of the 3 cytokines). To provide sufficient events for accurate phenotypic analysis, the data set was restricted to RM with specific response frequencies of >0.4%. (A) Analysis of CD4+ T cells. (B) Analysis of CD8+ T cells. Horizontal lines show medians. Significance assessed by Kruskall-Wallis test, with Dunn’s post-analysis. Of note, parametric statistical analysis by ANOVA with Bonferroni post-analysis revealed an age-related difference between the CD8+ T cell populations responding to SEB, at a significance of p<0.05, but not CD8+ T cell populations to IE, or any of the responding CD4+ T cell populations.
Function, phenotype and triggering thresholds of CD8+ T cell responses to immunodominant CMV peptide epitopes in adult and old monkeys
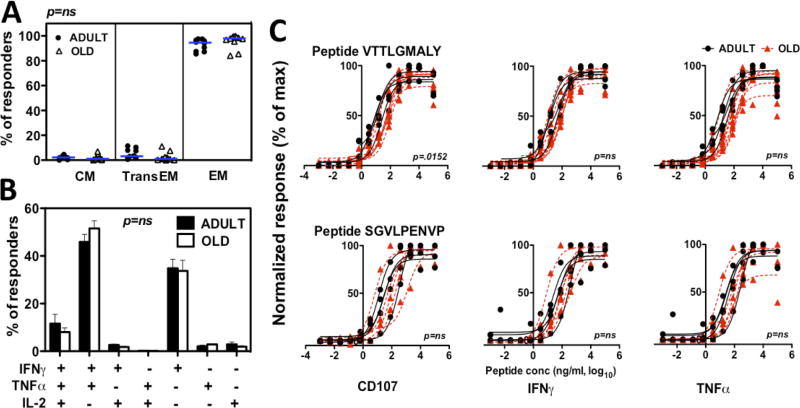
The 15-mer peptides comprising the IE-1/-2 peptide mixes are not optimal ligands for triggering CD8+ T cell responses (57) and the responses to these mixes likely include variable combinations of responses to different epitopes in each animal, factors that together might obscure differences in the function of RhCMV-specific CD8+ T cells in adult vs. old RM. To more precisely compare the functional responses in adult vs. old RM, we screened our cohorts for common CD8+ T cell responses to optimal IE-1 and IE-2–9-mer epitopes. Three epitopes with vigorous responses common to multiple RM in both age cohorts were identified (Table I). CD8+ T cells responding to these immunodominant optimal epitopes displayed an even higher EM bias than the overall RhCMV(IE)-specific CD8+ T cell responses, but again, the adult and old RM showed essentially identical phenotype profiles (Fig. 6A). In addition, the cytokine profile of the CD8+ T cells responding to these immunodominant optimal peptides were indistinguishable between the adult and old RM cohorts (Fig. 6B). As TCR bind to peptide-MHC complexes with varying avidity, and high-avidity responses provide better protection against viral pathogens (58), we next investigated the functional avidity of CD8+ T cells to these immunodominant peptides by determining the dependence of the cytokine and degranulation responses on the dose of added peptide. The normalized dose-response curves of CD107-, IFN-γ- or TNF-α-responding CD8+ T cells upon stimulation with graded peptide concentrations is shown in Fig. 6C. Notably, while dose dependence of the CD107 response to peptide VTTLGMALY showed a minor shift to the right in old monkeys, other functions triggered by this peptide, and all functions triggered by a different peptide (SGVLPENVP) manifested statistically indistinguishable dose-response profiles. Taken together, these data argue against a general decline in CD8+ T cell responsiveness to immunodominant RhCMV epitopes with aging in RM.
Figure 6. Comparison of the phenotype, cytokine profile and avidity of CD8+ T cells responding to immunodominant peptides in adult vs. old RM.
(A) CD8+ cells responding to the immunodominant 9-mer peptides shown in Table I were phenotyped by CD28 and CCR7 surface expression as in Fig. 5. Symbols show individual adult vs. old RM, horizontal lines are medians. Significance was assessed by Kruskall-Wallis test, with Dunns post-analysis. (B) Cytokine profiles of CD8+ T cells responding to immunodominant 9-mer peptides shown in Table I were determined as in Fig. 3. Histograms indicate average values for adult vs. old RM; error bars are SEM. Significance was assessed by mixed-effects regression model analysis. (C) PBMC were stimulated with serial dilutions of the VTTLGMALY (top panels) or SGVLPENVP (bottom panels) peptides. The % CD8+ T cells responding to these peptides with CD107 surface expression, or intracellular production of IFN-γ or TNF-α was determined as in Fig. 3, and was normalized to the maximum response for each RM. Dose-response curves were defined by regression analysis in each RM, and adult and old RM were compared by mixed model analysis.
Response of adult and old RM to CMV challenge
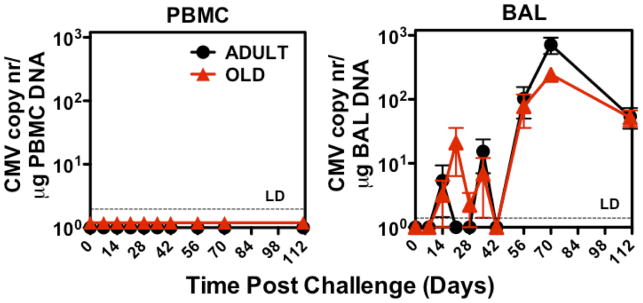
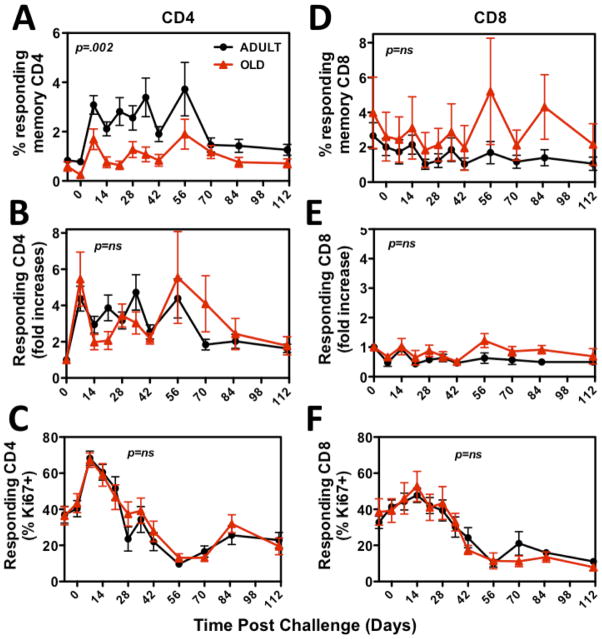
While ex vivo functional assays of RhCMV-specific T cells revealed few differences in the quantity or quality of these responses in adult vs. old RM, these data do not rule out a defect in the ability of aged RhCMV-specific T cells to respond to in vivo challenge. To directly address the in vivo capabilities of RhCMV-specific T cell responses, we challenged a subset of the adult (n=11) and old (n=9) RM cohorts with infectious RhCMV, and compared the ability of adult vs. old RM to respond to and control RhCMV super-infection (a super-physiologic challenge designed to reveal otherwise subclinical deficits in anti-RhCMV immunity). As a measure of such super-infection, levels of RhCMV DNA were monitored by quantitative PCR of both PBMC and BAL cells. RhCMV DNA was not detectable in either cell population in any RM prior to challenge, and even after challenge, PBMC remained negative in both adult and old RM, consistent with previous studies of RhCMV super-infection of adult RM (44). However, RhCMV DNA appeared in the BAL cells of both adult and old RM following challenge, starting at day 14 and peaking at post-infection day 70, prior to a slow decline (Fig. 7). Importantly, the kinetics and levels of RhCMV replication in BAL were remarkably similar in adult and old RM, strongly arguing that aging does not impair the ability of RhCMV-specific T cell responses to control RhCMV. This in vivo challenge also allowed assessment of the T cell proliferative response to RhCMV-antigen stimulation in vivo, as determined by the change in the frequency of RhCMV-specific T cells in blood and BAL, as well as the induction of the proliferation-associated marker Ki-67 on RhCMV-specific T cells post-challenge (41). In this regard, although the average frequency of RhCMV-specific CD4+ T cells in blood was slightly, but significantly, higher in adult RM as compared to old RM, both the fold increase in these frequencies and the kinetics and level of Ki-67 induction on these cells were indistinguishable in adult vs. old RM (Fig. 8A–C). In keeping with their marked EM bias (59), frequencies of RhCMV(IE)-specific CD8+ T cells did not increase following challenge, and Ki-67 induction was modest; however, these profiles were also essentially identical in the adult vs. old RM cohorts (Fig. 8D–F). Thus, the response to and control of RhCMV challenge was equivalent in the adult and old RM cohorts.
Figure 7. Comparison of RhCMV kinetics in adult and old monkeys after infectious RhCMV challenge.
Adult (n=11) or old (n=9) RM were challenged by subcutaneous injection of 107 plaque forming units of RhCMV. RhCMV genomes in PBMC or BAL cells were quantified by qPCR and standardized to genomic host DNA. Average ± SEM of RhCMV copy numbers (y-axis) at indicated post-challenge days (x-axis) are indicated. LD = limit of detection.
Figure 8. Comparison of the in vivo proliferation response of RhCMV-specific T cells in adult vs. old RM following infectious RhCMV challenge.
The adult and old RM challenged as shown in Fig. 7 were followed over time for frequencies of RhCMV-specific T cells in the circulating memory compartment by cytokine flow cytometry. Responding cells were defined by IFN-γ and/or TNF-α expression in CD69+ cells. Panels A–C show CD4+ T cells responding to whole RhCMV Ag preparations. Panels D–F show CD8+ T cells responding to RhCMV(IE) peptide mixes. Response frequencies are shown both as average ± SEM frequencies (A, D) and as average ± SEM fold change from the pre-challenge baseline (average of day -7 and day 0 values; B, E). Responding cells were simultaneously analyzed for expression of the proliferation marker Ki-67 (41) with results presented as average ± SEM for %Ki-67+ of the RhCMV-specific T cells (C-F). Statistical analysis was by repeated measures ANOVA.
DISCUSSION
Primate CMVs have evolved a unique immunobiologic relationship with their hosts. In the absence of disease and in most cases overt infection, these viruses elicit and maintain remarkably high frequency T cell responses, considerably higher than other common viral infections and similar to that of overtly pathogenic chronic infections such as HIV (18, 60–64). Moreover, these responses manifest a distinctive highly EM-biased phenotype (22–25, 28, 59). Although the basis of this unique immunogenicity remains to be experimentally confirmed, HCMV is thought to, and RhCMV is known to, persist as a very low-level continuous or frequently intermittent infection, rather than a completely or predominantly quiescent, latent infection (44, 65, 66). This characteristic would provide chronic, low-level Ag exposure, which might drive the characteristic EM differentiation of CMV-specific T cells and maintain the very high CMV-specific T cell response frequencies over the lifetime of the host. Note that this chronic Ag exposure is not so high as to induce the T cell “functional exhaustion” that characterizes pathogenic chronic viral infection (67, 68), as CMV-specific T cells are highly functional (23, 25, 46, 60, 69). Indeed, the rapidity of CMV reactivation after the onset of potent immunosuppressive therapy, the potential seriousness of CMV disease in this setting, and the fact that the CMV is almost invariably controlled without overt disease in immunocompetent individuals, even into old age, attests to the in vivo function of these T cell responses (30, 31, 47, 65). Moreover, the fact that the highly EM-biased SIV-specific T cell responses elicited by RhCMV vectors encoding SIV Ags have shown promising efficacy in protection against pathogenic SIV challenge in RM suggests that “CMV-like” T cell responses can effectively function against heterologous pathogens (46). However, as indicated above, the association of CMV infection and the IRP has led to the hypothesis that lifelong control of CMV might have adverse consequences in old age, with the massive immune responses against CMV possibly causing or materially contributing to the immune senescence and the consequent increased morbidity and mortality of infectious diseases in the elderly (7, 11–13, 15, 17, 21). More recently, it has also been suggested that CMV control itself might be compromised in the elderly, leading to occult infection and associated chronic inflammation that might compromise function of diverse organs and physiologic processes (14, 70).
Perhaps the most likely mechanism that could link CMV infection or anti-CMV immunity to aging pathogenesis would be the development in old age of an intrinsic senescence of the CMV-specific T cell response itself. Given that CMV is often acquired in early childhood and that CMV-specific T cells are, as suggested above, almost certainly exposed to chronic antigenic stimulation, it is reasonable to speculate that after decades of “use”, including many years without a functional thymus to provide new naïve cells to replenish and diversify these responses (71), CMV-specific T cells might develop critically short telomeres, accumulate mutations, and thus, simply wear out (15, 19, 20, 29, 72). Hypothetically, this might lead to diminished control of CMV replication, increasing the frequency and/or magnitude of reactivation episodes, which in turn might result in direct pathogenicity by frequent or continuous occult infection(s) and chronic inflammation in critical tissues and/or indirect pathogenicity by driving expansion of an abnormal CMV-specific T cell response or by stimulating chronic immune activation, thereby displacing or dysregulating the homeostasis of other crucial T cell populations. Conceivably, these conditions might set up a re-enforcing spiral of deterioration in which failing CMV-specific immunity leads to CMV replication, which further compromises both the CMV-specific response and, as collateral damage, the overall immune system, leading to further CMV replication (as well as other infectious complications), and further immune compromise.
This concept has been sustained by reports that either the CMV-specific T cell response itself and/or the CD28− EM population it dominates comprise the major proportion of peripheral blood lymphocytes in elderly individuals (particularly those with the highest short-term mortality), that these populations manifest replicative senescence and other functional “abnormalities”, that CMV detection in secretions is more common in the elderly, and that elderly individuals with the highest quartile of CMV-specific Ab levels have increased mortality (7, 13, 15–17, 19, 20, 29, 70, 72). There are, however, several crucial caveats in interpreting these observations. First, the domination of CMV-specific EM in the elderly has only been reported as a relative dominance in peripheral blood. The overall number of CMV-specific T cells in the body has not been enumerated in adult vs. elderly individuals, and thus the dominance of CMV-specific T cells might result from loss of non-EM populations around a stable EM that includes the vast majority of the CMV-specific T cells. CMV-seronegative individuals would lack the benchmark of the large CMV-specific population by which the relative loss of other populations in blood can be visualized. In tissue, CMV-specific T cells are present at high frequency in extra-lymphoid effector sites, but these cells constitute only a minor component of T cells in the lymph nodes supporting naïve and CM T cell homeostasis [(73) and L. Picker, unpublished data], arguing against a direct displacement of naïve and CM T cells by CMV-specific T cells. The replicative senescence and other “abnormal” functions attributed to CMV-specific T cells (low IL-2, etc.) are in fact intrinsic characteristics of EM differentiation, and typify CMV-specific T cell populations from individuals of all ages, including children, and even heterologous responses elicited by CMV vectors in the RM model (22, 46, 59). Moreover, the skewed clonotypic hierarchies of CMV-specific T cells are also characteristic of mature CMV-specific T cell responses regardless of age (22, 26, 44, 74). Finally, the association of CMV secretion or elevated levels of CMV Abs with sick or “soon to be sick” elderly individuals is not surprising given CMV replication can increase with stress (65, 75). It is therefore possible that CMV replication and increased levels of CMV-specific immunity in such end-of-life individuals is initiated by their other age-associated disease, rather than vice versa.
A crucial issue in the investigation of a possible causal role of CMV in immune senescence is the question of whether CMV-specific T cell populations do or do not become dysfunctional with age and therefore are less able to control the virus. In this paper, we addressed this question in the RM model, which includes both a human-like immune senescence syndrome and a CMV virus, RhCMV, which is biologically highly homologous to human CMV and which allows for infectious challenge with RhCMV. We compared the frequency of RhCMV-specific CD4+ and CD8+ T cells in blood and lung airspace (a representative mucosal interface effector site), the absolute number and differentiation phenotype of these cells in blood, and both ex vivo and in vivo function of these cells in cohorts of adult and old RM, the latter cohort showing laboratory and functional evidence of immune senescence. Overall, these studies failed to show biologically significant differences in adult vs. old CMV-specific T cell responses, most importantly including any difference in the response to and control of infectious RhCMV challenge. In a previously reported study, we found that the clonotypic hierarchies of RhCMV-specific CD4+ T cell responses were similar in adult and old RM (44). Taken together, these results are not consistent with a general deterioration of RhCMV-specific T cells with old age, and therefore argue against a major contribution of such deterioration to immune senescence in this model. There are, however, several caveats to these analyses. First, we did observe a minor, but statistically significant shift to the left in one functional response to one immunodominant peptide. Second, our analysis of RhCMV-specific CD8+ T cells was limited to those specific for IE-1 and -2. While this specificity is undoubtedly an important component of the overall RhCMV-specific response (55, 56), many other CMV proteins are targeted by CD8+ T cells (18), and the IE-1 and -2-specific CD8+ T cell response may not be representative of CD8+ T cell responses to all RhCMV proteins. However, given the equivalence of adult and old RM in the control of, and functional response to, a superphysiologic in vivo RhCMV challenge, we do not believe these caveats change our overall interpretation that cellular immunity to RhCMV is generally well preserved in old age. In this regard, it is notable that transcriptional analysis of highly purified CD8+/CD28− cells in young and elderly people revealed remarkable similarity, whereas analysis of CD8+/CD28+ populations revealed changes in the old donors (76), results which also suggest relative preservation of EM T cell integrity with age, as compared to naïve and CM T cells.
These data do not, by any means, rule out the participation of CMV infection in the pathogenesis of immune senescence in humans, but they do help refine the possible mechanisms by which such participation might occur. For example, since healthy elderly individuals may harbor CMV infection and the association between the IRP and aging mortality is not uniform across all human populations (32, 33), it is possible that other genetic or environmental predispositions might be required for failure of CMV-specific immunity or other age-associated contribution of CMV to aging pathogenesis. RM and human populations that lack such putative predispositions would lack CMV-mediated acceleration of immune senescence, but still would be susceptible to other age-related immune deterioration such as lack of thymopoiesis and loss of naïve populations (17). In addition, CMV might participate in the pathogenesis of immune senescence by mechanisms that do not require general deterioration of the CMV-specific T cell response. In this regard, chronic, non-pathogenic viral infections have been shown to reset the innate immune system in animal models, in some instances providing augmented innate immune-mediated protection against heterologous pathogens (77). This innate immune resetting may have a negative effect on immune homeostasis when maintained over long periods of time and/or when associated with other pathology such as thymic involution (78). Finally, CMV may only participate in aging pathogenesis when associated with multisystem failure, providing an additional insult to a collapsing overall physiology (75). In this scenario, dysfunction of the CMV-specific T cell response might only occur in old individuals with significant on-going disease, and would not be observed in otherwise healthy elderly subjects. As RM manifesting significant disease at time of assignment were excluded from the old RM cohort studied here, our study would not have identified such secondary failure of RhCMV-specific immunity.
The β-herpesviruses in general, and the CMV family of viruses in specific, emerged prior to mammalian radiation, and therefore, these viruses have co-evolved with their mammalian hosts, leading to an evolutionarily “negotiated” balance between the host immune system and the virus. With this balance, the virus enjoys pervasiveness in a large host population that could not occur with unchecked pathogenicity. For the host, the prevention of pathogenicity within reproductively relevant members of the population eliminates the evolutionary cost of carrying the infection. The unique high frequency of EM-biased T cell responses elicited by primate CMVs are clearly an integral part of this balance in these species, and should be considered normal and adaptive in children, adults and most elderly. This, of course, does not preclude the involvement of CMV or these protective T cell responses in disease in the elderly, a common theme in aging (7, 40), but does suggest that the mechanisms responsible for any such involvement are likely to be complex. Our data demonstrate a remarkable preservation of CMV-specific T cell responses in a primate model, arguing against a simple model in which progressive deterioration or dysregulation of these responses releases CMV from immune control in old individuals and/or otherwise contributes to immune dysfunction. Further work in both the human system and nonhuman primate models will be required to confirm or rule out other potential mechanisms linking CMV infection or CMV-specific immunity to age-related disease and mortality, and/or to define co-factors that might facilitate CMV-mediated pathogenesis in a subset of susceptible elderly individuals.
Acknowledgments
We gratefully acknowledge Nate Whizin for his cytometry assistance with data collection, and Tomi Mori for assistance with statistical analysis.
ABBREVIATIONS
- BAL
Bronchoalveolar lavage lymphocytes
- CM
central memory
- EM
effector memory
- IE
immediate early
- IRP
immune risk profile
- RM
Rhesus macaque
- RhCMV
Rhesus Cytomegalovirus
- SEB
Staphylococcus enterotoxin B
- TransEM
transitional effector memory
References
- 1.Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes Infect. 2001;3:851–857. doi: 10.1016/s1286-4579(01)01443-5. [DOI] [PubMed] [Google Scholar]
- 2.High KP. Infection as a cause of age-related morbidity and mortality. Ageing Res Rev. 2004;3:1–14. doi: 10.1016/j.arr.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 6.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 7.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21:440–445. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Kovaiou RD, Grubeck-Loebenstein B. Age-associated changes within CD4+ T cells. Immunol Lett. 2006;107:8–14. doi: 10.1016/j.imlet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102:187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 11.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 12.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–453. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 13.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 2010;184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 15.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 16.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol. 2004;39:607–613. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu H, Inui A, Sogo T, Fujisawa T, Nagasaka H, Nonoyama S, Sierro S, Northfield J, Lucas M, Vargas A, Klenerman P. Large scale analysis of pediatric antiviral CD8+ T cell populations reveals sustained, functional and mature responses. Immun Ageing. 2006;3:11. doi: 10.1186/1742-4933-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 25.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitmansour AD, Waldrop SL, Pitcher CJ, Khatamzas E, Kern F, Maino VC, Picker LJ. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J Immunol. 2001;167:1151–1163. doi: 10.4049/jimmunol.167.3.1151. [DOI] [PubMed] [Google Scholar]
- 27.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacre K, Carcelain G, Cassoux N, Fillet AM, Costagliola D, Vittecoq D, Salmon D, Amoura Z, Katlama C, Autran B. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 30.Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. doi: 10.1186/1743-422X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morunglav M, Theate I, Bertin G, Hantson P. CMV enteritis causing massive intestinal hemorrhage in an elderly patient. Case Report Med. 2010 doi: 10.1155/2010/385795. Epub2010Jul12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, Ammatuna P, Fletcher JM, Caruso C, Pawelec G. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol. 2007;42:995–1002. doi: 10.1016/j.exger.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Derhovanessian E, Maier AB, Beck R, Jahn G, Hahnel K, Slagboom PE, de Craen AJ, Westendorp RG, Pawelec G. Hallmark features of immunosenescence are absent in familial longevity. J Immunol. 2010;185:4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- 34.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 35.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wikby A, I, Mansson A, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9:299–308. doi: 10.1007/s10522-008-9138-6. [DOI] [PubMed] [Google Scholar]
- 38.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 40.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 42.Haberthur K, Engelman F, Barron A, Messaoudi I. Immune senescence in aged nonhuman primates. Exp Gerontol. 2010;45:655–661. doi: 10.1016/j.exger.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers C, Fruh K. Rhesus CMV: an emerging animal model for human CMV. Med Microbiol Immunol. 2008;197:109–115. doi: 10.1007/s00430-007-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price DA, Bitmansour AD, Edgar JB, Walker JM, Axthelm MK, Douek DC, Picker LJ. Induction and evolution of cytomegalovirus-specific CD4+ T cell clonotypes in rhesus macaques. J Immunol. 2008;180:269–280. doi: 10.4049/jimmunol.180.1.269. [DOI] [PubMed] [Google Scholar]
- 45.Kaur A, Daniel MD, Hempel D, Lee-Parritz D, Hirsch MS, Johnson RP. Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected rhesus macaques. J Virol. 1996;70:7725–7733. doi: 10.1128/jvi.70.11.7725-7733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Lippincott/The Williams and Wilkins Co; Philadelphia, PA: 2001. pp. 2675–2705. [Google Scholar]
- 48.Clarke LM, Duerr A, Feldman J, Sierra MF, Daidone BJ, Landesman SH. Factors associated with cytomegalovirus infection among human immunodeficiency virus type 1-seronegative and -seropositive women from an urban minority community. J Infect Dis. 1996;173:77–82. doi: 10.1093/infdis/173.1.77. [DOI] [PubMed] [Google Scholar]
- 49.Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis. 2010;202:1800–1803. doi: 10.1086/657412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. IL–15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Exp Gerontol. 2007;42:407–411. doi: 10.1016/j.exger.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosley RL, Koker MM, Miller RA. Idiosyncratic alterations of TCR size distributions affecting both CD4 and CD8 T cell subsets in aging mice. Cell Immunol. 1998;189:10–18. doi: 10.1006/cimm.1998.1369. [DOI] [PubMed] [Google Scholar]
- 54.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nickel P, Bold G, Presber F, Biti D, Babel N, Kreutzer S, Pratschke J, Schonemann C, Kern F, Volk HD, Reinke P. High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol. 2009;20:238–242. doi: 10.1016/j.trim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, Kern F. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, Bredt BM, McCune JM, Maino VC, Kern F, Picker LJ. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 58.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 59.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–866. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 62.Sester M, Sester U, Salvador SA, Heine G, Lipfert S, Girndt M, Gartner B, Kohler H. Age-related decrease in adenovirus-specific T cell responses. J Infect Dis. 2002;185:1379–1387. doi: 10.1086/340502. [DOI] [PubMed] [Google Scholar]
- 63.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 64.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reinke P, Prosch S, Kern F, Volk HD. Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transpl Infect Dis. 1999;1:157–164. doi: 10.1034/j.1399-3062.1999.010304.x. [DOI] [PubMed] [Google Scholar]
- 66.Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763–1779. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 67.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 68.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 69.Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, Hersperger AR, Dolfi D, Wherry EJ, Ferrari G, Betts MR. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6:e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vezys V, Masopust D, Kemball CC, Barber DL, O’Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2007;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28− CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 73.Ellefsen K, Harari A, Champagne P, Bart PA, Sekaly RP, Pantaleo G. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–3764. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 74.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lazuardi L, Herndler-Brandstetter D, Brunner S, Laschober GT, Lepperdinger G, Grubeck-Loebenstein B. Microarray analysis reveals similarity between CD8+CD28− T cells from young and elderly persons, but not of CD8+CD28+ T cells. Biogerontology. 2009;10:191–202. doi: 10.1007/s10522-008-9167-1. [DOI] [PubMed] [Google Scholar]
- 77.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 78.Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debre P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]