Abstract
The objective was to study the expression of zonula occludens-2, a tight junction protein, during preimplantation hamster embryonic development, to predict its possible localization, source, and roles in trophectoderm differentiation and blastocyst formation in this species. Comparison of zonula occludens-2 expression pattern between the hamster and mouse preimplantation embryos from the zygote up to the blastocyst stage was also an objective of this study. Zonula occludens-2 localization was noted in nuclei of blastomeres in all stages of hamster and mouse embryonic development. Compared to mice, where zonula occludens-2 was first localized in the interblastomere membrane at the morula stage, hamster embryos had membranous zonula occludens-2 localization from the 2-cell stage onwards. Based on combined results of immunolocalization study in parthenogenic embryos and ovarian and epididymal sections, and quantitative PCR done in oocytes and all developmental stages of preimplantation embryos, perhaps there was a carry-over of zonula occludens-2 proteins or mRNA from the dam to the embryo. Based on these findings, we inferred that maternally derived zonula occludens-2 was involved in nuclear functions, as well as differentiation of blastomeres and blastocoel formation during preimplantation embryonic development in the hamster.
Keywords: Zonula occludens-2, Preimplantation embryo, Oocyte, Hamster, Mouse
1. Introduction
Blastocyst formation occurs as a result of zygotic division and blastomere differentiation. The nascent blastocyst contains two committed cell subpopulations, the polarized trophectoderm (TE), and the pluriblast or inner cell mass (ICM). The establishment of these two cell lineages is critical, since the TE is required for initiation of implantation and placenta formation, whereas the ICM is required for formation of the fetus and extra-embryonic endodermal lineages. Blastomere differentiation to form the TE is a progressive process, and initiates from the 8-cell stage in the mouse conceptus. Adherens and tight junction (AJ and TJ) protein accumulation at the blastomere to blastomere contact sites from the 8-cell stage onwards is a prerequisite for normal TE formation [1–3].
Recently, we reported that the hamster conceptus became a blastocyst at the 16-cell stage, one cell cycle ahead of mice [4]. This early blastocyst formation phenomenon in hamsters motivated us to investigate TJ protein localization during zygotic development in this species. The tight junction (TJ) is the apical-most intercellular junction of the epithelial cell layer; it is responsible for maintenance of intramembranous apical-basal polarity (fence function) and creation of a physiological barrier (gate function) that limits the movements of solute and solvents in between cells. The TJ is formed when transmembrane proteins such as occludin and claudins bind to actin filaments via zonula occludens (ZO) proteins, ZO 1, ZO-2, and ZO-3 [5,6]. Since murine embryos null for ZO-2 died at gastrulation, perhaps it was not required for blastocyst formation in mice [7]. However, an siRNA-mediated ZO-2 knockdown approach in mouse embryos delayed blastocyst formation [8]; perhaps there was a ZO protein redundancy as a compensatory mechanism for blastocyst formation. However, zonula occludens-2 not only served as an organizer of TJ, but was also involved in transcriptional regulation, and cell proliferation and differentiation [6]. Similarly, blastomeres of the embryo not only undergo proliferation like their somatic brethren, but also differentiate to form TE. Gene transcription and translation for blastomere division and differentiation occur for the first time during zygotic development. Therefore, expression patterns of ZO-2 in cleaving hamster concepti were investigated in this study to determine their possible function during preimplantation embryo development.
2. Materials and methods
2.1. Eggs and embryo collection
Golden hamsters and CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and kept in a light-dark cycle (12 h light:12 h dark) in the Laboratory Animal Facility of the Vanderbilt University Medical Center (Nashville, TN, USA), with ad libitum access to water and food, according to the Institutional Guidelines on the Care and Use of Laboratory animals. The Vanderbilt University Institutional Animal Care and Use Committee approved all procedures.
Female hamsters were paired with males on the evening of proestrus for mating, except for females used for oocyte collection. The presence of sperm in vaginal secretions of hamsters the next morning indicated Day 1 of pregnancy. Female mice were caged with fertile males overnight for mating and the presence of a copulation plug the following morning indicated Day 1 of pregnancy. Oocytes and various stages of preimplantation embryos from hamsters and mice were collected by flushing oviducts or uteri with hamster embryo culture medium-9 (HECM-9), or potassium simplex optimized medium with amino acids (KSOMaa), respectively [9].
2.2. Embryo whole-mount immunofluorescence
Oocytes and embryos were fixed in cold paraformaldehyde (2%), permeabilized, preincubated with serum of the species from which the second antibody was derived, incubated with primary antiserum (a rabbit polyclonal anti-ZO-2 (cat. no. 71-1400; Invitrogen Corporation, Carlsbad, CA, USA) overnight at 4 °C, washed, and incubated again with FITC-conjugated goat anti-rabbit IgG (cat. no. 81-6111; Invitrogen Corporation) for 2 h at room temperature. Embryos were then stained with 4, 6’-diaminido-2-phenylindole, dihydrochloride (DAPI; cat. no. D9542; Sigma-Aldrich Corporation, St. Louis, MO, USA) to visualize nuclei. Non-specific staining was determined by processing embryos as described above, in the absence of primary antiserum. Embryos were mounted on a glass slide with Fluoromount G (cat. no. 0100-01; SouthernBiotech, Birmingham, AL, USA) and localization of ZO-2 was visualized under a Nikon Eclipse TE2000E inverted microscope equipped with X-Cite 120 for fluorescence and Opti-grid structured light confocal imaging system (Boyce Scientific, Inc., Gray Summit, MO, USA) [10].
2.3. Ethanol-induced parthenogenetic activation of oocyte
Hamsters were given PMSG (25 IU, ip; Sigma-Aldrich Corporation) and hCG (5 IU, ip; Sigma-Aldrich Corporation) to induce superovulation. Oocytes were collected approximately 17 h after hCG treatment, exposed to 7% ethanol for 5 min for parthenogenetic activation, and cultured overnight in HECM-9 [11,12]. Ethanol-activated oocytes containing two maternal pronuclei and cleaved to 2-cell stage embryos were processed for ZO-2 immunostaining, to determine whether nuclear ZO-2 in early embryos was maternally inherited.
2.4. Quantitative real-time polymerase chain reaction (qPCR)
Three independent batches of oocytes and various stages of developing preimplantation embryos (13 oocytes or embryos/group) were collected for isolation of total RNA, using the method previously described [10]. Total RNA from each batch was reverse transcribed with oligo-dT primer, and the RT-derived cDNA was then subjected to qPCR (LightCycler 2.0, Roche Diagnostics, Indianapolis, IN, USA). The Tjp2 gene specific primers were designed based on mouse Tjp2 mRNA sequence (GenBank accession number # NM_011597; forward: 5′-CCA TGG GCG CGG ACT ATC TGA-3′; reverse: 5′-CTG TGG CGG GGA GGT TTG ACT TG-3′). Primers used to detect the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (Hprt1; GenBank accession number NM_013556, forward: 5’-GCT TGC TGG TGA AAA GGA CCT CTC GAAG; reverse: CCC TGA AGT ACT CAT TAT AGT CAA GGG CAT-3’) were described by Mamo and coworkers [13]. Amplification products of Tjp2 and Hprt1 in control tissues were gel-purified, quantified (2100 BioAnalyzer; Agilent Technologies, Palo Alto, CA, USA), and serial 10-fold dilutions (10 pg to 0.001 fg) were made for use as standards in each real-time PCR analysis. FastStart Taq polymerase and SYBR green core mix (Roche Diagnostics Corporation) reactions were initially run to optimize primer and template concentrations, magnesium concentration and resolve melting curve artifacts. Quantification of Tjp2 expression was then determined in relation to serial dilutions of target sequence standards and normalized to Hprt1 expression (as a loading control). The reaction conditions were template denaturation at 95 ºC for 10 min followed by 45 cycles of 95 ºC for 10 s, 60 ºC for 5 s, and 72 ºC for 15 s. Results were analyzed with the LightCycler Software version 3.3 and GraphPad Prism 4 (GraphPad Software, La Jolla, CA, USA). Comparison among groups was made by Kruskal-Wallis one-way ANOVA and Dunn’s post-hoc test for multiple comparisons (P<0.05).
3. Results
3.1. Membranous, but not nuclear, expression of ZO-2 was stage- and species-specific
Evenly scattered dot-like ZO-2 protein localization was observed in the cytoplasm and membrane of unfertilized hamster eggs (Fig. 1). Dot-like ZO-2 localization was still noticed in the cytoplasm and surface membrane of the zygote, but at a reduced level. Surprisingly, both pronuclei of the zygote had ZO-2 expression. This nuclear ZO-2 staining was also observed in all blastomeres from 2-cell to blastocyst stages. Nuclear ZO-2 expression was not random; rather its expression appeared organized into discrete domains. In addition, ZO-2 was also localized at the membrane regions between adjacent blastomeres from the 2-cell stage onwards. At the blastocyst stage, whereas all blastomere nuclei had the speckled pattern of ZO-2, only outside TE cells had membranous localization of ZO-2 (Fig. 1). This nuclear and membranous staining of ZO-2 was specific, since no immunostaining was observed when zygotes and 2-cell stage embryos were incubated with secondary antibody in the absence of primary antibody (Fig. 1). Since both nuclear and membranous stainings of ZO-2 were observed in developing preimplantation hamster embryos, its expression was studied in preimplantation murine embryos. Nuclear ZO-2 expression pattern in developing mouse embryos was very similar to that observed in hamsters (Fig. 2). Zonula occludens-2 protein expression was noticed in nuclei of all blastomeres at all stages of mouse embryonic development. The striking difference in ZO-2 localization between the mouse and hamster was that ZO-2 localization in the membrane was not observed until the morula stage in mice (Fig. 2).
Fig. 1.
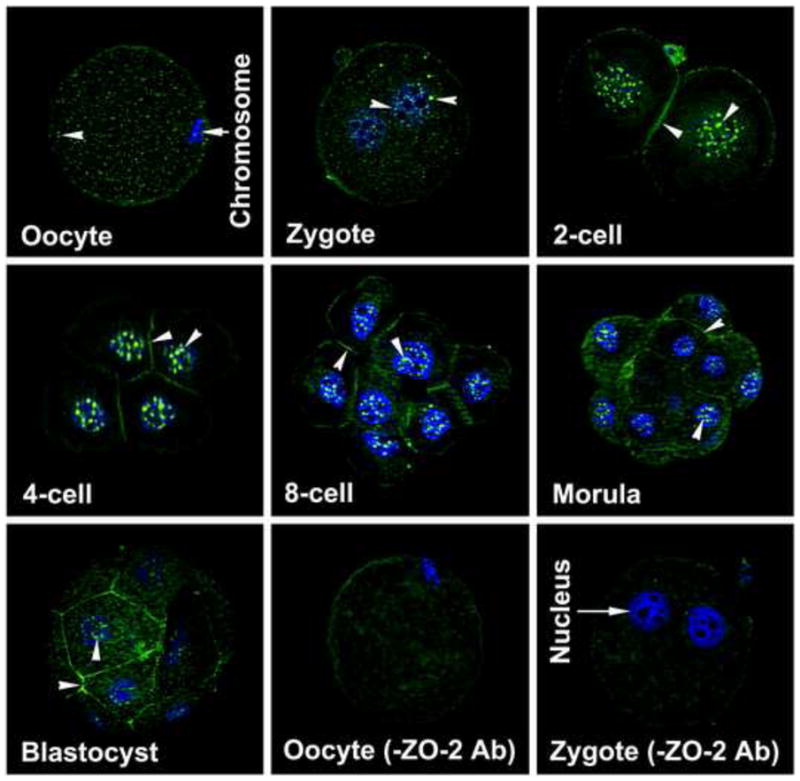
Localization of ZO-2 protein (FITC-labeling) in oocytes and all developing stages of preimplantation hamster embryos. These experiments were repeated at least three times, using 5 to 7 oocytes or embryos/experiment/stage. The nuclei were stained with DAPI. Nuclear and membranous localization of this protein is indicated by an arrow head.
TE, trophectoderm; ICM, Inner Cell Mass
Fig. 2.
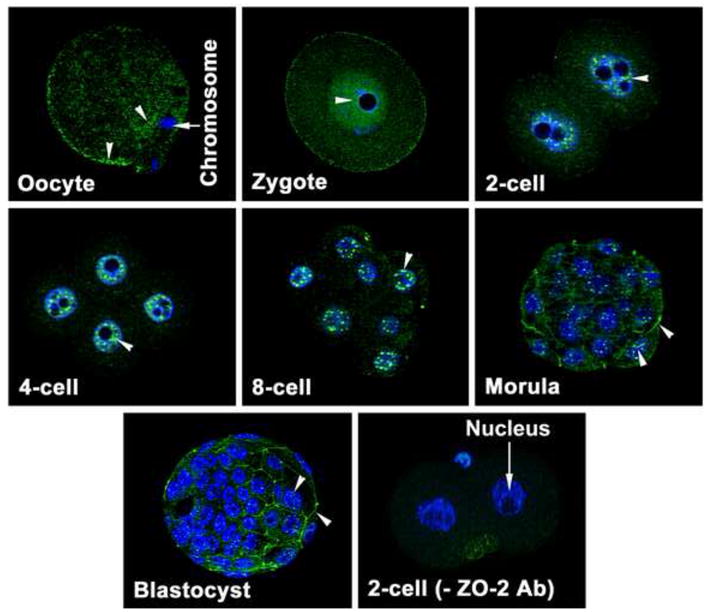
Localization of ZO-2 protein (FITC-labeling) in oocytes and all developing stages of preimplantation murine embryos. These experiments were repeated at least three times, using 5 to 7 oocytes or embryos/experiment/stage. The nuclei were stained with DAPI. Nuclear and membranous localization of this protein is indicated by arrow head.
TE, trophectoderm; ICM, Inner Cell Mass
3.2. ZO-2 could be parental or an early embryonic product
Since ZO-2 protein was present in unfertilized oocytes and fertilized zygotes of both species, we inferred that ZO-2 may have been residual parental product. Therefore, ZO-2 localization was examined in hamster and mouse ovarian follicles containing oocytes, and the distal part of the hamster epididymis containing sperm. As shown (Fig. 3), ZO-2 was localized in the oocyte plasma membrane and the germinal vesicle (nucleus). The germinal vesicle contained dot-like expression of ZO-2. In the epididymis, luminal epithelial cells were positive for ZO-2 staining, whereas ZO-2 expression was not noticed in sperm (Fig. 3). Following parthenogenetic activation, both pronuclei and nuclei of activated oocytes and 2-cell stage embryos, respectively, contained the speckled pattern of ZO-2 (Fig. 4). In addition, ZO-2 staining was present at the membrane regions between two blastomeres in parthenogenetically activated 2-cell stage embryos (Fig. 4).
Fig. 3.
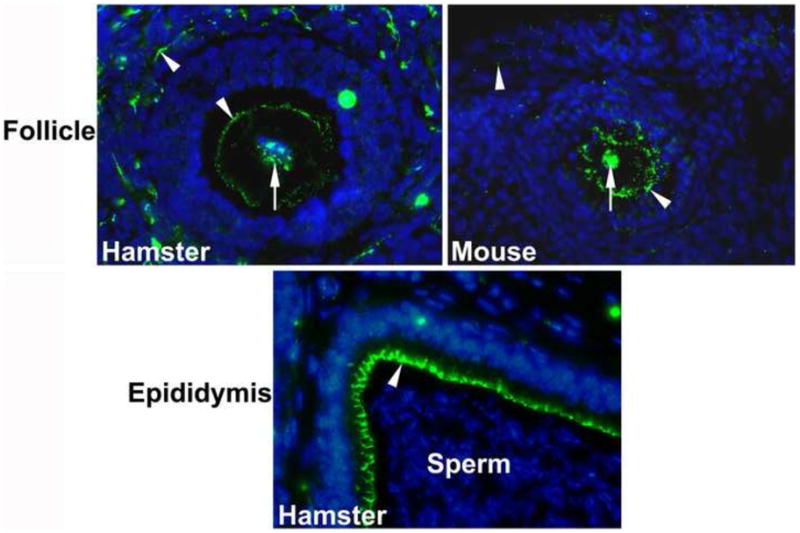
Immunolocalization of ZO-2 in sections from the epididymis and ovary. The nuclei were stained with DAPI. Nuclear and membranous localization of this protein are indicated by and arrow and arrow head, respectively.
Fig. 4.
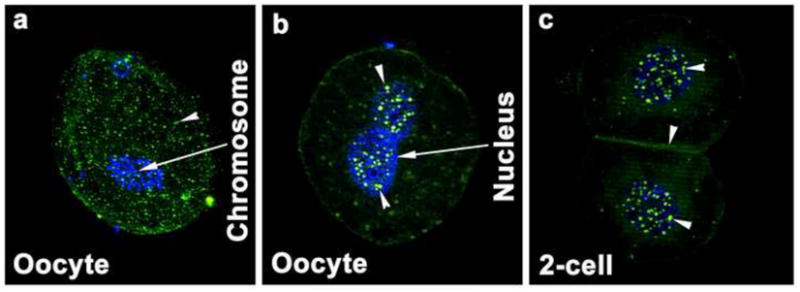
Localization of ZO-2 protein (FITC-labeling) in inactivated (a) and parthenogenetically activated oocytes (b) and 2-cell stage embryos (c). These experiments were repeated three times, using at least 10 oocytes. The nuclei were stained with DAPI. Nuclear and membranous localization of this protein is indicated by an arrow head.
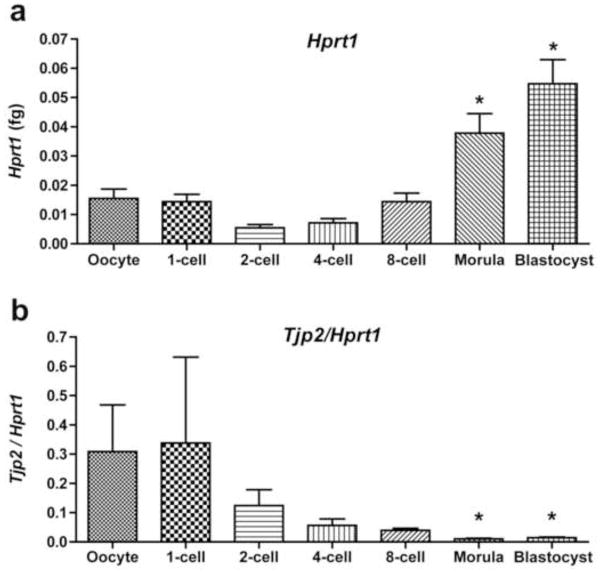
For further confirmation of maternal and/or embryonic origin of ZO-2, qPCR were performed on eggs and preimplantation stages of hamster embryos. Messenger RNA expression of the housekeeping gene Hprt1 (Fig. 5a) was performed for normalization of Tjp2 expression in eggs and embryos. Maximum levels of Tjp2 mRNA were present in the oocyte and zygote. Normalization of Tjp2 values to Hprt1 expression demonstrated a gradual decline of Tjp2 transcription from the egg and the 1-cell stage onwards (Fig. 5b). This was due to variability in the level of expression of Tjp2 and gradual increase of Hprt1 at various embryonic developmental stages, which were marked by progressive increases in cell numbers.
Fig. 5.
Relative Tjp2 mRNA expression profiles (b) detected by qPCR in the in vivo-derived hamster oocytes and embryos. These experiments were repeated three times. Tjp2 levels were normalized against Hprt1 (a). Bars are mean ± SEM.
* P<0.05 compared to 2-cell or 4-cell stage (a) or oocyte, or 1-cell stage embryos (b).
4. Discussion
In the present study, nuclear ZO-2 was localized in the oocyte and blastomere of all developmental stages of preimplantation embryos of hamsters and mice. In addition to its nuclear localization, ZO-2 in hamster embryos was also present in the membrane regions of two adjacent blastomeres from the 2-cell stage and onwards, whereas membranous localization of ZO-2 began from the morula stage onwards in mice. Therefore, we inferred that ZO-2 may participate in regulation of nuclear functions, in addition to its involvement in TJ formation between blastomeres.
Based on the presence of ZO-2 protein in the follicular oocyte of hamsters and mice, parthenogenetically activated oocyte and embryos of hamsters, and the abundance of Tjp2 transcripts in the oocyte and zygote, we inferred that ZO-2 could be a product of maternally inherited or newly synthesized transcripts of the zygote. In this regard, there are reports that 1-cell embryos were transcriptionally active [14]. Since sperm present in the distal part of the epididymis were not positive for ZO-2 staining, the male pronuclear ZO-2 might be either sequestered from the ooplasm, or synthesized after fertilization. It was suggested that transcription of paternal genes occurred in 1-cell stage embryos [15]. Embryonic ZO-2 protein synthesis from the 2-cell to blastocyst stages was questionable, since qPCR results in the present study had a gradual decrease in normalized Tjp2 mRNA expression pattern from the 2-cell stage onwards. Thus, it could be predicted that maternal or early embryonic ZO-2 protein persisted in the blastomere throughout zygotic development to the blastocyst stage; this could also account for normal development of preimplantation embryos to the blastocyst stage in the absence of embryonic ZO-2 [7].
Nuclear localization of ZO-2 in preimplantation embryos of hamsters was not surprising, since nuclear localization of ZO-2 had been documented in mouse embryos [8] and in sparse culture of both epithelial and endothelial cells [16]. Incorporation of ZO-2 inside the nucleus might have occurred due to the presence of nuclear localization and exportation signals in its sequence [17]. Several reports have suggested that specked nuclear ZO-2 is colocalized with splicing factor SC-35 and SAF-B [16,18]. Similarly, there are also reports that in the nucleus ZO-2, was associated with nuclear matrix and transcription factors such as JUN, FOS, and C/ERB [19,20]. Thus, based on the presence of ZO-2 in the nucleus of all blastomeres of all preimplantation developmental stages, including the inside and outside cells of the morula and the cells of the TE and pluriblast, we inferred that it may be involved in some common nuclear functions, e.g., chromatin reorganization, splicing of mRNAs, and gene transcription.
It has been reported that ZO-2 was localized in the nucleus when epithelial cells do not make tight contact in culture. However, at confluent culture, ZO-2 localized primarily in the plasma membrane [16]. Early development of murine zygotes could be very similar to sparse epithelial cell culture models, in that blastomeres of the embryo from the 2-cell stage to the early 8-cell stage loosely adhered to each other and were easily disaggregated [21]. Thus, ZO-2 localization only in nuclei of murine embryos from the 2- to 8-cell stages was not necessarily surprising. However, ZO-2 localization not only in the nucleus, but also in the interblastomere membranes from the 2-cell to the 8-cell stage in hamsters, was unexpected. Perhaps membrane assembly of ZO-2 initiated much tighter cell-cell adhesion from the 2-cell stage in hamster embryos than mouse embryos, where it began after the 8-cell stage. We inferred from this study that the beginning of accumulation of TJ proteins for possible TJ formation began from the 2-cell stage onwards in hamsters. There was no prior evidence of TJ formation in embryos so early in their developmental stage in any mammalian species, although there was one report of the presence of TJ in 6-cell human embryos [22]. Taken together, we speculated that embryos of different species have different adaptability for cellular adhesion and differentiation mechanisms.
In contrast to symmetrical early embryonic development, polarization of blastomeres begins at the late 8-cell stage of mouse embryos [1]. Division then takes place, such that at the 16-cell stage, daughter blastomeres that retain the polar properties of parental cells remain on the surface surrounding the daughter cells that lost their parental polar properties [23]. This inside-outside cell position was critical for blastocyst formation, since outside cells acquire the properties of epithelial cells to protect the inside pluriblast. The process of polarization of blastomeres from the 8-cell stage was associated with the beginning of formation of AJs and TJs in mouse embryos. Accumulation of ZO-1 α+ at interblastomere membranes has been reported [24]. In the present study, onset of ZO-2 protein accumulation at interblastomere membranes occurred from the morula stage of mouse embryos. Therefore, we inferred that like ZO-1, ZO-2 was also involved in blastomere polarization and TJ formation in the later stages of mouse embryonic development. However, at the morula and blastocyst stage of both species, membranous localization of ZO-2 was only noticed in outside cells. This cell-specific membranous expression of ZO-2 at the morula and the blastocyst stage may depend on simple cell positioning, or up- and down-regulation of unknown embryonic factor(s) from both cell lineages. Since ZO-2 in hamster embryos was inherited by daughter cells from their parental cells, perhaps early AJ formation induces shuttling of nuclear protein to the membrane for the initiation of TJ formation. In this regard, it has been demonstrated that AJ formation helps to form the TJ by accumulating ZO-1 at the AJ [5,25]. There are reports that both ZO-1 and ZO-2 can bind to the AJ protein α-catenin [5].
The blastocyst stage of the mammalian embryo is composed of the TE, ICM, and the blastocoel. Formation of the blastocoel allows separation of the TE and the ICM. Molecular mechanisms involved in the formation, expansion and maintenance of the blastocoel are not completely understood. Since water is the major component of the blastocoelic fluid, the involvement of several membrane transporters such as Na/K-ATPases [3,26], Na/H-exchangers [27] and aquaporins [28,29] has been proposed. However, accumulation of fluid in the blastocyst also requires a means of regulating egress of this fluid out of the blastocoel. In this regard, it has been demonstrated that a permeability barrier function of TJs among TE cells was required to maintain the integrity of the blastocoel cavity [9,30]. Thus, based on membrane assembly of ZO-2 protein in outside cells of the morula and blastocyst stage hamster embryo, we inferred its involvement in formation of TJs that regulate paracellular transport of fluid. Since ZO-1, ZO-2, and ZO-3 null mouse embryos developed to the blastocyst stage [7,31], either these genes were not critical for blastocoel formation, or the absence of one ZO gene was compensated by other ZO members. However, depletion of ZO-2 using ZO-2 siRNAs delayed mouse blastocyst formation in vitro [8]. Perhaps ZO-2 provided a supporting rather than a requisite role in regulation of fluid transport and accumulation during blastocyst formation, in addition to other ZO proteins (e.g. ZO-1 and ZO-3).
In conclusion, we demonstrated that ZO-2 was expressed in the nuclei of all embryonic blastomeres of both the hamster and mouse. However, while ZO-2 accumulated in the membrane regions of two adjacent blastomeres in hamster embryos from the 2-cell stage and onwards, its localization was not observed in the membrane of blastomeres until the morula stage in mice. We also demonstrated that hamster embryonic ZO-2 was a maternally-derived protein. Since ZO-2 was localized in all nuclei of blastomeres at all stages of embryonic development, its function may be to balance nuclear functions for cell division and differentiation .The accumulation of ZO-2 in interblastomere membranes from the 2-cell stage onwards in hamster embryos may be involved in initiating early biogenesis of TJ for the emergence of polar epithelial cells at the embryonic surface that represents the first cell diversification, and formation of the epithelial trophectoderm and the blastocoel. The current findings supported our earlier finding that blastocyst formation occurred at the end of the 4th cleavage division in hamsters, as compared to the 5th cleavage division in mice [4].
Acknowledgments
This work was supported by National Institutes of Health grants HD044741 and UO-1 HD042636
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson MH, McConnell JM. Lineage allocation and cell polarity during mouse embryogenesis. Semin Cell Dev Biol. 2004;15:583–97. doi: 10.1016/j.semcdb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–48. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 3.Watson AJ, Barcroft LC. Regulation of blastocyst formation. Front Biosci. 2001;6:D708–30. doi: 10.2741/watson. [DOI] [PubMed] [Google Scholar]
- 4.Reese J, Wang H, Ding T, Paria BC. The hamster as a model for embryo implantation: insights into a multifaceted process. Semin Cell Dev Biol. 2008;19:194–203. doi: 10.1016/j.semcdb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartsock A, Nelson WJ. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matter K, Balda MS. Functional analysis of tight junctions. Methods. 2003;30:228–34. doi: 10.1016/s1046-2023(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669–78. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheth B, Nowak RL, Anderson R, Kwong WY, Papenbrock T, Fleming TP. Tight junction protein ZO-2 expression and relative function of ZO-1 and ZO-2 during mouse blastocyst formation. Exp Cell Res. 2008;314:3356–68. doi: 10.1016/j.yexcr.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wang H, Matsumoto H, Roy SK, Das SK, Paria BC. Dual source and target of heparin-binding EGF-like growth factor during the onset of implantation in the hamster. Development. 2002;129:4125–34. doi: 10.1242/dev.129.17.4125. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Ding T, Brown N, Yamamoto Y, Prince LS, Reese J, Paria BC. Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse. Dev Biol. 2008;318:112–25. doi: 10.1016/j.ydbio.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Seshagiri PB. Heparin binding-epidermal growth factor improves blastocyst hatching and trophoblast outgrowth in the golden hamster. Reprod Biomed Online. 2000;1:87–95. doi: 10.1016/s1472-6483(10)61945-1. [DOI] [PubMed] [Google Scholar]
- 12.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2003. [Google Scholar]
- 13.Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC Dev Biol. 2007;7:14–25. doi: 10.1186/1471-213X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005;283:40–57. doi: 10.1016/j.ydbio.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Anzai M, Nakagata N, Takahashi A, Takahashi Y, Miyata K. Onset of paternal gene activation in early mouse embryos fertilized with transgenic mouse sperm. Mol Reprod Dev. 1994;39:136–40. doi: 10.1002/mrd.1080390203. [DOI] [PubMed] [Google Scholar]
- 16.Islas S, Vega J, Ponce L, Gonzalez-Mariscal L. Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp Cell Res. 2002;274:138–48. doi: 10.1006/excr.2001.5457. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Mariscal L, Ponce A, Alarcon L, Jaramillo BE. The tight junction protein ZO-2 has several functional nuclear export signals. Exp Cell Res. 2006;312:3323–35. doi: 10.1016/j.yexcr.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem. 2003;278:2692–2700. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- 19.Jaramillo BE, Ponce A, Moreno J, Betanzos A, Huerta M, Lopez-Bayghen E, Gonzalez-Mariscal L. Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells. Exp Cell Res. 2004;297:247–58. doi: 10.1016/j.yexcr.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res. 2004;292:51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 21.de Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–45. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 22.Dale B, Gualtieri R, Talevi R, Tosti E, Santella L, Elder K. Intercellular communication in the early human embryo. Mol Reprod Dev. 1991;29:22–8. doi: 10.1002/mrd.1080290105. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- 24.Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–37. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- 25.Eckert JJ, Fleming TP. Tight junction biogenesis during early development. Biochim Biophys Acta. 2008;1778:717–28. doi: 10.1016/j.bbamem.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33:492–504. doi: 10.1002/mrd.1080330417. [DOI] [PubMed] [Google Scholar]
- 27.Kawagishi R, Tahara M, Sawada K, Moriguchi K, Sakata M, Tasaka K, Murata Y. Na+/H+ exchanger-3 is involved in mouse blastocyst formation. J Exp Zool. 2004;301A:767–75. doi: 10.1002/jez.a.90. [DOI] [PubMed] [Google Scholar]
- 28.Barcroft LC, Offenberg H, Thomsen P, Watson AJ. Aquaporin proteins in murine trophectoderm mediate transepithelial water movements during cavitation. Dev Biol. 2003;256:342–54. doi: 10.1016/s0012-1606(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 29.Richard C, Gao J, Brown N, Reese J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrin. 2003;144:1533–41. doi: 10.1210/en.2002-0033. [DOI] [PubMed] [Google Scholar]
- 30.Moriwaki K, Tsukita S, Furuse M. Tight junctions containing claudin 4 and 6 are essential for blastocyst formation in preimplantation mouse embryos. Dev Biol. 2007;312:509–22. doi: 10.1016/j.ydbio.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S. Deficiency of zonula occludens-1causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–75. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]