Abstract
Application of 4-aminopyridine (4-AP, 100 μM) in a solution containing 0.6 mM Mg2+ and 1.2 mM Ca2+ to hippocampal-entorhinal-perirhinal slices of adult rat brain induced ictal-like epileptiform activity in entorhinal and perirhinal cortices as revealed by electrophysiological field potential recordings. The ictal-like activity persisted after washing out the 4-AP. This persistence indicated that a change had occurred in the slice so that it was now “epileptic” in the absence of the convulsant 4-AP. Induction of persistent ictal-like activity was dependent upon the concentration of divalent cations during 4-AP exposure; that is, although 4-AP caused ictal-like activity in approximately half the slices in solution containing 1.6 mM Mg2+ and 2.0 mM Ca2+, this ictal-like activity did not persist upon washout of the 4-AP. Expression of the persistent ictal-like epileptiform activity required ionotropic glutamate-mediated synaptic transmission: application of the AMPA/kainate receptor antagonist NBQX after 4-AP washout reduced persistent ictal-like activity, and the combined application of NBQX and the NMDA receptor antagonist D-AP5 completely blocked it. In order to investigate the mechanism of induction of persistent ictal-like activity, several agents were applied before the introduction of 4-AP. Application of D-AP5 did not block the onset of ictal-like activity upon introduction of 4-AP but did prevent the persistence of the ictal-like activity upon washout of the 4-AP. In contrast, induction of persistent ictal-like activity was not prevented by simultaneous application of the group I metabotropic glutamate receptor (mGluR) antagonists LY 367385 and MPEP or by application of the protein synthesis inhibitor cycloheximide. In conclusion, we have characterized a new in vitro model of epileptogenesis in which induction of ictal-like activity is dependent upon NMDA receptor activation but not upon group I mGluR activation or protein synthesis.
Keywords: epileptogenesis, brain slice, electrophysiology, NMDA, entorhinal cortex, perirhinal cortex, 4-aminopyridine, ictal-like
Introduction
Epileptogenesis is the process by which an initially normal area of the brain changes so that it becomes prone to having abnormal synchronous electrical activity that manifests as seizures. Though epileptogenesis normally applies only to whole animals, the term in vitro epileptogenesis can be applied to brain slice models in which a pharmacologic, ionic, or stimulation-based manipulation converts the brain slice to a state in which spontaneous or easily-evoked epileptiform activity persists after return to a normal environment (Clark and Wilson, 1999). The rationale behind the development of in vitro epileptogenesis models is that they offer an opportunity to uncover the mechanisms of epileptogenesis and to explore possible ways to inhibit epileptogenesis in an easily accessible preparation. Having multiple models is beneficial because there are many different types of epilepsy seen in patients, and these different types are likely the result of different epileptogenic processes (Engel and Schwartzkroin, 2006); using new models may allow us to uncover new and important epileptogenic mechanisms (Stables et al., 2002).
Epileptiform activity recorded in animal models of temporal lobe epilepsy is generally divided into ictal and interictal activity. In vivo, ictal activity is associated with behavioral seizures; whereas interictal activity is abnormal activity that occurs in the brain during the time the animal is not having a seizure. In vitro, an epileptiform event is typically classed as “ictal-like” if it is deemed to be long enough to cause a seizure if the organism were intact, and classed as “interictal-like” if it is deemed too short to have a behavioral correlate were the organism intact.
Stasheff et al. (1989) were among the first to make the important distinction between causing the onset of epileptiform activity that can be reversed upon removal of the causal agent, and initiating epileptogenesis, which results in a long-lasting change so that the epileptiform activity persists in the absence of the causal agent. While there are several in vitro models of ictal-like epileptiform activity, there are few in vitro models of epileptogenesis. Two well-studied brain slice models of epileptogenesis are the repeated stimulation model and the group I metabotropic glutamate receptor (mGluR)-dependent model, both employing hippocampal slices. The repeated stimulation model (Stasheff et al., 1989) involves using repeated electrical stimulation to induce spontaneous interictal and easily-evoked ictal-like epileptiform events. The group I mGluR-dependent model involves causing interictal-like events with the GABAA antagonist picrotoxin and then adding the group I mGluR agonist DHPG to convert the interictal-like activity to ictal-like activity. The ictal-like activity persists following the washout of the DHPG (Merlin and Wong, 1997), establishing the group I mGluR model as a model of in vitro epileptogenesis.
These two models differ from each other in their requirements for induction of persistent ictal-like activity, that is, in the receptors which must be activated during the induction period in order to cause epileptiform activity that persists following washout of the convulsant or cessation of the stimulation. In addition, within a model, the receptors required for induction of persistent ictal-like activity differ from those required for expression of ictal-like activity. In the repeated stimulation model, the induction of persistent epileptiform activity depends upon the NMDA subtype of ionotropic glutamate receptors; whereas the expression of persistent epileptiform activity is largely unaffected by NMDA receptor antagonists (Stasheff et al., 1989). In contrast, in the group I mGluR-dependent model, induction can take place in the presence of complete block of ionotropic glutamate receptors; whereas ionotropic glutamate-mediated synaptic transmission is required for the expression of the ictal-like activity (Merlin, 1999).
4-aminopyridine (4-AP) has been used to cause epileptiform activity in brain slices in hippocampus (e.g., Rutecki et al., 1987), entorhinal and perirhinal cortices (e.g., de Guzman et al., 2004) and neocortex (e.g., Aram et al., 1991). 4-AP typically causes interictal-like activity in hippocampus (Perreault and Avoli, 1991; Rutecki et al., 1987), but it can cause ictal-like activity in entorhinal cortex (Avoli et al., 1996) and perirhinal cortex (de Guzman et al., 2004). 4-AP elicits epileptiform activity at 50–100 μM; the mechanism is presumably its block of D-type (Storm, 1988), or Kv1.x (Coetzee et al., 1999), voltage-gated K+ currents, which leads to a broadening of the action potential in the axon terminal (Haas et al., 1983; Storm, 1987), increased calcium entry (Jones and Heinemann, 1987; Qian and Saggau, 1999), and a subsequent increase in transmitter release (Buckle and Haas, 1982). The increase in [Ca2+] in the presynaptic terminal may in turn decrease 4-AP-insensitive K+ currents leading to further broadening of subsequent action potentials and additional increase in transmitter release (Qian and Saggau, 1999b). Although biochemical changes that occur during the application of 4-AP have been investigated (Merlo et al,. 2004; Sanna et al., 2000), until now 4-AP has not been used as a model of in vitro epileptogenesis; that is, it has not been used as a model in which epileptiform activity persists following washout of the convulsant.
With the question of whether the induction of persistent ictal-like activity in brain slices always falls into one of two categories: 1) NMDA receptor-dependent or 2) group I metabotropic glutamate receptor-dependent, we decided to investigate ictal-like activity caused by 4-AP. Using 4-AP/reduced divalent cation solution, which had been shown to cause ictal-like activity in CA1 hippocampus (Ziburkus et al., 2006), we recorded ictal-like activity in entorhinal and perirhinal cortices, and we found that it persisted following washout of the 4-AP. Here we introduce this 4-AP/reduced divalent cation model of in vitro epileptogenesis, and we explore the mechanisms of expression and induction of this persistent ictal-like activity.
Methods
Slice preparation
Experiments were done in brain slices taken from adult (200–350 g) Sprague Dawley rats. In accordance with a protocol approved by the SUNY Downstate Animal Care and Use Committee, the animal was anesthetized with halothane, decapitated with a guillotine, and the brain was removed and placed in ice-cold vibratome solution. After cooling for 1–2 min, the brain was separated into right and left hemispheres. Then one hemisphere was placed cortex-side-down. Following a scalpel blade cut just rostral to the hippocampus, the rostral part of the brain was discarded. Then the brainstem was lifted off and discarded. Then a transverse cut of hippocampus was made to select the temporal third of hippocampus with attached entorhinal and perirhinal cortices. 350 μm transverse slices containing hippocampus, entorhinal cortex (EC) and perirhinal cortex (PRC) were cut on a Vibratome 3000. Slices were then stored in a holding chamber (Gibb and Edwards, 1987). The holding chamber was immersed in a 31.5°C water bath for 1 hour. At one hour the holding chamber was removed from the heated bath and thereafter was stored at room temperature.
Solutions
The vibratome solution (Ziburkus et al., 2006) contained (in mM) 205 sucrose, 2.6 KCl, 1.23 NaH2PO4, 24 NaHCO3, 20 D-glucose, 2 MgCl2, 0.1 CaCl2. The solution in the holding chamber where the slices were stored after cutting (Salah and Perkins 2008) contained (in mM) 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.6 MgCl2, 2.0 CaCl2, 11 D-glucose. The 1.6 mM Mg2+/2.0 mM Ca2+ extracellular recording solution contained (in mM) 130 NaCl, 24 NaHCO3, 2.5 NaH2PO4, 3.5 KCl, 1.6 MgSO4, 2.0 CaCl2, 10 D-glucose. The 0.6 mM Mg2+/1.2 mM Ca2+ reduced divalent cation extracellular recording solution (Ziburkus et al., 2006), our primary extracellular recording solution, contained (in mM) 130 NaCl, 24 NaHCO3, 2.5 NaH2PO4, 3.5 KCl, 0.6 MgSO4, 1.2 CaCl2, 10 D-glucose.
The divalent cation concentrations of our 1.6 mM Mg2+/2.0 mM Ca2+ extracellular recording solution are typical for brain slice experiments (e.g., Miles and Wong, 1987; cf. Stasheff et al., 1989 in which [Ca2+] was 1.3 mM and [Mg2+] was 0.9 mM), but are higher than those found in actual rat CSF (Somjen, 2004; Davson et al., 1987). The Mg2+ concentration of the 0.6 mM Mg2+/1.2 mM Ca2+ reduced divalent cation extracellular recording solution, our primary recording solution, is reduced compared to actual rat cerebrospinal fluid, which is 0.9 mM free and 1.3 mM total (Somjen, 2004), but not nearly as reduced as that in the solution used for the “low magnesium” model of epileptiform activity in brain slices, which is nominally zero (e.g., Dreir and Heinemann, 1991). The calcium concentration ([Ca2+]) of our 0.6 mM Mg2+/1.2 mM Ca2+ reduced divalent cation extracellular recording solution is lower than that historically found in brain slice experiments (2.0 or 2.4 mM; Dingledine and Gjerstad, 1980; Schwartzkroin, 1975; Yamamoto, 1972) but is similar to that of actual rat CSF, which is 1.0 mM free and 1.1 mM total (Somjen, 2004).
Electrophysiology
One slice was transferred from the holding chamber to the recording chamber (Fine Science Tools) where it was maintained at an interface between continuously perfusing oxygenated solution and humidified 95% O2/5% CO2 gas at 35°–36°C. Extracellular field recordings were performed with glass electrodes (0.2–2 MΩ) filled with 150 mM NaCl. Voltage was recorded in DC using a high-impedance amplifier (Warner IE-210; Harvard Apparatus) at 50 times gain and further amplified at 20 times gain using a Warner LPF-100B (Harvard Apparatus). Prior to digitization, voltage recording was filtered at 100 Hz using the low-pass Bessel filter on the Warner LPF-100B. Data points were collected at 500 Hz using pClamp software (Axon CNS, Molecular Devices). Recordings were made with one or two electrodes. When there were two recording electrodes, electrodes were placed at the EC and PRC or at one of those two locations along with the CA3 hippocampal area. In EC and PRC, the electrode was placed in layer II/III or layer V. In CA3 hippocampus, the electrode was placed in stratum lacunosum-moleculare (SLM) or at the border of SLM and stratum radiatum.
Drugs
The convulsant 4-AP was used at 100 μM. The ionotropic glutamate receptor antagonists used were the AMPA/kainate receptor antagonist 2,3-dioxo6-nitro-1,2,3,4,-tetrahydrobenzo[f] quinoxaline-7-sulfonamide disodium salt (NBQX, 10 μM) and the NMDA receptor antagonist D-(−)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 μM). The group I mGluR antagonists used were (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY 367385, 100 μM; Salah and Perkins, 2008), which preferentially blocks the mGlu1 receptor subtype, and 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP, 10 μM; Salah and Perkins, 2008), which preferentially blocks the mGlu5 receptor subtype. The general protein synthesis inhibitor cycloheximide (60 μM) was used in some experiments. The glutamate antagonists were purchased from Tocris (Ellisville, MO), and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
In the experiments that involved drug pre-incubation, the drug was already present when the slice was placed in the recording chamber. For subsequent drug applications or drug washouts, drug application time or washout time was measured with time zero being when the new solution was put in the bath solution reservoir.
Data analysis
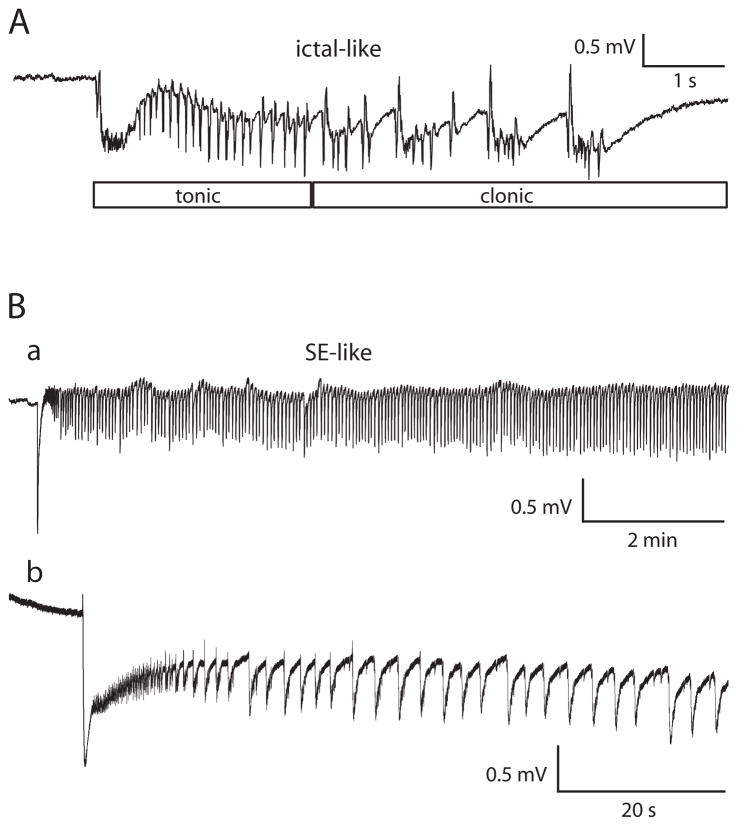
Epileptiform events in brain slices have typically been classified as ictal-like or interictal-like based on event duration. When stated, minimal event duration for being considered ictal-like has ranged from 1 – 4 s (e.g., D’Antuono et al., 2004, 4 s; de Guzman et al., 2004, 3 s; Köhling et al., 2000, 2 s; Merlin and Wong, 1997, 1 s). We considered an epileptiform event ≥ 4 sec but < 5 min to be ictal-like. Many of the ictal-like events recorded in these experiments, both during 4-AP exposure and after 4-AP washout, were composed of a tonic phase followed by a clonic phase (Fig. 1A). Although 4 s is a somewhat arbitrary cut-off point between interictal and ictal, we chose 4 s because a typical event in our experiments needed to be approximately that long in order to show a clonic component (e.g., Fig. 1A). An epileptiform event <4 s was considered to be an interictal-like event. When measuring the duration of an ictal-like event, discharges were considered to be part of a single ictal-like event as long as less than 2.5 s of baseline voltage separated the voltage deflections.
Fig. 1. Ictal-like and/or status epilepticus-like events could be recorded in entorhinal and perirhinal cortices after application of 4-aminopyridine.
Slices bathed in 0.6 mM Mg2+/1.2 mM Ca2+ solution. A: Ictal-like event illustrating tonic and clonic components. Field electrode in entorhinal cortex layer II/III. B: SE-like event in a different slice. Full event lasted over 29 min. Field electrode in perirhinal cortex layer II/III. a: Trace filtered after digitization using a 0.05 Hz high-pass RC filter. b: Same SE-like event on an expanded time scale. Trace in b not filtered after digitization.
Some slices experienced a period of status epilepticus-like activity (Fig. 1Ba). This activity, which we will refer to as SE-like activity or SE, typically began as an ictal-like event would begin, with a tonic phase, and then moved into an extended clonic phase (Fig. 1Bb). When measuring to determine if activity met the definition for SE-like, a stretch of activity was considered to be a single stretch of SE-like activity if clonic-like voltage deflections occurred continuously with no more than 2.5 s of baseline voltage separating the individual clonic-like voltage deflections and continued long enough so that the entire event was at least 5 min long.
Control 4-AP/reduced divalent cation recordings, in which no drugs other than 4-AP were used, were interspersed with the experiments addressing induction and expression mechanisms. Three 4-AP/reduced divalent cation control groups were used in data analysis. Control group A slices were exposed to 0.6 mM Mg2+/1.2 mM Ca2+ solution for 30–40 min before introduction of 4-AP. Control group A was used for comparison in experiments in which slices in the drug group were exposed to 0.6 mM Mg2+/1.2 mM Ca2+ solution before the introduction of 4-AP. Control group B slices were exposed to 0.6 mM Mg2+/1.2 mM Ca2+ solution and 4-AP simultaneously. Control group B was used for comparison in experiments in which slices in the drug group were exposed to 0.6 mM Mg2+/1.2 mM Ca2+ solution and 4-AP simultaneously. Control group C is a subset of control group B and includes only those slices from group B which did not experience periods of SE-like activity in 4-AP or after 30 minutes of 4-AP washout. Control group C was used when degree of ictal-like activity in 4-AP was tested as a predictor of ictal-like activity after 4-AP washout.
In order to measure time spent in ictal-like events, the duration of each ictal-like event was measured, and the individual event durations were summed. Similarly, each period of SE-like activity was measured. The percent time spent in SE + ictal-like events during 4-AP exposure (“in 4-AP”) and the longest epileptiform event during 4-AP exposure were measured for the time period beginning at 30 min of 4-AP exposure and continuing until commencement of 4-AP washout. (Two control group B slices were excluded from the “in 4-AP” analysis but retained for the “after 4-AP washout” analysis because washout was begun in those slices at 37 min of 4-AP exposure so that there was less than 10 min of data to measure “in 4-AP”.) 4-AP was considered to be completely washed out at 30 minutes of wash, based on data presented here (see Fig. 2 and the NMDA receptor antagonist effect on induction data), and additionally on control experiments in which giant GABA-mediated postsynaptic potentials (GPSPs), and the discharge deflections directly following each GPSP (as recorded in Salah and Perkins 2008) are completely gone by 23 ± 2 minutes of washing out the 4-AP (n=3, Salah and Perkins, unpublished data). Percent time spent in SE + ictal-like events after 4-AP washout was measured for the period encompassing 30–50 min of 4-AP washout. Longest epileptiform event was measured beginning at 30 min of 4-AP washout. Dual recordings from EC and PRC in hippocampal-entorhinal-perirhinal slices revealed that individual ictal-like events and periods of SE-like activity occurred synchronously between the two areas both before and after washout of the 4-AP (n = 31); therefore, measurements of percent time spent in SE + ictal-like events were made in only one of the two regions in each slice even when there were recordings from both regions, and EC and PRC data were pooled. Ictal-like events in the presence of ionotropic glutamate antagonists were not always synchronous between EC and PRC and were measured separately in those experiments.
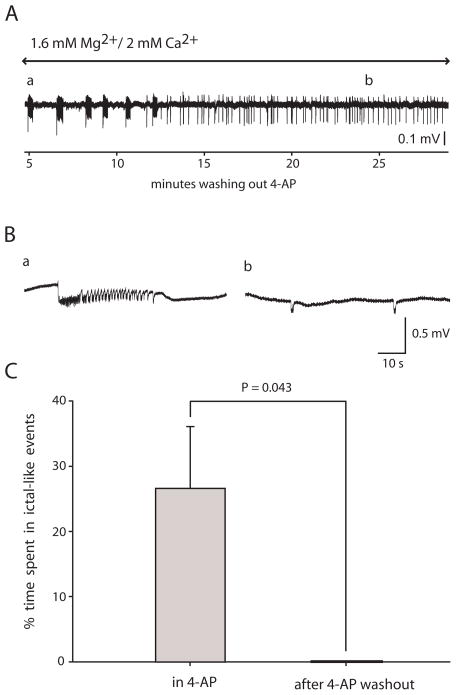
Fig. 2. Application of 4-AP in a solution containing 1.6 mM Mg2+ and 2.0 mM Ca2+ induced ictal-like epileptiform activity in entorhinal cortex which did not persist after washing out the 4-AP.
4-AP (100 μM) caused ictal-like activity in the entorhinal cortex in 1.6 mM Mg2+/2.0 mM Ca2+ solution in 10 of 22 slices; however, the ictal-like activity did not persist upon washout of the 4-AP (n = 5 of 5). A: In this slice the ictal-like activity was replaced with interictal-like activity at approximately 12 min of 4-AP washout. Trace filtered after digitization using a 0.5 Hz high-pass RC filter. B: Expanded traces from early (a) and late (b) in the washout showing an ictal-like event from point a in A and interictal-like events from point b in A. Traces in B were not filtered after digitization. C: Summary of 5 slices which had ictal-like activity in 4-AP, comparing percent time spent in ictal-like activity before and after 4-AP washout (Wilcoxon).
Values are reported as mean ± SD. n values in the text refer to the number of slices. The Wilcoxon signed ranks test (Wilcoxon), which is the nonparametric equivalent of the paired t-test, was used for statistics when each slice served as its own control. The Mann-Whitney U-test, which is the nonparametric equivalent of the t-test, was used when one group of slices was compared to another group of slices. P < 0.05 was considered significant. SigmaPlot 9.01 (Systat Software, Inc., Point Richmond, CA) was used for graphs and PASW Statistics 18 (SPSS, Inc.) was used for statistics. Portions of this study have appeared in abstract form (Salah and Perkins, 2008b).
Results
Application of 4-AP in 1.6 mM Mg2+/2.0 mM Ca2+ solution caused ictal-like activity in some slices, but it did not persist following 4-AP washout
Bathing the slices in the 1.6 mM Mg2+/2.0 mM Ca2+ solution and 4-AP, field recordings in EC showed ictal-like activity or mixed interictal and ictal-like activity in 10 of 22 slices. The other slices showed only interictal-like activity. In 5 of the slices with ictal-like activity, the 4-AP was washed out following 38–50 min in 4-AP (Fig. 2A). In the last 10 min before 4-AP washout, ictal-like events 33.6 ± 18.4 s duration occurred at a rate of 0.64 ± 0.45 events/min (n = 5 slices; mean of means; Fig. 2Ba). None of the five slices showed SE-like activity. After 4-AP washout, none of the 5 slices showed persistent ictal-like activity (Fig. 2C), but instead showed infrequent interictal-like events (237 ± 52 ms duration, 0.81 ± 0.88 events per min; n = 5; Fig. 2Bb). Time between beginning of washout and the end of the last ictal-like event ranged from 4 to 12 min, with a mean of 7 ± 4 min (n = 5). In three of the slices 4-AP was re-applied after 32–40 min of washout, and ictal-like activity reappeared in all three slices.
Application of 4-AP in a solution containing 0.6 mM Mg2+/1.2 mM Ca2+ induced ictal-like epileptiform activity in entorhinal/perirhinal cortex which persisted after washing out the 4-AP
The first group of reduced divalent cation/4-AP hippocampal-entorhinal-perirhinal slices (n=18, referred to as control group A) was first exposed to 0.6 mM Mg2+/1.2 mM Ca2+ solution alone for 30–40 min before 4-AP was added. 14 of 18 slices had no ictal-like epileptiform activity in EC/PRC in the 0.6 mM Mg2+/1.2 mM Ca2+ solution without 4-AP (Fig. 3A, left); no slices had SE-like activity. Percent time spent in ictal-like events in the last 10 minutes before addition of 4-AP was 0.5 ± 1.5 % (n=18). (An additional 4 slices were recorded from in 0.6 mM Mg2+/1.2 mM Ca2+ solution for 40–60 min, never adding 4-AP. One of those slices experienced some ictal-like activity.)
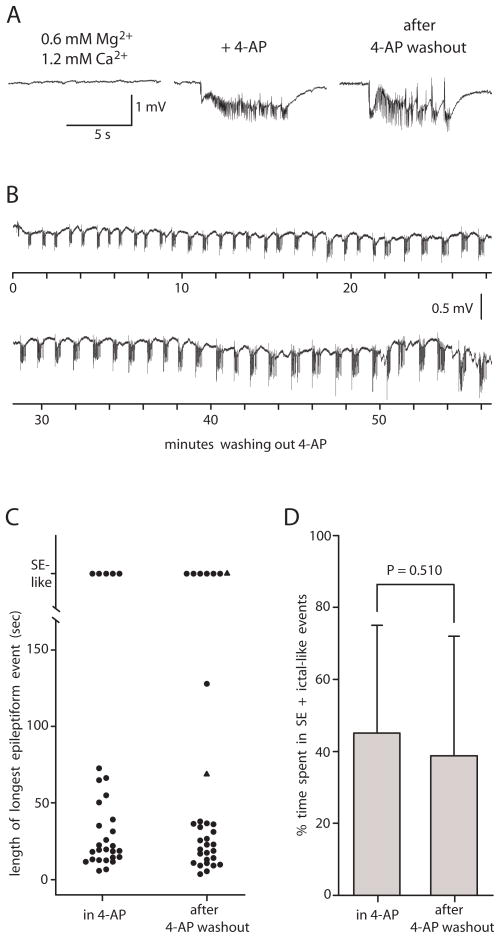
Fig. 3. Application of 4-AP in a solution containing 0.6 mM Mg2+/1.2 mM Ca2+ induced ictal-like epileptiform activity in entorhinal/perirhinal cortex which persisted after washing out the 4-AP.
A: Bathing slice in 0.6 mM Mg2+/1.2 mM Ca2+ solution did not cause epileptiform activity in this slice (left). Addition of 4-AP (100 μM) caused ictal-like epileptiform activity (middle). Ictal-like epileptiform activity persisted upon washout of 4-AP (right, same trace as Fig. 1A). Right trace taken 42 min after initiation of 4-AP washout. All traces in A recorded from same slice. Field electrode in A in entorhinal cortex layer II/III. B: A different slice showing persistence of ictal-like activity over 57 min of 4-AP washout. Field electrode in B in perirhinal cortex layer II/III. C: Scatter plot of control group B slices showing length of longest epileptiform event in each slice in 4-AP and after 4-AP washout. N=30 in 4-AP and n=32 after 4-AP washout. The two triangles denote the two slices in which the longest event after 4-AP washout was measured but the longest event in 4-AP was not measured due to a 4-AP exposure < 40 min. D: Bar graph of control group B slices comparing % time spent in SE + ictal-like events before and after 4-AP (Wilcoxon, n= 30).
Application of 4-AP (100 μM) caused the onset of (n =12) or increase in (n = 4) ictal-like epileptiform activity or mixed interictal and ictal-like epileptiform activity (Fig. 3A, middle) or the onset of SE-like activity (n = 2) in EC/PRC. In total, 8 slices showed SE-like activity at some point during the 4-AP application. After a period of 30 to 60 min in 4-AP, the 4-AP was washed out. Ictal-like activity (Fig. 3A, right), mixed interictal and ictal-like activity, and/or SE-like activity persisted in all slices following complete 4-AP washout. Percent time spent in SE + ictal-like events after 4-AP washout was 43.7 ± 31.8 % (n=18). There was no correlation between duration of 4-AP exposure and percent time spent in SE + ictal-like events after 4-AP washout (r2 = 0.0001, n = 18). Simultaneous recordings from CA3 hippocampus (n=5) revealed persistent interictal-like activity after 4-AP washout, but no ictal-like activity either before or after 4-AP washout.
The second group of reduced divalent cation/4-AP hippocampal-entorhinal-perirhinal slices (n = 32, referred to as control group B) was introduced to 0.6 mM Mg2+/1.2 mM Ca2+ solution and 4-AP simultaneously. The slices were exposed to 4-AP for 37–86 min. 4-AP was then washed out. Ictal-like activity, mixed interictal and ictal-like activity, and/or SE-like activity persisted in 31 of 32 slices after complete 4-AP washout and throughout the remainder of the recording (Fig. 3B). One of the slices showed only interictal-like activity. A scatter plot reveals that duration of the longest epileptiform event was very similar in 4-AP compared to after 4-AP washout (Fig. 3C). In addition, percent time spent in SE + ictal-like events was not significantly different in 4-AP vs. after 4-AP washout (Fig. 3D).
Among control group A and B slices, 7 slices were recorded from for at least 80 minutes following commencement of 4-AP washout. Comparing the period between 30 and 40 min of 4-AP washout to the period between 70 and 80 minutes of 4-AP washout, there was no difference in percent time spent in ictal-like events (P = 0.398, Wilcoxon).
Divalent cation concentration during 4-AP exposure determined persistence of ictal-like activity following 4-AP washout
Contrast the lack of persistent ictal-like activity in EC after 4-AP washout when the 4-AP was applied in 1.6 mM Mg2+/2.0 mM Ca2+ solution (Fig. 2A) to the persistence of ictal activity when the 4-AP was applied in 0.6 mM Mg2+/1.2 mM Ca2+ solution (Fig. 3B). In order to ask the question of whether the degree of ictal-like activity in the presence of 4-AP could account for the difference in persistence between the two groups, we compared the five 1.6 mM Mg2+/2.0 mM Ca2+ solution slices which had ictal-like activity in 4-AP to control group C (0.6 mM Mg2+/1.2 mM Ca2+ solution, see methods) slices (n=18). The same sets of slices were compared in 4-AP and then following 4-AP washout. There was no significant difference between the percent time spent in ictal-like events in 4-AP in the control group C slices vs. the 1.6 mM Mg2+/2.0 mM Ca2+ solution slices (P = 0.655); however the 1.6 mM Mg2+/2.0 mM Ca2+ slices showed zero ictal-like activity after 4-AP washout whereas the control group C slices spent 24.7 ± 13.8 % of the time in ictal-like events after 4-AP washout (P = 0.001).
Increasing divalent cation concentration after 4-AP washout
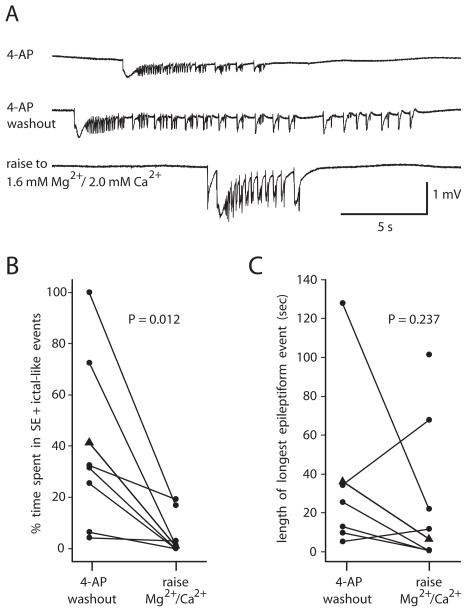
In 8 of the control group B slices the divalent cation concentrations were raised to 1.6 mM Mg2+ and 2.0 mM Ca2+ at 10–50 min after the complete washout of 4-AP (Fig. 4A). The increased divalent cation concentration was considered to have been fully reached at 30 min after the solution change. Ictal-like activity persisted after this point in 5 of 8 slices. The other three slices showed persistent interictal-like events. The mean percent time spent in SE + ictal-like events fell significantly upon raising the divalent cation concentration (Fig. 4B), but the longest epileptiform event was not significantly different before and after the increase in divalent cation concentration (Fig. 4C). The latest ictal-like event that was recorded occurred at 80 min after commencing the change to 1.6 mM Mg2+ and 2.0 mM Ca2+ solution, which was 2.2 hours after complete 4-AP washout (i.e., 2.7 hours after commencing the washout of 4-AP).
Fig. 4. Raising divalent cation concentration to 1.6 mM Mg2+/2.0 mM Ca2+ after complete 4-AP washout reduced but usually did not block ictal-like activity.
A: Recording from entorhinal cortex in 0.6 mM Mg2+/1.2 mM Ca2+ solution in 4-AP (top), after 4-AP washout for 32 min (middle) and after 31 min in 1.6 mM Mg2+/2.0 mM Ca2+ solution (bottom). B: % time spent in SE + ictal-like events after 4-AP washout fell upon raising the divalent cation concentration (n = 8, measured for the 10 min period immediately prior to initiation of the solution change and for the 10 min period between 30 and 40 min following initiation of the solution change). Triangles represent the slice in A. C: Longest epileptiform event in 4-AP washout in each slice before and after raising the divalent cation concentration. Three of eight slices had no ictal-length events after raising divalent cation concentration. The unpaired point represents the slice that had only SE-like activity before the divalent cation concentration was raised. Excluding unpaired point, P = 0.237, n = 7 (Wilcoxon).
Expression of persistent ictal-like epileptiform activity after 4-AP washout was dependent upon ionotropic glutamatergic synaptic transmission
We next investigated the role of ionotropic glutamatergic synaptic transmission in the expression of persistent ictal-like activity in entorhinal/perirhinal cortex after 4-AP washout. First we investigated the role of NMDA-mediated synaptic transmission. Following 37–47 min in 4-AP and 0.6 mM Mg2+/1.2 mM Ca2+ solution, 4-AP was washed out for 40–60 min. Ictal-like activity persisted as described above. D-AP5 was then added (n = 7), causing a reduction in time spent in SE + ictal-like events (Fig. 5A,C). D-AP5 was considered to have reached maximally effective concentration by 20 min. Ictal-like events were recorded beyond this point in all slices (Fig. 5E). Even though there was a significant reduction in time spent in SE + ictal-like events with the addition of D-AP5, ictal-like epileptiform activity was still robust in the presence of D-AP5 (Fig. 5A, C). Subsequent application of NBQX to the solution already containing D-AP5 blocked all remaining epileptiform activity in 16 min or less (n = 7; Fig. 5A, E; recording maintained for 30–50 min after addition of NBQX).
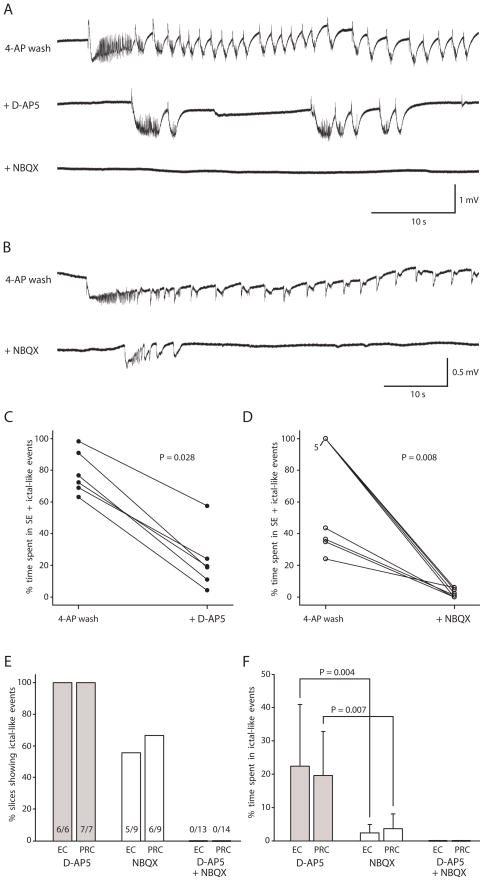
Fig. 5. Expression of persistent ictal-like activity after 4-AP washout was dependent upon ionotropic glutamatergic synaptic transmission.
A: Persistent, ictal-like epileptiform activity after 4-AP washout (top, full ictal-like event lasted 185 s) was reduced but still present after application of the NMDA receptor antagonist D-AP5 (middle), but all epileptiform activity was blocked after subsequent application of the AMPA/kainate antagonist NBQX (bottom). Data in A recorded in perirhinal cortex (PRC) in the same slice at 56 min of 4-AP washout (top), at 58 min of D-AP5 exposure (middle), and at 17 min of NBQX exposure (bottom). B: Persistent, ictal-like epileptiform activity after 4-AP washout (top, full ictal-like event lasted 168 s) was reduced but still present after application of the AMPA/kainate antagonist NBQX (bottom) in this slice. Data in B recorded in PRC in the same slice at 58 min of 4-AP washout (top) and at 37 min of NBQX exposure (bottom). C: Percent time spent in SE + ictal-like events in entorhinal cortex (EC) in each slice fell with addition of D-AP5 (n=6; measured for the last 10 min of 4-AP wash before the D-AP5 application and for the 10 minutes between 20 and 30 minutes of D-AP5 application). D: Percent time spent in SE + ictal-like events in EC in each slice fell with addition of NBQX (n=9; measured for the last 10 min of 4-AP wash before the NBQX application and for the 10 minutes between 20 and 30 minutes of NBQX application). E: Bar graph illustrating % of slices still showing ictal-like activity after D-AP5, after NBQX, and after both D-AP5 and NBQX in EC and PRC. F: Bar graph illustrating % time spent in ictal-like events after D-AP5, after NBQX, and after both D-AP5 and NBQX. (There was no SE-like activity.) Percent time spent in ictal-like events was significantly lower in the presence of only NBQX than in the presence of only D-AP5. Protocol was 4-AP, then 4-AP washout, then application of ionotropic glutamate antagonist, then sometimes application of second ionotropic glutamate antagonist. Slice bathed in 0.6 mM Mg2+/1.2 mM Ca2+ solution throughout protocol.
Next we investigated the role of AMPA/kainate-mediated synaptic transmission in the expression of persistent ictal-like activity. Following 36–49 min in 4-AP, the 4-AP was washed out for 40–60 min (n = 10). Ictal-like activity persisted, as described above, in 9 of 10 slices. One of the 10 slices was excluded from the rest of the experiment because only interictal activity occurred after complete 4-AP washout. Then NBQX was added, causing a reduction in epileptiform activity (Fig. 5B, D). NBQX was considered to have reached maximally effective concentration by 20 min. At least one ictal-like event was recorded beyond this point in 5 of 9 slices in EC and 6 of 9 slices in PRC (Fig. 5E). The other slices showed no epileptiform activity (n = 2) or 1.5 - <4 s long interictal-like events (n = 2 EC, n = 1 PRC). Subsequent application of the NMDA receptor antagonist D-AP5 to the solution already containing NBQX blocked all remaining epileptiform activity in 15 min or less (n = 7 of 7; recording maintained for 30–45 min after addition of D-AP5).
Comparing the slices exposed to NBQX first (n = 9) to those exposed to D-AP5 first (n = 7), the % time spent in ictal-like events in NBQX-only was significantly less than the % time spent in ictal-like events in D-AP5-only (Fig. 5F), and the longest epileptiform event in NBQX-only (4.8 ± 3.9 s in EC and 8.4 ± 8.8 s in PRC) was significantly shorter than the longest event in D-AP5-only (20.4 ± 10.3 s in EC, P = 0.003, and 24.6 ± 16.1 s in PRC, P = 0.013).
Induction of persistent ictal-like epileptiform activity was dependent upon NMDA receptor activation
Having established that ionotropic glutamate receptor-mediated synaptic activity was necessary for the expression of persistent ictal-like activity after 4-AP washout, we next tested whether NMDA receptor activation during 4-AP exposure would be necessary for the induction of persistent ictal-like activity (Fig. 6). First, the slice was pre-incubated with the NMDA receptor antagonist D-AP5 in 1.6 mM Mg2+/2.0 mM Ca2+ extracellular solution for 40 min. Then 4-AP was applied in 0.6 mM Mg2+/1.2 mM Ca2+ extracellular solution in the continued presence of D-AP5 for 60 min, which caused a mixture of interictal- and ictal-like activity in EC and ictal-like activity in PRC (n = 4). 4-AP was then washed out for 40 min in the continued presence of D-AP5. In all 4 slices all ictal-like activity stopped by 20 min of 4-AP washout (Fig. 6A, B,C), leaving only infrequent, interictal-like events (<1 s duration), indicating that D-AP5 blocked the induction of persistent ictal-like epileptiform activity.
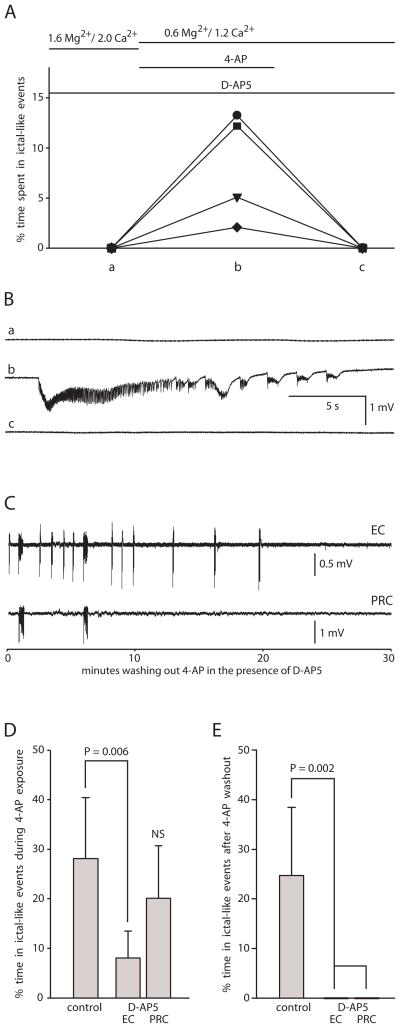
Fig. 6. NMDA receptor antagonist did not block the onset of ictal-like activity but did block the induction of persistent ictal-like activity.
A: Summary graph showing % time spent in ictal-like events in each successive condition in each slice in EC. (There was no SE-like activity.) Protocol of experiment shown at top. Each slice represented by a different symbol. Pre-incubation with the NMDA receptor antagonist D-AP5 in 1.6 mM Mg2+/2.0 mM Ca2+ solution (a), did not prevent the onset of ictal-like activity in 4-AP/0.6 mM Mg2+/1.2 mM Ca2+ solution (b), but did prevent persistence after 4-AP washout (c). B: Traces from EC in same slice (represented by square in A) in each condition. Lower case letters match letters on x-axis in A. Middle trace (b) was recorded in the presence of D-AP5 at 58 min of 4-AP application. C: Ictal-like epileptiform activity stopped within 20 min of 4-AP washout in continued presence of D-AP5 (recording continued for an additional 11 min. not shown). Simultaneous recording from EC and PRC from same slice as B. Traces filtered after digitization using a 0.3 Hz high-pass RC filter. D: Bar graph comparing D-AP5 slices to control group C slices showing a smaller percent time spent in ictal-like events during 4-AP exposure for D-AP5 slices in EC but no significant difference between D-AP5 slices and control slices in PRC (n=18 control, n= 4 D-AP5; NS = not significant compared to either of the other two bars). E: Bar graph comparing control group C slices to D-AP5 slices showing persistent ictal-like activity after 4-AP washout in control slices but none in D-AP5 slices, either in EC or PRC (n=18 control, n= 4 D-AP5).
In order to address whether a reduction in ictal-like activity during 4-AP exposure in the D-AP5 slices might account for lack of persistence following 4-AP washout, we compared the D-AP5 slices to control group C slices (Fig. 6D, E). There was no significant difference between the percent time spent in ictal-like events in 4-AP in the control slices vs. the D-AP5 ( Fig. 6D) slices in PRC; however the D-AP5 slices spent a significantly smaller percent time in ictal-like events in 4-AP in EC (Fig. 6D).
Induction of persistent ictal-like epileptiform activity was not dependent upon group I metabotropic glutamate receptor activation
Next we tested whether metabotropic glutamate receptor activation was necessary for the induction of persistent ictal-like epileptiform events in entorhinal cortex in the 4-AP/reduced divalent cation model. Slices were pre-incubated with the group I mGluR antagonists LY 367385 and MPEP for 30 min in 1.6 mM Mg2+/2.0 mM Ca2+ solution (Fig. 7). Then we applied 4-AP in 0.6 mM Mg2+/1.2 mM Ca2+ solution for 30–40 min. Ictal-like epileptiform activity was recorded in EC in all slices (PRC recordings not done), and it persisted after complete 4-AP washout in all slices (n = 4; Fig. 7A,B). The longest epileptiform event recorded after 4-AP washout was 137.4 ± 24.5 s (n = 4, Fig. 7C). Percent time spent in SE + ictal-like events after 4-AP washout did not differ between the control and LY/MPEP slices (Fig 7D). An additional 4 slices were tested using a similar protocol that varied only in that the initial LY/MPEP exposure occurred in the presence of 0.6 mM Mg2+/1.2 mM Ca2+ solution. As in the other protocol, there was no significant effect of LY/MPEP on induction of persistent ictal-like activity (P = 0.147, % time spent in SE + ictal-like events after 4-AP washout compared to control). Combining the two groups of LY/MPEP slices and comparing them to the combined controls, P = 0.901.
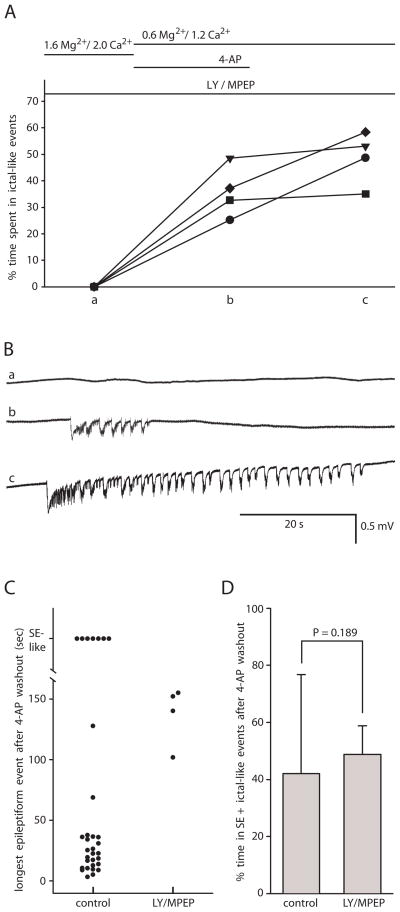
Fig. 7. Group I metabotropic glutamate receptor antagonists did not prevent the induction of persistent ictal-like epileptiform activity.
A: Summary graph showing percent time spent in ictal-like events in each successive condition in each slice in EC. Protocol of experiment shown at top. Each slice represented by a different symbol. Pre-incubation with the group I mGluR antagonists LY 367385 (LY) and MPEP in 1.6 mM Mg2+/2.0 mM Ca2+ solution for 40 min (a), did not prevent the onset of ictal-like epileptiform activity upon application of 4-AP in 0.6 mM Mg2+/1.2 mM Ca2+ solution (b), and the ictal-like epileptiform activity persisted after washing out 4-AP (c). B: Traces from EC in same slice (represented by circle in A) in each condition. Lower case letters match letters on x-axis in A. Top trace (a) recorded in 1.6 mM Mg2+/2.0 mM Ca2+ solution in the presence of LY/MPEP. Middle trace (b) recorded at 36 min of 4-AP application in the presence of LY/MPEP. Bottom trace (c) recorded at 53 min of 4-AP washout in the continued presence of LY/MPEP. C: Scatter plot comparing control group B slices and LY/MPEP slices showing length of longest epileptiform event in each slice after 4-AP washout. D: Bar graph shows no difference between control group B and LY/MPEP slices in percent time spent in SE + ictal-like events after 4-AP washout (n = 32 control and n = 4 LY/MPEP).
The protein synthesis inhibitor cycloheximide did not block induction of persistent ictal-like epileptiform activity
To further study the mechanism of induction of ictal-like activity in entorhinal/perirhinal cortex, we pre-incubated the slices with the general protein synthesis inhibitor cycloheximide (60 μM) for 30 min in 0.6 mM Mg2+/1.2 mM Ca2+ solution. Then we applied 4-AP for 50–60 min in the continued presence of cycloheximide, obtaining ictal-like epileptiform activity or mixed ictal and interictal-like epileptiform activity (Fig. 8A). Two of the slices experienced a period of SE-like activity. The 4-AP was then washed out. Recording was continued for 24 min or longer beyond complete 4-AP washout in the continued presence of cycloheximide. 6 of 6 slices (n = 4 rats) showed persistent ictal-like or mixed ictal- and interictal-like activity for the entire recording in both EC and PRC (Fig. 8). The longest epileptiform event after 4-AP washout was 111 ± 117 s (n = 6, Fig. 8B). Time spent in SE + ictal-like events after 4-AP washout did not differ between the control and cycloheximide slices (Fig. 8C).
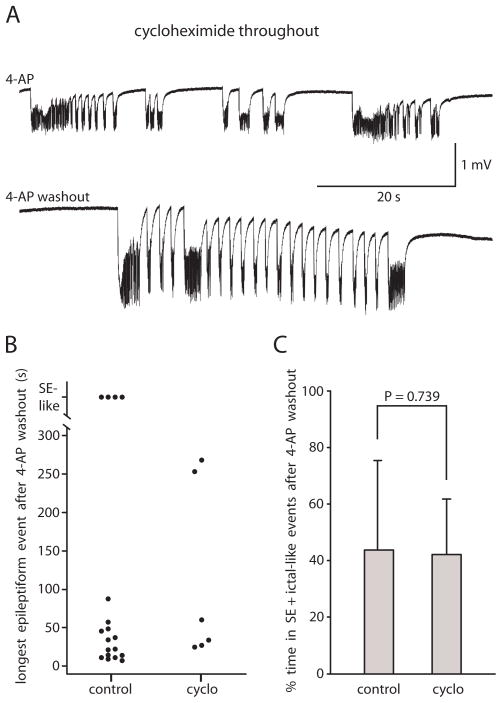
Fig. 8. The protein synthesis inhibitor cycloheximide did not prevent the induction of persistent ictal-like epileptiform activity.
A: Pre-incubation of slices in cycloheximide for 30 min in 0.6 mM Mg2+/1.2 mM Ca2+ solution before introduction of 4-AP did not prevent the onset of ictal-like activity in 4-AP (top) and did not prevent the persistence of ictal-like epileptiform activity upon washout of 4-AP (bottom). Top trace recorded in EC at 34 min of 4-AP exposure. Bottom trace recorded in EC in same slice at 48 min of 4-AP washout. B: Scatter plot comparing control group A slices and cycloheximide slices showing length of longest epileptiform event in each slice after 4-AP washout. C: Bar graph shows no difference between control group A slices and cycloheximide slices in percent time spent in SE + ictal-like events after 4-AP washout (n = 18 control and n = 6 cycloheximide). All slices bathed in 0.6 mM Mg2+/1.2 mM Ca2+ solution throughout time in recording chamber.
DISCUSSION
In this paper we have shown that application of 4-AP in a solution containing 0.6 mM Mg2+/1.2 mM Ca2+ induced ictal-like epileptiform activity in EC and PRC which persisted after washing out the 4-AP. This persistence of ictal-like activity indicated that a change had occurred in the slice so that it was now “epileptic” despite the removal of the convulsant. This 4-AP/reduced divalent cation model is thus a new model of in vitro epileptogenesis. We have shown that induction of persistent ictal-like activity in this model was 1) dependent upon the 0.6 mM Mg2+/1.2 mM Ca2+ extracellular solution, 2) dependent upon NMDA receptor activation, 3) not dependent upon group I mGluR activation, and 4) not dependent upon protein synthesis.
NMDA receptor involvement in ictal-like activity
Our experiments showed only a modest effect of NMDA receptor antagonist on the expression of ictal-like activity in the presence of 4-AP and on the expression of already-induced persistent epileptiform activity, and yet a complete block of the induction process. Our experiments showing only moderate effects of blocking NMDA receptors upon expression of ictal-like activity may be somewhat surprising given that brain slice experiments of Avoli and colleagues have shown that ictal-like activity in EC (Avoli et al., 1996) and PRC (de Guzman et al., 2004) in the presence of 4-AP and 2.0 mM Mg2+/2.0 mM Ca2+ is completely blocked by addition of NMDA antagonist. However, our results may be less surprising in light of recent experiments in whole isolated guinea pig brain which have shown that ictal activity in hippocampus/EC caused by arterial perfusion of 4-AP is not reduced by NMDA antagonists (Carriero et al., 2010). Our findings that NMDA receptor antagonist blocks induction of persistent ictal-like activity but has only a modest inhibitory effect on the expression of ictal-like activity is reminiscent of the results obtained in the repeated stimulation and status epilepticus models of epileptogenesis (see below).
Comparison to other models of epileptogenesis
Given our results, it remains a valid hypothesis that induction of persistent ictal-like epileptiform activity in brain slices is always dependent upon either NMDA receptor activation or group I mGluR receptor activation, but not both. Whereas work by several groups has indicated an important role for group I mGluR activation in the expression and/or induction of ictal-like activity in vitro (Arvanov et al., 1995; Martín et al., 2001; Merlin et al., 1997) and in vivo (Chapman et al., 1999, 2000; Smolders et al., 2004; Yan et al., 2005); in contrast, we showed here that in the 4-AP/reduced divalent cation model in EC and PRC, group I mGluR antagonists neither suppressed expression of ictal-like epileptiform activity nor blocked the induction of persistent ictal-like activity. Our data demonstrated that the 4-AP/reduced divalent cation model of epileptogenesis is clearly distinct from the group I mGluR in vitro model of epileptogenesis. In contrast to our model, the group I mGluR model requires group I mGluR activation for its induction (Merlin and Wong, 1997), can be induced in the presence of NMDA and AMPA/kainate receptor antagonists (Merlin, 1999), and is protein synthesis dependent (Chuang et al., 2005; Merlin et al., 1998).
On the other hand, our 4-AP/reduced divalent cation model of epileptogenesis shares features with NMDA-receptor-dependent epileptogenesis models. The repeated stimulation model of epileptogenesis in CA3 in hippocampal slices, in particular, shares features with our model: its induction is NMDA receptor-dependent (Stasheff et al., 1989), its expression is not NMDA receptor-dependent as long as AMPA/kainate-mediated transmission is intact (Stasheff et al., 1989), and its induction is not blocked by protein synthesis inhibitor (Jones et al., 1992).
Our model also shares its dependence on NMDA receptor activation for induction with the kindling and status epilepticus models of in vivo epileptogenesis in rats. In the kindling model, brief electrical stimulation repeated over days gradually lowers the threshold for triggering a seizure and leads eventually to spontaneous seizures. Administration of NMDA antagonists just before each electrical stimulation suppresses this epileptogenesis (e.g., Sutula et al., 1996). In the status epilepticus models, pilocarpine or kainic acid is used to induce status epilepticus which is followed some time later by the onset of spontaneous seizures. If NMDA receptor antagonist is administered prior to the pilocarpine or kainic acid injection, status epilepticus is not prevented, but the subsequent development of spontaneous ictal activity and concurrent behavioral seizures is suppressed (Rice and DeLorenzo, 1998; Stafstrom et al., 1993).
Ictal-like activity in 4-AP is not sufficient for induction of persistent ictal-like activity
Our 1.6 mM Mg2+/2.0 mM Ca2+ experiments allowed us to address whether ictal-like activity in 4-AP is sufficient for the induction of persistent ictal-like activity after 4-AP washout. We found that ictal-like activity occurred in the presence of 4-AP in approximately half of the 1.6 mM Mg2+/2.0 mM Ca2+ slices (the ictal group). When comparing the ictal group of 1.6 mM Mg2+/2.0 mM Ca2+ slices to the 0.6 mM Mg2+/1.2 mM Ca2+ control slices, there was no significant difference in percent time spent in ictal-like activity in the presence of 4-AP. Despite no difference in percent time spent in ictal-like events in the presence of 4-AP as compared to control, the ictal group of 1.6 mM Mg2+/2.0 mM Ca2+ slices showed no persistent ictal-like activity upon washout of 4-AP. This lack of persistence following 4-AP washout indicates that something other than, or something in addition to, the ictal-like activity itself is required for achieving persistence.
Mechanism of induction of persistent ictal-like activity in 4-AP/reduced divalent cation model
The dependence of epileptogenesis in our experiments upon 0.6 mM Mg2+/1.2 mM Ca2+ solution and NMDA receptor activation suggests that a key step in induction of persistent ictal-like activity is the opening of NMDA receptor channels. In this scenario, reduced [Mg2+] and the depolarization caused by enhanced AMPA/kainate receptor activation in 4-aminopyridine would promote removal of the Mg2+ block of the NMDA receptor channel (Nowak et al., 1984). In addition, the general reduction in extracellular divalent cation concentration may increase cellular excitability by shifting the activation curve for voltage-gated Na+ channels (Hille, 2001.) Increased opening of NMDA receptor channels can lead to Ca2+ influx through the channels and to a large number of potential downstream effects. Because the induction of persistent ictal-like activity in the 4-AP/reduced divalent cation model was not protein synthesis-dependent, at least in its initial stages, the hypothesized mechanism of induction should not require a newly made protein. That leaves us to hypothesize post-translational modification of a protein and/or translocation of a protein that has already been made.
One of the outcomes of NMDA receptor activation and a rise in intracellular [Ca2+] can be a dephosphorylation-dependent suppression of GABAergic inhibition (Chen et al., 1990; Chen and Wong, 1995, Stelzer and Shi, 1994). Suppressed GABAergic inhibition generally promotes epileptiform activity (e.g., Miles and Wong, 1987; Stelzer et al., 1987; however, c.f. Kantrowitz et al., 2005 and Lopantsev and Avoli, 1998). Another possible downstream effect following NMDA receptor activation and Ca2+ entry would be the postsynaptic trafficking of NMDA and AMPA receptors to the synapses involved in producing the ictal-like events; an increased number of glutamate receptors at the synapse would create larger depolarizing responses to released transmitter. There is evidence that trafficking of AMPA and/or NMDA receptors to the activated synapse may account for the protein-synthesis-independent early phase of NMDA receptor-dependent long-term potentiation in the hippocampus and dentate gyrus (Grosshans et al., 2002; Shi et al., 1999; Williams et al., 2007). A third downstream effect of NMDA receptor activation and a rise in intracellular calcium can be the sustained opening of pannexin hemichannels (Locovei et al., 2006; Thompson et al., 2008) or post-exposure-current-(Ipe) channels (Chen et al. 1997, 1998; Ipe shares many features with current through pannexin hemichannels, suggesting that Ipe channels are pannexin hemichannels). This step would not require protein synthesis because closed hemichannels are constitutively present in the cell membrane (Zoidl et al., 2007). Current through open pannexin hemichannels may provide a sustained depolarization that itself may augment epileptiform activity (Thompson et al., 2008) or, alternatively, the large Ca2+ influx through the Ipe channels may promote cell death (Chen et al., 1997) which would change the neural network and possibly promote epileptiform activity (Kobayashi et al., 2003; Du et al., 1993; Du et al., 1995).
In conclusion, we have characterized a new in vitro model of epileptogenesis which is clearly distinct from the group I mGluR-dependent model, but which shares features with the repeated stimulation model. In this 4-AP/reduced divalent cation model, induction is dependent upon NMDA receptor activation but not upon group I mGluR activation or protein synthesis.
Acknowledgments
The authors thank RKS Wong, DS Ling, H Moreno, H Valsamis, LR Merlin, M Stewart, and A Bibbig for helpful discussion. The authors thank K Acker for assistance with data analysis.
Grants
This project was sponsored by National Institute of Neurological Disorders and Stroke Grant NS-047435 to KL Perkins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aram JA, Michelson HB, Wong RK. Synchronized GABAergic IPSPs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J Neurophysiol. 1991;65:1034–1041. doi: 10.1152/jn.1991.65.5.1034. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Holmes KH, Keele NB, Shinnick-Gallagher P. The functional role of metabotropic glutamate receptors in epileptiform activity induced by 4-aminopyridine in the rat amygdala slice. Brain Res. 1995;669:140–144. doi: 10.1016/0006-8993(94)01243-b. [DOI] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero G, Uva L, Gnatkovsky V, Avoli M, de Curtis M. Independent Epileptiform Discharge Patterns in the Olfactory and Limbic Areas of the In Vitro Isolated Guinea Pig Brain During 4-Aminopyridine Treatment. J Neurophysiol. 2010;103:2728–2736. doi: 10.1152/jn.00862.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AG, Yip PK, Yap JS, Quinn LP, Tang E, Harris JR, Meldrum BS. Anticonvulsant actions of LY 367385 ((+)-2-methyl-4-carboxyphenylglycine) and AIDA ((RS)-1-aminoindan-1,5-dicarboxylic acid) European Journal of Pharmacology. 1999;368:17–24. doi: 10.1016/s0014-2999(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Nanan K, Williams M, Meldrum BS. Anticonvulsant activity of two metabotropic glutamate group I antagonists selective for the mGlu5 receptor: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), and (E)-6-methyl-2-styryl-pyridine (SIB 1893) Neuropharmacology. 2000;39:1567–1574. doi: 10.1016/s0028-3908(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Chen QX, Perkins KL, Choi DW, Wong RKS. Secondary activation of a cation conductance is responsible for NMDA toxicity in acutely isolated hippocampal neurons. J Neurosci. 1997;17:4032–4036. doi: 10.1523/JNEUROSCI.17-11-04032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QX, Perkins KL, Wong RKS. Zn2+ blocks the NMDA- and Ca2+ -triggered postexposure current Ipe in hippocampal pyramidal cells. J Neurophysiol. 1998;79:1124–1126. doi: 10.1152/jn.1998.79.2.1124. [DOI] [PubMed] [Google Scholar]
- Chen QX, Stelzer A, Kay AR, Wong RK. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QX, Wong RKS. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol. 1995;482:353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RKS. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, Wilson WA. Mechanisms of epileptogenesis. Adv Neurol. 1999;79:607–30. [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Poutney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy Sciences. 1999;68:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Davson H, Welch K, Segal MB. Physiology and Pathophysiology of the Cerebrospinal Fluid. Edinburgh: Churchill Livingstone; 1987. p. 24. [Google Scholar]
- D’Antuono M, Louvel J, Köhling R, Mattia D, Bernasconi A, Olivier A, Turak B, Devaux A, Pumain R, Avoli M. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain. 2004;127:1626–1640. doi: 10.1093/brain/awh181. [DOI] [PubMed] [Google Scholar]
- De Guzman P, D’Antuono M, Avoli M. Initiation of electrographic seizures by neuronal networks in entorhinal and perirhinal cortices in vitro. Neuroscience. 2004;123:875–886. doi: 10.1016/j.neuroscience.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 1980;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Köhler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Jr, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Engel J, Schwartzkroin PA. What should be modeled? In: Pitkanen A, et al., editors. Models of Seizures and Epilepsy. Chapter 1. Elsevier Inc; 2006. [Google Scholar]
- Gibb AJ, Edwards FA. Microelectrode techniques. The Plymouth workshop handbook. Cambridge: The Company of Biologists Limited; 1987. Patch-clamp recording from cells in sliced tissues. [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Haas HL, Wieser HG, Yaşargil MG. 4-Aminopyridine and fiber potentials in rat and human hippocampal slices. Experientia. 1983;39:114–115. doi: 10.1007/BF01960661. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. chapter 20 Sinauer Associates, Inc; Sunderland, MA: 2001. [Google Scholar]
- Jones LS, Grooms SY, Lapadula DM, Lewis DV. Protein synthesis inhibition blocks maintenance but not induction of epileptogenesis in hippocampal slice. Brain Res. 1992;599:338–344. doi: 10.1016/0006-8993(92)90410-b. [DOI] [PubMed] [Google Scholar]
- Jones RS, Heinemann U. Pre- and postsynaptic K+ and Ca2+ fluxes in area CA1 of the rat hippocampus in vitro: effects of Ni2+, TEA and 4-AP. Exp Brain Res. 1987;68:205–209. doi: 10.1007/BF00255246. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Francis NN, Salah A, Perkins KL. Synaptic depolarizing GABA response in adults is excitatory and proconvulsive when GABAB receptors are blocked. J Neurophysiol. 2005;93:2656–2667. doi: 10.1152/jn.01026.2004. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Wen X, Buckmaster PS. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J Neurophysiol. 1998;79:352–360. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- Martín ED, Araque A, Buño W. Synaptic regulation of the slow Ca2+-activated K+ current in hippocampal CA1 pyramidal neurons: Implications in epileptogenesis. J Neurophysiol. 2001;86:2878–2886. doi: 10.1152/jn.2001.86.6.2878. [DOI] [PubMed] [Google Scholar]
- Merlin LR. Group I mGluR-mediated silent induction of long-lasting epileptiform discharges. J Neurophysiol. 1999;82:1078–1081. doi: 10.1152/jn.1999.82.2.1078. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Wong RKS. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RKS. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Merlo D, Cifelli P, Cicconi S, Tancredi V, Avoli M. 4-Aminopyridine-induced epileptogenesis depends on activation of mitogen-activated protein kinase ERK. J Neurochem. 2004;89:654–659. doi: 10.1111/j.1471-4159.2004.02382.x. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Activity-dependent modulation of K+ currents at presynaptic terminals of mammalian central synapses. J Physiol. 1999b;519:427–437. doi: 10.1111/j.1469-7793.1999.0427m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Saggau P. Modulation of transmitter release by action potential duration at the hippocampal CA3-CA1 synapse. J Neurophysiol. 1999;81:288–298. doi: 10.1152/jn.1999.81.1.288. [DOI] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782:240–247. doi: 10.1016/s0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FL, Johnston D. 4-aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Salah A, Perkins KL. Effects of subtype-selective group I mGluR antagonists on synchronous activity induced by 4-aminopyridine/CGP 55845 in adult guinea pig hippocampal slices. Neuropharmacology. 2008;55:47–54. doi: 10.1016/j.neuropharm.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah A, Perkins KL. 2008 Neuroscience Meeting Planner. Washington, D.C: Society for Neuroscience; 2008b. In vitro epileptogenesis in entorhinal and perirhinal cortices. Program No. 449.26. Online. [Google Scholar]
- Sanna PP, Berton F, Cammalleri M, Tallent MK, Siggins GR, Bloom FE, Francesconi W. A role for Src kinase in spontaneous epileptiform activity in the CA3 region of the hippocampus. Proc Natl Acad Sci U S A. 2000;97:8653–8657. doi: 10.1073/pnas.140219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975;85:423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Smolders I, Lindekens H, Clinckers R, Meurs A, O’Neill MJ, Lodge D, Ebinger G. In vivo modulation of extracellular hippocampal glutamate and GABA levels and limbic seizures by group I and II metabotropic glutamate receptor ligands. J Neurochem. 2004;88:1068–1077. doi: 10.1046/j.1471-4159.2003.02251.x. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ions in the Brain: Normal Function, Seizures, and Stroke. Oxford University Press; New York, NY: 2004. p. 16. [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Holmes GL, Thompson JL. MK801 pretreatment reduces kainic acid-induced spontaneous seizures in prepubescent rats. Epilepsy Res. 1993;14:41–48. doi: 10.1016/0920-1211(93)90073-g. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989;245:648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- Stelzer A, Shi H. Impairment of GABAA receptor function by N-methyl-D-aspartate-mediated calcium influx in isolated CA1 pyramidal cells. Neuroscience. 1994;62:813–828. doi: 10.1016/0306-4522(94)90479-0. [DOI] [PubMed] [Google Scholar]
- Stelzer A, Slater NT, ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326:698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Sutula T, Koch J, Golarai G, Watanabe Y, McNamara JO. NMDA receptor dependence of kindling and mossy fiber sprouting: evidence that the NMDA receptor regulates patterning of hippocampal circuits in the adult brain. J Neurosci. 1996;16:7398–7406. doi: 10.1523/JNEUROSCI.16-22-07398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- Williams JM, Guévremont D, Mason-Parker SE, Luxmanan C, Tate WP, Abraham WC. Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals. J Neurosci. 2007;27:14171–14178. doi: 10.1523/JNEUROSCI.2348-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto C. Intracellular study of seizure-like afterdischarges elicited in thin hippocampal sections in vitro. Exp Neurol. 1972;35:154–164. doi: 10.1016/0014-4886(72)90066-0. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff FJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience. 2007;146:9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]