Abstract
Maintenance of circulating, functional neutrophils and their robust recruitment to tissues in response to injury and/or microbial infection are critical for host defense. Equally important, though less well understood, are the processes for removal of these short-lived cells. Here we review recent findings of novel neutrophil characteristics that determine removal. These neutrophil-derived signals, in turn, can shape the responses of other cells and surrounding tissues and promote a return to homeostasis. If not removed, dying neutrophils disintegrate and release phlogistic cargo that can further contribute to ongoing inflammation, tissue destruction, or autoimmunity.
Removal of neutrophils from circulation and following tissue deployment
Neutrophils, the most numerous of circulating leukocytes, are readily mobilized innate immune cells that are critical for defense against bacteria and fungal pathogens [1]. These terminally differentiated cells are short-lived; their lifespans are generally measured in terms of hours, though a recent and provocative report suggests a circulation time in the blood of several days [2–4]. Neutrophils are continuously replenished during homeostasis, and their provision is augmented during infection or significant tissue injury, such that much of the bone marrow may become devoted to their production. Homeostatic removal of neutrophils from the circulation must match production and is mediated by macrophages in the liver, bone marrow stroma, and marginal zone of the spleen [4,5]. The kinetics of neutrophil production, release, equilibrium between circulating and marginated pools, and their removal have been recently reviewed [3,4,6,7].
Following injury, with or without infection, neutrophils are recruited to tissues by chemotactic factors presented in a temporally and spatially defined manner [8,9]. Within tissues, the neutrophils orchestrate many mononuclear cell activities, and progressively unleash an arsenal of diverse constituents for host defense, e.g. reactive oxygen species (ROS), antimicrobial peptides and serine proteases, many with potential for inflicting further tissue injury [1,10,11]. These recruited neutrophils are largely removed in situ by macrophages, although to a small extent also by dendritic cells and/or exodus to draining lymph nodes and following migration outside the body as in airways, gut, gingival crevice, etc. [12]. Despite their large numbers relative to tissue macrophages, neutrophil disappearance in resolving inflammation is often dramatic, such that detection of morphologically dead neutrophils is relatively infrequent in tissues during resolution (Fig. 1). In this context, it is important to recognize that neutrophil numbers at any given time represent simultaneous recruitment and removal combined, and few studies address the absolute flux of these cells within a given lesion. Thus, as we will discuss, in the absence of defects in clearance, removal is an efficient, high capacity process that in fact actively promotes resolution and restoration of tissue structure and function.
Fig. 1. Time course of tissue neutrophilia in acute inflammation.
a). Following tissue injury or infection (e.g. zymosan-induced peritonitis), neutrophils (N) are simultaneously recruited to the site and removed in situ by macrophages (M). Neutrophilia often resolves dramatically with accumulation of few apoptotic neutrophils supporting mechanisms for efficient, high-capacity clearance. b) Time course of efferocytosis by macrophages in peritonitis.
What marks a neutrophil for clearance? The focus of this review is to highlight emerging data regarding different mechanisms for neutrophil demise, their repertoire of signals to engage the macrophages, and to some extent, the consequences of these interactions. We start with three underlying concepts: i) neutrophil removal from either the circulating pool, or from tissues, depends on signals resulting from neutrophil death, activation or aging (referred to here as neutrophil “fates”), rather than by stochastic processes; ii) different neutrophil “fates” have unique phenotypic signatures, but also share an overlapping repertoire of signals that are important for recognition; and iii) the consequences of recognition and removal, or its failure, depend on the “mix” of neurophil-derived signals both quantitatively and qualitatively. Two caveats also deserve mention: much of the data come from in vitro studies and murine models, hence, extrapolating their relevance in human health and disease is often speculative.
Neutrophil “fates”: dead, activated or aged
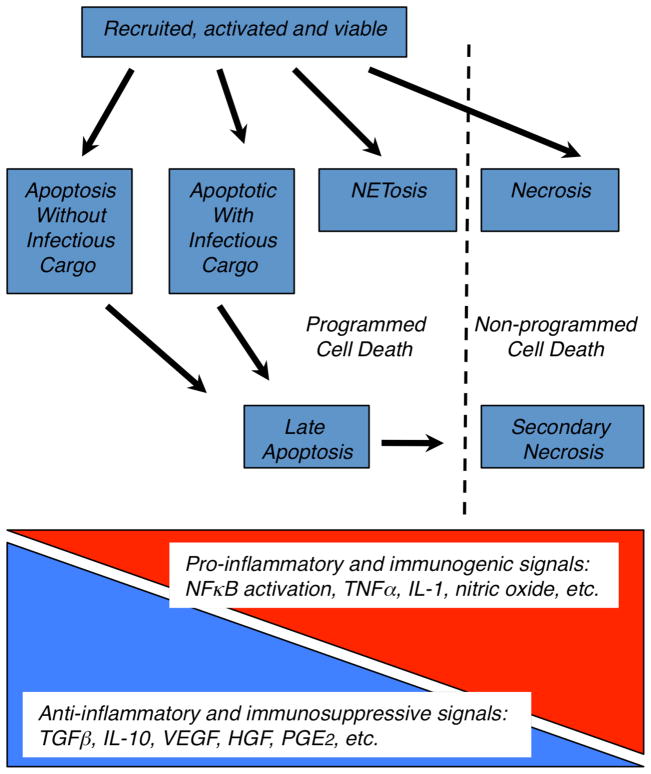
Cell death is an irreversible endpoint, but lacking a precise definition, may be considered to have occurred when i) plasma membrane integrity is lost, ii) the cell fragments into discrete bodies, or iii) the cell is engulfed by an adjacent cell [13,14]. While neutrophil longevity during inflammation may be extended by various stimuli including pattern recognition signals, growth factors or chemokines, it can also be shortened following phagocytosis of a number of pathogens (reviewed in [2,7]). Ultimately, however, neutrophils succumb via an expanding repertoire of possible death pathways (Fig. 2 and text box). Most importantly, these all result in the expression and/or exposure of signaling and recognition structures that can, under normal conditions, promote removal of the dying cells.
Fig. 2. Possible death pathways for neutrophils and impact on resolution or perpetuation of inflammation.
Recruited neutrophils succumb to various death pathways within tissues (blue boxes). Based on signaling for their recognition by macrophages, and the stage at which they are removed, inflammatory consequences will vary across a continuum from anti-inflammatory and immunosuppressive to pro-inflammatory and immunogenic.
Conceptually, neutrophils may also signal for removal before actually dying. Recent investigation has found that brief activation of the neutrophil NADPH oxidase generates surface ligands similar to those on apoptotic neutrophils, that enhance removal both in vitro and in vivo [15,16] (see below). These findings are consistent with an older report (in need of reinvestigation) that over-expression of the pro-survival protein, Bcl-2, to inhibit neutrophil apoptosis, nevertheless, did not delay their normal clearance [17]. Similarly, aging of neutrophils in the circulation may generate signals prior to obvious apoptosis/cell death. Murine studies that identfy a senescent neutrophil phenotype implicate CXCR4 re-expression in neutrophil removal in the bone marrow [4,6]. Whether such processes apply to the bulk removal of the cells in the liver and spleen is unknown. However, mathematical modeling in man suggests that neutrophil clearance, is not exclusively senescence-based, supporting the possible role of signals such as those associated with activation and/or apoptosis.
Although death endpoints are depicted as discrete in Fig. 2, overlap is considerable (text box). For example, neutrophils ingesting community-associated methicillin-resistant Staphylococcus aureus or Streptococcus pyogenes can undergo necrosis or accelerated apoptosis which culminates in cell lysis, so-called secondary necrosis [2,18]. Further, as outlined above, the underlying mechanisms driving death processes, often overlap. For example ROS from the NADPH oxidase may be essential for NETosis and autophagy, and often play a predominant role in apoptosis [19,20]. The latter is especially evident following pathogen ingestion [2], and the lack of ROS can prolong survival of neutrophils from chronic granulomatous disease patients [21]. ROS from various sources are also heightened in necrotic tissue. Hence the signals deriving from these different forms of neutrophil “death” are also significantly overlapping.
Neutrophil death: several ways to go.
Cellular necrosis follows an insult so devastating that influx of extracellular ions is rapid, and causes cell and organelle swelling, permeabilization of the plasma membrane and release of intracellular constituents.
Programmed cell death on the other hand is a genetically determined orchestrated death, and several processes have been described in neutrophils:
Neutrophil apoptosis can be mediated via both extrinsic and intrinsic pathways, and roles for ROS from the NADPH oxidase and/or mitochondria, increased calcium flux, activation of caspases, cathepsins and calpains, and pro-apoptotic proteins of the Bcl-2 family have been variously demonstrated [2,7]. Apoptosis is generally recognized by characteristic chromatin fragmentation, chromatin and cytoplasmic condensation with the formation of small apoptotic bodies. However, while neutrophils do exhibit the nuclear changes and undergo cell shrinkage, they do not release blebs or fragment to the extent of many cells. They do shed receptors and become functionally effete, demonstrating impaired chemotaxis, degranulation and ROS generation (reviewed in [2]).
A relatively newly described form of programmed cell death is called NETosis, for the striking generation of neutrophil extracellular traps (NETs) from decondensed nuclear chromatin. The process involves NADPH oxidase-dependent dissolution of the nucleus and intracellular membranes and rupture of the plasma membrane [20]. Notably, viable, intact neutrophils can also produce NETs depending on the conditions of stimulation; in this case, by ROS-dependent extrusion of mitochondrial DNA [22]. Thus, the appearance of NETs does not necessary signify neutrophil death. NETs are thought to both physically contain microbes and concentrate neutrophil antimicrobial products for enhanced killing and has been demonstrated in a number of in vivo systems [20,23].
Neutrophils also undergoing autophagy-associated death have been recently recognized. Autophagy is a recycling process prompted by conditions of nutrient limitation, in which a cell ingests and degrades redundant or damaged organelles and macromolecular components into double- or single-membrane lysosomal-derived vesicles. Large vacuoles in neutrophils associated with infection and autoimmunity likely represent autophagosomes [24]. In vitro, such structures can be identified following stimulation, in some cases in the presence of pro-survival factors such as GM-CSF to inhibit caspase activation and apoptosis [24]. These data suggest potential branch points in death pathways in which autophagy represents a survival pathway but when it fails, leads to apoptosis [25]. In neutrophils, however, these pathways have not been clearly delineated, though a requirement for a functioning NADPH oxidase has been demonstrated in autophagy [19].
Although a number of other types of programmed cell death have been described, e.g. pyroptosis, and oncosis [2,23], it is not clear at this point how relevant they are to neutrophil death processes.
An ever-expanding repertoire of signals are released from, or exposed on the surface of dying (or activated) neutrophils. These are then recognized by macrophages and other cells of the inflammatory milieu, and can lead to removal of the neutrophils as well as both anti- and pro-inflammatory consequences. Many of the intracellular constituents released during neutrophil necrosis are recognized by macrophages via TLRs and NLRs as damage-associated molecular patterns (DAMPs), e.g. cationic proteins, uric acid, HMGB-1, chromatin-bound IL-1a, etc. [26,27]. Typically such signals result in activation of NFκB resulting in pro-inflammatory cytokine production. Released proteases, such as neutrophil elastase can also be proinflammatory [18]. However, other neutrophil intracellular proteins such as annexin 1, α-defensin, and S100A9 are anti-inflammatory and may modulate the effects ordinarily associated with the recognition of DAMPs [28–30].
By striking contrast, apoptotic cells, including neutrophils, are more generally anti-inflammatory, anti-immunogenic and tissue restorative in that their recognition by macrophages and other cells can stimulate to various degrees production and secretion of factors such as TGFβ, IL-10, VEGF, etc. [31–33]. Notably, the plasma membrane of apoptotic cells remains intact initially. With time, if removal of the apoptotic cell is delayed, plasma integrity is lost (secondary necrosis, Fig. 2) but it is noteworthy that the apoptotic neutrophil seems to maintain its intact membrane for longer than most cells, possibly because of its reliance on glycolysis as an ATP source [12,34]. This persistence as an intact cell may importantly allow time for complete removal of a cell type that is so richly endowed with potentially tissue-damaging constituents. Further, in vitro, and most certainly in vivo, death and dying of a population of neutrophils is neither synchronous, nor homogeneous. For example, following optimized activation in vitro, only approximately a third of neutrophils can be induced to undergo death by NETosis [23].
“Palatability” of neutrophils, and their efferocytosis by macrophages
The process of cell corpse removal, called efferocytosis (“to carry to the grave”) has been best studied with regard to the signals and mechanics involved in the removal of apoptotic cells, including apoptotic neutrophils. Colloquially, these signals have been designated as “find me” signals that attract macrophages to the site of cellular demise, and “eat me” signals on the apoptotic cell surface that often cluster into signaling platforms (Table 1, Fig. 3) [12,35]. Of known “eat me” signals, many are bound “bridge” molecules, soluble pattern-recognition proteins of the innate immune system present in the milieu that opsonize the apoptotic target cell (reviewed in [36]). A number of changes on the dying cell surface lead either to binding of such molecules or to direct engagement of macrophage receptors. These include externalized phosphatidylserine (PS), altered lipids, changes in surface charges likely attributed to amino sugars, and exposure of intracellular molecules such as calreticulin, mitochondrial and nuclear constituents (Table 1). Amongst these signals, some ligand combinations promote adherence or “tethering” to macrophages, while others “tickle” the macrophage to induce uptake, or others do both, in a two-step “tether and tickle” model (Fig. 3) [12]. Further, these effects are opposed by “don’t eat me” systems, repulsive signals found on viable cells that must be removed, sequestered or otherwise disabled for uptake to occur.
Table 1.
Ligands, bridge molecules, and receptors for the recognition of dying cellsa
| Ligand on dying cell | Bridge molecules | Macrophage receptor |
|---|---|---|
| “Eat me” signals | ||
| PS b | PS recognition structures (BAI1, TIM-4, TIM-1, TIM-3, Stablin-2); scavenger receptors (SRA, SRB, etc.) | |
| “ | Annexins 1 and 2 | Homophilic interaction |
| “ | C1q | C1q receptors; Calreticulin/LRP (CD91) |
| “ | β2 glycoprotein 1 | ? |
| PS; oxidized PS c | Gas6, Protein S | TAM Rs (Tyro, Axl, Mer) |
| “ | MFG-E8, Del-1 | αvβ3/β5 integrins |
| “ | Collectins (SPA, SPD) | LRP (CD91) |
| “ | CD36 | |
| Oxidized PC c | CD36; other scavenger receptors; LOX-1; CD14; TLR4; PAFR | |
| Oxidized PC c; phosphorylcholine | Pentraxins (e.g. CRP); IgM natural antibodies; deposited complement after activation; | Complement Rs (CR3, CR4, CR1); |
| IgG autoantibodies | FcR | |
| Cardiolipin; oxidized cardiolipin | IgM natural antibodies; deposited complement after activation | Complement Rs |
| Calreticulinb | LRP (CD91) | |
| Mitochondrial and nuclear nucleic acids and associated constituents (histones, etc.) | Collectins, C1q, pentraxins (e.g. CRP), C4b binding protein, properdin, deposited complement after activation | LRP (CD91); complement Rs |
| Altered carbohydrates, nucleic acids, phospholipids | Ficolins; MBL; deposited complement after activation | MMR; lectin receptors; LRP; complement Rs |
| C3b and iC3b deposition | CR3, CR4, CR1 | |
| CD31 c | fibronectin | αvβ1/CD31 |
| CD43, polyglycosamine side chains | Nucleolin | |
| CD14 c | ICAM3 | |
| PS; unknown | Thrombospondin b | CD36/αvβ3/β5; LRP |
| ? | adiponectin | Calreticulin/LRP |
| -- | Crosslinking antibody | CD44 b |
| PTX3 translocated to the surface of late apoptotic neutrophils | C1q and deposited complement after activation | LRP (CD91); complement Rs |
| “Don’t eat me” signals | ||
| CD47 b | SIRPα | |
| CD31 | CD31 (homophilic interaction) | |
| PAI-1 b | ||
| uPAR b | uPAR (homophilic interaction) | |
| PR3 b | ? | |
| Nuclear debris | Soluble PTX3 | ? |
Specifically shown to play a role in neutrophil removal.
Demonstrated for other cell types, and studied in neutrophil removal, but role in neutrophil removal not demonstrated.
Abbreviations: PS, phosphatidylserine; BAI1, brain-specific angiogenesis inhibitor 1; TIM, T cell Ig and mucin; SRA, scavenger receptor A; SRB, scavenger receptor D; LRP, lipoprotein receptor-related protein; TAM, Tyro Axl Mer; MFG-E8, milk fat globule-EGF factor 8 (lactadherin); Del-1, developmental endothelial locus-1; SPA, surfactant protein A; SPD, surfactant protein D; PC, phosphatidylcholine; LOX-1, oxidized LDL receptor 1; PAFR, platelet activating factor receptor; TLR, Toll-like receptor; CRP, C-reactive protein; MBL, mannose binding lectin; MMR, macrophage mannose receptor; PTX3, pentraxin-related protein 3; SIRPα, signal-regulatory protein alpha; PAI-1, plasminogen activator inhibitor-1; uPAR, urokinase plasminogen activator receptor; PR3, proteinase 3.
Fig. 3. Signaling for efferocytosis.
An apoptotic cell presents signals on its surface or recruits bridge molecules from the milieu that serve as “eat me” signals to macrophages. “Eat me” signals both tether the apoptotic cell to the macrophage and “tickle” or signal for it to reorganize its cytoskeleton for uptake. Uptake is opposed by “don’t eat me” signals usually cleared from the cell or segregated from “eat me” signals as the neutrophil undergoes apoptosis. Other factors serve as enhancers to improve efficiency of uptake, or as inhibitors to suppress uptake. Figure published with permission of the Journal of Leukocyte Biology.
The engulfment process, under the control of the RhoGTPases, Rac1 and Cdc42, is thought to result in the formation of a spacious phagosome surrounding the apoptotic cell and imbibed fluid from the extracellular milieu by a process akin to macropinocytosis [12]. Others have suggested that the macrophage membrane surrounds the apoptotic cell by a “zipper” mechanism where ligands on the apoptotic cell are sequentially engaged to form a tight-fitting phagosome, at least with cells early in the apoptotic process [37]. The overall processes almost certainly depend on the state of the cell and the combined repertoire and presentation of signals. For instance, necrotic neutrophils may release intracellular constituents acting as “find me” signals, but have little chance to assemble “eat me” signaling platforms or disable “don’t eat me” signals. NETosising neutrophils may be recognized by exposed DNA and histones, but their efferocytosis may be slowed by PR3 (Table 1; see below) and degradation of the NETs by DNAses may be required for their removal. Further descriptions of individual molecules, particularly where information is available to implicate roles in the removal of dead, activated or aged neutrophils, are described in greater detail below and shown in Table 1.
“Find me” signals
A number of diffusible signals, e.g. ATP and UTP, and DAMPS released from permeabilized cells and to some extent apoptotic cells [35,38], are chemoattractants for many types of inflammatory cells. Other “find me” signals released from apoptosing cells are more selective for monocytes/macrophages and dendritic cells (e.g. fractalkine, lysophosphatidylcholine, and sphingosine-1-phosphate) [35,38], and others, such as lactoferrin, appear to actively inhibit neutrophil migration [39]. The roles of such “find me” signals in orchestrating neutrophil removal under various inflammatory circumstances have not been thoroughly investigated, and it might be argued that such signals are unnecessary: monocytes and macrophages are invariably recruited in acute inflammation by monokines and products released by activated neutrophils or other inflammatory cells, e.g. eicosanoids and alarmins such as α-defensin [10,40], as well as products of inflammatory tissue injury. Notably, although the lipid signal, lysophosphatidylcholine has been described as a “find me” signal for late apoptotic cells of various cell types, we have been unable to identify this lipid product in apoptosing neutrophils after prolonged culture [15].
“Eat me” signals
The best described “eat me” signal is phosphatidylserine, PS. Normally sequestered in the plasma membrane inner leaflet, PS exposure on the cell surface leads to recognition of its headgroup by an increasing number of bridge molecules and macrophage receptors (Table 1) [12,36]. The near-universal appearance of PS on apoptotic cells, and its detection by annexin V, has become a standard assay for detection of apoptosis. However, PS is also exposed, at least to some degree, as neutrophils (and other cell types) undergo activation [16,41] and by definition, after membrane disruption as in necrosis, and death associated with NETosis [42]. PS exposure, even in the absence of evidence of apoptosis by TUNEL staining, was shown to mediate neutrophil uptake into the liver of rats following LPS injection [4]. Maintenance of PS on the inner leaflet of unstimulated, viable cells requires ATP and aminophospholipid translocases. However, translocase function is impaired by oxidative or nitrosative stress, ATP depletion or high levels of intracellular calcium during cell death [12]. Also, as a separate event, increased transbilayer flip-flop of all phospholipid classes (which leads to PS exposure) occurs during neutrophil activation as well as apoptosis. Whether phospholipid flip-flop represents a membrane repair process or is the result of specific “scramblases” or “floppases” is not yet clear [43,44]. Calreticulin is an additional “eat me” signal recognized by LDL receptor related protein (LRP) on macrophages or by collectins as bridge molecules [45]. A number of additional bridge molecules have been associated with neutrophil removal. These include thrombospondin, annexin 1, deposited complement, and long pentraxin (PTX3) stored in neutrophils and translocated to the surface during apoptosis [2,12,30,46].
Oxidized phospholipids (oxPL), of both the PS and phosphatidylcholine (PC) subclasses, are generated in some dying cells, and enhance binding of certain bridge molecules to facilitate recognition through scavenger receptors (e.g. CD36) expressed on macrophages [47]. Considering the oxidizing capacity of the neutrophil NADPH oxidase, it was hypothesized that oxPL species would comprise “eat me” signals during both activation and apoptosis. Surprisingly, oxPL species were not produced by human neutrophils in vitro or murine neutrophils in vivo in a peritonitis model [15,16]. In contrast, up to 20% of the PS pool was converted to a PS species containing only one fatty acyl constituent, lysophosphatidylserine (lyso-PS) in an NADPH oxidase-dependent manner. Lyso-PS remained cell-associated and signaled to macrophages via the G-protein receptor, G2A, to enhance, but not initiate, neutrophil removal in vitro and in vivo [15,16]. As such, lyso-PS may be a key signal for high capacity uptake of activated neutrophils even before they show signs of programmed cell death.
“Don’t eat me” signals and suppressors of efferocytosis
A number of molecules have been identified as “don’t eat me” signals on viable neutrophils. CD47 on viable cells signals to macrophages via the inhibitory receptor, SIRPα, and such signaling is diminished with apoptosis leading to enhanced uptake [45,48]. Similarly, PAI-1, a member of the SERPIN family of serine protease inhibitors, appears to colocalize with calreticulin on viable neutrophils, where it is thought to block or diminish its “tickling” signal to macrophages, but it segregates from calreticulin during neutrophil apoptosis [49]. Genetic deficiency or blockade of PAI-1 rendered both viable and apoptotic neutrophils more palatable to macrophages, an effect abrogated by its replenishment. Other candidates include the urokinase receptor (uPAR) and CD31 both of which have altered expression during neutrophil activation and/or apoptosis [50–52].
Suppressors of neutrophil removal are also described, e.g. PR3, which is mobilized from neutrophil secretory vesicles to the cell surface along with PS during early apoptosis [53]. Following genetic deletion of PR3, efferocytosis of neutrophils doubled in vitro. Though enzymatic activity of PR3 was not required, hydrophobic interactions of PR3 to signaling lipids was hypothesized. Likewise, released neutrophil elastase and cathepsins may serve to limit efferocytosis by cleaving macrophage surface receptors or ligands on the surface of the apoptosing cell, leading to inefficient clearance as seen, for example, in cystic fibrosis [54].
The milieu and macrophage programming in efferocytosis of neutrophils
Aside from signals on the neutrophil itself, efferocytosis obviously depends on factors in the microenvironment and the status of macrophage programming which determines their “readiness” to engulf [55–57]. First, availability of bridge molecules is critical. For example, efferocytosis is much more efficient in the presence of serum in vitro, and presumably proteins in the interstitial fluid and inflammatory exudate contribute in vivo. Notably, some bridge molecules are tissue specific, e.g. SPA and SPD are found primarily (though not exclusively) in the lung, and other bridge molecules such as MFG-E8 are produced by macrophages themselves. Genetic manipulation has demonstrated significant roles for individual bridge molecules (e.g. MFG-E8 and C1q [12]). Other efferocytosis enhancers in the milieu have also been described. Annexin 1 and derived peptides (released by activated and apoptosing neutrophils themselves as well as other cells [30,58]), adiponectin, and the lipids, lipoxins, protectins, resolvins, and maresins, enhance efferocytose or general phagocytic capability [59,60]. In the opposite direction, inhibitors such as TNFα and oxidants likely add to the balance that controls overall efficiency of neutrophil removal [12,61].
Macrophages programmed towards so-called “alternative activation” are more efficient at efferocytosis. Pivotal roles for the nuclear receptors, LXR, PPARγ and/or PPARδ, which in turn, upregulate efferocytic receptors and bridge molecules, have been demonstrated [55,57,62]. Interestingly, PS itself can drive PPARγ upregulation [63] as can oxidation products which act either as PPARγ activators or via Nrf2 [64], suggesting that apoptotic cells in general, and neutrophils in particular, might influence their own uptake by macrophages. However, under certain circumstances, exposure to apoptotic cells can also lead to macrophage deactivation via adenylyl cyclase activation leading to their inability to efferocytose [65]. Conversely, programming of macrophages towards “classical activation” is associated with decreased efferocytosis, and its association with a growing list of chronic autoimmune states disease states, for example, SLE, COPD, CF, severe asthma and CGD [31,54,66,67], raises intriguing questions about the role in these circumstances for defective neutrophil clearance. Notably, macrophage programming can be modulated therapeutically. For example glucocorticoids, statins, azithromycin all enhance efferocytosis [54,58,68], and therapeutic IFNγ nonspecifically enhances general phagocytic capacity [69].
Consequences of apoptotic neutrophil recognition
The consequences of neutrophil recognition and removal depend on neutrophil fate, the signals generated, programming of macrophages, and the playing field in which these events take place. Given the size of this subject, only a few outcomes are highlighted below and the reader is directed to several recent references [18,31,32,54,67,70,71].
Physical removal of neutrophils is the most obvious consequence of recognition by macrophages, and is required to return tissues to their normal architecture and function. While it is thought that neutrophils and their debris are ultimately digested within the macrophage phagolysosome, macrophages have also been shown to co-opt ingested neutrophil granule proteins for antimicrobial defense, e.g. against mycobacteria [72]. Removal also serves to protect tissues from further injury due to neutrophil constituents. Conversely, failure of removal, allows further deterioration of neutrophils regardless of mechanism of death [18]. If enough disintegrate in a given locale, tissue liquefaction results in formation of a puss-filled abscess [1]. In other instances, failure of neutrophil removal results in the so-called “nuclear dust” characteristic of lesions of ANCA positive polyarteritis [73]. Significantly, autoimmunity is often associated with clearance defects, and in particular, the production of anti-neutrophil antibodies [18,67]. Specifically the generation of ANCA directed against neutrophil PR3 and other cytoplasmic epitopes are both a marker of defects in clearance and are pathogenic by heightening neutrophil death and their inflammatory recognition [74].
A major role of macrophages is the production of molecules, e.g. cytokines and chemokines that “shape” inflammatory, as well as homeostatic tissue events [5,75]. The recognition of dying cells, results in profound alterations in macrophage responses. As noted above, responses to neutrophils, dying, activated or aged, represent a continuum (Fig. 2). At one extreme, TGFβ and/or IL-10 production by macrophages exposed to apoptosing neutrophils suppresses pro-inflammatory cytokine, chemokine and eicosanoid production (Fig. 2). While anti-inflammatory signaling is thought of as important for resolution of inflammation, the consequences are not always without detriment. Over-exuberant fibrotic responses driven by TGFβ are an example. Another is the immunosuppression of macrophages that accompanies ingestion of infected neutrophils, the so-called Trojan horse effect, which results in enhanced infection by certain microbes [12]. Under homeostatic conditions, neutrophil recruitment into tissues and their recognition by phagocytes is thought to provide a “sensor” for regulating a neutrophil “turnstile,” matching bone marrow production and release with removal [5,6,76]. Interestingly, depending on the model, divergent macrophage “sensor” populations appear to respond differently with regard to production of G-CSF, a growth factor essential for neutrophil production and release from the bone marrow. On the one hand, recognition and engulfment of senescent neutrophils by bone marrow stromal macrophages reportedly enhances G-CSF production [6]. Alternatively, recognition of neutrophils by peripheral tissue macrophages reportedly suppresses production of G-CSF to “tune down” neutrophil production [5,76]. In these latter models, blockade of neutrophil migration into tissues and/or their recognition by macrophages resulted in heightened G-CSF-driven neutrophil production. In other models, phagocyte recognition of neutrophils containing infected material enhances their production of TGFβ, but also of IL-6 and IL-23, which together generate Th17 cells that subsequently enhance G-CSF for “stress” neutrophil production [70]. The resultant neutrophilia insures reinforcements for sterilizing tissues. As hypothesized, the signals produced on or by neutrophils, aged, activated or dying, and the character of pertinent factors in the milieu, including the programming of the responding macrophages, are likely key in determining the varying outcomes of their interaction.
Concluding remarks
Undeniably, the primary function of neutrophils is host defense that, in turn, depends on adequate numbers in circulation and timely recruitment to sites of bacterial and fungal invasion. Once sterilization is accomplished, removal of neutrophils is imperative for the return of tissues to their functional state and to prevent further injury as these short-lived cells die and lose integrity. Aside from antimicrobial duties, neutrophils are also recruited during acute inflammation (e.g. in ischemia-reperfusion) and various chronic disorders (e.g. chronic obstructive pulmonary disease), where they contribute to tissue injury and underlying pathology. Emerging data provide new insights into the expanding array of mechanisms by which these cells are recognized and removed by phagocytes. The repertoire of signals generated by and on neutrophils as they age, are activated or ultimately die, either in the circulation or on the battlefield of injured or infected tissues, helps to titrate their numbers, modulate their activities and determine their legacy. Though much remains to be learned, an increasingly sophisticated understanding of the nature of these signals, their interpretation by other cells within the tissues, and their roles in the orchestration of subsequent host responses, may allow us unique opportunities to intervene and modify such responses where needed to either enhance host defense, or conversely, prevent inappropriate injury.
Acknowledgments
The authors wish to thank Ms. Brenda Sebern for preparation of the manuscript. This work was funded by HL34303, GM61031, HL68864, HL81151.
Abbreviations
- ANCA
anti-neutrophil cytoplasmic antibodies
- ATP
adenosine triphosphate
- BAI1
brain-specific angiogenesis inhibitor 1
- CF
cystic fibrosis
- CGD
chronic granulomatous disease
- COPD
chronic obstructive pulmonary disease
- CRP
C-reactive protein
- Del-1
developmental endothelial locus-1
- FcR
Fc receptor
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte macrophage-colony stimulating factor
- HMGB-1
high-mobility group protein B1
- IL-6
interleukin 6
- IL-10
interleukin 10
- IL-23
interleukin 23
- IFNγ
interferon gamma
- LDL
low-density lipoprotein
- LOX-1
oxidized LDL receptor 1
- LPS
lipopolysaccharide
- LRP
lipoprotein receptor-related protein
- LXR
liver X receptor
- Lyso-PS
lysophosphatidylserine
- MBL
mannose binding lectin
- MFG-E8
milk fat globule-EGF factor 8 (lactadherin)
- MMR
macrophage mannose receptor
- NADPH
nicotinamide adenine dinucleotide phosphate
- NETs
Neutrophil Extracellular Traps
- NFκB
nuclear factor kappa B
- NLR
Nod-like receptor
- Nrf2
NF-E2-related factor 2
- PAFR
platelet activating factor receptor
- PAI-1
plasminogen activator inhibitor-1
- PC
phosphatidylcholine
- PPARδ
peroxisome proliferator-activated receptor delta
- PPARγ
peroxisome proliferator-activated receptor gamma
- PR3
proteinase 3
- PS
phosphatidylserine
- PTX3
pentraxin-related protein 3
- ROS
reactive oxygen species
- SERPIN
serine proteinase inhibitors
- SIRPα
signal-regulatory protein alpha
- SLE
systemic lupus erythematosus
- SPA
surfactant protein A
- SPD
surfactant protein D
- SRA
scavenger receptor A
- SRB
scavenger receptor D
- TAM
Tyro Axl Mer
- TGFβ
transforming growth factor beta
- Th17
T helper 17
- TIM
T cell Ig and mucin
- TNFα
tumor necrosis factor alpha
- TLR
Toll-like receptor
- TUNEL
terminal deoxynucleotide transferase dUTP neck end labeling
- uPAR
urokinase plasminogen activator receptor
- UTP
uridine triphosphate
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Donna L. Bratton, National Jewish Health, 1400 Jackson Street, Room A540, Denver, CO 80206
Peter M. Henson, National Jewish Health, 1400 Jackson Street, Room A539, Denver, CO 80206
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 3.Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 4.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordy C, et al. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2011;117:618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witko-Sarsat V, et al. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011 doi: 10.1016/j.it.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Billadeau DD. PTEN gives neutrophils direction. Nat Immunol. 2008;9:716–718. doi: 10.1038/ni0708-716. [DOI] [PubMed] [Google Scholar]
- 9.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 10.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 11.Soehnlein O, et al. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Henson PM, Bratton DL. Recognition and removal of apoptotic cells. In: Russell DG, Gordon S, editors. Phagocyte-pathogen interactions: macrophages and the host responses to infection. ASM Press; 2009. pp. 341–365. [Google Scholar]
- 13.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 14.Mevorach D, et al. What do we mean when we write “senescence,” “apoptosis,” “necrosis,” or “clearance of dying cells”? Ann N Y Acad Sci. 2010;1209:1–9. doi: 10.1111/j.1749-6632.2010.05774.x. [DOI] [PubMed] [Google Scholar]
- 15.Frasch S, et al. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of RAC activity. J Biol Chem. 2011 doi: 10.1074/jbc.M110.181800. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frasch SC, et al. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J Biol Chem. 2008;283:33736–33749. doi: 10.1074/jbc.M807047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva MT, et al. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463–482. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitroulis I, et al. Regulation of the autophagic machinery in human neutrophils. Eur J Immunol. 2010;40:1461–1472. doi: 10.1002/eji.200940025. [DOI] [PubMed] [Google Scholar]
- 20.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Sanmun D, et al. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am J Physiol Cell Physiol. 2009;297:C621–631. doi: 10.1152/ajpcell.00651.2008. [DOI] [PubMed] [Google Scholar]
- 22.Yousefi S, et al. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 23.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Gunten S, Simon HU. Autophagic-like cell death in neutrophils induced by autoantibodies. Autophagy. 2007;3:67–68. doi: 10.4161/auto.3436. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, et al. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen I, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peter C, et al. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis. 2010;15:1007–1028. doi: 10.1007/s10495-010-0472-1. [DOI] [PubMed] [Google Scholar]
- 28.De Lorenzo BH, et al. Macrophage suppression following phagocytosis of apoptotic neutrophils is mediated by the S100A9 calcium-binding protein. Immunobiology. 2010;215:341–347. doi: 10.1016/j.imbio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Miles K, et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scannell M, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- 31.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox S, et al. Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun. 2010;2:216–227. doi: 10.1159/000284367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Regulation of interleukin-10 gene expression in macrophages engulfing apoptotic cells. J Interferon Cytokine Res. 2010;30:113–122. doi: 10.1089/jir.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunn AV, et al. Characterisation of secondary metabolites associated with neutrophil apoptosis. FEBS Lett. 1996;392:295–298. doi: 10.1016/0014-5793(96)00839-3. [DOI] [PubMed] [Google Scholar]
- 35.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litvack ML, Palaniyar N. Review: Soluble innate immune pattern-recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation. Innate Immun. 2010;16:191–200. doi: 10.1177/1753425910369271. [DOI] [PubMed] [Google Scholar]
- 37.Krysko DV, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 38.Chekeni FB, Ravichandran KS. The role of nucleotides in apoptotic cell clearance: implications for disease pathogenesis. J Mol Med. 2011;89:13–22. doi: 10.1007/s00109-010-0673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bournazou I, et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soehnlein O, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frasch SC, et al. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirnikjoo B, et al. Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis. J Biol Chem. 2009;284:22512–22516. doi: 10.1074/jbc.C109.022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki J, et al. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 45.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Jaillon S, et al. Endogenous PTX3 translocates at the membrane of late apoptotic human neutrophils and is involved in their engulfment by macrophages. Cell Death Differ. 2009;16:465–474. doi: 10.1038/cdd.2008.173. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg ME, et al. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence DW, et al. Decreased CD47 expression during spontaneous apoptosis targets neutrophils for phagocytosis by monocyte-derived macrophages. Early Hum Dev. 2009;85:659–663. doi: 10.1016/j.earlhumdev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park YJ, et al. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci U S A. 2008;105:11784–11789. doi: 10.1073/pnas.0801394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown S, et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 51.Park YJ, et al. Participation of the urokinase receptor in neutrophil efferocytosis. Blood. 2009;114:860–870. doi: 10.1182/blood-2008-12-193524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pliyev BK. Activated human neutrophils rapidly release the chemotactically active D2D3 form of the urokinase-type plasminogen activator receptor (uPAR/CD87) Mol Cell Biochem. 2009;321:111–122. doi: 10.1007/s11010-008-9925-z. [DOI] [PubMed] [Google Scholar]
- 53.Kantari C, et al. Proteinase 3, the Wegener autoantigen, is externalized during neutrophil apoptosis: evidence for a functional association with phospholipid scramblase 1 and interference with macrophage phagocytosis. Blood. 2007;110:4086–4095. doi: 10.1182/blood-2007-03-080457. [DOI] [PubMed] [Google Scholar]
- 54.Krysko O, et al. Impairment of phagocytosis of apoptotic cells and its role in chronic airway diseases. Apoptosis. 2010;15:1137–1146. doi: 10.1007/s10495-010-0504-x. [DOI] [PubMed] [Google Scholar]
- 55.Majai G, et al. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37:1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 56.Martinez FO, et al. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 57.Mukundan L, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 59.Serhan CN, et al. Novel anti-inflammatory - pro-resolving mediators and their receptors. Curr Top Med Chem. 2011 doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takemura Y, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borges VM, et al. TNFalpha inhibits apoptotic cell clearance in the lung, exacerbating acute inflammation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L586–595. doi: 10.1152/ajplung.90569.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Boyanapalli RF, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho HY, et al. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medeiros AI, et al. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez-Boyanapalli R, et al. PPARgamma activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood. 2010;116:4512–4522. doi: 10.1182/blood-2010-02-272005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munoz LE, et al. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 68.Mukaro VR, Hodge S. Airway Clearance of Apoptotic Cells in COPD. Curr Drug Targets. 2010 doi: 10.2174/138945011794751609. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez-Boyanapalli R, et al. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brereton CF, Blander JM. The unexpected link between infection-induced apoptosis and a Th17 immune response. J Leukoc Biol. 2011 doi: 10.1189/jlb.0710421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green DR, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan BH, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 73.Lynch JM, Barrett TL. Collagenolytic (necrobiotic) granulomas: part 1--the “blue” granulomas. J Cutan Pathol. 2004;31:353–361. doi: 10.1111/j.0303-6987.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 74.Lande R, et al. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goren I, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]