Abstract
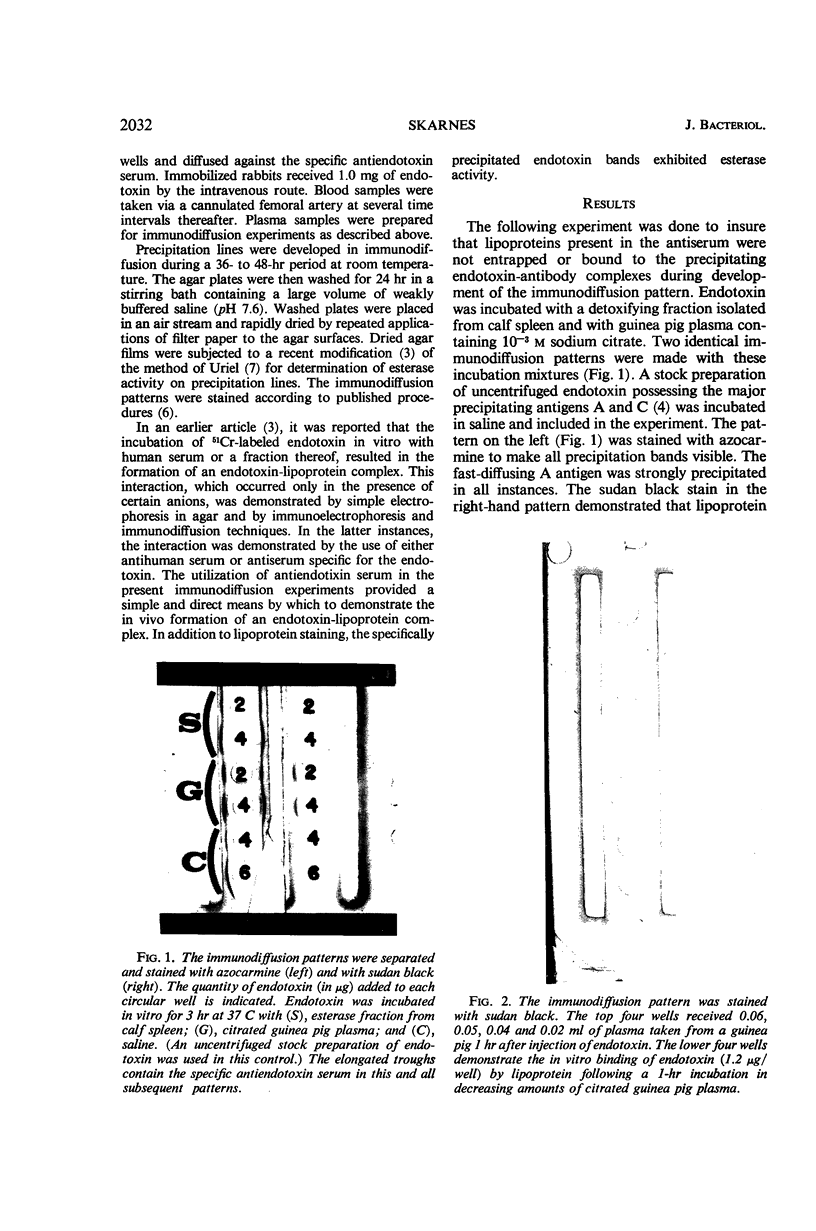
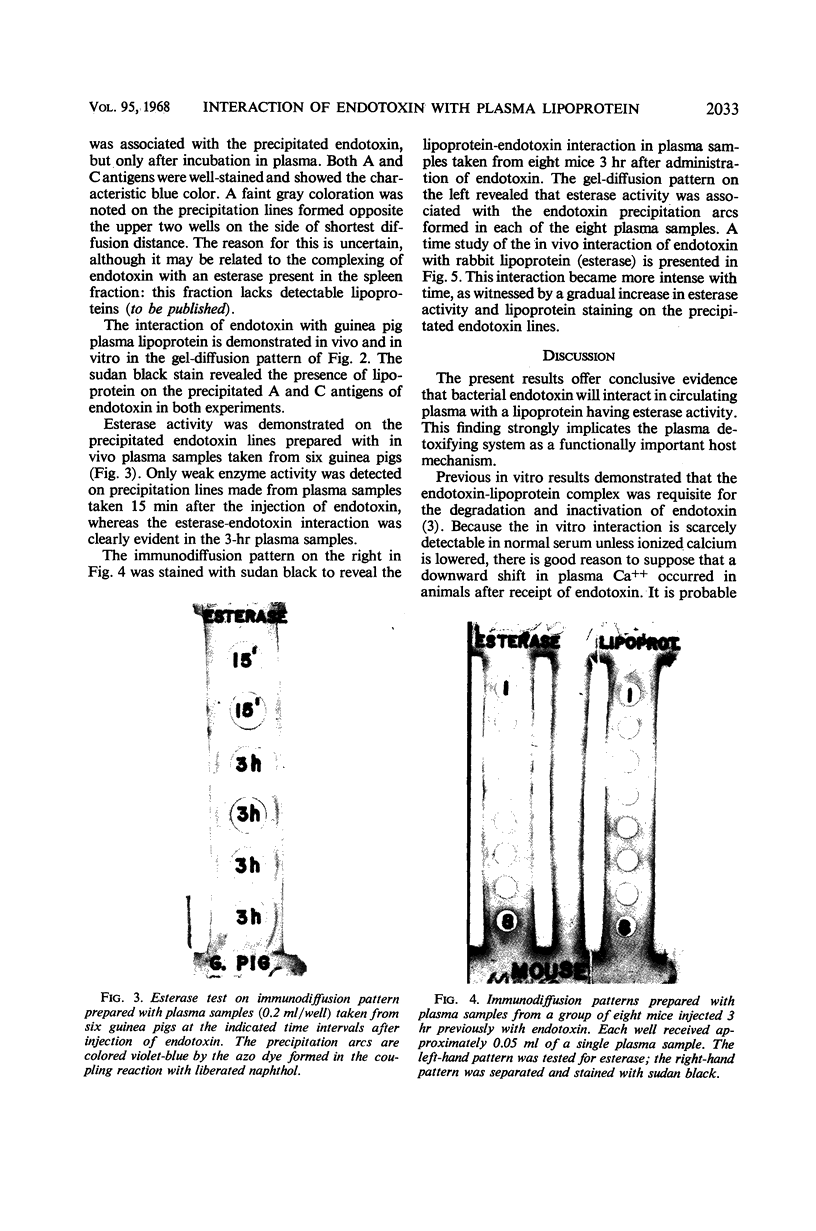
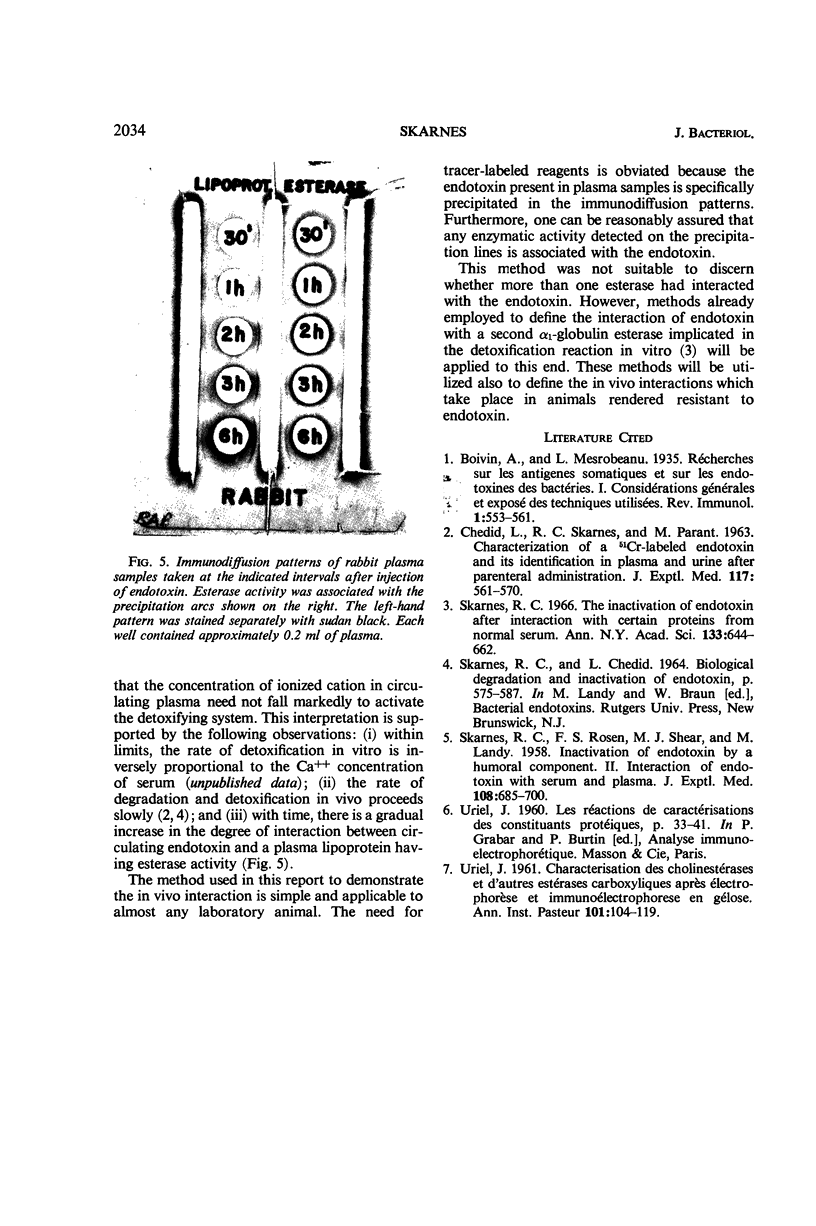
Circulating endotoxin was specifically precipitated from plasma samples withdrawn from three different animal species subsequent to parenteral injection of the toxin. Lipoprotein-positive staining and esterase activity were demonstrated on the precipitation lines formed in immunodiffusion, thus establishing the in vivo interaction of endotoxin with a plasma lipoprotein having esterase activity. Evidence was given to show that the intensity of this interaction in circulating plasma increased gradually with time. The concordance of this in vivo inter-action with the in vitro degradation and inactivation of endotoxin by plasma esterases is discussed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHEDID L., SKARNES R. C., PARANT M. Characterization of a Cr51-labeled endotoxin and its identification in plasma and urine after parenteral administration. J Exp Med. 1963 Apr 1;117:561–571. doi: 10.1084/jem.117.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKARNES R. C., ROSEN F. S., SHEAR M. J., LANDY M. Inactivation of endotoxin by a humoral component. II. Interaction of endotoxin with serum and plasma. J Exp Med. 1958 Nov 1;108(5):685–699. doi: 10.1084/jem.108.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. The inactivation of endotoxin after interaction with certain proteins of normal serum. Ann N Y Acad Sci. 1966 Jun 30;133(2):644–662. doi: 10.1111/j.1749-6632.1966.tb52395.x. [DOI] [PubMed] [Google Scholar]
- URIEL J. [Characterization of cholinesterase and other carboxylic esterases after electrophoresis and immunoelectrophoresis on agar. I. Application to the study of esterases of normal human serum]. Ann Inst Pasteur (Paris) 1961 Jul;101:104–119. [PubMed] [Google Scholar]