Abstract
Phrenic motoneurons (PMNs) provide a synaptic relay between bulbospinal respiratory pathways and the diaphragm muscle. PMNs also receive propriospinal inputs, although the functional role of these interneuronal projections has not been established. Here we review the literature regarding PMN discharge patterns during breathing and the potential mechanisms that underlie PMN recruitment. Anatomical and neurophysiological studies indicate that PMNs form a heterogeneous pool, with respiratory-related PMN discharge and recruitment patterns likely determined by a balance between intrinsic MN properties and extrinsic synaptic inputs. We also review the limited literature regarding PMN bursting during respiratory plasticity. Differential recruitment or rate modulation of PMN subtypes may underlie phrenic motor plasticity following neural injury and/or respiratory stimulation; however this possibility remains relatively unexplored.
Keywords: phrenic, diaphragm, motoneuron, respiratory
1. Introduction
Phrenic motoneurons (PMNs) provide efferent motor input to the diaphragm, and are located in the ventral horn of cervical spinal segments C3–C6 (see Lane, this issue). The total number of cells in the PMN pool has been reported as between 200–700, and both species and methodological differences likely contribute to this variability (Berger et al., 1984; Boulenguez et al., 2007; Goshgarian and Rafols, 1981; Johnson and Getting, 1988; Qiu et al., 2010; Webber et al., 1979). Both neurophysiological and morphological data indicate that PMNs form a heterogeneous motor pool. In particular, whenever populations of PMNs have been studied, distinct differences in burst pattern (Hilaire et al., 1983; Kong and Berger, 1986; Lee et al., 2009; St John and Bartlett, 1979), recruitment order (Hilaire et al., 1983; Hilaire et al., 1972; Torikai et al., 1996), morphology (Torikai et al., 1996), and membrane potential (Berger, 1979; Hayashi and Fukuda, 1995) have been reported. This article provides an overview of the synaptic inputs to PMNs, and the discharge patterns of these cells during respiratory-related behaviors. In our opinion, the available evidence suggests that the recruitment order and discharge patterns of PMNs during breathing reflects a balance of both intrinsic MN properties and extrinsic synaptic inputs (Hilaire et al., 1983). However, confirmation of this hypothesis awaits further experimentation. The neural control of PMNs has been described in several previous review articles (Berger, 1990; Feldman, 1986; Monteau and Hilaire, 1991; Rekling et al., 2000; van Lunteren and Dick, 1992).
2. Synaptic inputs to PMNs
2.1 Bulbospinal projections
The respiratory-related discharge of PMNs is driven primarily by bulbospinal inputs (de Castro et al., 1994; Dobbins and Feldman, 1994; Ellenberger et al., 1990; Tian et al., 1998). Corticospinal control of the phrenic pool has been studied (Rikard-Bell et al., 1985; Rikard-Bell et al., 1986), but this topic is not reviewed here. The brainstem location of phrenic premotor neurons has been evaluated using both neurophysiological and neuroanatomical methods (reviewed in (Bianchi et al., 1995; Duffin et al., 2000)). Collectively, prior work has established that inspiratory PMN bursting is driven by neurons located within the medullary rostral ventral respiratory group (rVRG) and dorsal respiratory group (DRG). The relative importance of bulbospinal projections from the rVRG vs. DRG appears to be species specific. For example, DRG neurons provide inspiratory drives to contralateral PMNs in cats (Dick et al., 1988; Otake et al., 1989; Rikard-Bell et al., 1984) but do not appear to drive inspiratory PMN bursting in rats (Tian and Duffin, 1998). The anatomical distribution of bulbospinal projections to PMNs also shows variability between species. In rats and rabbits, rVRG projections are located in the ipsilateral spinal cord with only minimal inputs to contralateral PMNs (de Castro et al., 1994; Dobbins and Feldman, 1994; Ellenberger et al., 1990; Tian and Duffin, 1998). The small bilateral descending innervation on PMNs reflects “crossed pathways” which decussate in the brainstem and/or spinal cord (Duffin and Li, 2006; Goshgarian et al., 1991). In contrast to the rat and rabbit, inspiratory inputs to cat PMNs appear to originate mainly from the contralateral rVRG (Feldman et al., 1985; Rikard-Bell et al., 1984). To our knowledge, the origin of bulbospinal inputs to PMNs has not been described other species.
Within the cervical spinal cord, axons from bulbospinal projections to PMNs are found throughout the ventral white matter (Feldman et al., 1985; Fuller et al., 2009; Lipski et al., 1994). However, these inputs are concentrated within the lateral and ventral cervical funiculi (see Fig. 2 in (Feldman et al., 1985)). Within these funiculi, the location of axons from the ipsilateral and contralateral rVRG is different. Lipski et al. (Lipski et al., 1994) demonstrated that rVRG axons projecting to the ipsilateral phrenic nucleus are located in the lateral funiculus of the rat spinal cord. In contrast, rVRG axons innervating the contralateral phrenic nucleus were seen predominately in the ventral and ventromedial spinal cord.
In addition to excitatory inputs associated with inspiration, PMNs also receive inhibitory synaptic input during the expiratory phase (Ellenberger and Feldman, 1988; Merrill and Fedorko, 1984; Schreihofer et al., 1999; Tian et al., 1998). This inhibitory expiratory input derives from the Bötzinger complex in the rostral medulla, and the expiratory bulbospinal axons are located primarily in the ipsilateral dorsolateral spinal cord (Tian et al., 1998). Parkis et al. (Parkis et al., 1999) demonstrated that neonatal rat PMNs also receive an inhibitory input which can reduce their excitability during inspiration. However, the source of this inhibitory input has not yet been identified.
2.2 Cervical interneurons
Several studies indicate that PMNs receive synaptic inputs from propriospinal interneurons (INs), but establishing the precise functional significance of interneuronal input to PMNs presents an experimental challenge. The possibilities that PMN bursting is modulated by Renshaw cells or pre-phrenic cervical INs are considered next.
2.2.1 Renshaw cells and recurrent inhibition
Anatomical and neurophysiological evidence suggests that at least some PMNs are subject to recurrent inhibition (Goshgarian and Rafols, 1984; Hilaire et al., 1983; Hilaire et al., 1986; Lipski et al., 1985). For example, Lipski et al. found that 40% (11/28) PMNs showed inhibitory potentials during stimulation of the ventral phrenic nerve roots (Lipski et al., 1985). The source of this inhibition was hypothesized to be inhibitory interneurons that were activated via axon collaterals from the phrenic nerve (i.e. recurrent inhibition). Consistent with this hypothesis, phrenic axon collaterals were noted in 12% (6/49) PMNs intracellularly labeled with horseradish peroxidase (Lipski et al., 1985). Hilaire and colleagues demonstrated that phrenic nerve stimulation caused INs in the immediate vicinity of PMNs to burst at high frequencies. This result indicates that these cells are receiving a synaptic input from phrenic axons. Since a portion of these INs (18/33) also showed inspiratory burst activity, the authors speculated that inspiratory PMN bursting may be modulated, in part, by synaptic inputs from cervical INs acting as Renshaw cells (Hilaire et al., 1986). It should be noted, however, that other published reports have failed to provide evidence for recurrent inhibition of PMNs (Cameron et al., 1983; Gill and Kuno, 1963b). The discrepancy probably reflects the fact that the phrenic pool consists of approximately 500 PMNs (Berger et al., 1984; Goshgarian and Rafols, 1981), and the data described above indicates that only a subset of these neurons show recurrent inhibition. Since it is impossible to sample the entire phrenic pool during neurophysiological experiments, the relatively low sample sizes (e.g. <15 cells) used in many studies may make it very difficult to detect evidence of recurrent inhibition.
2.2.2 Pre-phrenic cervical INs
The primary source of inspiratory drive to PMNs arises from monosynaptic bulbospinal projections (Ellenberger and Feldman, 1988; Ellenberger et al., 1990). However, a population of pre-phrenic cervical INs has been described in both cats (Lois et al., 2009) and rats (Dobbins and Feldman, 1994; Lane et al., 2009; Lane et al., 2008b). The function of these INs to the control of PMNs is not clear, and their chemical phenotype (e.g. glutamatergic, GABAergic) has not been established. However, anterograde tracing experiments indicate that at least a subset of these INs are innervated by inspiratory rVRG neurons (Lane et al., 2008b). Neurophysiological studies confirm that cervical INs can display inspiratory-related phasic bursting (Duffin and Iscoe, 1996; Hayashi et al., 2003; Hilaire et al., 1986; Lane et al., 2009; Palisses and Viala, 1987) and, at least some of these cells have direct input from the VRG (Duffin and Iscoe, 1996). However, cross-correlation studies have failed to provide strong evidence that inspiratory INs convey central respiratory drive to PMNs during spontaneous breathing (Duffin and Iscoe, 1996). Therefore, the functional role of INs remains an open question. We have suggested that under some circumstances pre-phrenic INs may act as a synaptic relay between the brainstem and PMNs (Lane et al., 2008a; Lane et al., 2009; Lane et al., 2008b; Sandhu et al., 2009). For example, we recently used a cross-correlation method to show that PMNs ipsilateral and caudal to high cervical spinal cord injury can be activated synchronously with contralateral phrenic cells, but with a slight delay (Sandhu et al., 2009). The delay could be explained by several factors (Sandhu et al., 2009), but is consistent with the possibility of an IN relay of respiratory drive to PMNs after chronic spinal cord injury. In addition, the possibility that cervical INs contribute to phrenic motor plasticity in spinal intact animals (e.g. phrenic long-term facilitation, LTF (Mitchell et al., 2001)) has to our knowledge not been explored.
Cervical INs may also play a role in coordinating phrenic and intercostal motor outputs (Bellingham, 1999; Decima et al., 1967). For example, several studies have documented reflex changes in phrenic activity after intercostal muscle activation (Bellingham, 1999; Remmers, 1973), and Lane et al. used a dual labeling method to show that cervical INs are synaptically coupled to both intercostal MNs and PMNs (Lane et al., 2008b). In summary, the precise functional role of cervical INs in the control of PMNs is an important topic that requires further study.
3. PMN discharge patterns
3.1. Earliest reports
PMN burst patterns were first reported by Adrian and Bronk in 1928 (Adrian and Bronk, 1928) using extracellular recordings of phrenic neurofilaments in rabbits and cats. Their key observations were that PMN interspike intervals were longer at the onset vs. end of inspiration, and that PMN discharge frequency increased from approximately 20–30 Hz during quiet breathing to 50–80 Hz during a severe respiratory challenge. Subsequently, Gesell et al. (1941) used electromyogram (EMG) recordings to measure diaphragm motor unit activity and reported that “many fibers are inactive during eupnea, that some fibers twitch throughout the entire period of inspiration while others twitch but once or twice at the very end of inspiration, that the remaining active fibers contract variable period of time and fill in the intervening gap”. Pitts (Pitts, 1942) observed similar PMN discharge patterns, and confirmed that previously inactive phrenic motor units could be recruited by respiratory stimulation. These initial investigations established that inspiratory-related PMN discharge patterns are not homogenous, and this conclusion has been upheld by many subsequent studies in animal models. Table 1 provides a comprehensive list of prior descriptions of PMN bursting as studies in a range of experimental preparations. We note, however, that a recent report (Saboisky et al., 2007) suggests that PMN bursting patterns in spontaneously breathing humans may be more homogenous than has been noted in animal preparations. Below we provide a more comprehensive review of PMN discharge during conditions of “quiet breathing” (3.2) or respiratory challenge (3.3).
Table 1. Summary of prior studies describing respiratory-related PMN bursting in animal models.
This table is provided to give the reader a comprehensive reference of prior studies which have described PMN burst properties. A brief summary of the results of each study is provided under the header of “description of phrenic motoneuron bursting”. We have presented the original classification schemes used by each author, and the numbers in parentheses show the overall proportion of PMNs exhibiting a particular burst pattern. In some cases, only qualitative descriptions of PMN bursting were provided by the authors. “Onset time” refers to the interval between the beginning of inspiration and PMN spike activity. In some cases, the relative PMN burst onset time is given as a percentage of the total inspiratory duration (TI). For example, “onset time < 10 % TI” indicates that the cell began bursting in the first 10 % of the inspiratory period. “Neurofilament” refers to the method of recording “single phrenic fibers” with extracellular electrodes; see Fig. 1 for example of data obtained with this approach.
| Author | Animal model | Method for PMN recording | Description of Phrenic Motoneuron Bursting |
|---|---|---|---|
| (Adrian and Bronk, 1928) | Anesthetized adult rabbit | Neurofilament | No detailed descriptions were provided |
| (Gesell et al., 1941) | * Dog | Diaphragm electromyogram | Qualitative: some fibers discharge throughout inspiration and some only fired at end of inspiration; silent motor units could be recruited during hypercapnia. |
| (Pitts, 1942) | Anesthetized adult cat | Neurofilament | Qualitative: silent motor units could be recruited by vagotomy or hypercapnia. |
| (Gill, 1963) | Decerebrated adult cat | Neurofilament | Qualitative: silent motor units could be recruited by hypercapnia. |
| (Nail et al., 1972) | Anesthetized adult cat | Neurofilament | Low threshold (20/35): discharged throughout TI High threshold (15/35): discharged at end of TI or recruited during hypercapnia/asphyxia |
| (Iscoe et al., 1976) | Anesthetized adult cat | Neurofilament | Early-recruited (74/101):< 30 % TI Late-recruited (27/101): 30–80 % TI |
| (Berger, 1979) | Anesthetized adult cat | Intracellular | Type A (59/78): onset time was 696 ± 76 ms; linear membrane potential trajectory during expiration Type B (13/78): onset time was 4 ± 9 ms; non-linear membrane potential trajectory during expiration Type A/B (6/78): onset time was 603 ± 222 ms. |
| (St John and Bartlett, 1979) | Decerebrated adult cat | Neurofilament | Early (13/25): Onset time < 19 % TI Late (12/25): Onset time between 40–79 % TI Silent: Recruited by hypercapnia |
| Bishop 1981 | Anesthetized adult cat | Diaphragm electromyogram | Qualitative: both early and late onset PMNs were noted |
| (St John and Bartlett, 1981) | Decerebrated adult cat | Neurofilament | Early (13/25): Onset time < 24 % TI Late (12/25): Onset time > 36 % TI |
| (Hilaire et al., 1983) | Anesthetized adult cat | Neurofilament | Early (32/60): Onset time < 10 % TI Late (28/60): Onset time > 10 % TI |
| (Donnelly et al., 1985) | Anesthetized and decerebrated adult cat | Extracellular PMN and neurofilament | Early (25/42): Onset time within 80 ms Late (17/42): Onset time > 80 ms |
| (St John and Bartlett, 1985) | Decerebrated adult cat | Neurofilament | Early (45/74): Onset time < 20 % TI Late (29/74): Onset time > 20 % TI |
| (Kong and Berger, 1986) | Anesthetized adult rat | Neurofilament | Early-I (19/33): Onset time < 12.5 % TI Late-I (14/33): Onset time > 20 % TI |
| (Prabhakar et al., 1986) | Anesthetized adult cat | Neurofilament | Early (26/45): discharges at the same time as whole phrenic discharge Late (19/45): discharge 20–700 ms following whole phrenic bursting |
| (Hwang et al., 1987) | Decerebrated adult cat | Neurofilament | Early (55/78): Onset time < 30 % TI Late (23/78): Onset time > 36 % TI |
| (Jodkowski et al., 1987) | Anesthetized adult cat | Intracellular | Type H (18/38): RN > 1.3 MΩ Type L (20/38): RN < 1.3 MΩ |
| (Macefield and Nail, 1987) | Anesthetized adult rabbit | Neurofilament | Low threshold (18/27): Onset time < 10 % TI High threshold (9/27): Onset time > 10 % TI or recruited during asphyxia |
| (Lee et al., 1990) | Anesthetized adult dog | Neurofilament | Early (53/110): Onset time < 17 % TI Late (57/110): Onset time > 25 % TI |
| (Cameron et al., 1991) | Anesthetized cat | Intracellular | Adult Early (7/29): Onset time < 33 % TI Late (8/29): Onset time > 33 % TI Quiescent (14/29): Recruited during nasopharyngeal stimulation Neonate Early (8/16): Onset time < 33 % TI Late (7/16): Onset time > 33 % TI Quiescent (1/16): Recruited during nasopharyngeal stimulation |
| (Hwang and St John, 1993) | Decerebrated adult cat | Neurofilament | Early (67/95): Onset time < 30 % TI Late (28/95): Onset time > 30 % TI |
| (Hayashi and Fukuda, 1995) | Anesthetized adult rat | Intracellular | Early recruited (21/90): Onset time < 10 % TI Late recruited (22/90): Onset time during 12.5–37.5% TI Very late recruited (16/90): Onset time > 45% TI Quiescent (31/90): did not fire spontaneously |
| (Torikai et al., 1996) | Anesthetized adult rat | Intracellular | Early recruited (21/90): Onset time < 10 % TI Late recruited (22/90): Onset time> 12.5 % TI Quiescent (31/90): did not fire spontaneously |
| (Road et al., 1995) | Anesthetized adult rabbit | Neurofilament | Active (40/68): fired during unloaded inspiration Silent (27/68): recruited by increasing inspiratory load |
| (Road and Cairns, 1997) | Anesthetized adult rabbit | Neurofilament | Active (74/117): fired during unloaded inspiration Silent (41/117): recruited by increasing inspiratory load |
| (Su et al., 1997) | In vitro neonatal rat | Patch | Active (107/127): spikes during TI Silent (20/127): silent for all or most TI |
| (Lee et al., 2009) | Anesthetized adult rat | Neurofilament | Early-I (15/32): Onset time < 20 % TI Late-I (8/32): Onset time > 20 % TI Silent (9/32): Recruited during hypoxia |
no details provided regarding age or anesthesia
3.2. Baseline conditions of quiet breathing
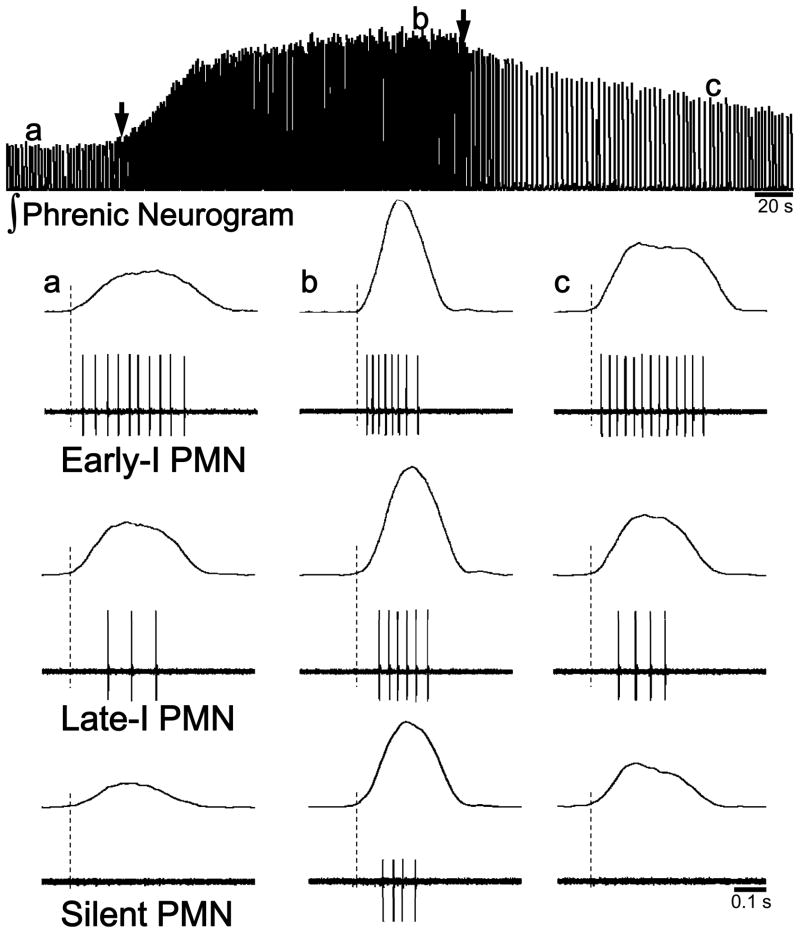
PMNs can be classified based on their bursting patterns as early-inspiratory (Early-I) or late-inspiratory (Late-I) (Kong and Berger, 1986; Lee et al., 2009; St John and Bartlett, 1979). It should be emphasized that this classification is based on neural output; however, as discussed subsequently (see 4. Factors determining PMN recruitment order), PMN biophysical properties (e.g. resting membrane potential) are generally consistent with this classification scheme. Generally, Early-I PMNs begin to discharge during the initial part of inspiration, whereas Late-I PMNs begin bursting after ~ 10–40 % of the inspiratory period has elapsed (see Fig. 1). Early-I PMNs show more action potential spikes per breath, and have a longer overall discharge duration compared to Late-I cells. However, the mean discharge frequency and the interspike interval are similar between Early-I and Late-I PMNs (Hilaire et al., 1983; Hwang and St John, 1993; Kong and Berger, 1986; Lee et al., 1990; Lee et al., 2009; Prabhakar et al., 1986; St John and Bartlett, 1979, 1981). A few studies have reported that Early-I, but not Late-I PMNs can continue bursting during the post-inspiratory period (Kong and Berger, 1986; Prabhakar et al., 1986).
Figure 1. PMN bursting patterns during normoxia and hypoxia.
This figure illustrates the three general types of PMN bursting that have been described in animal preparations, and how PMN discharge is altered during and following respiratory stimulation with hypoxia. These examples were compiled from recordings made in anesthetized, vagotomized and ventilated rats. The top panel shows a typical integrated efferent phrenic neurogram (∫Phrenic) response to hypoxia (onset and offset indicated by the arrows). The bottom panel shows examples of Early-I, Late-I and silent PMN discharge during normocapnic baseline (a), the end of isocapnic hypoxia (PaO2~40 mmHg) (b) and three minutes following hypoxia (c). The vertical dashed lines in the bottom panels represent the onset of the efferent phrenic nerve burst. Note that following hypoxia, the number of spikes per breath remains elevated in both Early-I and Late-I cells, but the silent PMN stops bursting (see (Lee et al., 2009)).
Intracellular recordings have confirmed the existence of PMN subtypes based on electrophysiological properties (Berger, 1979; Hayashi and Fukuda, 1995; Jodkowski et al., 1989; Jodkowski et al., 1987, 1988; Torikai et al., 1996). While the first intracellular PMN recordings were conducted by Gill and Kuno (Gill and Kuno, 1963a, b), their analyses did not include a description of PMN burst characteristics across the inspiratory duration. In 1979, Berger (Berger, 1979) published a landmark paper in which he described three PMN subpopulations that were designated as type A, type B, or type A/B. The classification scheme was based on differences in the membrane potential during expiration. Based on this scheme, Berger reported that type B cells were activated early in inspiration (onset delay of 4 ± 9 ms), and had both reduced conduction velocity and greater input resistance as compared to cells activated later in inspiration (type A cells, onset delay of 696 ± 76 ms). Type A/B PMNs were late-onset cells and had electrophysiological properties that were a mixture of the A and B neurons. Another key finding of the Berger study was the PMNs are actively inhibited during the expiratory period (Berger, 1979). In a subsequent paper, Jodkowski et al. (Jodkowski et al., 1987) demonstrated that the input resistance of PMNs had a bimodal distribution. Low resistance PMNs had high rheobase current, and conversely high resistance PMNs showed a low rheobase (Jodkowski et al., 1987). Hayashi and Fukuda (Hayashi and Fukuda, 1995) used intracellular recordings in adult rats to describe four types of PMNs which were designated as early-, late-, very-late recruited and quiescent. They demonstrated that recruitment order of PMNs is negatively correlated with the end-expiratory membrane potential and firing threshold, and positively correlated with the central respiratory drive potential. In addition, both early- and late-recruited MNs had inhibitory inputs during expiration. In neonatal rat in vitro preparation, two types of PMN (active and silent) could be observed by whole-cell patch-clamp recording (Su et al., 1997).
3.3. Respiratory challenge
Only a portion of the PMN pool is active during quite breathing (e.g. 30–50 %; (Mantilla et al., 2010; Road et al., 1995; Sieck and Fournier, 1989); also see Mantilla and Sieck, this issue). The phrenic motor response to response to acute respiratory challenge (e.g. hypercapnia, hypoxia) represents a mixture of rate coding of the discharge frequency in active PMNs and recruitment of previously silent cells (Gesell et al., 1941; Iscoe et al., 1976; Kong and Berger, 1986; Lee et al., 2009; Pitts, 1942). One of the first description of the PMN response during increased respiratory drive was published by Gill (Gill, 1963). This study demonstrated that discharge frequency of the PMN was progressively enhanced when end-tidal CO2 was increased above the CO2 PMN recruitment threshold. Although the discharge frequency of all PMNs is increased by hypercapnia, differential responses of Early-I vs. Late-I cells have been described in both cat and rat (Kong and Berger, 1986; Prabhakar et al., 1986; St John and Bartlett, 1979). These studies indicated that hypercapnia results in an earlier discharge onset of Late-I PMN with no change in Early-I PMN burst onset. Hypoxia, as expected, increases the discharge frequency of both Early-I and Late-I PMNs and recruit previously silent cells. Late-I PMNs also show an earlier burst onset during hypoxic stimulation (Lee et al., 1990; Lee et al., 2009; Prabhakar et al., 1986; St John and Bartlett, 1979). We recently observed that hypoxia induces differential responses in Early-I vs. Late-I PMNs (Lee et al., 2009). For example, the total number of action potential spikes per breath was increased during hypoxia in Late-I cells, but was decreased in Early-I PMNs. In addition, hypoxia reduced the overall discharge duration of Early-I but not Late-I PMNs (Lee et al., 2009).
3.4. Mechanical Stimuli
A few studies have investigated the impact of lung inflation and/or airway pressure on PMN discharge patterns (Bishop et al., 1981; Hwang and St John, 1993; Hwang et al., 1987; Road et al., 1995; Road and Cairns, 1997). Pulmonary stretch receptor activation has complex effects on PMN bursting (Hwang and St John, 1993). Facilitation of phrenic output can occur during lung inflation, and this response is associated with an earlier burst onset of Late-I PMNs (Hwang and St John, 1993). Bishop et al. manipulated airway pressures during spontaneous breathing in anesthetized cats (Bishop et al., 1981). Positive airway pressures increased PMN burst onset latency and diminished the duration of discharge. In addition, Late-I PMNs were often silenced during positive pressure breathing. Negative pressure breathing reduced PMN burst onset latency, increased burst duration and causes recruitment of previously silent cells. In some cases, negative pressure resulted in PMN bursting throughout the expiratory phase (Bishop et al., 1981). Road and colleagues demonstrated that inspiratory resistive loading increases both the burst frequency and duration of active PMNs (Road et al., 1995; Road and Cairns, 1997). However, the relative impact of loading on Early-I vs. Late-I PMN bursting was not described. Silent PMNs are also recruited during loading, and upon activation these cells show a much steeper increase in discharge frequency as compared to previously active PMNs (Road et al., 1995; Road and Cairns, 1997). During prolonged exposure to inspiratory resistive loads (e.g. 30 min), PMNs show a progressive increase in burst frequency, and this may serve to offset the onset of diaphragm muscle fatigue (Road and Cairns, 1997).
3.5. Gasping and apneusis
PMN bursting has also been examined during both gasping and apneusis. Gasping can be evoked by severe hypoxia and transform phrenic discharge from an augmenting to a decrementing pattern (Richter, 2003; St -John and Paton, 2003). St. John and Bartlett (St John and Bartlett, 1981) demonstrated that Early-I and Late-I PMN discharges were synchronized during gasping. In addition, this study also reported that Late-I PMNs are preferentially excited during gasping. Apneusis can be induced by lesion of rostrolateral pons and is characterized by elongation of inspiration and slow rise of phrenic activity (St-John and Paton, 2000; Wang et al., 1993). During apneusis, Early-I PMNs maintain a similar discharge frequency with a longer overall discharge duration. In contrast, apneusis causes a substantial delay in the onset of Late-I PMN bursting, and in some cases Late-I cells cease bursting entirely (St John and Bartlett, 1985).
3.6. Neuroplasticity
It is unknown whether PMN subpopulations (i.e. Early-I, Late-I, silent) have different capacities for neuroplasticity (Johnson and Mitchell, 2002). Indeed, despite intensive study of respiratory related plasticity (reviewed in (Feldman et al., 2003; Mitchell et al., 2001)), very little information is available regarding basic respiratory motoneuron discharge mechanisms (i.e. recruitment vs. rate coding) during plasticity. Below we review existing data based on plasticity evoked by spinal cord stimulation or hypoxia.
McCrimmon and colleagues investigated PMN discharge during repetitive electrical stimulation of the C1–C2 lateral funiculus (Hayashi et al., 2003; McCrimmon et al., 1997). This procedure potentiates inspiratory phrenic nerve activity in a manner similar to the more well described short term potentiation (STP) that follows hypoxia exposure (Powell et al., 1998). Their results indicated that PMNs have heterogeneous responses to electrical activation of descending synaptic inputs (Hayashi et al., 2003). Specifically, PMNs responded to high frequency stimulation in different ways: 1) depolarization peaking a few seconds post-stimulation; 2) depolarization during stimulation followed by rapid repolarization, or 3) depolarization that was maintained post-stimulation (termed bi-stable behavior; (Hayashi et al., 2003). These data suggest that the capacity for plasticity - at least in the short term - is heterogenous across the phrenic motor pool.
Exposure to hypoxia has been widely used to induce respiratory neuroplasticity (Mitchell et al., 2001; Powell et al., 1998). Two of the more well-established of hypoxia-induced phrenic plasticity include the brief (i.e. 3–5 minutes) increase in motor output following hypoxia (i.e., STP) and the more persistent increase following episodes of hypoxia (i.e., long term facilitation or LTF). We recently found that STP can be evoked in most, if not all, PMNs that are active prior to hypoxia (see Fig. 1). Specifically, both Early-I and Late-I PMNs showed STP of discharge frequency following hypoxic challenge. However, previously silent PMNs (i.e. recruited cells not active prior to hypoxia) ceased bursting immediately upon removal of the hypoxic stimulus. Accordingly, silent PMNs do not appear to make a significant contribution to post-hypoxia phrenic STP.
Despite intensive study of respiratory LTF in a range of species and conditions (reviewed in (Fuller et al., 2000; Mitchell et al., 2001)), there are no reports of PMN discharge patterns during in vivo LTF. Accordingly it is unknown if LTF (or any other form of prolonged respiratory plasticity) is associated with rate coding of active PMNs, or recruitment of additional “silent” PMNs. It is also unknown if the ability to increase bursting during LTF is similar between cells activated early vs. late in the respiratory cycle (Kong and Berger, 1986; Lee et al., 2009). In preliminary experiments, we have observed that phrenic LTF in anesthetized rats primarily reflects increased burst frequency in Late-I PMNs (KZ Lee and DD Fuller, unpublished observations).
Neural injury can also induce phrenic motor plasticity. For example, the response of the phrenic motor system to spinal cord injury has been studied extensively (Lane et al., 2008a; Sandhu et al., 2009). In the majority of published studies, recordings of phrenic nerve compound action potentials (“neurograms”) or diaphragm EMG signals have been used to examine the recovery process after spinal cord injury (reviewed in (Goshgarian, 2003; Sandhu et al., 2009)). This work shows that phrenic output ipsilateral to a cervical (C2) hemisection lesion gradually and spontaneously increases over time post-injury. This response reflects the so called crossed phrenic phenomenon (CPP). There is a single prior report of PMN burst patterns after cervical spinal cord injury (El-Bohy and Goshgarian, 1999). This work by Goshgarian and colleagues showed that discharge frequency of both Early-I and Late-I PMNs was increased during asphyxia-induced CPP following acute C2HS (~ 3hrs). In addition, some silent PMNs were also recruited during induction of CPP. To our knowledge, the impact of chronic cervical spinal cord injury on the control and discharge patterns of PMNs has never been explored. This information is necessary to help interpret the morphological plasticity occurring in and around the phrenic nucleus after spinal cord injury (Goshgarian, 2003; Mantilla and Sieck, 2003; Rowley et al., 2005), and to provide a baseline for interventional studies intended to enhance PMN recovery.
4. Factors determining PMN recruitment order
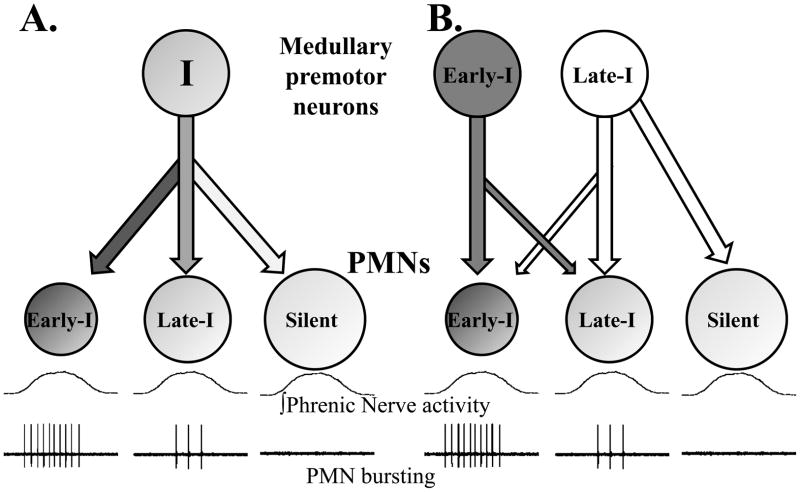
There are two general mechanisms that could explain the documented recruitment patterns of PMNs during inspiration (Berger, 1990). First, burst onset differences between Early- and Late-I PMNs could reflect intrinsic motoneuron properties (Berger, 1979; Dick et al., 1987; Webber and Pleschka, 1976). Thus, in accordance with Henneman’s size principle of motoneuron recruitment (Henneman et al., 1965), Early-I PMNs may be high resistance cells that are more likely to depolarize for a given synaptic input. The alternate mechanism is differential recruitment of PMN subtypes via pre-synaptic inputs (i.e., an “organized central command”; (Monteau et al., 1985)). Proving either hypothesis would be difficult, and data that are consistent with both possibilities have been published. Below we briefly review the evidence for each of these mechanisms.
A considerable amount of data supports the idea that orderly PMN recruitment is driven by intrinsic properties. Berger (Berger, 1979) used intracellular recordings in cats to demonstrate that PMNs recruited early in the inspiratory period have slower conduction velocity and higher input resistance as compared to late-recruited cells. Similarly, Dick et al. (Dick et al., 1987) found that the recruitment order of diaphragm motor units significantly correlates with axon conduction velocity in spontaneously breathing cats. Webber and Pleschka (Webber and Pleschka, 1976) observed that PMNs with slower conduction velocities show greater depolarization during inspiration as compared to faster conducting PMNs. Intracellular recordings in rats also indicate that Early-I PMNs have both larger membrane resistance and smaller rheobase current relative to Late-I cells (Hayashi and Fukuda, 1995). Descriptions of PMNs which are inactive during quiet breathing (i.e. silent cells) are also consistent with the size principle. For example, Torikai et al. (Torikai et al., 1996) found that total neuronal surface area is greater in silent compared to active PMNs. Finally, compared to Early-I cells, silent PMNs in adult rats have lower membrane resistance and higher rheobase current (Hayashi and Fukuda, 1995); a similar finding has been reported in neonatal rats (Su et al., 1997).
Recent work by DiMarco and colleagues also suggests that PMNs can “integrate” synaptic inputs (DiMarco, 2009; DiMarco and Kowalski, 2009). High frequency stimulation (e.g. 300 Hz) of the ventral surface of the thoracic spinal cord in spinalized (C1–2) dogs resulted in PMN bursting similar to what was observed during spontaneous breathing in spinal intact dogs. DiMarco et al. speculate that PMNs may be appropriately “integrating” propriospinal inputs during the stimulation, and the result is orderly PMN recruitment. In summary, it seems likely that intrinsic PMN properties play a major role in shaping the pattern of phrenic motor output during breathing. However, available data do not rule out a potential contribution of differential descending inputs to PMN recruitment patterns, and as reviewed below several studies have provided data that are consistent with this notion (Hilaire et al., 1983; Hilaire and Monteau, 1979; Monteau et al., 1985; Saboisky et al., 2007; St John and Bartlett, 1981).
Based on cross-correlation experiments, Hilaire et al. (1983) suggested that common synaptic inputs to Early-I and Late-I PMNs – as would be predicted by the size principle – are relatively rare. In their experiments, a pair of phrenic neurofilaments was recorded simultaneously and the degree of synchronization was examined by cross-correlation. Homogenous PMN pairs (i.e., Early-I:Early-I or Late-I:Late-I) had greater synchronization as compared to heterogenous pairs (Early-I:Late-I) (Hilaire et al., 1983). Monteau et al. (Monteau et al., 1985) simultaneously recorded from brainstem DRG neurons and PMNs in cats. They observed that synchrony between brainstem inspiratory activity and excitatory post-synaptic potentials (EPSPs) in PMNs was more likely to occur when pairing cells with similar onset times (e.g. Early-I brainstem:Early-I PMN vs. Early-I brainstem:Late-I PMN). In other words, early-recruited inspiratory brainstem neurons seemed to preferentially drive Early-I PMN, whereas late-recruited inspiratory brainstem neurons tended to excite Late-I PMN. Accordingly, the pattern of activation of these cell groups may reflect distinct innervation of Early-I and Late-I neurons vs. exclusively intrinsic neuronal properties. However, as pointed out by Berger (Berger, 1990), the DRG provides “only a small fraction of the total inspiratory-phase depolarization required by a phrenic motoneuron to reach spike threshold”, and therefore the Monteau study cannot be viewed as conclusive evidence for specific connectivity between brainstem neurons and PMNs. Berger (Berger, 1990) also notes that several additional studies have not found evidence for “preferential connectivity” between brainstem cells and PMN subpopulations (Dick et al., 1987; Donnelly et al., 1985). There is also recent, albeit indirect, evidence that may argue against the size principle for PMN recruitment (Saboisky et al., 2007). Saboisky et al. noted that diaphragm motor units show a unimodel distribution bursting patterns during spontaneous breathing in humans. Based on these data, they suggested that “motoneurons receive inspiratory drive that is unevenly distributed within and between each muscle” (Saboisky et al., 2007).
A few additional comments are warranted regarding PMN recruitment order. First, the possibility that cervical INs are involved in shaping the overall pattern of PMN recruitment should be considered (Monteau and Hilaire, 1991). Direct evidence for this possibility is lacking (Duffin and Iscoe, 1996), but results of spinal cord stimulation experiments suggest that some PMNs are preferentially activated via polysynaptic spinal inputs (Hayashi et al., 2003; McCrimmon et al., 1997). Given that at least some cervical INs show respiratory modulation (Hayashi et al., 2003; Lane et al., 2008b), it is possible that these cells may be involved in determining PMN recruitment order during breathing. Second, the relative degree of active inhibition of PMNs could influence recruitment patterns. For example, Berger noted that Early-I PMNs showed the highest degree of active inhibition during expiration in the cat (Berger, 1979). Therefore, the earlier recruitment of these cells could, in theory, partially reflect a “post-inhibitory rebound excitation” (Berger, 1979). However, a study in rats found that the expiratory inhibition is stronger in Late-I compared to Early-I PMNs (Hayashi and Fukuda, 1995).
Fig. 2 presents two possible models of PMN recruitment during inspiration. In the first example (Fig. 2a), synaptic inputs conveying inspiratory drive are constant across the phrenic motor pool. In this scenario, orderly recruitment of PMNs occurs as a result of differences in intrinsic membrane properties. As reviewed above, a considerable amount of experimental data are consistent with this model. Accordingly, it is highly likely that intrinsic properties are fundamentally important to the determination of PMN recruitment order during breathing (Berger, 1990). However, it seems unlikely that intrinsic properties are the only factor contributing to recruitment order, and in Fig. 2b we present a revised model which accounts for both intrinsic PMN properties and the potential for selective pre-synaptic inputs. We favor the model presented in Fig. 2b because it accounts for all available experimental data. The possible mechanisms and/or anatomical substrate underlying the control of the “upper motor neurons” which drive phrenic bursting are not considered here. Rather, we simply point out that at least some variability in PMN bursting probably reflects differential regulation via bulbospinal inputs, but the control of these inputs is beyond the scope of this review. In any case, confirming the hypothesis that both intrinsic motoneuron properties and pre-synaptic inputs contribute to orderly recruitment of PMNs will be experimentally challenging. Obtaining definitive data would likely require recordings from a large sample of brainstem premotor neurons coupled with simultaneous intracellular PMN recordings. The key issue is that it will be difficult to record a neuronal sample size that is large enough to test for preferential synaptic inputs to Early-I vs. Late-I PMNs. However, the use of multi-array extracellular electrode technology (Nuding et al., 2009) could help answer this question. Simultaneous recording of neural populations within the medulla and phrenic motor nucleus could provide a large enough sample size to determine if selective connectivity exists (e.g. Fig. 2b).
Figure 2. Two models of the mechanisms underlying orderly recruitment of PMNs during inspiration.
Panel A depicts the “size principle” model of PMN recruitment (see text). In this model, all PMNs receive a similar synaptic input from inspiratory premotor neurons. Neural recruitment thus reflects intrinsic PMN properties (e.g., cell size, membrane resistance). Silent PMNs are inactive during eupneic breathing but can be recruited by increasing inspiratory drive (i.e. hypercapnia, hypoxia) or during non-ventilatory behaviors (i.e. cough, sneeze). Panel B allows for the possibility that both intrinsic PMN properties and selective inputs contribute to PMN recruitment order. In this example premotor neurons firing during early-inspiration primarily innervate Early-I PMNs and premotor neurons discharging during late-inspiration mainly project to Late-I and silent cells. We do not discount the potential for cervical INs and/or inhibitory expiratory inputs to modulate PMN recruitment order. However, for simplicity these factors are not included in these diagrams but rather are reviewed in the text. Similarly, the potential for divergent inputs to the bulbospinal premotor neurons is not depicted in the figure.
5. Summary
In this review article we have provided an historical overview of studies of PMN discharge, and have highlighted a few intriguing questions regarding the regulation of PMN bursting during breathing. First, the potential contribution of propriospinal INs to the control of PMNs merits further study. In particular, these cells are strong candidates for modulating PMN bursting after spinal cord injury and are also well positioned to coordinate bursting between PMN and thoracic motoneuronpools. Second, very little is known regarding the PMN bursting and/or recruitment patterns underlying different forms of phrenic motor plasticity. Is there a pool of silent PMNs which are recruited during prolonged phrenic plasticity (e.g. hypoxia-induced LTF), or are previously active cells subject to rate coding? Do those phenotypically described PMNs which are active during eupnea (e.g. late-I, early-I) have a differential capacity for plasticity, and if so what is the cellular and molecular basis for this? Lastly, the relative contribution of intrinsic motoneuronproperties vs. extrinsic synaptic inputs to PMN recruitment has not been definitively established. Here we suggest that both intrinsic and extrinsic factors are responsible for shaping phrenic output during breathing; however, the relative contribution of each factor is not clear. Further debate and discussion of this topic is warranted. For example, the usefulness of defining PMNs based on bursting patterns could legitimately be questioned (Saboisky et al., 2007). However, this classification scheme is supported by both biophysical and anatomical descriptions (Berger, 1979; Hayashi and Fukuda, 1995; Torikai et al., 1996) and can provide the basis for testing hypotheses about the control of PMNs. Definitively establishing if phenoytypically defined subpopulations of PMNs are anatomically or biochemically distinct will require difficult experiments. For instance, the relative expression of membrane receptors (e.g. glutamatergic, serotonergic, etc.) across subpopulations of PMNs has to our knowledge not been examined. Studies using the “single cell capture” method (Perrin et al., 2006) could prove useful in this regard, although this will be complicated by the need to first electrophysiologically characterize the cell of interest. In conclusion, substantial progress has been made in the area of PMN control since the original publications in first half of the 20th century (Adrian and Bronk, 1928; Gesell et al., 1941; Pitts, 1942). The application of new and emerging technologies to the study of respiratory neural control should enable even greater progress in the coming decades.
Acknowledgments
We thank Drs. A.J. Berger and T.L. Baker-Herman for comments on aspects of this manuscript. KZ Lee was supported by the Paralyzed Veterans of America Research Foundation (grant 2691). Dr. Fuller’s laboratory was supported by NIH grant (1R01HD052682-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibres: Part I. Impulses in single fibres of the phrenic nerve. J Physiol. 1928;66:81–101. doi: 10.1113/jphysiol.1928.sp002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82:1224–1232. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Orderly recruitment of phrenic motoneurons. In: Binder MD, Mendell LM, editors. The segmental Motor System. Oxford University Press; New York: 1990. [Google Scholar]
- Berger AJ, Cameron WE, Averill DB, Kramis RC, Binder MD. Spatial distributions of phrenic and medial gastrocnemius motoneurons in the cat spinal cord. Exp Neurol. 1984;86:559–575. doi: 10.1016/0014-4886(84)90089-x. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bishop B, Settle S, Hirsch J. Single motor unit activity in the diaphragm of cat during pressure breathing. J Appl Physiol. 1981;50:348–357. doi: 10.1152/jappl.1981.50.2.348. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Gestreau C, Vinit S, Stamegna JC, Kastner A, Gauthier P. Specific and artifactual labeling in the rat spinal cord and medulla after injection of monosynaptic retrograde tracers into the diaphragm. Neurosci Lett. 2007;417:206–211. doi: 10.1016/j.neulet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Averill DB, Berger AJ. Morphology of cat phrenic motoneurons as revealed by intracellular injection of horseradish peroxidase. J Comp Neurol. 1983;219:70–80. doi: 10.1002/cne.902190107. [DOI] [PubMed] [Google Scholar]
- de Castro D, Lipski J, Kanjhan R. Electrophysiological study of dorsal respiratory neurons in the medulla oblongata of the rat. Brain Res. 1994;639:49–56. doi: 10.1016/0006-8993(94)91763-9. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature. 1967;214:312–313. doi: 10.1038/214312a0. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol. 1987;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- Dick TE, Viana F, Berger AJ. Electrophysiological determination of the axonal projections of single dorsal respiratory group neurons to the cervical spinal cord of cat. Brain Res. 1988;454:31–39. doi: 10.1016/0006-8993(88)90800-1. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol. 2009;169:200–209. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol. 2009;107:662–669. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Cohen MI, Sica AL, Zhang H. Responses of early and late onset phrenic motoneurons to lung inflation. Respir Physiol. 1985;61:69–83. doi: 10.1016/0034-5687(85)90029-5. [DOI] [PubMed] [Google Scholar]
- Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res. 1996;112:35–40. doi: 10.1007/BF00227175. [DOI] [PubMed] [Google Scholar]
- Duffin J, Li YM. Transmission of respiratory rhythm: midline-crossing connections at the level of the phrenic motor nucleus? Respir Physiol Neurobiol. 2006;153:139–147. doi: 10.1016/j.resp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Duffin J, Tian GF, Peever JH. Functional synaptic connections among respiratory neurons. Respir Physiol. 2000;122:237–246. doi: 10.1016/s0034-5687(00)00162-6. [DOI] [PubMed] [Google Scholar]
- El-Bohy AA, Goshgarian HG. The use of single phrenic axon recordings to assess diaphragm recovery after cervical spinal cord injury. Exp Neurol. 1999;156:172–179. doi: 10.1006/exnr.1999.7013. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988;269:47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Vera PL, Haselton JR, Haselton CL, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Res Bull. 1990;24:163–174. doi: 10.1016/0361-9230(90)90201-a. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of Breathing in Mammals. In: Bloom FE, editor. Handbook of Physiology; Section I: The Nervous System; Volume IV: Intrinsic Regulatory Systems of the Brain. Am. Physiol. Soc; Bethesda, MD: 1986. pp. 463–524. [Google Scholar]
- Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesell R, Atkinson AK, Brown RC. The gradation of intensity of inspiratory contractions. Am J Physiol. 1941;128:615–628. [Google Scholar]
- Gill PK. The Effects of End-Tidal Co2 on the Discharge of Individual Phrenic Motoneurones. J Physiol. 1963;168:239–257. doi: 10.1113/jphysiol.1963.sp007190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PK, Kuno M. Excitatory and Inhibitory Actions on Phrenic Motoneurones. J Physiol. 1963a;168:274–289. doi: 10.1113/jphysiol.1963.sp007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PK, Kuno M. Properties of Phrenic Motoneurones. J Physiol. 1963b;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of th albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol. 1984;13:85–109. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Fukuda Y. Electrophysiological properties of phrenic motoneurons in adult rats. Jpn J Physiol. 1995;45:69–83. doi: 10.2170/jjphysiol.45.69. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol. 2003;94:1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Gauthier P, Monteau R. Central respiratory drive and recruitment order of phrenic and inspiratory laryngeal motoneurones. Respir Physiol. 1983;51:341–359. doi: 10.1016/0034-5687(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Khatib M, Monteau R. Central drive on Renshaw cells coupled with phrenic motoneurons. Brain Res. 1986;376:133–139. doi: 10.1016/0006-8993(86)90907-8. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R. Size and inspiratory input timing as factors determining the recruitment order of the phrenic motoneurones (author’s transl) J Physiol (Paris) 1979;75:765–781. [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Dussardier M. Pattern of recruitment of phrenic motor neurons. J Physiol (Paris) 1972;64:457–478. [PubMed] [Google Scholar]
- Hwang JC, St John WM. Facilitation and inhibition of phrenic motoneuronal activities by lung inflation. J Appl Physiol. 1993;74:2485–2492. doi: 10.1152/jappl.1993.74.5.2485. [DOI] [PubMed] [Google Scholar]
- Hwang JC, St John WM, Bartlett D., Jr Influence of pulmonary inflations on discharge patterns of phrenic motoneurons. J Appl Physiol. 1987;63:1421–1427. doi: 10.1152/jappl.1987.63.4.1421. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol. 1976;26:113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Guthrie RD, Cameron WE. The activity pattern of phrenic motoneurons during the aspiration reflex: an intracellular study. Brain Res. 1989;505:187–194. doi: 10.1016/0006-8993(89)91441-8. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Repetitive firing properties of phrenic motoneurons in the cat. J Neurophysiol. 1988;60:687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Getting PA. Phrenic motor nucleus of the guinea pig: dendrites are bundled without clustering of cell somas. Exp Neurol. 1988;101:208–220. doi: 10.1016/0014-4886(88)90004-0. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. Activity-dependent plasticity in descending synaptic inputs to respiratory spinal motoneurons. Respir Physiol Neurobiol. 2002;131:79–90. doi: 10.1016/s1569-9048(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol. 1986;61:1999–2004. doi: 10.1152/jappl.1986.61.6.1999. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008a;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BP, Green J, Chiang ST. Responses of single phrenic motoneurons to altered ventilatory drives in anesthetized dogs. J Appl Physiol. 1990;68:2150–2158. doi: 10.1152/jappl.1990.68.5.2150. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. J Neurophysiol. 2009;102:2184–2193. doi: 10.1152/jn.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Fyffe RE, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci. 1985;5:1545–1555. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol. 2009;106:138–152. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Invited review: Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Botzinger neurons. J Neurosci. 1984;4:2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Monteau R, Khatib M, Hilaire G. Central determination of recruitment order: intracellular study of phrenic motoneurons. Neurosci Lett. 1985;56:341–346. doi: 10.1016/0304-3940(85)90266-6. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Baekey DM, Dick TE, Solomon IC, Shannon R, Morris KF, Lindsey BG. Pontine-ventral respiratory column interactions through raphe circuits detected using multi-array spike train recordings. J Neurophysiol. 2009;101:2943–2960. doi: 10.1152/jn.91305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake K, Sasaki H, Ezure K, Manabe M. Axonal trajectory and terminal distribution of inspiratory neurons of the dorsal respiratory group in the cat’s medulla. J Comp Neurol. 1989;286:218–230. doi: 10.1002/cne.902860207. [DOI] [PubMed] [Google Scholar]
- Palisses R, Viala D. Existence of respiratory interneurons in the cervical spinal cord of the rabbit. C R Acad Sci III. 1987;305:321–324. [PubMed] [Google Scholar]
- Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin FE, Boisset G, Lathuiliere A, Kato AC. Cell death pathways differ in several mouse models with motoneurone disease: analysis of pure motoneurone populations at a presymptomatic age. J Neurochem. 2006;98:1959–1972. doi: 10.1111/j.1471-4159.2006.04024.x. [DOI] [PubMed] [Google Scholar]
- Pitts RF. The function of components of the respiratory complex. J Neurophysiol. 1942;5:403–413. [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Mitra J, Overholt JL, Cherniack NS. Analysis of postinspiratory activity of phrenic motoneurons with chemical and vagal reflexes. J Appl Physiol. 1986;61:1499–1509. doi: 10.1152/jappl.1986.61.4.1499. [DOI] [PubMed] [Google Scholar]
- Qiu K, Lane MA, Lee KZ, Reier PJ, Fuller DD. The phrenic motor nucleus in the adult mouse. Exp Neurol. 226:254–258. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K, Lane MA, Lee KZ, Reier PJ, Fuller DD. The phrenic motor nucleus in the adult mouse. Exp Neurol. 2010;226:254–258. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE. Extra-segmental reflexes derived from intercostal afferents: phrenic and laryngeal responses. J Physiol. 1973;233:45–62. doi: 10.1113/jphysiol.1973.sp010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Commentary on eupneic breathing patterns and gasping. Respir Physiol Neurobiol. 2003;139:121–130. doi: 10.1016/s1569-9048(03)00196-4. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK, Nail BS. Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Res Bull. 1984;12:469–477. doi: 10.1016/0361-9230(84)90162-x. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK, Nail BS. Cells of origin of corticospinal projections to phrenic and thoracic respiratory motoneurones in the cat as shown by retrograde transport of HRP. Brain Res Bull. 1985;14:39–47. doi: 10.1016/0361-9230(85)90175-3. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Tork I, Bystrzycka EK. Distribution of corticospinal motor fibres within the cervical spinal cord with special reference to the phrenic nucleus: a WGA-HRP anterograde transport study in the cat. Brain Res. 1986;379:75–83. doi: 10.1016/0006-8993(86)90257-x. [DOI] [PubMed] [Google Scholar]
- Road J, Osborne S, Cairns A. Phrenic motoneuron firing rates during brief inspiratory resistive loads. J Appl Physiol. 1995;79:1540–1545. doi: 10.1152/jappl.1995.79.5.1540. [DOI] [PubMed] [Google Scholar]
- Road JD, Cairns AM. Phrenic motoneuron firing rates before, during, and after prolonged inspiratory resistive loading. J Appl Physiol. 1997;83:776–783. doi: 10.1152/jappl.1997.83.3.776. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respir Physiol Neurobiol. 2005;147:235–251. doi: 10.1016/j.resp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–780. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol. 2000;123:201–213. doi: 10.1016/s0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- St -John WM, Paton JF. Defining eupnea. Respir Physiol Neurobiol. 2003;139:97–103. doi: 10.1016/s1569-9048(03)00193-9. [DOI] [PubMed] [Google Scholar]
- St John WM, Bartlett D., Jr Comparison of phrenic motoneuron responses to hypercapnia and isocapnic hypoxia. J Appl Physiol. 1979;46:1096–1102. doi: 10.1152/jappl.1979.46.6.1096. [DOI] [PubMed] [Google Scholar]
- St John WM, Bartlett D., Jr Comparison of phrenic motoneuron activity during eupnea and gasping. J Appl Physiol. 1981;50:994–998. doi: 10.1152/jappl.1981.50.5.994. [DOI] [PubMed] [Google Scholar]
- St John WM, Bartlett D., Jr Comparison of phrenic motoneuron activity in eupnea and apneusis. Respir Physiol. 1985;60:347–355. doi: 10.1016/0034-5687(85)90062-3. [DOI] [PubMed] [Google Scholar]
- Su CK, Mellen NM, Feldman JL. Intrinsic and extrinsic factors affecting phrenic motoneuronal excitability in neonatal rats. Brain Res. 1997;774:62–68. doi: 10.1016/s0006-8993(97)81688-5. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. The role of dorsal respiratory group neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1998;121:29–34. doi: 10.1007/s002210050433. [DOI] [PubMed] [Google Scholar]
- Tian GF, Peever JH, Duffin J. Botzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp Brain Res. 1998;122:149–156. doi: 10.1007/s002210050502. [DOI] [PubMed] [Google Scholar]
- Torikai H, Hayashi F, Tanaka K, Chiba T, Fukuda Y, Moriya H. Recruitment order and dendritic morphology of rat phrenic motoneurons. J Comp Neurol. 1996;366:231–243. doi: 10.1002/(SICI)1096-9861(19960304)366:2<231::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Dick TE. Intrinsic properties of pharyngeal and diaphragmatic respiratory motoneurons and muscles. J Appl Physiol. 1992;73:787–800. doi: 10.1152/jappl.1992.73.3.787. [DOI] [PubMed] [Google Scholar]
- Wang W, Fung ML, St John WM. Pontile regulation of ventilatory activity in the adult rat. J Appl Physiol. 1993;74:2801–2811. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- Webber CL, Jr, Pleschka K. Structural and functional characteristics of individual phrenic motoneurons. Pflugers Arch. 1976;364:113–121. doi: 10.1007/BF00585178. [DOI] [PubMed] [Google Scholar]
- Webber CL, Jr, Wurster RD, Chung JM. Cat phrenic nucleus architecture as revealed by horseradish peroxidase mapping. Exp Brain Res. 1979;35:395–406. doi: 10.1007/BF00236759. [DOI] [PubMed] [Google Scholar]