Abstract
The analysis of the aerial parts of Bonannia graeca led to the isolation and characterization of two new polar geranylated flavonoids (6 and 7). The structure elucidation was performed by extensive spectroscopic methods (1D and 2D NMR) and comparison with literature data. All natural flavonoids isolated from B. graeca (1–7) and some synthetic derivatives (8–11) were tested for cytotoxic activity against four human tumor cell lines. Preliminary structure-activity relationship correlations are discussed.
Keywords: Bonannia graeca, Apiaceae, Geranylflavonoids, Cytotoxic Activity
1. Introduction
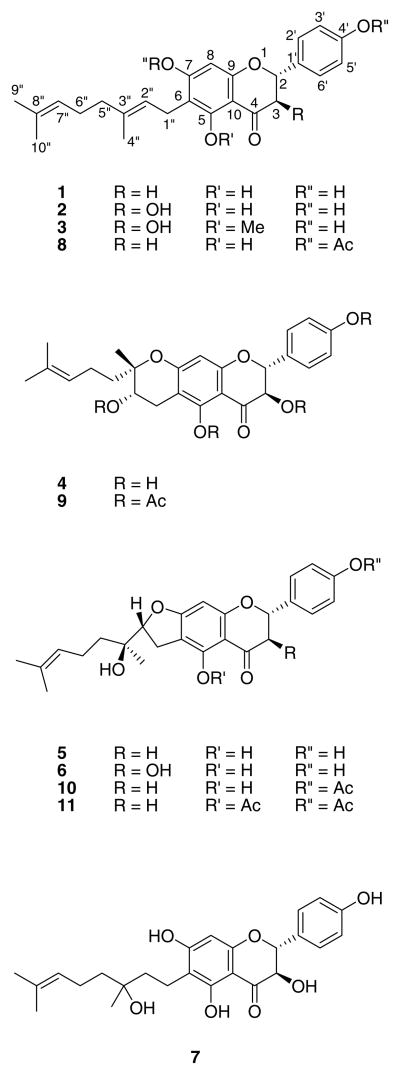
Bonannia graeca (L.) Halácsy, belonging to the Apiaceae family, is a rare plant growing in several Mediterranean areas, such as the southern parts of Italy and Greece. It is known for its toxicity against herbivores and for causing the death of lambs feeding on the aerial parts during its blossoming season (June–July). Our previous phytochemical investigations of this species allowed us to isolate a new irregular diterpene, bonandiol (Bruno et al., 1984), and five new C-geranylated flavonoids bonannione A (1), bonanniol A (2), bonanniol B (3), bonanniol C (4) and bonannione B (5) (Figure 1) (Bruno et al., 1985; Rosselli et al., 2007a). Compound 1 occurs in plants of Macaranga genus (Euphorbiaceae), including M. pleiostemona (Schuetz et al., 1995) and M. alnifolia (Yoder et al., 2007), and in Schizolaena hystrix (Sarcolaenaceae) (Murphy et al., 2005). Other papers also report its isolation from Paulownia tomentosa (Scrophulariaceae) and P. fortunei (Smejkal et al., 2008; Duan et al., 2007) and from Mimulus (Phrymaceae) species, such as M. aurantiacus (Hare, 2008) and M. clevelandii (Phillips et al., 1996). In these reports, 1 is named mimulone. Compound 2 has been isolated from Monotes africanus (Dipterocarpaceae) (Meragelman et al., 2001) and, along with 1, from Macaranga alnifolia (Yoder et al., 2007) and Schizolaena hystrix (Murphy et al., 2005). Several papers by various researchers have reported on the biological properties of these compounds, including the antibacterial activity of 1 (Schuetz et al., 1995), anti-HIV activity of 2 (Meragelman et al., 2001) and cytotoxic properties of 1 (Murphy et al., 2005; Smejkal et al., 2008).
Fig. 1.
Structures of compounds 1–11.
Herein we report the isolation and characterization of two new polar flavonoids (6 and 7), occurring as minor compounds, from B. graeca. Structure elucidation was performed by extensive spectroscopic methods (1D and 2D NMR) and comparison with literature data. Furthermore, as part of our ongoing research on compounds with cytotoxic activity (Bruno et al., 2002; Bruno et al., 2005a; Rosselli et al., 2008; Bruno et al., 2005b; Rosselli et al., 2007b; Rosselli et al., 2009), we evaluated the natural flavonoids isolated from B. graeca (1–7) and some synthetic derivatives (8–11) (Bruno et al., 1985; Rosselli et al., 2007a) against four human tumor cell lines.
2. Result and Discussion
The elemental analysis and ESI-MS data of compound 6 were in agreement with a formula of C25H28O7, and its IR spectrum showed the presence of hydroxy groups (3265 cm−1), a chelated carbonyl group (1659 cm−1), and aromatic rings (1596 cm−1). The 1H and 13C NMR (Table 1) spectra showed a similar flavonoid ring B/C pattern to that of 5, co-occurring in the same plant. Signals were present for a para-substituted aromatic ring (δH = 7.40, 2H, brd, H-2′ and H-6′; δC = 129.0, d, C-2′ and C-6′; δH = 6.87, 2H, brd, H-3′ and H-5′; δC = 115.7, d, C-3′ and C-5′) bearing an oxygenated moiety (δC = 156.7, s, C-4′), an aromatic methine group (δH = 6.00, 1H, s, H-8; δC = 91.1, d, C-8), other aromatic carbon atoms characteristic of a 5,7-dioxygenated flavonoid, an oxygenated methine group (δC = 83.3, d, C-2) at δH = 4.99 (1H, d, H-2β) coupling with the proton at δH = 4.54 (1H, d, H-3α) on the oxygenated methine group at δC = 72.2 (d, C-3), and for a carbonyl group. The 1H and 13C NMR signals of the side chain indicated the presence of a trisubstituted double bond (δH = 5.13, 1H, brt, H-7″; δC = 123.8, d, C-7″; δC = 132.4, s, C-8″) bearing two methyl groups (δH = 1.70, 3H, s, Me-9″; δC = 25.7, q, C-9″; δH = 1.64, 3H, s, Me-10″; δC = 17.7, q, C-10″), a third methyl group (δH = 1.30, 3H, s, Me-4″; δC = 22.7, q, C-4″) linked to an oxygenated quaternary carbon atom (δC = 73.9, s, C-3″), and a methylene group (δH = 3.09, 2H, d, H-1″; δC = 25.9, t, C-1″) whose protons coupled with the proton (δH = 4.79, 1H, dd, H-2″) of an oxygenated methine group at δC = 91.6 (d, C-2″). The deshielding of the latter carbon signal indicated the presence of a dihydrofuran ring, which was confirmed by a correlation in the HMBC spectrum between C-7 and H-2″. HSQC and HMBC experiments allowed us to unequivocally assign all carbon atoms and to confirm that the geranyl chain is linked at C-6 and not at C-8, as clearly indicated by the correlation between C-5 and H-1″. Consequently, the side chain was the same as that of 5, another geranylated flavonoid co-occurring in the same species. We had previously determined the relative configuration of the two stereogenic centers at C-2″ and C-3″ of 5 by using a combined approach of extensive spectroscopic and quantum mechanical methods (Rosselli et al., 2007a). Because the 1H and 13C chemical shifts of the side chain in 6 were almost identical with respect to those of 5, we can presume that the two compounds have the same relative configurations at C-2″ and C-3″. The absolute configuration of the dihydroflavonol moiety was ascertained as shown based on an identical CD curve compared with that of 2 (Bruno et al., 1985) and literature data (Gaffield, 1970). Consequently, this compound was assigned C-2(R), C-3(R), C-2″ (R), C-3″ (S) stereochemistry, or the diastereoisomeric C-2(R), C-3(R), C-2″ (S), C-3″ (R) configuration, and given the trivial name bonanniol D (6).
Table 1.
NMR spectroscopic data of compound 6a
| Position. | δH mult. (J in Hz) | δCc | HMBC (H) |
|---|---|---|---|
| 2β | 4.99 d (12.0) | 83.3 d | 2′, 6′, 3 |
| 3α | 4.54 d (12.0) | 72.2 d | 2 |
| 4 | - | 195.7 s | 2, 3 |
| 5 | - | 157.8 s | OH, 1″ |
| 6 | - | 106.5 s | OH, 8, 1″ |
| 7 | - | 169.4 s | 8, 2″, 1″ |
| 8 | 6.00 s | 91.1 d | |
| 9 | - | 163.7 s | 8 |
| 10 | - | 100.9 s | OH, 8 |
| 1′ | - | 127.8 s | 3′, 5′, 2, 3 |
| 2′ | 7.40 brd (8.4) | 129.0 d | 2 |
| 3′ | 6.87 brd (8.4) | 115.7 d | |
| 4′ | - | 156.7 s | 2′, 6′, 3′, 5′ |
| 5′ | 6.87 brd (8.4) | 115.7 d | |
| 6′ | 7.40 brd (8.4) | 129.0 d | 2 |
| 1″ | 3.09 d (2H) (8.7) | 25.9 t | |
| 2″ | 4.79 dd (8.7, 8.7) | 91.6 d | 1″, 5″, 4″ |
| 3″ | - | 73.9 s | 1″, 5″, 4″ |
| Me-4″ | 1.30 (3H) s | 22.7 q | 2″, 5″ |
| 5″ | 1.53b (2H) | 36.6 t | 2″, 6″, 4″ |
| 6″ | 2.13 (2H) m | 21.9 t | |
| 7″ | 5.13 brt (6.3) | 123.8 d | 6″, 9″, 10″, 5″ |
| 8″ | - | 132.4 s | 6″, 9″, 10″ |
| Me-9″ | 1.70 (3H) s | 25.7 q | 7″, 10″ |
| Me-10″ | 1.64 (3H) s | 17.7 q | 7″, 9″ |
| OH | 11.32 s |
CDCl3 solution.
Overlapped signal.
Multiplicity has been determined by DEPT experiments and assignments have been made by HSQC.
The elemental analysis and ESI-MS data of the more polar compound 7 were in agreement with a formula of C25H30O7. Because the compound was not soluble in CHCl3, its 1H and 13C NMR spectra were run in MeOD (Table 2). An identical flavonoid ring B/C pattern was found to that of 6, with signals for a para-substituted aromatic ring (δH = 7.35, 2H, brd, H-2′ and H-6′; δC = 130.3, d, C-2′ and C-6′; δH = 6.83, 2H, brd, H-3′ and H-5′; δC = 116.2, d, C-3′ and C-5′) bearing an oxygenated functionality (δC = 159.2 s, C-4′), an aromatic methine group (δH = 5.85, 1H, s, H-8; δC = 96.9, d, C-8), other aromatic carbon atoms characteristic of a 5,7-dioxygenated flavonoid, an oxygenated methine group (δC = 84.9, d, C-2) at δH = 4.95 (d, H-2β) coupling with the proton at δH = 4.49 (d, H-3α) on the oxygenated methine group at δC = 73.7 (t, C-3), and a carbonyl group (δC = 197.3, s, C-4). Also, similarly to 6, the 1H and 13C NMR signals of the side chain indicated the presence of a trisubstituted double bond (δH = 5.14, 1H, brt, H-7″; δC = 126.1, d, C-7″; δC = 131.9, s, C-8″) bearing two methyl groups (δH = 1.69, 3H, s, Me-9″; δC = 25.9, q, C-9″; δH = 1.65, 3H, s, Me-10″; δC = 17.8, q, C-10″) and a third methyl group (δH = 1.24, 3H, s, Me-4″; δC = 27.2, q, C-4″) linked to an oxygenated quaternary carbon atom (δC = 73.7, s, C-3″). However, in 7, the protons of the benzylic methylene group (δH = 2.58, 2H, dd, H-1″; δC = 17.8, t, C-1″) were coupled with the protons (δH = 1.62, 2H, o.s., H-2″) of a methylene group at C-2″ (δC = 40.9, t). HSQC and HMBC experiments allowed us to unequivocally assign all carbon atoms and to confirm that the geranyl chain is linked at C-6 and not at C-8, as clearly indicated by the correlation between C-5 and H-1″ as well as the absence of a dihydrofuran ring and the presence of a free hydroxy group at C-3″. The absolute configuration of the dihydroflavonol moiety was ascertained similarly as reported above for 6. Consequently, because it was not possible to assign the absolute configuration of carbon C-3″ either by spectroscopic (NOESY, Mosher) or chemical (Horeau) methods, compound 7 was assigned the C-2(R), C-3(R), C-3″ (S) or the diastereoisomeric C-2(R), C-3(R), C-3″ (R) configuration, and also was given the trivial name bonanniol E.
Table 2.
NMR spectroscopic data for compound 7a
| Position. | δH mult. (J in Hz) | δCc | δCc,d | HMBC (H) |
|---|---|---|---|---|
| 2β | 4.95 d (12.0) | 84.9 d | 83.2 d | 2′, 6′, 3 |
| 3α | 4.49 d (12.0) | 73.7 d | 72.3 d | 2 |
| 4 | - | 197.3 s | 195.9 s | 2, 3 |
| 5 | - | 162.4 s | 160.7 s | |
| 6 | - | 111.3 s | 109.7 s | 8, 1″, 2″ |
| 7 | - | 162.3 s | 164.9 s | 8, 1″ |
| 8 | 5.85 s | 96.9 d | 95.6 d | |
| 9 | - | 162.3 s | 160.7 s | 8 |
| 10 | - | 101.0 s | 100.1 s | 8 |
| 1′ | - | 129.7 s | 127.4 s | 3′, 5′, 2, 3 |
| 2′ | 7.35 brd (8.4) | 130.3 d | 128.9 d | 2 |
| 3′ | 6.83 brd (8.4) | 116.2 d | 115.5 d | |
| 4′ | - | 159.2 s | 157.5 s | 2′, 6′, 3′, 5′ |
| 5′ | 6.83 brd (8.4) | 116.2 d | 115.5 d | |
| 6′ | 7.35 brd (8.4) | 130.3 d | 128.9 d | 2 |
| 1″ | 2.58 (2H) dd (9.6, 6.6) | 17.8 t | 15.9 t | |
| 2″ | 1.62b (2H) | 40.9 t | 39.5 t | 1″, 4″ |
| 3″ | - | 73.7 s | 73.2 s | 5″, 4″ |
| Me-4″ | 1.24 (3H) s | 27.2 q | 26.4 q | |
| 5″ | 1.51 (2H) dd (11.4, 5.1) | 42.4 t | 41.6 t | 6″, 4″ |
| 6″ | 2.10 (2H) m | 23.8 t | 22.7 t | |
| 7″ | 5.14 brt (6.3) | 126.1 d | 124.3 d | 6″, 9″, 10″ |
| 8″ | - | 131.9 s | 131.7 s | 6″, 9″, 10″ |
| Me-9″ | 1.69 (3H) s | 25.9 q | 25.6 q | 10″ |
| Me-10″ | 1.65 (3H) s | 17.8 q | 17.5 q | 9″ |
MeOD solution.
Overlapped signal.
Multiplicity has been determined by DEPT experiments and assignments have been made by HSQC.
CDCl3/MeOD 9:1.
All of the natural compounds (1–7) isolated from B. graeca and four synthetic derivatives (8–11), prepared as previously reported (Bruno et al., 1985; Rosselli et al., 2007a), were screened against four human tumor cell lines including KB (nasopharyngeal), KB-VIN (multidrug-resistant KB subline), A549 (lung), and DU145 (prostate), in order to explore their cytotoxic selectivity and critical drug-resistance profiles. The results of the in vitro anticancer assays (Table 3) can be correlated with certain structural features of the compounds. In particular, the hydroxy group on C-3 generally decreased the cytotoxic activity (1 vs 2), but only about twofold. Decreased potency also occurred when the geranyl side chain was cyclized to a dihydrofuran ring. In fact, compounds 5, 6, 9, and 10 generally showed lower activity and were inactive against some cell lines. On the other hand, compounds with a cyclized dihydropyran ring (4 and 11) exhibited the highest, but modest, cytotoxic activity. Finally, the geranyl side chain seemed to play an important role, because the hydration of 2″–3″ double bond of this moiety produced a decrease in activity (2 vs 7).
Table 3.
Effects of 1–11 against tumor cell line replication
| compound | GI50 (μM)
|
|||
|---|---|---|---|---|
| KB | KB-VIN | A549 | DU145 | |
| 1 | NA | 8.7 | 9.3 | 8.4 |
| 2 | NA | 15.8 | 14.6 | 18.3 |
| 3 | 16.3 | 10.8 | NA | 20.3 |
| 4 | 10.0 | 8.2 | 7.9 | 9.9 |
| 5 | NA | 18.2 | NA | NA |
| 6 | 17.4 | 21.3 | 20.7 | 22.5 |
| 7 | NA | NA | NA | NA |
| 8 | 14.1 | 8.7 | 11.0 | 12.6 |
| 9 | 21.2 | 13.8 | NA | NA |
| 10 | NA | NA | NA | NA |
| 11 | 13.3 | 9.0 | 9.5 | 8.1 |
| Paclitaxel | 5.9 nM | >1000 nM | 6.5 nM | 3.3 nM |
Cell line: KB = nasopharynx; KB-VIN = nasopharynx MDR, A549 = lung; DU145 = prostate.
NA = not active
3. Concluding remarks
To our knowledge, compounds 1 and 2 have been isolated only from one species belonging to the family of Apiaceae, i.e. B. graeca. This plant contains many C-geranylated flavonoids and compounds with six- or five-membered rings arising from a cyclization of the geranyl chain. These structurally intriguing compounds have shown cytotoxic activity allowing us to deduce some structure-activity relationships.
4. Experimental
4.1. General experimental procedures
Optical rotations were determined on a JASCO P-1010 digital polarimeter. IR spectra were obtained on a Shimadzu FTIR-8300 spectrophotometer. 1H and 13C NMR spectra were recorded on a Bruker Avance series 300 MHz spectrometer, using the residual solvent signal (δ = 7.27 in 1H and δ = 77.00 in 13C for CDCl3 and δ = 3.31 in 1H and δ = 49.00 in 13C for MeOD) as reference. 13C NMR assignments were determined by DEPT spectra. ESIMS was obtained with an Applied Biosystem API-2000 mass spectrometer. Elemental analysis was carried out with a Perkin-Elmer 240 apparatus. CD data were obtained by a Jasco J-715 spectropolarimeter. Merck Si gel (70–230 mesh), deactivated with 15% H2O, was used for column chromatography. Preparative TLC was performed using Merck glass plates (product code 1.13895.0001).
4.2. Plant material
The aerial parts of Bonannia graeca (L.) Halácsy were collected at Quacella, Piano Battaglia, 80 km SE of Palermo, Sicily, Italy, in July 2006. A typical specimen was identified by Prof. F. M. Raimondo, University of Palermo, and has been deposited in the Herbarium of the Botanical Science Department, Palermo, Italy (voucher number PAL 06/326).
4.3. Extraction and isolation
The dried and finely powdered aerial parts of B. graeca (800 g) were extracted with Me2CO (3 × 5 L) at room temperature for one week. After filtration, the solvent was evaporated to yield a gum (50 g), which was subjected to chromatography on a dry-packed silica gel column eluting with a solvent gradient from 100% petroleum ether to 100% EtOAc. The fraction eluted with petroleum ether/EtOAc (4:1) yielded bonandiol (4 g). The fraction eluted with petroleum ether/EtOAc (3:2), was further purified by column chromatography with petroleum ether/EtOAc (7:3) as eluent to afford, in order of increasing polarity, bonannione A (1, 1.8 g), bonanniol C (4, 60 mg), bonannione B (5, 40 mg), bonanniol A (2, 3.2 g) and bonanniol B (3, 0.9 g). The most polar sub-fraction was further purified by preparative TLC with diethyl ether/petroleum ether (1:1) yielding 20 mg of bonanniol D (6). The fraction eluted with petroleum ether/EtOAc (1:1) was subjected to preparative TLC with diethyl ether/petroleum ether (4:1) as eluent to afford 2 mg of bonanniol E (7).
4.3.1. Bonanniol D (6)
Amorphous solid; [α]D25 −5.8° (c 1.03, CHCl3); IR (film) νmax 3265, 2924, 1659, 1596, 1462, 1377, 1113, 1094, cm−1; 1H NMR (CDCl3, 300 MHz) see Table 1; 13C NMR (CDCl3, 75.4 MHz) see Table 1; ESIMS (positive mode) m/z 479 [M+K]+ (26), 463 [M+Na]+ (100); ESIMS (negative mode) m/z 439 [M-H]− (100); CD (MeOH) [θ]332nm +710, [θ]294nm −33093; anal. C 60.20%, H 6.37%, calcd for C25H28O7, C 68.17%, H 6.41%.
4.3.2. Bonanniol E (7)
Amorphous solid; [α]D25 −30.0° (c 0.21, MeOH); IR (film) νmax 3280, 2935, 1661, 1600, 1472, 1365, 1080, cm−1; 1H NMR (MeOD, 300 MHz) see Table 2; 13C NMR (MeOD, 75.4 MHz) see Table 1; 13C NMR (CDCl3/MeOD, 9:1, 75.4 MHz) see Table 2; ESIMS (positive mode) m/z 465 [M+Na]+ (100); ESIMS (negative mode) m/z 441 [M−H]− (100); CD (MeOH), [θ]294nm −43098; anal. C 67.83%, H 6.85%, calcd for C25H30O7, C 67.86%, H 6.83%.
4.4. Synthesis of Compounds 8–11
Compounds 8–11 were synthesized as previously reported (Bruno et al., 1985; Rosselli et al., 2007a).
4.5. In vitro cytotoxicity assay
An in vitro anticancer assay established by NCI was used as described briefly below (Rubinstein et al., 1990). All stock cultures were grown in T-25 flasks. Freshly trypsinized cell suspensions were seeded in 96-well microliter plates with compounds added from DMSO-diluted stock. The plates were incubated for an additional 72 h after attachment and test compound addition, and the assay was terminated by addition of 10% trichloroacetic acid. Then, 0.4% sulforhodamine B (SRB) dye in 1% HOAc was added to stain the cells for 10 min. Unbound dye was removed by repeated washing with 1% HOAc and the plates were air dried. Bound stain was subsequently dissolved with 10 mM trizma base, and the absorbance read at 515 nm. Fifty percent growth inhibition (GI50) was calculated as the concentration of agent that caused a 50% reduction in the net protein increase in control cells during the incubation. The mean GI50 is the concentration of agent that reduces cell growth by 50% under the experimental conditions and is the average from at least three independent determinations. Variation between replicates was no more than 5% of the mean. The following human tumor cell lines were used in the assay: KB (nasopharynx), KB-VIN (vincristine-resistant KB subline), A549 (lung), DU145 (prostate).
Acknowledgments
This work was supported by Italian Government project MIUR-PRIN and in part by a NIH grant CA-17625 from the National Cancer Institute (K.H. Lee).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bruno M, Lamartina L, Lentini F, Pascual C, Savona G. Bonandiol: a new, irregular, monocyclic diterpene from Bonannia graeca (L.) Halacsy (Umbelliferae) Tetrahedron Lett. 1984;25:4287–4290. [Google Scholar]
- Bruno M, Savona G, Lamartina L, Lentini F. New flavonoids from Bonannia graeca (L.) Halacsy. Heterocycles. 1985;23:1147–1153. [Google Scholar]
- Bruno M, Rosselli S, Pibiri I, Kilgore N, Lee KH. Anti-HIV agents derived from the ent-kaurane diterpenoid linearol. J Nat Prod. 2002;65:1594–1597. doi: 10.1021/np020029b. [DOI] [PubMed] [Google Scholar]
- Bruno M, Rosselli S, Maggio A, Raccuglia RA, Bastow KF, Lee KH. Cytotoxic activity of some natural and synthetic guaianolides. J Nat Prod. 2005a;68:1042–1046. doi: 10.1021/np0500575. [DOI] [PubMed] [Google Scholar]
- Bruno M, Rosselli S, Maggio A, Raccuglia RA, Bastow KF, Wu CC, Lee KH. Cytotoxic activity of some natural and synthetic sesquiterpene lactones. Planta Med. 2005b;71:1176–1178. doi: 10.1055/s-2005-873139. [DOI] [PubMed] [Google Scholar]
- Duan W, Zhang J, Xie G, Zhang C, Li C. Chemical constituents from the flower of Paulownia fortunei (Seem) Hemsl Zhongyaocai. 2007;30:168–170. [PubMed] [Google Scholar]
- Gaffield W. Circular dichroism, optical rotatory dispersion, and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides. Tetrahedron. 1970;26:4093–4108. [Google Scholar]
- Hare JD. Inheritance of leaf geranylflavanone production and seed production within and among chemically distinct populations of Mimulus aurantiacus. Biochem Syst Ecol. 2008;36:84–91. [Google Scholar]
- Meragelman KM, McKee TC, Boyd MR. Anti-HIV prenylated flavonoids from Monotes africanus. J Nat Prod. 2001;64:546–548. doi: 10.1021/np0005457. [DOI] [PubMed] [Google Scholar]
- Murphy BT, Cao S, Norris A, Miller JS, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DGI. Cytotoxic flavanones of Schizolaena hystrix from the Madagascar rainforest. J Nat Prod. 2005;68:417–419. doi: 10.1021/np049639x. [DOI] [PubMed] [Google Scholar]
- Phillips WR, Baj NJ, Gunatilaka AAL, Kingston DGI. C-Geranyl compounds from Mimulus clevelandii. J Nat Prod. 1996;59:495–497. doi: 10.1021/np960240l. [DOI] [PubMed] [Google Scholar]
- Rosselli S, Bruno M, Maggio A, Bellone G, Formisano C, Mattia CA, Di Micco S, Bifulco G. Two new flavonoids from Bonannia graeca: a DFT-NMR combined approach in solving structures. Eur J Org Chem. 2007a:2504–2510. [Google Scholar]
- Rosselli S, Bruno M, Maggio A, Bellone G, Chen TH, Bastow KF, Lee KH. Cytotoxic activity of some natural and synthetic rnt-kauranes. J Nat Prod. 2007b;70:347–352. doi: 10.1021/np060504w. [DOI] [PubMed] [Google Scholar]
- Rosselli S, Maggio A, Eiroa C, Formisano C, Bruno M, Irace C, Maffettone C, Mascolo N. Cytotoxic activity of diterpenoids isolated from the aerial parts of Elaeoselinum asclepium subsp meoides. Planta Med. 2008;74:1285–1287. doi: 10.1055/s-2008-1074579. [DOI] [PubMed] [Google Scholar]
- Rosselli S, Maggio AM, Faraone N, Spadaro V, Morris-Natsche SL, Bastow KF, Lee KH, Bruno M. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat Prod Commun. 2009;4:1701–1706. [PubMed] [Google Scholar]
- Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- Schuetz BA, Wright AD, Rali T, Sticher O. Prenylated flavanones from leaves of Macaranga pleiostemona. Phytochemistry. 1995;40:1273–1277. [Google Scholar]
- Smejkal K, Babula P, Slapetova T, Brognara E, Dall’ Acqua S, Zemlicka M, Innocenti G, Cvacka J. Cytotoxic activity of C-geranyl compounds from Paulownia tomentosa fruits. Planta Med. 2008;74:1488–1491. doi: 10.1055/s-2008-1081339. [DOI] [PubMed] [Google Scholar]
- Yoder BJ, Cao S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, Kingston DGI. Antiproliferative prenylated stilbenes and flavonoids from Macaranga alnifolia from the Madagascar rainforest. J Nat Prod. 2007;70:342–346. doi: 10.1021/np060484y. [DOI] [PMC free article] [PubMed] [Google Scholar]