Abstract
Our oral cancer chemoprevention trial data implied that patient-specific differences in local retention and metabolism of freeze-dried black raspberries' (BRB) components affected therapeutic responsiveness. Subsequent studies have confirmed that anthocyanins are key contributors to BRB's chemopreventive effects. Consequently, functional assays, immunoblotting and immunohistochemical analyses to evaluate levels and distribution of BRB anthocyanin-relevant metabolic enzymes in human oral tissues were performed. LC-MS/MS analyses of time course saliva samples collected following BRB rinses were conducted to assess local pharmacokinetics and compare the capacities of three different BRB rinse formulations to provide sustained intraoral levels of anthocyanins. Protein profiles demonstrated the presence of key metabolic enzymes in all 15 oral mucosal tissues evaluated while immunohistochemistry confirmed these enzymes were distributed within surface oral epithelia and terminal salivary ducts. β-glucosidase assays confirmed that whole and microflora-reduced saliva can deglycosylate BRB anthocyanins, enabling generation of the bioactive aglycone, cyanidin. LC-MS/MS analyses demonstrated retention of parent anthocyanins and their functional, stable metabolite, protocatechuic acid, in saliva for up to 4 hours after rinsing. Furthermore, post-rinse saliva samples contained glucuronidated anthocyanin conjugates, consistent with intracellular uptake and Phase II conversion of BRB anthocyanins into forms amenable to local recycling. Our data demonstrate that comparable to the small intestine, the requisite hydrolytic, Phase II and efflux transporting enzymes necessary for local enteric recycling are present and functional in human oral mucosa. Notably, inter-patient differences in anthocyanin bioactivation and capacities for enteric recycling would impact treatment as retention of bioactivated chemopreventives at the target site would sustain therapeutic effectiveness.
Keywords: anthocyanins, enteric recycling, oral chemoprevention, bioactivation, sustainability
Results from our recent Phase I/II pilot chemopreventive trial revealed that approximately one third of the trial participants were “high level responders” (1, 2). As such, their premalignant oral lesions responded to topical application of a gel containing 10% w/w freeze dried black raspberries (BRB) (0.5 gm gel applied 4 times, total 2 gm daily) by improvement in all parameters assessed including histopathology, gene expression and reduction in loss of heterozygosity indices (1, 2). The study design accommodated for the extensive inter-patient heterogeneity in oral dysplastic lesions by inclusion of pretreatment incisional biopsies to establish pretreatment baseline lesional parameters and provide a confirmed histopathologic diagnosis (1,2). The beneficial therapeutic responsiveness did not correlate with a lower baseline histologic grade as some of the optimal effects were obtained in persons with higher grade, previously recalcitrant-to-treatment dysplastic lesions (1,2). These findings imply that patient-specific differences in local pharmacokinetics i.e. target tissue absorption, metabolic bioactivation, and local retention of the BRB constituents affected chemopreventive responsiveness. Subsequent BRB gel pharmacokinetic analyses conducted in volunteers with healthy oral mucosa, which revealed extensive inter-participant differences with regard to gel absorption, distribution and local BRB compound retention, support this premise (3). Identification of the pharmacokinetic and metabolic parameters that modulate BRB gel effectiveness could help to optimize chemopreventive strategies. For example, if a BRB metabolite is determined to deliver enhanced chemopreventive effects, then subsequent formulations could incorporate the more bioactive metabolite instead of the parent compound. This approach would provide equal therapeutic benefits to those patients with low bioactivating enzyme activities. Furthermore, identification of key therapy-modulating pharmacokinetic parameters such as absorption or tissue penetration could direct future topical agent formulations e.g. addition of penetration enhancing compounds. Finally, patient-specific pretreatment metabolic profiling, assessed relative to the identified BRB modulating parameters, could also be used to predict clinical outcomes. Supplemental strategies, such as introduction of additional chemopreventive compounds or different delivery systems, could then be employed on patients' lesions that show poor uptake and fail to bioactivate BRB chemopreventive compounds.
BRB, which served as the active agents in the gel, contain numerous compounds with potential chemopreventive activities, including anthocyanins, ellagic, ferulic, and p-coumeric acids, vitamins (ascorbic acid, α and β-carotene and folate), and minerals (4). It is, however, the BRB anthocyanins i.e. cyanidin 3-rutinoside, cyanidin 3-xylosylrutinoside, cyanidin 3-glucoside and cyanidin 3-sambubioside, which comprise the predominant (3 to 5% BRB weight) polyphenolic compounds in BRB and provide a large component of the chemopreventive impact (5). Our laboratories have shown that the anthocyanin-enriched fraction is the BRB component responsible for inhibition of benzo-a-pyrene diol epoxide (ultimate tobacco-associated carcinogen), redox-mediated activation of the pleiotropic transcription activating factors NF-κB and AP-1 (6). Subsequent studies by Wang et al. which established that BRB anthocyanins prevent esophageal tumors in rats, confirmed the in vivo applicability of Hecht et al.'s in vitro bioassay results (7). Due to their obvious importance in BRB chemoprevention, anthocyanin metabolism is the focus of this study.
Deglycosylation of the parent BRB anthocyanin to its respective aglycone enhances chemopreventive impact by removing the bulky sugar groups which could cause steric hindrance, replacing the glucosidic linkage by another antioxidant-scavenging hydroxyl group and facilitating transport independent cell uptake (8). Previous studies by Walle et al. demonstrated that human saliva and oral microflora generate aglycones from intact flavonoid glycosides e.g. genisten, with β-glucosidase representing the key responsible enzyme (9). Additional studies by Fleschhut et al. showed that gastrointestinal flora not only metabolize monoglycosylated anthocyanins (such as the recognized less stable cyanidin 3-glucoside) but also deglycosylate the more complex diglycosylated and acetylated anthocyanins (10). In addition to deglycosylation, anthocyanins can be metabolized by Phase II enzymes [UDP-glucuronosyl transferases (UGTs) and sulfotransferases (SULTs)] and also undergo methylation via catechol-O-methyltransferase (11). Phase II enzymes are typically associated with enhancing solubility to facilitate compound elimination. Recent investigations by Hu et al., which imply that Phase II metabolites of flavonoids undergo enteric recycling within the gastrointestinal tract, suggest an expanded role for Phase II enzymes (12, 13). Oral mucosal anthyocyanin recycling would augment chemopreventive efficacy by increasing contact time of the BRB chemopreventives with the target premalignant oral epithelial cells. As the mouth is contiguous with the more distal components of the gastrointestinal tract, oral cavity enteric recycling is logical and plausible.
Potential contributors to intraoral anthocyanin metabolism include oral tissues, saliva, and oral microflora. Accordingly, this study investigated the effects of these oral components on parameters likely to modulate BRB anthocyanins' chemopreventive efficacy. Tissue analyses included a comprehensive profiling of enzymes of interest that could affect anthocyanin bioactivation and local intraoral levels. Saliva and oral microflora contributions to anthocyanin metabolism were assessed via pharmacokinetic analyses of three different BRB rinse formulations as well as functional activity assays. Collectively, our data support the prospect that enteric recycling of anthocyanins occurs in the mouth. Furthermore, inter-patient variations in the extent of this process could directly impact the effects of locally delivered chemopreventives.
Materials and Methods
Participation of human subjects
Human subject participation in these studies was in accordance with Ohio State University Institutional Review Board approval and followed the tenets of the Declaration of Helsinki 1964. Ten clinically healthy, nonsmoking volunteers between the ages of 19 to 61 participated in the rinse study. These same 10 individuals plus an additional participant provided saliva samples for the functional β-glucosidase activity assay. This eleventh saliva donor was also a non-smoking, clinically healthy male aged 30. Fifteen donor tissues, obtained from 15 consented individuals undergoing elective oral maxillofacial surgical procedures were employed for the enzyme metabolic profiling studies by Western blot analyses. As only 10 of the tissues employed for immunoblotting contained adequate tissue for immunohistochemical analyses (IHC), five additional clinically normal oral tissues for the IHC studies were obtained from consented donors. All human subjects participants had uncomplicated medical histories which were characterized by no hospitalizations with the exception of elective procedures, ASA PS1 health status, healthy oral tissues and no mucosal or intrabony pathologies.
Preparation of the BRB rinse formulations
Three BRB rinse formulations were evaluated in this study (See Table 1 for description of the three rinses). All constituents were pharmaceutical grade, and the rinses were prepared fresh on the day of the assay under a laminar flow sterile hood. Rinses were prepared with the same amount of BRB (10% w/w) that was used in our gel formulation (See Table 1). (1–3). Rinse II also included chlorhexidine gluconate (0.12% final concentration). Chlorhexidine gluconate persists in human saliva for at least 12 hours after rinsing (14, 15) and its intraoral sustainability is felt to reflect the cationic salt's absorption to oral surfaces (14, 15). The purpose for chlorhexidine inclusion was to determine whether or not chlorhexidine could augment BRB absorption and sustainability. Secondly, as chlorhexidine gluconate is antibacterial, inclusion of chlorhexidine gluconate permitted determination of the relative contribution of salivary and oral tissue metabolizing enzymes in the presence of reduced amounts of oral microflora.
Table 1.
Composition of BRB Oral Rinses.
| Purified water-BRB (Rinse I) | Chlorhexidine gluconate rinse* (Rinse II) | Augmented rinse +BRB (Rinse III) |
|---|---|---|
| Ingredient | Ingredient | Ingredient |
| Purified water | Ora-Plus™ | Ora-Plus™ |
| BRB (10% final concentration) | Ora-Sweet SF™ | Ora-Sweet SF™ |
| Glycerin | Glycerin | |
| Chlorhexidine gluconate (0.12% final concentration) | BRB (10% final concentration) | |
| BRB (10% final concentration) |
Rationale for selection and description of the solution components.
Ora-Plus™: This is an oral suspending vehicle, manufactured by Paddock Laboratories, Minneapolis, MN. Its composition is: water (97%), sodium phosphate monobasic (<1%), sodium carboxymethylcellulose (<1%), microcrystalline cellulose (<1%), xanthan gum (<1%), carrageenan (<1%). Any other ingredients are <0.1% and are considered GRAS. This vehicle is frequently used in pediatric pharmaceutical preparations.
Ora-Sweet SF™: This is a sugar and alcohol-free oral syrup vehicle, manufactured by Paddock Laboratories, which is used to provide a sweet fruity odor and taste to liquid medications. Its component ingredients are: sorbitol (10%), glycerin (9%), and sodium saccharin (0.1%). Ora-Sweet SF™ is used in conjunction with Ora-Plus™ in the preparation of liquid pediatric pharmaceutical preparations.
Glycerin: This is a GRAS compound that is used as an antimicrobial, preservative emollient. Glycerin was included in the enhanced rinse formulations to augment rinse viscosity and therefore increase opportunity for the adherence of BRB compounds to oral mucosal tissues.
Chlorhexidine gluconate: This is the antimicrobial agent used in oral health care products for treatment of periodontal disease e.g. Peridex®, Gum® chlorhexidine gluconate oral rinse. Chlorhexidine gluconate has been shown to demonstrate substantivity in the mouth (approximately 30% of chlorhexidine is retained in the oral cavity following rinsing), with the retained drug slowly being released into oral fluids. Chlorhexidine gluconate's substantivity is felt to reflect binding of this positively charged compound to negative charges located on oral mucosal tissues.
Pharmacokinetics of chlorhexidine gluconate. Following rinsing, approximately 30% of chlorhexidine gluconate is retained in the oral cavity, and the retained compound is slowly released into oral fluids. This compound is poorly absorbed from the gastrointestinal tract, with the mean peak plasma levels of chlorhexidine gluconate obtained at 30 minutes (0.206 μg/g) after ingestion of a 300 mg dose (14).
BRB Rinse Pharmacokinetic Analyses
In this crossover study, ten participants rinsed with all three preparations, with a seven day “wash out” period between the testing of different rinse formulations (Table 1). Baseline saliva samples (obtained >1 h following any eating, drinking, or oral hygiene such as tooth brushing or mouth rinses) were obtained prior to rinsing. Participants vigorously rinsed with 15 ml of the test solution for 3 minutes and volume of the expectorated rinse recorded. Samples were shielded from the light and immediately acidified with formic acid (5% final concentration) followed by storage at −80°C until LC-MS/MS analyses. Additional saliva samples were obtained at 15, 30, 60, 120, 180 and 240 minutes after rinsing. All participants refrained from eating, drinking or oral hygiene during the time course saliva collections. Samples were used to determine: 1) pre-rinse saliva levels of BRB anthocyanins and metabolites, 2) salivary levels of the parent BRB anthocyanins, the respective aglycone (cyanidin) and the stable phenolic acid end-product (protocatechuic acid) at timed collection points (5, 30, 60, 120, 180 and 240 minutes after rinsing and expectorating with the three BRB rinse formulations), 3) levels of BRB anthocyanins and the aglycone (cyanidin) in the expectorated rinse. Both intra-donor and inter-donor data comparisons of the BRB anthocyanin parent compounds and metabolites were conducted.
Determination of residual levels of the four BRB anthocyanins in post-rinse human saliva samples by LC-MS/MS
The four BRB anthocyanins i.e. cyanidin 3-rutinoside, cyanidin 3-xylosylrutinoside, cyanidin 3-sambubioside, and cyanidin 3-glucoside and their aglycone metabolite, cyanidin, in human saliva were quantified in accordance with the method described in our recent manuscript (16). The minimum quantifiable levels for the 4 BRB anthocyanins were 1 ng/ml; the cyanidin level was 2 ng/mL, with standards prepared in 0.2 ml of diluted saliva. The analytical methods were validated for quantification of the BRB anthocyanins and cyanidin in human saliva by using an internal standard (malvidin 3-glucoside) and BRB standards “spiked” with human saliva. Due to the high concentrations of four BRB anthocyanins in the early time point samples, these samples were diluted with water to ensure their final concentrations fell within the linear concentration range (1–1000 ng/mL) of the corresponding standard curve.
LC-MS/MS analyses to determine the presence of the stable cyanidin degradatory product, protocatechuic acid (PCA), in human saliva following BRB rinses
Due to the importance of PCA formation in anthocyanin metabolism and bioactivation, a LC-MS/MS method was developed to determine residual PCA levels in post-BRB rinse saliva. Briefly, PCA and the internal standard 4-hydroxy-3-nitrobenzoic acid (HNBA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The standards in the human samples were analyzed using a API-3000 triple quadruple mass spectrometer (Applied Biosystem, Sciex, Ontario, Canada) under an electro-spray ionization (ESI) negative mode (standard curves ranged from 1 to 2000 ng/ml, R2>0.99). The minimum quantifiable level for PCA is 1 ng/ml while the detectable level is 0.2 ng/mL in human saliva. Prior to sample analyses, the analytical methods were validated for quantification of PCA in human saliva by using the PCA standard “spiked” with human saliva.
Determination of β-glucosidase function in human saliva
Eleven individuals (10 rinse participants donors plus an additional person) provided a baseline (≥3 hours n.p.o.) saliva sample then rinsed for 3 minutes with phosphate buffered saline (PBS) that contained chlorhexidine gluconate (0.12% final concentration). Fifteen minutes after rinsing with the PBS-chlorhexidine solution, another saliva sample was obtained and volumes recorded. Sample β-glucosidase activities were determined by the QuantiChrom β-Glucosidase Assay Kit (BioAssay Systems, Hayward, CA), which evaluates β-glucosidase hydrolysis of p-nitrophenyl-β-D-glucopyranoside to a chromagenic product. The rate of the reaction is directly proportional to the enzyme activity. β-glucosidase activity was reported as units/liter (U/L).
Evaluation of anthocyanin-related metabolic enzymes in human oral mucosa by immunoblot analyses
Fifteen clinically normal oral mucosal tissues were obtained from consented donors. Tissues were snap frozen in liquid nitrogen and stored at −80°C prior to analyses. Tissues were homogenized in ice-cold RIPA Lysis Buffer with protease inhibitor cocktail plus PMSF (Santa Cruz Biotechnology, CA), followed by protein determination (Bio-Rad Bradford protein assay, Hercules, CA). SDS-PAGE was conducted on 10% acrylaminde/bis gels and used the following primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA.) and working dilutions: mouse anti-β-glucosidase monoclonal antibody (1:200), rabbit anti-COMT polyclonal antibody (1:1000), rabbit anti-UGT1A polyclonal antibody (1:400), goat anti-UDP-GlcDH polyclonal antibody (1:200), mouse anti-MRP-1 monoclonal antibody (1:100). mouse anti MRP-2 monoclonal antibody (1:2,000), mouse anti β-actin monoclonal antibody (1:20,000). Additional antibodies purchased from Abcam (Cambridge, MA.) were: mouse anti-P-glycoprotein monoclonal antibody (1:2,000), rabbit anti-SGLT1 polyclonal antibody (1:500), rabbit anti-SULTA1 polyclonal antibody (1:200). Mouse anti-BCRP monoclonal antibody (1:500, Chemicon, Temecula, CA) was also used. Secondary antibodies were goat anti-mouse IgG-HRP, goat anti-rabbit IgG-HRP, and donkey anti-goat IgG-HRP, which were purchased from Santa Cruz Biotechnology. Proteins were visualized using ECL Plus Western Blotting Detection system (GE Healthcare/Amersham, Piscataway, NJ) followed by exposure to Kodak films (Kodak, Rochester, NY) and densitometery analyses (Kodak 1D3 image analysis software, Kodak, Rochester, NY). Results were normalized relative to endogenous β-actin expression.
Confirmation of enzyme distribution within the surface epithelium by immunohistochemical staining
Although surface epithelium was present in each specimen, connective tissue stroma was the predominant tissue present in the mucosal specimens. Consequently, tissue-specific immunohistochemical analyses were conducted to assess levels of metabolic enzymes at the target tissue epithelial site. Fifteen clinically and histologically normal oral mucosal specimens were used in these studies. The antibodies used for immunohistochemical staining were: mouse monoclonal anti-β-glucosidase (1:100) and rabbit polyclonal anti-UGT1A (1:200) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA.), rabbit polyclonal anti-LCT (1:100, Sigma, St. Louis, MO), rabbit polyclonal anti-COMT (1:200, Sigma, St. Louis, MO), mouse monoclonal anti-BCRP (1:20, Abcam, Cambridge, MA) and rabbit polyclonal anti-SGLT1 (1:500, Abcam). Blocking buffer (negative control) was used in place of primary antibody. Samples were incubated with their respective biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) followed by application of Vectastain ABC reagent (Vector Laboratories). Immunoreactions were visualized using the DAB substrate, followed by hematoxylin counterstaining. Images were captured using a Nikon DS-Fi-1 high-resolution digital camera and analyzed using Image-Pro Plus 6.2 software (Media Cybernetics, Bethesda, MD, USA). IHC negative controls consisted of the exclusive addition of secondary antibody in the absence of primary antibody.
Detection of residual glucuronidated Phase II anthocyanin conjugates in post-rinse human saliva samples by LC-MS/MS
Studies were conducted to evaluate whether or not anthocyanin conjugates are detectable in saliva following BRB rinses. These initial studies evaluated selected donors' (based on high levels of salivary anthocyanins) saliva samples from the Rinse III group (highest levels of anthocyanin metabolites). Glucuronidation was selected as the initial Phase II enzyme pathway to pursue due to the high levels and uniform distribution of UGTs in all of the tissue donors' mucosa. The LC-MS/MS method employed a Finnigan TSQ Quantum EMR Triple Quadruple mass spectrometer (Thermo Fisher Scientific Corporation, San Jose, CA) coupled to a Shimadzu HPLC system (Shimadzu, Columbia, MD). BRB anthocyanins, cyanidin and potential glucuronidated conjugates were eluted within 60 minutes using an gradient program with a flow rate 0.20 ml/min on an Aquasil C18 (5 μm) column (2.1 mm i.d. × 150mm), which consisted of water containing 0.1 % formic acid as mobile phase A and acetonitrile containing 0.1% formic acid as mobile phase B. The gradient, which is initially 0% B, was increased to 100 % B in 60 min, and then reverted back to 0% B in 1 min, followed by equilibration at 0% B for 14 min, for a total running time of 75 minutes. The tandem mass spectra of the following ions: mz 625, 757, 771 corresponding to the molecular ions of glucuronides of C3GLU, C3SAM, C3RUT, respectively, were utilized.
Determination if saliva and/or human oral microflora generate glucuronidated anthocyanin conjugates in the absence of oral tissues
To evaluate whether or not saliva and/or oral microflora possess functional UGT 1A1 enzymatic activity, ex vivo incubations (37°C, 5% CO2) of 10% BRB with saliva from six different donors were conducted. Additional samples which consisted of saliva-BRB supplemented human liver microsomes were also evaluated. These samples were analyzed under the conditions as described in the previous section Detection of residual glucuronidated Phase II anthocyanin conjugates in post-rinse human saliva samples by LC-MS/MS.
Detection of protocatechuic acid O-glucuronide (PCAOG) in post Rinse III saliva samples
As the PCA data demonstrated that Rinse III resulted in significantly higher levels of salivary PCA, additional timed harvest post Rinse III saliva samples were collected from two donors. The triple play (Full Scan, Zoom Scan and Collision-induced Dissociation Product ion Scan) LC-MS analysis for the synthetic protocatechuic acid O-glucuronide (PCAOG) standard and samples was conducted using an LCQ system (Thermo) equipped with Shimadzu Class 10Vp HPLC. An aquasil C18 column coupled to an aquasil C18 2mm precolumn filter were used for the separations. The triple play mode was chosen for analysis of these reconstituted solutions as full mass scan in the range of 150–1000 Th, zoom scan of and data-dependent MS/MS of the most intense peak from the full analytical scan. The mass spectrometer was tuned to its optimum sensitivity by infusion of PCAOG. All operations were controlled by Finnigan Xcaliber software in a Windows NT 4.0 system. For detection of PCAOG in saliva samples, the following transitional ionic reaction with parameters established at 329.0>153.0 @35% energy was monitored.
Statistical Analyses
The Kruskal-Wallis ANOVA, followed by the Dunns' Multiple Comparison test, was used to evaluate the anthocyanin levels at respective time points in the donor population as a whole. Assessment of individual donor responsiveness to the respective rinses i.e. which rinse provided the highest intra-donor levels of the parent anthocyanin at time points was determined by the Friedman Two-Way Analysis of Variance by Ranks. A two tailed Mann Whitney U test was used to compare rinse salivary levels of BRB anthocyanins relative to saliva levels obtained from the 10% BRB gel (3). The Repeated Measures Analysis of Variance, followed by the Bonferroni Multiple Comparisons Test, was used to evaluate salivary retention of PCA. The effects of chlorhexidine gluconate on salivary β-glucosidase activities were assessed via the chi square one-way classification. The normality of the data distribution determined whether parametric or nonparametric analyses were utilized. Findings with p values ≤ 0.05 were considered significant.
Results
BRB rinses provide sustained levels of BRB anthocyanins in saliva and demonstrate inter-rinse differences
Post-rinse anthocyanin levels showed rinse-related differences, with saliva collected following Rinse I containing the highest levels of BRB parent anthocyanins over the time course of the study. Inter-donor differences in salivary anthocyanin levels were also apparent. The greatest inter-donor variability was noted at the early (0, 5 and 30 minutes) time points, and most apparent in the cyanidin 3-rutinoside (predominant BRB anthocyanin) and protocatechuic acid (stabilized metabolite) samples. Average inter-donor early time point differences were 5 fold, with ranges from 3.6 to 10.3 fold. Inter-rinse comparisons demonstrated that Rinse I provided significantly higher (p≤ 0.05) BRB anthocyanin levels relative to Rinse III at several time points: time 0 (cyanidin 3-rutinoside, cyanidin 3-glucoside, cyanidin 3-sambubioside), 30 minutes (cyanidin 3-xylosylrutinoside, cyanidin 3-sambubioside), 60 minutes (cyanidin 3-xylosylrutinoside), 240 minutes (cyanidin 3-rutinoside, cyanidin-3 xylosylrutinoside, cyanidin-3 sambubioside). These significant differences were detected in both the inter-donor and intra-donor comparisons. Rinse II anthocyanin levels were only significantly lower than Rinse I at one time point: time 0, cyanidin-3 glucoside, for both inter and intra donor comparisons. As would be anticipated, levels of all measured compounds decreased over time e.g. rutinoside levels in saliva ranged from a high (mean ± S.D.) 2287.12 ± 489.55 μg/ml at initial time point (Rinse I) to 0.05 ± 0.06 μg/ml_at the final (240 minute) time point (Rinse III). Overall, parent BRB anthocyanins showed the greatest decrease in Rinse III over time. In addition, Rinse III, which contained the food-mimic sweeteners but not the antimicrobial chlorhexidine gluconate, showed the highest levels of anthocyanin metabolism and/or degradation. Finally, while early (up to 5 minute post rinse) saliva samples obtained from all three rinse groups showed detectable levels of the aglycone, cyanidin, in all donors, cyanidin levels rapidly decreased and were undetectable in all samples from all rinses by 60 minutes (data not shown).
BRB rinses provide significantly higher levels of salivary anthocyanins relative to the BRB gel
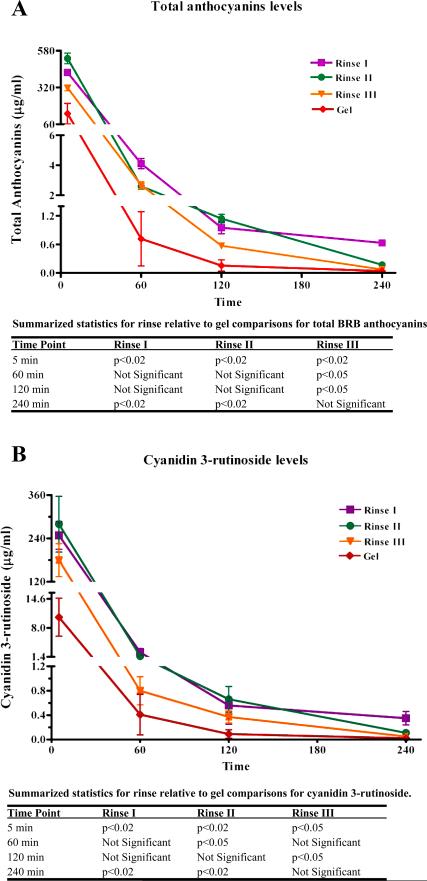
Previous studies have confirmed that saliva is the intraoral compartment that retains the highest BRB anthocyanin levels following topical intraoral gel application (3). Gel to BRB rinse comparisons of salivary levels of cyanidin 3-rutinoside (most prevalent BRB anthocyanin) and total anthocyanins (collective chemopreventive compounds) were therefore performed at all shared saliva collection time points (5, 60, 120 and 240 minutes). BRB rinses provided higher salivary levels of both total BRB anthocyanins as well as the predominant anthocyanin (cyanidin-3 rutinoside) relative to topical gel application (Figure 1.A. and 1.B.). Notably, 9 of 10 participants retained detectable rutinoside levels at 240 minutes (Rinses I and III), and 10 out of 10 donors showed detectable rutinoside at 240 minutes after Rinse II. In contrast, only 60% (3 out of 5) donors retained any salivary anthocyanins, including rutinoside, at the 240 minute time point following gel application.
Figure 1.
Figure 1 data depict comparisons of the mean BRB anthocyanin levels ± s.e.m. obtained from the 10 participants in the rinse studies relative to data obtained from 5 individuals from the gel pharmacokinetic study (3). Figure 1. A. Comparison of salivary levels of total anthocyanins detected over time following rinsing with Rinses I, II or III or topical intraoral placement of a 10% bioadhesive BRB gel.
Figure 1. B. Comparison of salivary levels of the predominant BRB anthocyanin (cyanidin 3-rutinoside) detected over time following rinsing with Rinses I, II or III or topical intraoral placement of a 10% bioadhesive BRB gel.
The functional antioxidant and stable phenolic acid derivative of BRB anthocyanins i.e. protocatechuic acid (PCA), persists in the oral cavity following use of BRB rinses
Sustained intraoral levels of stable BRB metabolites that retain chemopreventive properties could markedly impact chemopreventive efficacy. Pilot studies to assess salivary levels of the antioxidant, stable anthocyanin metabolite, protocatechuic acid (PCA), were therefore also conducted. Our data show that Rinse III provided statistically significantly higher levels of PCA over the time course of the assay (p<0.05, relative to Rinse I, p<0.01 relative to Rinse II). At the final (240 minute) time point, 6 of 10 Rinse III donors' saliva contained detectable PCA, whereas only 2 of 10 donors' saliva from either Rinses I or II retained detectable PCA levels (0.2 ng/ml detection limit).
Oral microflora, salivary enzymes and surface oral epithelium can all contribute to intraoral bioactivation of anthocyanins via B-glucosidase activity
Comparison of saliva samples obtained before and after rinsing with the antimicrobial chlorhexidine gluconate demonstrated a decrease in β-glucosidase in all donors' saliva following rinsing [69.43% ±7.24 (mean ± s.e.m), extent of inhibition ranged from 25 to 100%, n=11, p<0.001]. These data clearly show that oral microflora contribute the majority of salivary β-glucosidase activity. Notably, saliva and/or oral epithelia also contributed to β-glucosidase activity in 10 of 11 donors, as indicated by retention of appreciable functional activity following rinsing with chlorhexidine gluconate. Finally, high levels of inter-donor differences in β-glucosidase activities were observed in both whole saliva samples (including oral microflora, 80 fold) as well as after rinsing with chlorhexidine gluconate (38-fold).
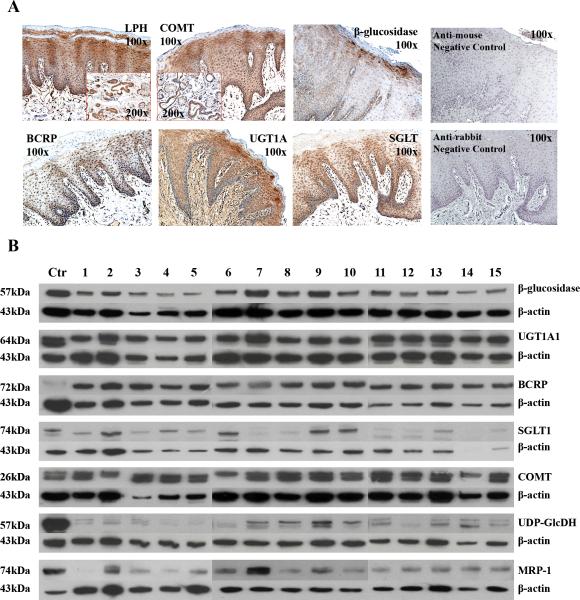
Results from the Western blots and IHC analyses of oral mucosal tissues confirmed the presence of β-glucosidase protein in human oral mucosa. Western blots also confirmed that all 15 tissues analyzed contained modest to high (18% to 66% of control protein) levels of β-glucosidase with a 3.7 fold inter-donor protein level differences observed (Table 2). Complementary IHC analyses to identify the sites for cellular distribution revealed β-glucosidase is concentrated in the spinous and granular layers of stratified squamous surface epithelium. Notably, while salivary gland acini and intercalated ducts were negative, terminal salivary ducts demonstrated intense staining for β-glucosidase (Figure 2). These data imply that the non-microbial β-glucosidase reflects contributions of both saliva as well as enzyme release into saliva from both the surface epithelium and the terminal ducts through which saliva passes en route to the surface.
Table 2.
Tissue distributions and inter-donor variations in oral mucosal anthocyanin-relevant metabolic enzymes.
| Enzyme | Anthocyanin Relevant Function | Western Data | IHC Data-Localization |
|---|---|---|---|
| Sodium dependent glucose co-transporter (SGLT1) | Would facilitate uptake of BRB anthocyanins. | 15/15; 14.2 fold (5%–71%) | Surface epithelia, prominent granular layer |
| β-glucosidase | Removes sugar moieties from parent anthocyanins. | 15/15; 3.7 fold (18%–66%) | granular, surface epithelial layer, ducts |
| UDP-glucuronosyl-transferase (UGTs) | Increase water solubility by glucuronide attachment. Augment local retention via enteric recycling of glucuronidated anthocyanins. | 15/15; 3.5 fold (66%–228%) | Granular, spinous, epithelial layer, salivary gland, ducts |
| Catechol-O-methyltransferase (COMT) | Transfers a methyl group to polyphenols, facilitates compound elimination by increasing water solubility. | 15/15; 9.3 fold (40%–372%) | spinous, granular epithelial layer, salivary gland,ducts |
| Sulfotransferases (SULTs) | Transfers a sulfur moiety, thereby enhancing water-solubility. | 0/15 | N.D.* |
| UDP-glucose dehydrogenase (UDP-Glu-DH) | Generates glucuronides for use by UGTs. | 15/15; 7.4 fold (7%–54%) | N.D. |
| Lactase phlorizin hydrolase (LPH) | Regenerates aglycone from UGT or SULT conjugates. | •N.D. | •Surface epithelia layer, salivary gland, ducts |
| β-glucuronidase | Removes glucuronides attached by UGTs, functions in enteric recycling. | While N.D. in surface epithelia, β-glucuronidase present in human saliva (17). | |
| Breast cancer resistance protein (BCRP) | ATP-dependent efflux transporter that could participate in the efflux of Phase II enzyme conjugates. | 15/15; 7 fold (48%–338%) | spinous, granular, epithelial layer, ducts |
| Quinoid anhydrolase | Generates the corresponding phenolic acid and aldehyde from the parent anthocyanin. | N.D. | N.D. |
| Permeability-glycoprotein (P-gp) | ATP-dependent efflux transporter that could participate in the efflux of glucuronidated compounds. | 0/15 | N.D. |
| MRP1 | ATP-dependent efflux transporters that could participate in the efflux of glucuronidated compounds. | 15/15 63.4 fold (1.8%–114.2%) | Spinous epithelial layer |
| MRP2 | ATP-dependent efflux transporters that could participate in the efflux of glucuronidated compounds. | 0/15 | N.D. |
N.D.: Not detected. Arylsulfatase-removes sulfurs attached via SULTs, functions in local enteric recycling. Due to absence of SULTs in oral tissues, IHC studies not conducted. Arylsulfatase is present in human saliva (17).
Localization of LPH to surface and terminal duct epithelium and the proportionately low amounts of epithelium relative to connective tissue in oral mucosal biopsies used for the Westerns likely attributes for the discrepancy regarding negative Western data with corresponding positive IHC findings.
Table 2 presents oral mucosal tissue levels and distribution of enzymes associated with anthocyanin metabolism. The Western data are organized to depict: number of tissues that contain the specific protein e.g. x/15, the fold difference between the highest and lowest protein levels among the 15 tissues evaluated, and lastly the percentile range in respective protein level expression relative to the housekeeping protein, β-actin. Arylsulfatase is another anthocyanin and local enteric recycling-relevant metabolic enzyme that removes sulfurs attached via SULTs. Due to lack of SULT detection in oral tissues, arylsulfatase-based IHC studies were not conducted. Notably, arylsulfatase has been detected in human saliva (17).
Figure 2. Tissue distribution and protein levels of anthocyanin-related metabolic enzymes.
Immunohistochemical images (2.A.) demonstrate high levels of key enzymes for anthocyanin metabolism [β-glucosidase, UDP-glucuronosyl-transferases 1A (UGT1A), breast cancer resistance protein, sodium dependent glucose co-transporter, lactase phlorizin hydrolase (LPH), catechol-O-methyltransferase (COMT)] within the surface oral epithelial target tissues. Enzymes were primarily distributed in basilar and spinous layers, with sparing of the cornified outer layer. UGT1A was also present, albeit at lower levels, in the underlying connective tissue. The specialized epithelia in the salivary ducts also contained LPH and COMT. Representative negative controls (no primary antibodies) which were stained with anti-mouse (upper panel) or anti-rabbit (lower panel) secondary antibody only are displayed in the two right panels. Immunoblotting data (2.B.) depict total protein levels in oral mucosal tissues. Results of densitometry analyses, normalized to endogenous levels of β-actin, are presented in Table 2.
Oral mucosal tissues possess moderate to high levels of the enzymes that are requisite for flavonoid enteric recycling
Table 2 depicts a summary of the immunoblotting and immunohistochemical profiling of enzymes that can affect anthocyanin metabolism in human oral mucosa. Notably, all tissues evaluated (15/15) showed the presence of key Phase II, drug egress and associated enzymes: UDP-glucuronosyl transferases (UGTs), catechol-O-methyltransferase (COMT), UDP-glucose-dehydrogenase UDP-Glu-DH), breast cancer resistance protein (BCRP) and β-glucosidase. Fourteen out of 15 tissues contained sodium dependent glucose co-transporter (SGLT1) Inter-donor differences in levels of protein expression ranged from 3.5 fold (UGTs) to 14.2 fold (SGLT1). Concurrent IHC analyses confirmed tissue distributions of six of these enzymes (including β-glucosidase) within stratified squamous surface epithelia (Figure 2) as well as within the terminal ducts of the salivary glands. While not detected by Westerns, IHC analyses confirmed the presence of lactase phlorizin hydrolase in both the stratified squamous surface epithelia as well as the terminal minor salivary gland ducts. No immunoreactivity was detected in the IHC negative control slides (data not shown). Two additional key enzymes i.e. arylsulfatase and β-glucuronidase are known to be present in human saliva (17).
Glucuronidated anthocyanin conjugates are detectable in post BRB rinse saliva samples
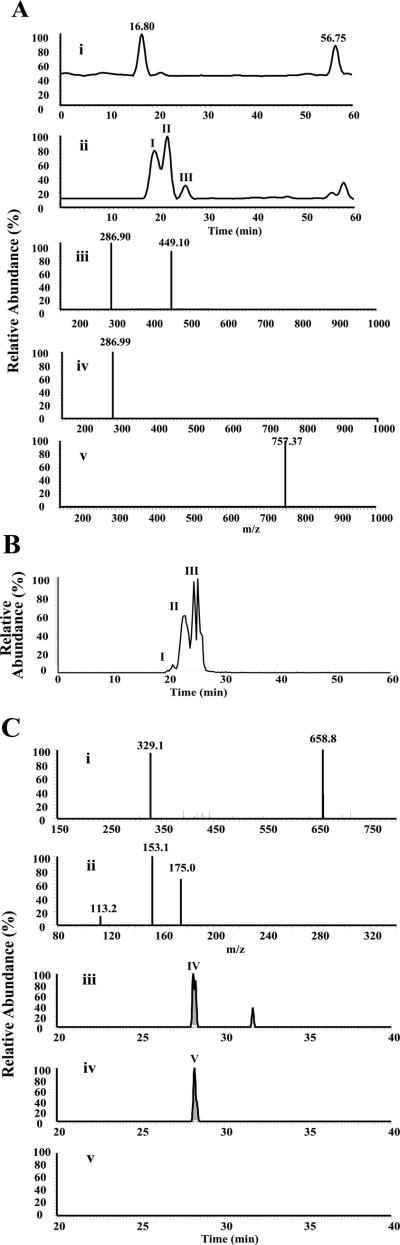
To enhance the sensitivity of monitoring for the presence of glucuronidated anthocyanins in these saliva samples, the expected ions of these anticipated glucuronidates of at the following m/zs were monitored. As shown in Figure 3.A.i, the selection ion chromatograms (SIM) of glucoside (m/z 625), cyanidin-3 rutinoside (m/z 771) and cyanidin-3 sambubioside (m/z 757) in the five minute Rinse III saliva samples did not reveal any novel peaks that demonstrated the appropriate molecular weight and their product ions corresponding to the glucuronides of cyanidin-3 glucoside and cyanidin-3 rutinoside (m/z 771) (Data not shown). There were, however, three peaks with the retention time of 19.22, 21.28 and 25.65 min labeled as Peak I, II and III in the SIC of cyanidin-3 sambubioside glucuronides detected in 6 of the 6 five minute time point samples and 3 of 6 samples from the 30 minute samples (Figure 3.A.ii.), but absent in the original Rinse III solution (Figure 3.A.i.). Also, the tandem mass spectra of Peak I and II provide the two expected product ions of m/z 449 and 287 (Figure 3.A.iii. and 3.A.iv.). These results imply that the components of these two peaks are cyanidin-3 sambubioside glucuronides. Further confirmation of these data was obtained following incubation of cyanidin-3 sambubioside with rat liver microsomes, which generated cyanidin-3 sambubioside glucuronides with the similar retention time and tandem mass spectra as previously reported (18). The tandem mass spectrum of Peak III showed a single peak of m/z 757.37 (Figure 3.A.v.). As retention times for glucuronidated anthocyanins are shorter relative to the parent anthocyanins, this peak does not represent cyanidin-3 sambubioside glucuronide. Studies are ongoing to further elucidate the structure of this novel metabolite.
Figure 3.
A. Glucuronidated anthocyanin conjugates are detectable in post BRB rinse saliva samples. The selected ion chromatograms (SIC) of the tandem mass spectrum of the specific ion of m/z 757 in the Rinse III solution (i) and the 5 min saliva sample post rinsed with Rinse III solution (ii). In contrast to the Rinse III solution (i.), there are three peaks with a retention time of 19.22, 21.88 and 25.65 labeled as Peak I, II and III in the SIC of the saliva samples. The tandem mass spectra of Peak I and II provide two expected product ions of m/z 287 and 449 (iii. and iv.), which are consistent with the presence of sambubioside glucuronides. The tandem mass spectrum of peak III showed a single peak of m/z 757.37 (v.), which shows a longer retention time relative to sambubioside, represents a novel metabolite.
Figure 3. B. The SIC of m/z 757 from the ex-vivo incubation (15 min) of 10% FBR and human liver microsomes in saliva collected from one of six healthy volunteers. Similar to data depicted in Figure 3.A., three peaks are also observed in the saliva-BRB-liver microsomal incubations. There are, however, slight variations in peak retention times and relative intensities which likely reflect different time frames when the samples were analyzed on the column as well as tissue specific variations i.e. liver relative to oral mucosal UGT polymorphisms.
Figure 3. C. Protocatechuic acid glucuronide (PCAOG) is also detectable in post-rinse saliva. The full scan (i) and collision induced dissociation (ii) mass spectra of protocatechuic acid O-glucuronide (PCAOG) under a negative mode demonstrated two predominant peaks with m/z 329.1 and 658.8, which correspond to the deprotonated ion of PCAOG and its dimer, respectively. The data dependent collision-induced dissociation spectrum of the ion of m/z 329.1 (ii) showed the most abundant peak with m/z 153, corresponding to the deprotonated protocatechuic acid formed via the labile cleavage of glycosidic bond, and two minor peaks with m/z 175.0 and 113.2. The total ion chromatograms (iii) of the ionic transition 329>153 of PCAOG (100 ng/mL) in 1% formic acid aqueous solution, the 5-min saliva sample (iv), and the 30 min saliva sample (v) from a healthy volunteer after rinse with rinse formulation III. A peak with retention time of 28.12 min labeled as Peak IV and a relative small peak with the retention time of 31.5 min labeled at Peak V was shown in PCAOG (iii), an identical peak IV was shown in the XIC 5 min saliva samples, but no peaks were shown in the 30 min saliva sample (v).
No glucuronidated conjugates of the other BRB anthocyanins were detected within any of the post rinse saliva samples and no cyanidin 3-sambubioside glucuronides were observed after the 30 minute post rinse samples. To test the hypothetic scenario that the detected cyanidin-3-sambubioside glucuronides were generated intracellularly from within oral surface epithelial cells and then transported to saliva, an ex-vivo 15 minute incubation of 10% FBR (37 °C, 5% CO2) with saliva collected from 6 volunteers was conducted. The mixture was then analyzed under the conditions as described in the Section LC-MS/MS Detection of Anthocyanin Conjugates. Peak I and II were not observed in the saliva incubates of 6 out of these 6 samples. Addition of human liver microsomes to the ex vivo saliva samples from these same donors + 10% BRB incubates did result in peaks corresponding to a sambubioside glucuronidated conjugate in 2 of the 6 samples (Figure 3.B.).
Protocatechuic acid glucuronide is also detectable in post-rinse saliva
The total ion chromatogram of synthetic protocatechuic acid O-glucuronide (PCAOG generated from the hydrolysis of protocatechuic acid methyl ester O-glucuronide) in situ showed one peak with retention time of 28.12 min in the total ion chromatogram (Data not shown). The mass spectrum (Figure 3.C.ii.) of PCAOG under negative mode demonstrated two predominant peaks with m/z 329.1 and 658.5, corresponding to the deprotonated ion of PCAOG and its dimer, respectively. These data are consistent with a recently reported mass spectrum of PCAOG (19). The data dependent collision-induced dissociation spectrum (Figure 3.C.ii.) of the ion of m/z 329.1 has shown the most abundant peak with m/z 153, corresponding to the deprotonated protocatechuic acid via the labile cleavage of glycosidic bond, along with two minor peaks with m/z 175.0 and 113.2. The ionic transition m/z 329>153 was then used to monitor PCAOG in order to increase the detection limit. Preliminary study demonstrated that this method can detect 100ng/mL PCAOG with a retention time of 28.12 min as shown in Figure 3.C.iii. Also, an additional small peak at the retention time of 31.5 min was observed, which could be a region-isomer of PCAOG. Notably, a peak with the same retention time of 28.2 min was detected in the 5 min saliva sample from 1 of the 2 donors (Figure 3.C.iv.). Later time point saliva samples (30, 60, 120 and 240 min) did not reveal any peaks corresponding to PCAOG in the total ionic chromatogram in saliva samples of either donor (Figure 3.C.v.).
Discussion
Determination of chemopreventive compound levels achieved at the treatment site and correlation between lesional tissue metabolism and therapeutic efficacy are not currently assessed in chemoprevention trials. These aspects, however, are likely among the prime effectors that determine chemopreventive outcomes. The goal of this study was therefore to characterize the oral cavity's anthocyanin-relevant metabolic enzyme profile and to evaluate in situ anthocyanin metabolism.
The entire oral mucosa of persons who develop oral cancer is speculated to have undergone field cancerization (20). Oral cancer chemopreventive strategies should therefore incorporate formulations which address both site-specific and field coverage components. A topical agent plus rinse combination, which would deliver high intra-lesional levels and also disperse chemopreventives throughout the mouth, would fulfill these requirements. Consequently, the purpose of the BRB rinse studies was two-fold i.e. assess human oral mucosal anthocyanin metabolism and determine if rinses can provide sustainable chemopreventive levels in the mouth.
Formulation-associated differences in post-rinse salivary BRB anthocyanin levels were noted. Rinse I (dH2O only) provided the overall highest post-rinse salivary levels of parent BRB anthocyanins. In contrast, Rinses II and III, which incorporated flavoring agents, resulted in lower salivary anthocyanin levels. These data suggest that rinses perceived as food stimulated release of saliva and associated salivary β-glucosidase, thereby increasing anthocyanin deglycosylation. Recorded salivary volumes, which showed higher saliva volumes in the Rinse II and III samples, support this premise (data not shown). As these data reflect total anthocyanins and account for saliva volumes, the differences don't reflect volume dilutions in the Rinse II and III samples. Furthermore, the higher anthocyanin levels detected in post Rinse II saliva relative to Rinse III, likely reflect Rinse II's chlorhexidine gluconate-mediated reduction in oral microflora. The reduced bacterial β-glucosidase and lactase phylorizin hydrolase activities in the Rinse II samples would decrease deglycosylation, enabling increased retention of parent BRB anthocyanins in saliva.
Ideally, locally delivered chemopreventives persist at the treatment site. While the saliva data confirmed BRB retention, the real question is whether these levels are clinically relevant. Previous studies have confirmed that the BRB topical gel elicited chemopreventive effects (1, 2) and that the highest levels of the parent BRB anthocyanins were detected in saliva (3). Post-rinse BRB salivary anthocyanin levels were therefore compared to salivary levels achieved following gel application. All of the rinses delivered higher salivary BRB anthocyanin levels at the initial time point, and also provided greater sustainability over time relative to levels achieved with gel application. The mechanism of rinse-mediated anthocyanin retention is under investigation. For example, chlorhexidine gluconate interacts with salivary films, and adsorbs to teeth and oral mucosal surfaces (14, 21). Local adsorption enables retention of approximately 30% of chlorhexidine gluconate, which is then slowly released over time (21). Although BRB anthocyanins are structurally distinct from chlorhexidine gluconate, the post-rinse data, which show sustained salivary levels of anthocyanins, suggest that BRB anthocyanins may also be adsorbed to oral tissues and/or oral microflora present in plaque. Notably, although the rinses and gel both contained the identical 10% BRB concentration, the larger rinse volume delivered a greater amount of BRB per dose.
Two enzymes i.e. β-glucosidase and lactase phlorizin hydrolase (LPH) are responsible for deglycosylation of anthocyanins, resulting in formation of the aglycone, cyanidin. Our β-glucosidase data, which demonstrated that oral microflora provide a significant amount of salivary β-glucosidase activity, are consistent with previous studies by Walle et al. (21). Furthermore, while the presence of β-glucosidase is well-recognized in human intestinal epithelium (22), only a single reference that describes β-glucosidase in keratinizing epithelia was identified (23). Our findings, which demonstrate β-glucosidase protein in human oral mucosa by immunoblotting and confirm the presence of both β-glucosidase and LPH localization in surface oral epithelium by immunohistochemistry, clarify the distribution of these relevant metabolic enzymes in human oral tissues. These findings confirm that oral microflora, salivary enzymes and oral epithelium can all contribute to generation of the bioactive aglycone from parent anthocyanins.
The strong correlation between smoking and alcohol use and the development of oral cancer implies that the mouth is an active site for xenobiotic metabolism. Intraoral xenobiotic enzyme characterization studies to date, however, have focused at the transcriptional level (24). Consequently, little is known regarding inter-donor variations in protein levels and in which cell population(s) the enzymes are distributed. Our protein profiles of clinically and histologically normal oral mucosa demonstrated that the majority of the metabolic and recycling enzymes necessary for anthocyanin enteric recycling were present in every oral tissue specimen evaluated. Furthermore, enzyme distribution, as determined by IHC analyses, confirmed the relevant enzymes were almost exclusively distributed within the stratified squamous surface epithelium or within the terminal salivary ducts i.e. target tissues. Also apparent were the fairly extensive inter-donor differences in levels of enzyme present (ranging from 3.5 to 63 fold) and enzyme activities (over 30 fold differences in β-glucosidase function). Finally, two additional key enzymes essential for recycling of Phase II anthocyanin conjugates i.e. β-glucuronidase and arylsulfatase, are known to be present in human saliva (17).
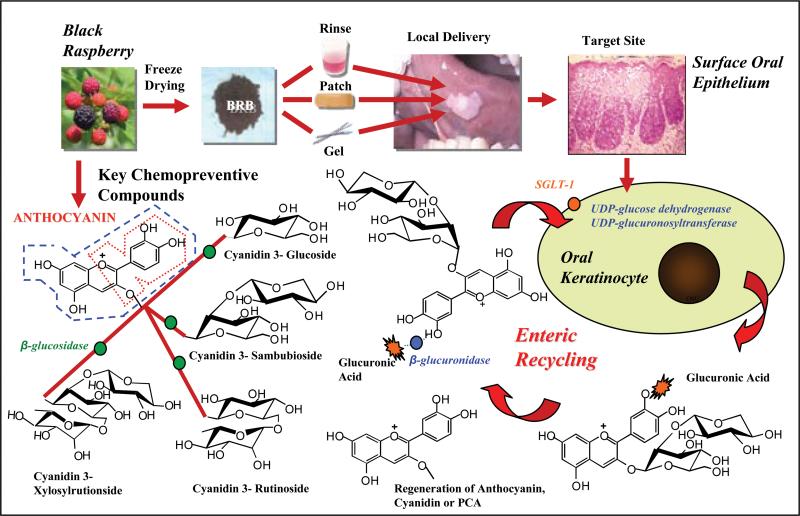
Although detection of the necessary bioactivating, Phase II and efflux transporter enzymes in human oral tissues supports the feasibility for intraoral anthocyanin enteric recycling, their mere presence does not confirm ongoing activity. The detection of glucuronidated anthocyanin-derived conjugates in saliva implies anthocyanin epithelial uptake, metabolism, and release of a stabilized metabolite capable of undergoing recycling via deglucuronidation and cellular re-entry (Figure 4). As UGTs are present in organisms ranging from bacteria to humans, conceptually the detected conjugate could reflect oral bacteria-associated glucuronidation and not entail intracellular uptake, glucuronidation and egress via UGT-efflux transporters (25). Notably, results obtained from the ex-vivo saliva-BRB incubations, which did not reveal any glucuronidated conjugates in whole saliva without the addition of exogenous liver microsomal enzymes, imply that the requisite UGT1A activity does not exist in saliva. Furthermore, gastrointestinal microflora-conjugate interactions are commonly associated with deglucuronidation via bacterial β-glucuronidase. Detection of a glucuronidated conjugate is therefore consistent with anthocyanin epithelial uptake (26), metabolism, and release of a metabolite capable of undergoing recycling via deglucuronidation and cellular re-entry (Figure 4). Cyanidin-3 sambubioside, which is found in relatively low levels in BRB (5, 7), was the exclusive glucuronidated anthocyanin conjugate identified. Di and triglycosylated anthocyanins are more stable than the monoglycosylated forms and are therefore present at higher levels (4, 5). Furthermore, as enteric recycling is a dynamic process, all glucuronidated species are likely present in small quantities and for a very transient time.
Figure 4. Concept of enteric recycling of BRB anthocyanins in oral cavity.
Sodium-dependent glucose co-transporter (SGLT1), which is present in surface oral epithelia, facilitates internalization of the relatively bulky anthocyanins. Intraorally, anthocyanins are deglycosylated via enzymes (β-glucosidase and lactase phylorizin hydrolase) which are contained in oral microflora, saliva and surface oral epithelium. Although the aglycone, cyanidin (depicted in blue dashed line) is a superior antioxidant (due to its additional hydroxyl group), it is unstable and readily degrades to its corresponding phenolic acid, protocatechuic acid (PCA, shown in red dashed line). PCA, which has a higher chemical and microbial stability relative to cyanidin, is also an antioxidant and may represent the chemical constituent responsible for sustained chemopreventive effects. While anthocyanins are substrates for sulfotransferases, methylation and glucuronidation reactions represent the primary Phase II metabolic routes. Cyanidin and PCA can also undergo glucuronidation. As carboxy-O-methlytransferase (COMT)-mediated methylation occurs at the B ring, cyanidin and PCA should also represent COMT substrates. Both COMT and UDP-glucuronosyltransferases are present in human surface oral epithelia.
Interestingly, our data also confirmed the presence of a PCA-glucuronide. These data are consistent with findings by Woodward et al., which imply that PCA glucuronides represent a predominant anthocyanin species following simulated gastrointestinal digestion (18). Our data imply that the major glucuronidated PCA isomer is formed shortly after rinsing and provide the proof of concept that PCA glucuronides are generated intraorally after anthocyanin administration. Studies to elucidate the intraoral glucuronidation kinetics of intraoral anthocyanin and related compounds are ongoing.
Enterohepatic recycling is a well-accepted mechanism by which parent compounds and their metabolites re-enter systemic circulation following first-pass metabolism in the liver. For many tissues and organs e.g. breast, prostate, this is the exclusive route by which exposure to chemopreventives occurs. In contrast, gastrointestinal mucosa, which is in direct contact with orally administered agents, avoids first pass metabolism by the liver and directly absorbs, bioactivates and metabolizes chemopreventives (27). The requisite components that enable gastrointestinal enteric recycling of flavonoid compounds are: 1) hydrolytic enzymes (present in either GI bacteria and/or GI epithelial cells) which de-glycosylate parent compounds to generate aglycones, thereby facilitating absorption by the lining epithelial cells, 2) Intracellular conjugation of aglycones to glucuronic acid or sulfate by intestinal UGTs or sulfotransferases (SULTs), respectively, 3) Presence of efflux transporters such as breast cancer resistance protein (BCRP) or multidrug resistance protein (MRP1) which can transport the conjugated metabolites into the intestinal lumen (or oral cavity), 4) Once in the lumen (or mouth), the conjugates are subject to hydrolysis by salivary and/or bacterial β-glucuronidase or arylsulfatase to regenerate the aglycones or their corresponding phenolic acids and sustain the enteric cycling (12, 28). Our data demonstrate that comparable to the small intestine, the requisite hydrolytic, Phase II and efflux transporting enzymes are present in the oral cavity.
Although glucuronidation is presumed to be the primary route for anthocyanin Phase II metabolism (29), the requisite components for other routes of anthocyanin metabolism e.g. methylation or glutathionylation (COMT and glutathione, respectively) are also present in oral mucosa. Similar to glucuronic acid, conjugation with methyl groups or glutathione could also affect anthocyanin stability and local retention. Notably, inter-patient differences in anthocyanin bioactivation and enteric recycling would impact treatment as retention of bioactivated chemopreventives at the target site would sustain therapeutic effectiveness. Pretreatment metabolic profiling of key anthocyanin bioactivating and enteric recycling enzymes and local pharmacokinetics could potentially be used to predict patients most likely to respond favorably to an anthocyanin-based chemopreventive. Such studies are currently ongoing as a component of our BRB gel chemoprevention trial. At the conclusion of the trial, pretreatment profiles will be compared to post treatment effects to determine the “predictive potential” of anthocyanin metabolic profiles with prospective clinical outcomes.
Supplementary Material
Acknowledgements
The authors would like to thank Mary Lloyd and Mary Marin for their expertise in preparation and sectioning of the histologic sections.
Financial Support: NIH grants: R01 CA129609, RC2 CA148099, R21 CA132138
References
- 1.Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, et al. Topical Application of a Bioadhesive Black Raspberry Gel Modulates Gene Expression and Reduces Cyclooxygenase 2 Protein in Human Premalignant Oral Lesions. Cancer Res. 2008;68:4945–57. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shumway BS, Kresty LA, Larsen PE, Zwick JC, Lu B, Fields HE, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–30. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ugalde CM, Liu Z, Ren C, Chan KK, Rodrigo KA, Ling Y, et al. Fields HW, Mallery SR. Distribution of Anthocyanins Delivered from a Bioadhesive Black Raspberry Gel Following Topical Intraoral Application in Normal Healthy Volunteers. Pharm Res. 2009;26:977–86. doi: 10.1007/s11095-008-9806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoner GD, Wang LS, Zikri N, Chen T, Hecht SS, Huang C, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–10. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–90. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht SS, Huang C, Stoner GD, Li J, Kenney PMJ, Sturla SJ, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide induced NFκB and AP-1 activity. Carcinogenesis. 2006;27:1617–26. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LS, Hecht S, Carmella S, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prevention Research. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Wallace TC, Keatley KE, Failla ML, Giusti MM. Stability of Black Raspberry Anthocyanins in the Digestive Tract Lumen and Transport Efficiency into Gastric and Small Intestinal Tissues in the Rat. J. Agric. Food Chem. 2009;57:3141–8. doi: 10.1021/jf900567t. [DOI] [PubMed] [Google Scholar]
- 9.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity. J Nutr. 2005;135:48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE. Stability and biotransformation of various anthocyanins in vitro. Eur J Nutr. 2006;45:7–18. doi: 10.1007/s00394-005-0557-8. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes I, Azevedo J, Faria A, Calhau C, Freitas V, Mateus N. Enzymatic Hemisynthesis of Metabolites and Conjugates of Anthocyanins. J. Agric. Food Chem. 2009;57:735–45. doi: 10.1021/jf802844p. [DOI] [PubMed] [Google Scholar]
- 12.Wang SW, Kulkarni KH, Tang L, Wang JR, Yin T, Daidoji T, et al. Disposition of Flavonoids via Enteric Recycling: UDPGlucuronosyltransferase(UGT) 1As Deficiency in Gunn Rats IsCompensated by Increases in UGT2Bs Activities. J Pharm Exp Ther. 2009;329:1023–31. doi: 10.1124/jpet.108.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Hu M. Absorption and Metabolism of Flavonoids in the Caco-2 Cell Culture Model and a Perfused Rat Intestinal Model. Drug Disp Metab. 2002;30:370–7. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 14.Monograph for chlorhexidine gluconate. http://www.drugs.com/monograph/chlorhexidine-gluconate-eent.html.
- 15.Gjermo P. Some Aspects of Drug Dynamics as Related to Oral Soft Tissue. J Dent Res. 1975;54:B44–B56. doi: 10.1177/00220345750540022401. [DOI] [PubMed] [Google Scholar]
- 16.Ling Y, Ren C, Mallery SR, Ugalde CM, Pei P, Saradhi UV, et al. A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J Chromatography. 2009;877:4027–34. doi: 10.1016/j.jchromb.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauncey HH, Lionetti F, Winer RA, Lisanti VF. Enzymes of Human Saliva. The Determination, Distribution and Origin of Whole Saliva Enzymes. J Dent Res. 1954;33:321–34. doi: 10.1177/00220345540330030501. [DOI] [PubMed] [Google Scholar]
- 18.Woodward GM, Needs PW, Kay CD. Anthocyanin-derived phenolic acids form glucuronides following simulated gastrointestinal digestion and microsomal glucuronidation. Mol. Nutr. Food Res. 2010 doi: 10.1002/mnfr.201000355. DOI 10.1002/mnfr.201000355 1. [DOI] [PubMed] [Google Scholar]
- 19.Slaughter DP, Southwick HW, Smejkat W. Field cancerization in oral stratified squamous epithelium. Clinical Implications of Multicentric Origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Freitas LB, Vassilakos N, Arnebrant T. Interactions of chlorhexidine with salivary films adsorbed at solid/liquid and air/liquid interfaces. J Periodontal Res. 1993;28:92–7. doi: 10.1111/j.1600-0765.1993.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 21.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid Glucosides Are Hydrolyzed and Thus Activated in the Oral Cavity in Humans. J. Nutr. 2005;135:48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 23.Chang F, Wertz PW, Squier CA. Localization of beta-glucosidase activity within keratinizing epithelia. Comp Biochem Physiol Comp Physiol. 1993;105:251–3. doi: 10.1016/0300-9629(93)90204-h. [DOI] [PubMed] [Google Scholar]
- 24.Boyle JO, Gümüş ZH, Kacker A, Choksi VL, Bocker JM, Zhou XK, et al. Effects of Cigarette Smoke on the Human OralMucosal Transcriptome. Cancer Prev Res. 2010;3:266–78. doi: 10.1158/1940-6207.CAPR-09-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bock KW. Vertebrate UDP-glucuronosyltransferases: functional and evolutionary aspect. Biochem Pharmacol. 2003;66:691–6. doi: 10.1016/s0006-2952(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 26.Oyama Y, Yamano H, Ohkuma A, Ogawara K, Higaki K, Kimura T. Carrier-mediated transport systems for glucose in mucosal cells of the human oral cavity. J Pharm Sci. 1999;88:830–4. doi: 10.1021/js980298f. [DOI] [PubMed] [Google Scholar]
- 27.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High Absorption but very low bioavailability of oral resveratrol in humans. Drug Metabolism Disposition. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Hu M. Natural Polyphenol Disposition via Coupled Metabolic Pathways. Expert Opin Drug Metab Toxicol. 2007;3:389–406. doi: 10.1517/17425255.3.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prior RL, Wu X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Res. 2006;40:1014–28. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- 30.Cell Signaling PhosphoSite Plus. http://www.phosphosite.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.