Abstract
Purpose of review
As genetic testing for long QT syndrome (LQTS) has become readily available, important advances are being made in understanding the exact link between ion channel mutation and observed phenotype. This paper reviews recent findings in the literature.
Recent findings
Congenital LQTS is an important cause of sudden cardiac death. To date, 12 genes have been identified as the cause of congenital LQTS. With increasing availability of genetic testing, subtype-specific management of LQTS has become the standard of care. Detailed correlative studies between LQTS mutations and clinical phenotypes are leading the field towards ‘mutation-specific’ management within LQTS subtypes. A clear link between the distinct functional/biophysical defect in each LQT mutation and disease phenotype is complicated by the variable penetrance and pleiotropic expression of clinical phenotype. This is especially evident with the overlap syndrome now documented for several sodium channel (SCN5A) mutations.
Summary
The management of LQTS has become subtype-specific due to the availability of genotype information. Review of recent literature suggests that ‘mutation-specific’ management is possible based upon distinct functional/biophysical characteristics of mutations within each LQT gene. Further research is required to clearly delineate the molecular and cellular mechanisms underlying variable penetrance, and pleiotropic expression of LQTS mutations.
Keywords: genetics, ion channels, long QT syndrome, mutation-specific management, sudden cardiac death
Introduction
Long QT syndrome (LQTS) is a rare (1:2500–1:10000) inherited disorder that is associated with an increased propensity to arrhythmogenic syncope, polymorphous ventricular tachycardia, and sudden cardiac death [1]. LQTS was probably first reported in 1856 by Meissner [2], who described a deaf girl who collapsed and died while being publicly reprimanded at school. Jervell and Lange-Nielsen [3] provided the first complete description of congenital LQTS in 1957; they reported a family with four deaf-mute children with fainting spells, sudden death, and prolonged QT intervals (Jervell Lange-Nielsen syndrome or JLN). Romano et al. [4] and Ward [5] independently reported patients with autosomal dominant cardiac disorder identical to JLN, but without the deafness (Romano–Ward syndrome or RWS). In the contemporary literature, RWS is used interchangeably with LQTS. Our understanding of this disorder changed dramatically in the 1990s, when three genes were identified to be linked to autosomal dominant forms of LQTS: KvLQT1 (KCNQ1), human ether-à-go-go related gene (hERG) (KCNH2), and SCN5A [6]. Consistently with the autosomal recessive inheritance, JLN was subsequently found to be a homozygous mutation of KvLQT1 and subsequently KCNE1 [7].
These and subsequent findings established LQTS as a heterogeneous congenital cardiac channelopathy, a disease caused by mutations in genes coding for cardiac ion channel subunits or channel-associated proteins [1]. In this manuscript, we will provide an overview of congenital LQTS as well as reviewing the major advances over recent years. Acquired LQTS will not be extensively discussed.
LQTS is associated with delayed repolarization of ventricular cells in the heart detected as abnormally long QTc [heart rate (HR) corrected QT] intervals on ECG. The details of the ionic currents responsible for cellular cardiac action potentials have been reviewed elsewhere [8]. QTc/action potential duration prolongation can arise from either a decrease in repolarizing current (potassium channel current) or an increase in excitatory membrane current (sodium or calcium channel currents or both) during the action potentials plateau phase. Most commonly, QTc prolongation is produced by delayed repolarization due to loss of function of cardiac potassium (K+) currents, IKr or IKs [9]. Less commonly, QT prolongation results from prolonged depolarization due to an increase in inward current (via inactivation disruption), carried by either the principal cardiac sodium channel (Nav1.5, INa) or L-type calcium (Ca2+) channels (Cav1.2, ICa,L) [10,11].
Long QT syndrome genetics
Congenital LQTS currently is associated with mutations in 12 different genes, with the majority of the known mutations located in the first three: LQT1 (KCNQ1) mutations account for 42–45% of genetically positive LQTS, LQT2 (KCNH2) for 35–45%, and LQT3 (SCN5A) for 8–10% [12,13]. All of the LQT genes except LQT4, LQT9, LQT11, and LQT12 code for ion channel subunits. LQT4 (ANKB or ANK2) encodes a structural protein, ankyrin-B, which anchors ion channels to specific domains in the plasma membrane [14]. LQT9 (CAV3) is a gene encoding caveolin 3, a membrane scaffolding protein that interacts with a variety of signaling proteins including sodium channels [15]. LQT11 (AKAP9 or yotiao) encodes an A-kinase anchoring protein, shown to be an integral part of the IKs macromolecular complex, whose presence is necessary for the physiological response of the IKs channel to β-adrenergic stimulation [16,17]. LQT12 (SNTA1) codes for α-1 syntrophin, known to associate with the Nav1.5 cardiac sodium channel as part of the neuronal nitric oxide synthase complex that appears to regulate ion channel function [18••]. Other than LQT7 and LQT8, which are part of multisystemic discorders, Andersen–Tawil (periodic paralysis, dysmorphic features, and long QTU) [19,20] and Timothy syndromes (autism, syndactyly, and LQT) [21], all LQTS genes appear to be functionally related to three cardiac ion currents: IKs, IKr, and INa. Dysfunction of these three ionic currents, caused by either channel subunit or accessory protein mutations, appears to be the ‘final common pathways’ for LQTS (Table 1).
Table 1.
Long QT subtypes classified by final common pathways
| Current | Long QT subtype |
Genes | Protein | Function |
|---|---|---|---|---|
| IKs | LQT1 | KCNQ1 | Kv7.1 | α-Subunit IKs |
| LQT5 | KCNE1 | minK | β-Subunit IKs | |
| LQT11 | AKAP9 | Yotiao | Regulatory adaptor IKs |
|
| IKr | LQT2 | KCNH2 | Kv11.1 | α-Subunit IKr |
| LQT6 | KCNE2 | MiRP1 | β-Subunit IKr | |
| INa | LQT3 | SCN5A | Nav1.5 | α-Subunit INa |
| LQT4 | ANK2 | Ankyrin B | Adaptor INaK INa–Ca, INa |
|
| LQT9 | CAV3 | M-Caveolin | Adaptor INa | |
| LQT10 | SCN4B | Navb4 | β-Subunit INa | |
| LQT12 | SNTA1 | a-1-Syntrophin | Scaffolding protein INa |
|
| Ik1 | LQT7 | KCNJ2 | Kir2.1 | α-Subunit IK1 |
| ICaL | LQT8 | CACNA1C | Cav1.2 | α-Subunit ICaL |
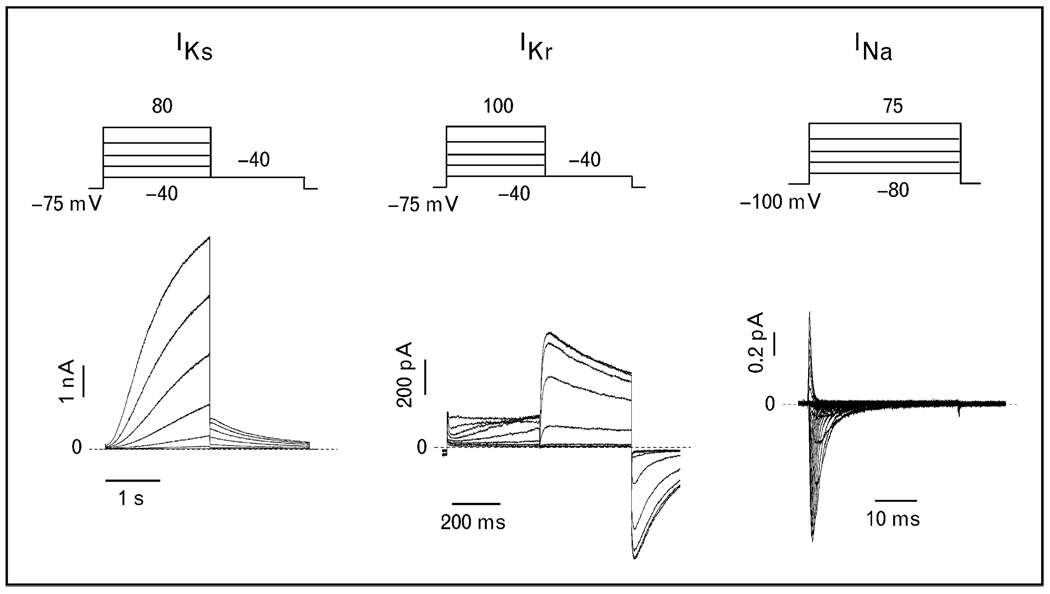
Depending on the stringency of clinical phenotype assessment, the yield for positive genetic results in LQTS ranges from 50 to 78% [12,13]. The cohorts with longer average QTc intervals and more symptoms have a higher yield of clinical testing [22]. Interestingly, about 10% of gene-positive LQTS had multiple mutations in the tested genes [13]. This leaves at least 22% of clinically positive LQTS without a genetic explanation. We believe that studies of phenotypically robust, but genotype-negative, LQTS patients will yield additional LQTS genes and novel insights into the fundamental mechanism of cardiac ion channel physiology. However, additional LQTS subtypes will likely each account for only a minute percentage of the total congenital LQTS population, and point to interacting proteins and modifiers that affect the final common pathways: IKs, IKr, and INa (Fig. 1).
Figure 1. Long QT syndrome common final pathway current traces IKs, IKr, and INa recorded using patch clamp studies.
Stimulation protocols are indicated above the current traces.
Gene/mutation-specific arrhythmia risk factors and management
The discovery that distinct LQTS variants are associated with genes coding for different ion channel subunits has had a major impact on the diagnosis, analysis, and treatment of LQTS patients. Clinical data have revealed distinct risk factors associated with the different LQTS genotypes, and that genotypic information must be taken into account during patient evaluation, diagnosis, and management. Current prophylactic and preventive therapy for LQTS to reduce the incidence of syncope and sudden death include β-blockers, implanted defibrillators, and, less frequently, left cervicothoracic sympathetic ganglionectomy [23]. The utility of sodium channel blockers such as flecainide, mexilitine, and more recently ranolazine in LQT3 is being actively investigated [24,25•]. It is becoming clear that the efficacies of the therapies are variable according to the LQTS genotype [26]. With the increasing availability of LQTS genetic testing, gene-specific patient management is now generally considered standard of care and information regarding mutation-specific risk stratification and therapy is rapidly evolving [23]. Given the rarity of patients with LQT4–12, extensive genotype–phenotype correlation studies have not been performed. Until such data become available, it is logical to manage patients according to their affected currents. This review is thus organized according to the affected final common pathway currents IKs, IKr, and INa.
IKs
The LQT variants where IKs is dysfunctional include LQT1, LQT5, and LQT11; the majority are of the LQT1 variant. Homozygous mutations of KCNQ1 and KCNE1 are the genetic basis for JLN, where affected patients are also deaf [7]. LQT1 patients are at greatest risk for cardiac events during exercise or conditions associated with elevated sympathetic nerve activity [26]. β-Blocking drugs, despite minimal effects on the QTc interval, are associated with a significant reduction in cardiac events and are considered the first-line treatment in LQTS. Critical evaluation of genotyped LQTS patients treated with β-blockers has made it clear that patients with LQT1 subtype derive the most significant benefit, whereas this treatment is less effective for LQT2 and neutral for LQT3 patients [27].
Most insights into the causal effects of mutations affecting IKs have generally revealed mutation-dependent loss of function and loss of responsiveness to adrenergic regulation. However, two studies also have addressed ‘mutation–phenotype’ correlation in KCNQ1 mutations. In a first study by Zareba et al. [28] mutations were classified by structural localization within the ion channel (prepore, pore, and postpore); however, no significant difference was found in ECG characteristics, cardiac events, and response to β-blockade according to the location of the mutations. In a second, larger study by Moss et al. [29] in which LQT1 mutations were classified by location (N-terminus, C-terminus, and transmembrane), mutation type (missense vs. nonmissense), and biophysical function (haploinsufficiency vs. dominant negative), the transmembrane location, missense mutation, and dominant negative biophysical profiles were found to be independent predictors of higher frequency of cardiac events. This study contributes to building clinical predictors of disease severity based on mutation and biophysical properties, thus representing an important step towards mutation-specific management of LQTS.
IKr
The LQT variants where IKr is dysfunctional include LQT2 and LQT6; the majority are of LQT2. It is worth noting that unintended inhibition of IKr accounts for the majority of the drug-induced LQTS [30]. Cardiac events in LQT2 are associated with arousal, conditions in which patients are startled, or both [26]. More than 200 putative disease-causing mutations have been identified for KCNH2; most appear to disrupt the maturation and trafficking process, thereby reducing the number of functional ion channels at the cell surface membrane [31]. In a report from the International LQTS Registry [32], KCNH2 mutations were classified according to pore vs. nonpore localization; patients with pore mutations experienced a higher frequency of arrhythmia-related cardiac events at an earlier age than did patients with nonpore mutations. A larger, more recent study [33••], which combined KCNH2 patients and mutations from three LQTS registries, found that in missense mutations the location of mutation significantly influenced the clinical phenotype, whereas the risk of nonsense mutations did not appear to be modulated by the location of the mutation. This was the first demonstration of the location–type interaction for the production of clinical phenotype.
Despite structural similarities between the two six-transmembrane spanning potassium ion channels KCNQ1 and KCNH2, the level of risk conferred by mutation location-type appears to be highly specific and cannot be extrapolated from one potassium channel to another. These studies tell us that LQT mutations are not created equal, and it is necessary to go beyond the simple classification of LQT subtypes for more accurate risk stratification and treatment.
INa
LQT variants with dysfunctional INa include LQT3, LQT9, LQT10, and LQT12, with the majority of patients having LQT3 (SCN5A) mutations. The greatest contrast in LQT subtype risk factors can be seen by comparing LQT3 with LQT1 patients. In SCN5A mutation carriers, risk of cardiac events is highest during rest (bradycardia) when sympathetic activity is low. As might be expected, treatment with β-blockers does not appear to afford LQT3 patients much benefit [26]. In fact, it is surprising that mimicking the LQT3 risk condition by β-blockade is not more harmful. It is now clear that genotype–phenotype correlation studies of SCN5A mutations are complicated by the fact that SCN5A mutations are thought to be causal in other arrhythmia syndromes, including Brugada syndrome, sinus node dysfunction, and conduction disease [34–36]. Previously, the SCN5A arrhythmia syndromes have been considered clinical entities distinguishable by the biophysical profiles of the mutated channels. In general, most LQT3 mutations disrupt channel inactivation and cause abnormal sustained sodium current (INaL) during the action potential plateau phase, referred to as a ‘gain of function’ [10]. In contrast, Brugada and conduction disease SCN5A mutations diminish sodium current, resulting in a ‘loss of function’ [35]. Recent studies have demonstrated that the clinical and biophysical overlap among the various types of SCN5A mutations is greater than previously appreciated [37]. The SCN5A ‘overlap’ syndrome was first reported in a family with SCN5A 1795insD mutation, an insertion mutation in the c-terminus. The large multigenerational family presented with the full spectrum of arrhythmic syndromes, including sinus node dysfunction, conduction disease, Brugada syndrome, and LQT3 [38,39]. This mutation was shown to have a dual effect with inappropriate sodium entry at slow HRs (LQTS ECG pattern), and reduced sodium entry at fast HRs (Brugada ECG pattern) [38], and distinct mutations of the same residue (Y1795) cause either Brugada syndrome (Y1795H) or LQT3 (Y1795C) [40]. Interestingly, transgenic mice expressing the murine equivalent of SCN5A 1795insD mutation revealed a full spectrum of LQTS, conduction disorder, and right ventricle conduction delay (Brugada) syndrome, but the severity, as well as the magnitude, of sodium current reduction was greatly modulated by the background strain of mice [41•,42]. There are now two reports [43,44••] of families with ΔKPQ mutations (deletion of lysine, proline, and glutamine in residues 1505–1507; first LQT3 mutation described and considered the prototype for gain-of-function mutation) that manifest as LQT3, conduction disease, and possibly cardiomyopathy.
The SCN5A overlap syndrome presents a management conundrum for LQT3 patients. Na+ channel blockers such as lidocaine, mexiletine, and flecainide with local anesthetic properties are currently believed to be the most promising treatment for LQT3 patients due to preferential inhibition of INaL [1,12,13]. Ranolazine, a drug developed as an antianginal agent and affecting a number of cardiac ion channels, has also been found to be highly selective for blocking INaL [45,46]. Accumulating knowledge of the overlap syndrome raises concern over use of these sodium channel blockers for LQT3, which may unintentionally unmask Brugada and other SCN5A arrhythmia syndromes.
It is intriguing that the effect of treatment with β-blockers for LQT3 patients appears to be clinically neutral despite simulation of risk conditions by reducing HR and adrenergic input. This paradox may be explained by a recent study [47••] that showed that β-blockers such as propranolol, metoprolol, and carvedilol also inhibit INaL. These studies may have implications beyond LQT3. Given that INaL is present in myocytes of failing heart, it is possible that the local anesthetic-like effect may be an underappreciated mechanism that contributes to the clinical benefit of β-blockers.
Conclusion
Despite LQTS being a relatively rare disease, insights derived from the investigations of LQT ion channel mutations have markedly advanced our understanding of human cardiac electrophysiology. Through LQTS studies, for example, it is now absolutely clear that the same clinical phenotype (delayed ventricular repolarization) can be caused by alterations in any one of a growing number of genes. Further, it is clear from the work described above that it is now possible to manage LQTS patients utilizing a subtype-specific strategy. As our ability to understand the link from genotype to phenotype in LQTS increases, so does our potential to pharmacologically treat these disorders based primarily on genotype rather than phenotype. Ongoing investigations are laying the foundation for the novel paradigm for mutationspecific risk stratification and management based on the biophysical, topographical, and structural characteristics of the mutation.
What are the major obstacles to achieving the goal of mutation-specific or personalized management of patients with congenital LQTS? One major hurdle is the variable expression of LQT mutations in individual patients. This is particularly problematic for LQT3/ SCN5A mutations, in which it is becoming increasingly clear that multiple arrhythmic syndromes called overlap syndrome can occur even with mutations (ΔKPQ) that were previously identified to have biophysical profiles consistent with only LQT3. There is evidence that, for some mutations, the variable phenotypic expression of the SCN5A mutation is not only a consequence of the specific mutation but also due to modifiers that may be present in the patients [41•,42]. Without more complete understanding of the link between mutation and pheno-type in LQTS patients, we will be limited in our ability to risk stratify and derive pharmacologic treatment based upon the mutation and modifiers.
Current advances in stem cell technology have developed the breakthrough technology to allow us to manipulate the pluripotency of differentiated somatic cells by widely available molecular and cell culture techniques. It is possible to derive induced pluripotent stem cells (iPSCs) from patient skin biopsies [48]. This technology, combined with our ability to differentiate iPSCs into cardiomyocyte lineage, empowers us with unprecedented opportunities to directly examine patient-specific cardiomyocytes. We believe this will be a particularly powerful technique because cardiac myocytes derived from patient-specific iPSCs will have the full complement of individual genetic variations, including modifiers of LQTS phenotypes. By performing functional studies of patient-specific cardiomyocytes, we will have the opportunity to record and examine the effect of a given mutation in the native cardiomyocyte environment. We believe these studies are the novel paradigm that will allow us to reach mutation-specific, and ultimately patient-specific, management of LQTS.
Acknowledgements
This work was supported by grants from the National Institutes of Health (1R01 HL-56810–15) for R.S.K. and NYSTEM NO8T-034 for J.T.L We wish to thank Cecil Terrenoire for assistance with figure preparation, and Kevin J. Sampson for manuscript input.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 286).
- 1.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meissner F. Deaf and Deaf Education [in German] Leipzig and Heidelberg. 1856:119–120. [Google Scholar]
- 3.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 4.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age. II. Syncopal attacks due to paroxysmal ventricular fibrillation (Presentation of 1st case in Italian pediatric literature) [in Italian] Clin Pediatr. 1963;45:656–661. [PubMed] [Google Scholar]
- 5.Ward OC. A new familial cardiac syndrome in children. Irish Med J. 1964;54:103–106. [PubMed] [Google Scholar]
- 6.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Spazzolini C, Crotti L, et al. The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–790. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 8.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 9.Anderson CL, Delisle BP, Anson BD, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 10.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 11.Splawski I, Timothy KW, Sharpe LM, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Splawski I, Shen J, Timothy KW, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 13.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Mohler P, Splawski I, Napolitano C, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Marquardt ML, Tester DJ, et al. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci USA. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx SO, Kurokawa J, Reiken S, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1 -KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 18. Ueda K, Valdivia C, Medeiros-Domingo A, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci USA. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. This study identified a patient with LQTS phenotype and a mutation in SNTA1 (syntrophin) and provided biochemical and biophysical evidence that this mutation causes LQTS by markedly increasing late sodium current (INaL), establishing SNTA1 as the 12th LQTS susceptibility gene.
- 19.Sansone V, Griggs RC, Meola G, et al. Andersen’s syndrome: a distinct periodic paralysis. Ann Neurol. 1997;42:305–312. doi: 10.1002/ana.410420306. [DOI] [PubMed] [Google Scholar]
- 20.Plaster NM, Tawil R, Tristani-Firouzi M, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 21.Splawski I, Timothy KW, Decher N, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Effect of clinical phenotype on yield of long QT syndrome genetic testing. J Am Coll Cardiol. 2006;47:764–768. doi: 10.1016/j.jacc.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Moss AJ. Long QT Syndrome. JAMA. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 24.Windle JR, Geletka RC, Moss AJ, et al. Normalization of ventricular repolarization with flecainide in long QT syndrome patients with SCN5A:DeltaKPQ mutation. Ann Noninvasive Electrocardiol. 2001;6:153–158. doi: 10.1111/j.1542-474X.2001.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moss AJ, Zareba W, Schwarz KQ, et al. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. This study showed that intravenous infusion of ranolazine, a drug previously shown to preferentially inhibit late sodium current (INaL), was able to shorten QTc in five patients with genetically defined ΔKPQ mutation in SCN5A
- 26.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 27.Priori SG. From trials to guidelines to clinical practice: the need for improvement. Europace. 2004;6:176–178. doi: 10.1016/j.eupc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Zareba W, Moss AJ, Sheu G, et al. Location of mutation in the KCNQ1 and phenotypic presentation of long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1149–1153. doi: 10.1046/j.1540-8167.2003.03177.x. [DOI] [PubMed] [Google Scholar]
- 29.Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 31.Robertson GA, January CT. HERG trafficking and pharmacological rescue of LQTS-2 mutant channels. Handb Exp Pharmacol. 2006:349–355. doi: 10.1007/3-540-29715-4_14. [DOI] [PubMed] [Google Scholar]
- 32.Moss AJ, Zareba W, Kaufman ES, et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105:794–799. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 33. Shimizu W, Moss A, Wilde A, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052–2062. doi: 10.1016/j.jacc.2009.08.028. This study investigated the impact of the location, mutation type, and topology of the KCNH2 (or hERG) mutation in 858 patients including 162 different mutations. This was a collaborative effort between three LQT registries and showed that missense mutations located in the transmembrane S5-loop-S6 region are associated with the greatest risk.
- 34.Benson DW, Wang DW, Dyment M, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J Clin Invest. 2003;112:1019–1028. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Q, Kirsch GE, Zhang D, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 36.Schott JJ, Alshinawi C, Kyndt F, et al. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet. 1999;23:20–21. doi: 10.1038/12618. [DOI] [PubMed] [Google Scholar]
- 37.Remme C, Wilde A. SCN5A overlap syndromes: no end to disease complexity? Europace. 2008;10:1253–1255. doi: 10.1093/europace/eun267. [DOI] [PubMed] [Google Scholar]
- 38.Veldkamp MW, Viswanathan PC, Bezzina C, et al. Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ Res. 2000;86:E91–E97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 39.Bezzina C, Veldkamp MW, van den Berg MP, et al. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999;85:1206–1213. doi: 10.1161/01.res.85.12.1206. [DOI] [PubMed] [Google Scholar]
- 40.Rivolta I, Abriel H, Tateyama M, et al. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem. 2001;276:30623–30630. doi: 10.1074/jbc.M104471200. [DOI] [PubMed] [Google Scholar]
- 41. Remme CA, Scicluna BP, Verkerk AO, et al. Genetically determined differences in sodium current characteristics modulate conduction disease severity in mice with cardiac sodium channelopathy. Circ Res. 2009;104:1283–1292. doi: 10.1161/CIRCRESAHA.109.194423. Investigators of this study engineered a human SCN5A mutation into two different strains of mice, and demonstrated that the genetic background of the mice had a significant impact on the phenotypic expression of the channelopathy.
- 42.Remme CA, Verkerk AO, Nuyens D, et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–2594. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- 43.Zareba W, Sattari MN, Rosero S, et al. Altered atrial, atrioventricular, and ventricular conduction in patients with the long QT syndrome caused by the DeltaKPQ SCN5A sodium channel gene mutation. Am J Cardiol. 2001;88:1311–1314. doi: 10.1016/s0002-9149(01)02097-5. [DOI] [PubMed] [Google Scholar]
- 44. Shi R, Zhang Y, Yang C, et al. The cardiac sodium channel mutation delQK 1507–1509 is associated with the expanding phenotypic spectrum of LQT3, conduction disorder, dilated cardiomyopathy, and high incidence of youth sudden death. Europace. 2008;10:1253–1255. doi: 10.1093/europace/eun202. This study showed that the SCN5A ΔKPQ, considered by many to be the prototypical LQT3 mutation, could also clinically manifest as conduction disease as well as cardiomyopathy.
- 45.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006;148:16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L, Shryock JC, Song Y, et al. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 47. Bankston J, Kass R. Molecular determinants of local anesthetic action of beta-blocking drugs: Implications for therapeutic management of long QT syndrome variant 3. J Mol Cell Cardiol. 2010;48:246–253. doi: 10.1016/j.yjmcc.2009.05.012. This study revealed that many beta-blockers could inhibit late sodium current, thought to be the biophysical determinant of LQT3. The results of these studies may help explain why beta-blockers have not been found to be harmful in the treatment of LQT3 patients.
- 48.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]