Abstract
Our previous study comparing inhalation and aspiration to administer agents directly to lung indicated that aspiration route is as effective as inhalation while reducing costs for equipment and chemopreventive agent. This study evaluated the chemopreventive efficacy and mechanism of Licofelone, a dual inhibitor of cyclooxygenase-2 (Cox-2) and 5-lipoxygenase (5-Lox), via oropharyngeal aspiration against mouse lung adenoma. Eight week old female A/J mice were given three doses of benzo[a]pyrene (B[a]P, 2 mg/dose, gavage) to induce lung adenomas. After dysplasia developed, the mice were given Licofelone (0, 0.03, 0.1 or 0.3 mg/kg) for 16 weeks and tumor incidence and multiplicity in lung were measured. In addition, the expression of a series of biomarkers in lung cancer progression was evaluated at 2 and 16 weeks. Licofelone showed dose-related inhibition of B[a]P-induced tumor incidence and multiplicity at 0.03 and 0.1 mg/kg following 16-week treatment. Licofelone also showed dose-dependent inhibition of Cox-2 (25–41%) and 5-Lox (35–61%) at 2 and 16 weeks and proliferating cell nuclear antigen (PCNA, 41–61%) at 16 weeks. A dose-dependent increase in apoptosis (1.5–2.4-fold) was also observed in Licofelone groups. A marginal inhibition of survivin was observed at one dose. In conclusion, this study demonstrated that Licofelone via aspiration showed chemopreventive efficacy against mouse lung adenoma with good correlation to early and late biomarkers of lung cancer progression. This is the first study to show that the aspiration route can be an excellent inexpensive alternative to inhalation for direct delivery of drugs to rodent lungs for efficacy testing of potential chemopreventive agents.
Keywords: Chemoprevention, Lung Tumor, Licofelone, A/J Mice, Biomarkers
Introduction
While the number of active smokers in this country has diminished over the last decade, there are currently over 50 million ex-smokers, who have a 12- to 15-fold greater risk of developing lung cancer. Given these startling numbers, chemoprevention may be the only approach that could significantly impact the development of new cases (1–4). Chemoprevention refers to the administration of chemical agents that inhibit one or more stages of neoplastic development and, as a result, decrease the incidence of cancer. Pre-clinical studies in mice on over sixty compounds have led to the identification of a few groups of chemicals as potential chemopreventive agents for lung cancer such as retinoids, glucocorticoids, green tea polyphenols, anti-inflammatory agents, isothiocyanates, and farnesyl transferase inhibitors (4, 5). Unfortunately, none of these agents have been successful in clinical trials to date. In fact, a large scale clinical study had to be terminated when it was discovered that beta-carotene actually increased the risk of lung cancer among smokers. (6). There are some preliminary indications from a small nonrandomized Phase I trial of former smokers that myo-inositol may reverse bronchial dysplasia (7). Clearly, discovery of new agents that prevent lung cancer in former smokers and their critical evaluation in pertinent preclinical models are urgently needed.
Our interest in testing Licofelone ([2,2-dimethyl 1-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid), a nonsteroidal anti-inflammatory drug (NSAID), in a prototype ex-smoker model for lung cancer is based on it having dual Cox/Lox inhibitory activity. Dual inhibitors that block both Cox and 5-Lox could synergize their individual anti-inflammatory effects and also decrease gastrointestinal side effects associated with NSAIDs (8). Licofelone is one of the most promising compounds in this category and the first compound that is currently on Phase III clinical trial for treatment of pain and inflammation associated with osteoarthritis (9). Multiple preclinical studies reported that licofelone has anti-inflammatory, analgesic, antipyretic and antiplatelet activities (10–13).
Licofelone also showed anti-arthritic activity in rats at the dose range of 20–80 mg/kg, showing significant reduction in erythema, edema and arthritis-associated splenomegaly (14). Licofelone is known to be well tolerated at pharmacologically active doses in terms of general toxicity associated with autonomic nervous system, CNS and cardiovascular system (15) as well as in genotoxicity (16) in preclinical studies. More importantly, licofelone showed much lower level of gastric mucosal damage compared to other NSAIDs on the market (17) and actually decreased ulcerogenic potential by aspirin that was used in combination with licofelone (18) in preclinical studies. The low gastric toxicity of licofelone was also confirmed in clinical studies when licofelone was co-administered with enteric-coated aspirin (19). Another clinical study reported that licofelone was equally effective compared to celecoxib, one of the Cox-2 specific inhibitors on the market, but showed better gastrointestinal tolerability (20). The mechanism of action for low gastric damage and anti-arthritic activity by licofelone is presumably attributed to the reduced leukotriene production, especially leukotriene-B4, by blocking 5-Lox signaling pathway (21, 22). Inhibiting 5-Lox pathway, one of the major arachidonic acid metabolic pathways, and the subsequent reduction of pro-inflammatory and gastrotoxic leukotrienes is an additional benefit of licofelone over other NSAIDs and Cox-2 specific inhibitors.
Interestingly, many preclinical studies implied that dual inhibitory effects on Cox and 5-Lox could reduce the incidence and the progression of various cancers (e.g., 23). Rao and colleagues (24) reported that licofelone in diet inhibited azoxymethane-induced rat colon cancer in a dose-dependent manner with associated reduction in PCNA and increased apoptosis. Narayanan and colleagues (25) reported that licofelone suppressed prostate cancer cell growth via apoptosis induction. A significant down-regulation on Cox-2 and 5-Lox expression was found along with a weak inhibitory effect on Cox-1 protein in this study. Apoptosis induction was also observed in colon cancer cells, HCA-7, by licofelone treatment in a dose- and time-dependent manner (26).
Strategically, a direct delivery of a drug to its target tissue is maximizing efficacy while limiting systemic exposure and the associated side effects. Delivery of agents to the respiratory tract can be accomplished by nose only inhalation route as aerosols (27). Our previous efficacy study (28) showed a significant inhibition of B[a]P-induced mouse lung tumors by employing nose-only inhalation to deliver a chemopreventive agent, Oltipraz, to the respiratory tract directly. However, aerosolization of agents to obtain appropriate particle size for delivery by inhalation requires large amounts of drug which is a potential problem in drug development. Our data comparing inhalation and aspiration methods of administration of an agent to lung airway epithelia indicated that aspiration can be an equally effective route of exposure while reducing the need for inhalation equipment and amount of chemopreventive agent required (unpublished). The aspiration method has been used in administering virus, bacteria or soluble antigens directly to the respiratory tract in animal models and is a very non-invasive method compared to intratracheal instillation (29, 30).
Budesonide (16,17-butylidenebis(oxy)-11,21-dihydroxypregna-1,4-diene-3,20-dione), an anti-inflammatory glucocorticoid, is currently on the market and has been reported to be very effective in inhibiting chemically-induced mouse lung tumors by inhalation or dietary formulation (31, 32). Budesonide was used as a positive tumor inhibitor for this study. The present study is also the first study to test the aspiration route as an alternative, replacing costly inhalation methods, for assessing chemopreventive efficacy of agents in an animal model for lung cancer.
Materials & Methods
Chemicals and reagents
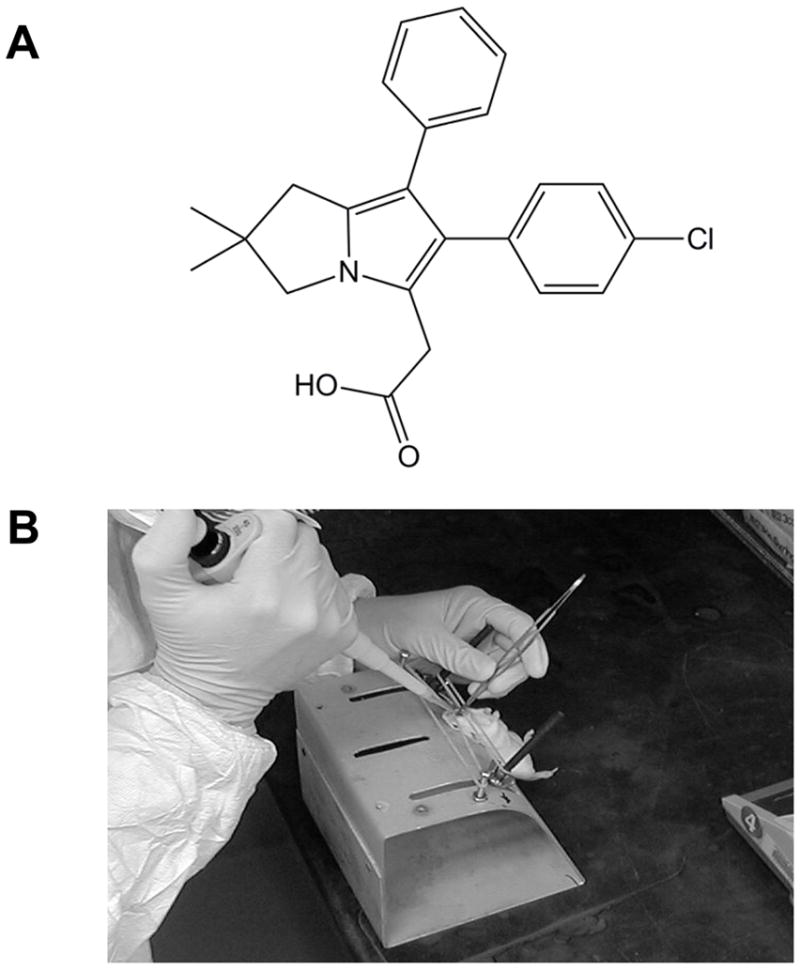
Licofelone (purity: >99% - Fig. 1A) and labrasol were obtained from the NCI Chemical Repository (Rockville, MD). Budesonide (purity: >99%), B[a]P, mouse anti-PCNA monoclonal and other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit anti-Cox-2 and anti-5-Lox polyclonal antibody were purchased from Cayman Chemical Co.(Ann Arbor, MI) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Chicken anti-survivin polyclonal antibody and mouse anti-β-actin antibody were purchased from Aves Labs, Inc. (Tigard, OR) and GenScript (Piscataway, NJ), respectively. ECL Plex™ goat anti-rabbit IgG Cy™5, ECL Plex™ goat anti-chicken IgG Cy™5 and ECL Plex™ goat anti-mouse IgG Cy™3 were purchased from GE Healthcare (Piscataway, NJ). ApopTag® Peroxidase In Situ Apoptosis Detection Kit was purchased from Millipore (Billerica, MA). Normal horse serum and biotinylated anti-mouse IgG were purchased from Vector Lab (Burlington, CA). Peroxidase-conjugated Streptavidin and 3-amino-9-ethylcarbazole (AEC) substrate kit were purchased from Invitrogen (Carlsbad, CA).
Figure 1.
Animals and diet
Seven-week-old, viral antigen- and specific pathogen-free Strain A/J female mice were purchased from the Jackson Laboratory (Bar Harbor, ME), and quarantined for up to two weeks in a temperature-controlled, pathogen-free animal facility before use.
All study animals were provided with fresh deionized, chlorinated water, and pelletized, semipurified AIN-76A diet (casein 20%, DL-methionine 0.3%, cornstarch 52%, dextrose 13%, corn oil 5%, alphacel 5%, AIN mineral mixture 3.5%, AIN vitamin mixture, 1.0%, and choline bitartrate 0.2%), purchased from Dyets, Inc. (Bethlehem, PA) on an ad libitum basis. Before the end of a 14-day acclimation/quarantine period prior to use, all mice were tail tattooed with permanent black ink, each with a unique number. Body weights were recorded weekly during chemopreventive aspiration exposure and monthly thereafter.
Aspiration
Mice were anesthetized with isoflurane vapor via an anesthesia machine. Upon verification of loss of muscular tension, the mice were secured on restraint board by hooking the upper and lower incisors with thin rubber bands, thereby holding the mouth open. Blunt forceps were used to gently pull the tongue to one side of the mouth to prevent swallowing. A pipette tip filled with licofelone suspension was gently released into the distal part of oropharynx and by natural reflex the licofelone suspension was aspirated into the lower respiratory tract (Fig. 1B). The volume of the dosing suspension did not exceed 0.15 ml/100g (~50μl/mouse) of the body weight.
Dose selection (MTD) of Licofelone for chemoprevention bioassay
The maximum tolerated dose (MTD) was determined by changes in animal body weight. Animals were dosed with Licofelone in a fine suspension with Labrasol (0.02 %) at 4 different concentrations (3, 10, 30 and 75 mg/kg) for 6 weeks and sacrificed. Body weights were measured daily and before necropsy. Organ weights (liver, lungs, heart, and kidneys) were also measured at the time of gross necropsy for observation and to determine the organ/body weight ratio.
Chemoprevention bioassay and mechanism-based biomarker assays
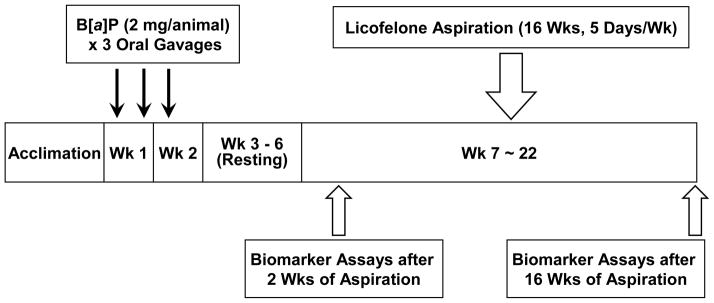
For chemoprevention studies, 0.01 MTD (0.3 mg/kg) of Licofelone was used as the highest dose followed by two half-log doses (0.1 and 0.03 mg/kg). The experimental design is shown in Table 1 and Fig. 2. Lung adenomas were induced in 8-week old female strain A/J mice by three oral gavages of B[a]P (2 mg in 0.2 ml cotton seed oil per animal). The mice were left for 4 weeks to develop dysplasia. One animal from Group 2, 3, and 6 were randomly selected after 4 weeks after B[a]P treatment to verify dysplasia in the alveolar region of the lung tissues. The lungs were inflated with 10% neutral buffered formalin and fixed for 24 hrs. Tissue sections were prepared and stained with Hematoxylin & Eosin (HE) and then examined microscopically to observe the presence of hyperplasia or dysplasia in the alveolar region of the lung tissues. Licofelone was administered in 0.02% labrasol in saline by aspiration. Animals were given one of three doses of Licofelone (0.03, 0.1 and 0.3 mg/kg) 5 days per week, for 16 weeks. Fresh solution was made prior to each aspiration. Control animals received same amount of labrasol by aspiration. The toxicity of Licofelone was determined by animal body weight change by weighing animals once a week during the sixteen weeks of Licofelone exposure and before necropsy. At this time, detailed clinical observations for signs of toxicity were performed on each study animal. The animals were also observed once a day for morbidity and mortality.
Table 1.
Experimental Design for Chemoprevention Study with Licofelone
| * Group | Treatment | Exposure Duration (weeks) | B[a]P (2 mg/dose) | Agent Dose |
|---|---|---|---|---|
| 1 | Vehicle Control | 16 | − | None |
| 2 | B[a]P alone | 2 | + | None |
| 3 | Budesonide (Pos. ctrl.) | 16 | + | 0.075 mg/kg |
| 4 | Licofelone Dose 1 | 16 | + | 0.03 mg/kg |
| 5 | Licofelone Dose 2 | 16 | + | 0.1 mg/kg |
| 6 | Licofelone Dose 3 | 16 | + | 0.3 mg/kg |
33 Animals/group
Figure 2.
At 2 and 16 weeks of dosing, the expression of Cox-2 and 5-Lox in the lung was evaluated by Western blot. After completing 16 weeks of dosing, lungs were harvested from 14–22 mice per group and fixed in Tellyesniczky’s fixative (1:2:20=acetic acid:formalin:70% ethanol) to measure tumor incidence and multiplicity and to evaluate effect on additional biomarkers including survivin, PCNA and apoptosis by Western blot and immunohistochemistry. The effect of Licofelone on tumor inhibition and expression of biomarkers was compared to B[a]P only group. Budesonide was used as a positive control for tumor inhibition.
Analysis of biomarkers by Western blot (Cox-2, 5-Lox, and survivin)
Lung tissue lysates were processed for Western blot which was performed using the fluorescent ECL Plex™ multiplex detection method. Fifty μg of protein was loaded in each lane and separated on 4–20% gradient Tris-HCl gels (BioRad, Hercules, CA) before transfer to Hybond LFP PVDF membrane (GE Healthcare, Piscataway, NJ). After incubating with primary antibodies against Cox-2, 5-Lox, or survivin (1:2000) and βactin (1:5000) at 4°C overnight and secondary antibodies (ECL Plex™ goat anti-rabbit IgG Cy™5 and goat anti-mouse IgG Cy™3, 1:10,000, 2 hrs at RT), the Hybond LFP PVDF membrane was imaged using a Typhoon 9410 fluorescent scanner and quantitated using ImageQuant software. Data was normalized to β-actin.
Immunohistochemistry (PCNA and apoptosis)
The rate of cell proliferation in lung tissues was assessed by PCNA staining. Briefly, lung tissues fixed in 10% neutral buffered formalin for 24 hrs, were sectioned (5 μm), deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval in citrate buffer (10 mM, pH 6.0) for 8 min at 120°C. Endogenous peroxidase was quenched with 3% hydrogen peroxide, nonspecific binding sites were blocked with normal horse serum and antibody against PCNA (1:500) was applied for 30 min at RT followed by secondary antibody (biotinylated anti-mouse IgG, 1:200). The expression PCNA was visualized using AEC substrate kit and counterstained with Mayer’s hematoxylin. PCNA-positive stained cells were quantitated in both alveolar and bronchiolar epithelial cells and the scored values were combined for the final data.
Apoptotic cells were indentified by terminal deoxynucleotidyl transferase- mediated dUTP nick end labeling (TUNEL) staining using ApopTag® Peroxidase In Situ Apoptosis detection kit and visualized using DAB and 0.5% methyl green as counter stain in 0.1 M sodium acetate (pH 4.0). Scoring for apoptosis-positive cells was performed in both alveolar and bronchiolar epithelial cells and combined for the final quantitation.
Statistical analysis
Numerical study data from the Licofelone chemoprevention bioassay were analyzed statistically by ANOVA one-way Student’s t-test for tumor multiplicity, biomarkers and body weight changes, Cochran-Armitage test for trend in tumor incidence (33, 34) using a p < 0.05 level of significance. Data files generated from spreadsheets were used for statistical analysis using a SAS program.
Results
Effect of Licofelone treatment on body weight
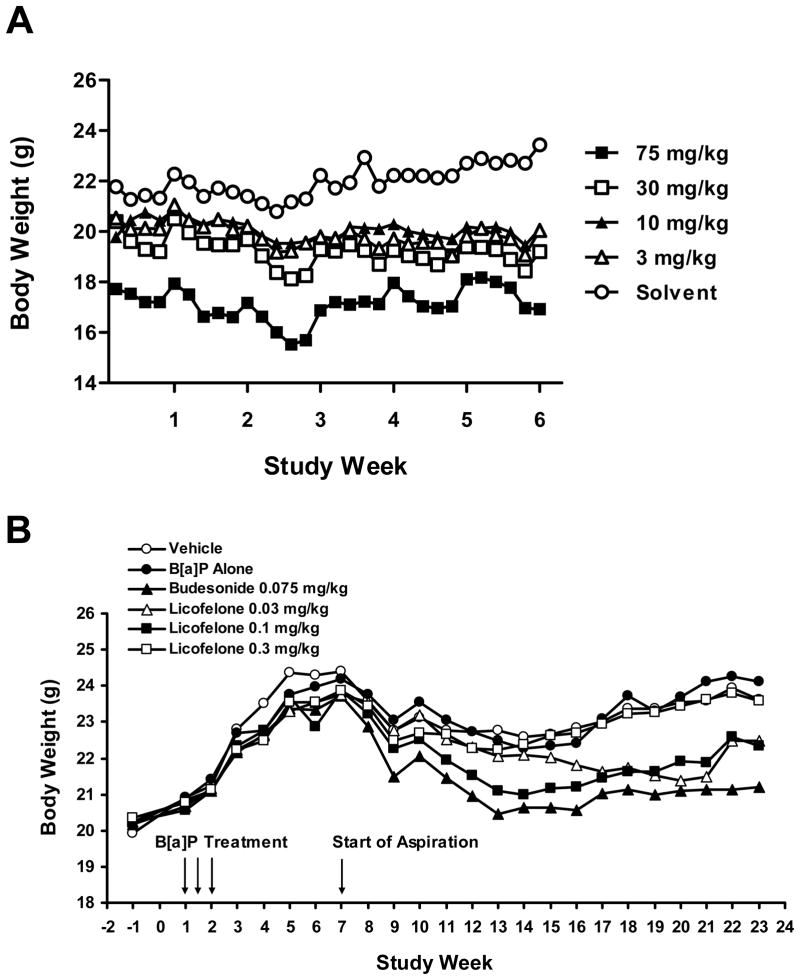
The body weight change in animals treated with Licofelone from the 6-week MTD study is shown in Fig. 3A. There was no significant change in body weight over the study period in all dose groups. However, since the data from the organ/body weight ratio indicated that at 75 mg/kg there was a significant difference in the kidney/body weight ratio (p=0.04) compared to the solvent control (data not shown), the next dose (30 mg/kg) was selected as the MTD.
Figure 3.
During the chemoprevention bioassay, when the animals were exposed to different doses of Licofelone by aspiration for 16 weeks, a temporary body weight loss was observed after the exposure (aspiration) started but recovered within few weeks of aspiration (Fig. 3B). There was no mortality or any sign of toxicity observed during the study and body weight gain in exposed animals at the end of exposure was comparable to the control animals. However, a significant body weight reduction was observed in Budesonide (positive control)-treated animals.
Effect of Licofelone exposure on B[a]P-induced lung tumors
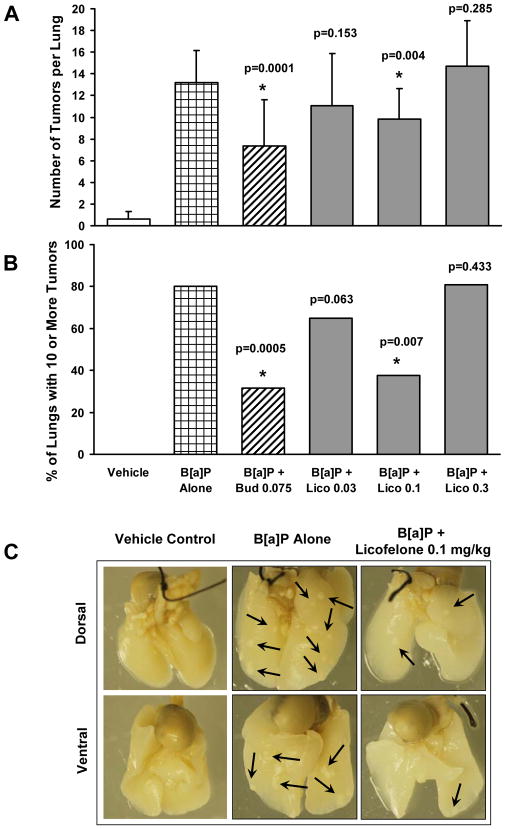
In order to determine the effect of 16 week Licofelone aspiration on tumor multiplicity, an average of three independent tumor counts (by three different scorers) per animal was used for statistical analysis. Comparisons were performed between vehicle control and B[a]P ± Licofelone-treated groups. There was very low spontaneous tumor development in A/J mice (average 0.6 tumors per lung) in vehicle control (labrasol) group, whereas, there was an average of 13.2 tumors per lung (22-fold induction) in B[a]P- treated group (Table 3 and Fig. 4A). Licofelone was effective only at 0.1 mg/kg for reducing tumor multiplicity by 26% compared to B[a]P alone group (Fig. 4A).
Table 3.
Tumor Multiplicity Data in Mouse Lung Following 16-Week Exposure of Licofelone
| Vehicle Control | B[a]P Alone | B[a]P + Budesonide 0.075 mg/kg | B[a]P + Licofelone 0.03 mg/kg | B[a]P + Licofelone 0.1 mg/kg | B[a]P + Licofelone 0.3 mg/kg | |
|---|---|---|---|---|---|---|
| Tumors per Lung | 0.6 ± 0.6 | 13.2 ± 3.0 | 7.3 ± 4.3 | 11.1 ± 4.8 | 9.8 ± 2.8 | 14.7 ± 4.2 |
| % Inhibition (vs. B[a]P) | 45 | 16 | 25 | -12 | ||
| p-Value a | 0.0001 | 0.1528 | 0.0039 | 0.2848 |
Tumor multiplicity comparison vs. B[a]P alone group was conducted using one-way ANOVA Student’s t-test.
Figure 4.
The tumor incidence data were based on the analysis of mice with tumors (Table 2) after they were subdivided into 4 categories; 0-3, 4-6, 7-10 and >10 tumors. The statistical analysis by the (exact) Cochran-Armitage test for trend was done by comparing the B[a]P-treated group to the Licofelone-treated groups. Analysis of the tumor counts in B[a]P only group showed that, 86 % of the animals had 10 or more tumors, whereas, in Licofelone-exposed (0.03-0.1 mg/kg) animals, there was a significant reduction (24–56%) in the percentage of animals with 10 or more tumors (Fig. 4B and Table 2). Most importantly, Licofelone has affected the tumor incidence trend significantly at 0.03 and 0.1 mg/kg (p=0.063 and 0.007, respectively) compared to B[a]P alone group (Table 2). Interestingly, the highest dose of 0.3 mg/kg was not effective. Representative tumors in the mouse lung from different treatment groups are shown in Fig. 4C. It is very evident that there is a significant decrease in the number of tumors (6 tumors) in lungs of mice treated with 0.1 mg/kg of Licofelone compared to those treated with B[a]P alone (15 tumors). Budesonide (positive control) inhibited both tumor multiplicity (45%) and incidence (63%) significantly compared to B[a]P alone group, validating the integrity of the current aspiration model for testing potential chemopreventive agents (Table 2 and Fig. 4).
Table 2.
Tumor Incidence Data in Mouse Lung Following 16-Week Exposure of Licofelone
| Tumor Incidence | Vehicle Control | B[a]P Alone | B[a]P + Budesonide 0.075 mg/kg | B[a]P + Licofelone 0.03 mg/kg | B[a]P + Licofelone 0.1 mg/kg | B[a]P + Licofelone 0.3 mg/kg |
|---|---|---|---|---|---|---|
| 0–3 a | 20 | 0 | 4 | 3 | 0 | 0 |
| 4–6 a | 0 | 0 | 5 | 1 | 2 | 0 |
| 7–10 a | 0 | 2 | 4 | 3 | 8 | 5 |
| > 10 a | 0 | 12 | 6 | 13 | 6 | 17 |
|
| ||||||
| Percentage of Animals with >10 Tumors | 0 | 86 | 32 | 65 | 38 | 77 |
| p-Value b | 0.0005 | 0.063 | 0.007 | 0.433 | ||
Number of animals containing 0–3, 4–6, 7–10 or more than 10 tumors in the lung.
Tumor incidence comparison vs. B[a]P alone group was conducted using Cochran-Armitage Trend Test.
Effect of Licofelone exposure on biomarker expression
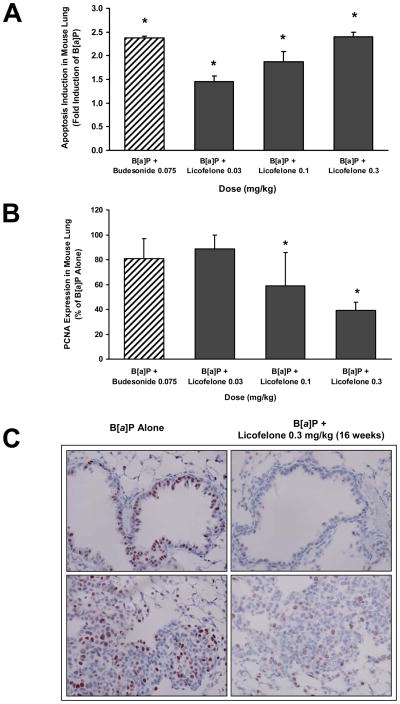
Lung tissues were harvested from 3 mice per group after 2 or 16 weeks of aspiration and processed for Western blot or immunostaining and the data from different biomarkers is shown Figs. 5 and 6.
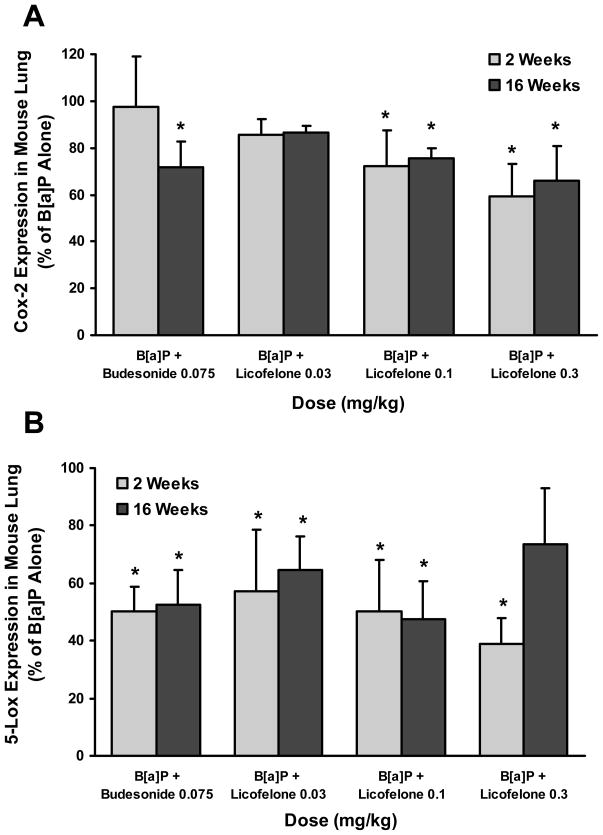
Figure 5.
Figure 6.
Inhibition of Cox-2 and Lox-5
Licofelone inhibited Cox-2 expression in the lung by 15–41% compared to B[a]P alone group after 2 weeks and by 13–34% after 16 weeks of aspiration in a dose-dependent manner (Fig.5A). Licofelone also showed dose-dependent inhibition of 5-Lox expression by 43–61% compared to B[a]P alone group after 2 weeks of aspiration. A similar inhibition profile (35–53%) of 5-Lox was also observed at two low doses (0.03 and 0.1 mg/kg) after 16 weeks of aspiration (Fig. 5B). Licofelone showed only a marginal inhibition of survivin at one dose (0.3 mg/kg) and showed no effects at lower doses (data not shown).
Induction of apoptosis by Licofelone
A dose-dependent induction of apoptosis as measured by TUNEL assay was observed in the lungs of animals treated with Licofelone compared to B[a]P alone group. The increase in induction ranged from 1.5 - 2.4 fold from low to high doses (Fig. 6A).
Inhibition of cell proliferation (PCNA)
An excellent dose-dependent inhibition of cell proliferation measured by PCNA staining was observed in lung samples from Licofelone treated animals. Licofelone reduced PCNA expression significantly in the lung (p < 0.05) compared to B[a]P only treatment group by 41–61% at 0.1–0.3 mg/kg (Fig. 6B). Representative images of PCNA expression in lung sections of B[a]P alone or B[a]P animals treated with Licofelone is shown in Fig. 6C. There was a significant reduction in the number of PCNA positive nuclei in lung sections of Licofelone-treated animals compared to B[a]P alone treated animals.
Discussion
This is the first study that tested the feasibility of administering chemopreventive agents by aspiration for efficacy evaluation in a mouse lung tumor model. This tumor model was designed in such a way that it mimicked morphological changes that are probably present in lungs of former and current smokers and is more relevant to chemopreventive use in clinical trials. For example, after administering three low doses of B[a]P in two weeks, animals were left for 4 weeks to ensure that they all have precancerous lesions in the form of dysplasia in the alveolar region, verified by pathology, before initiating the chemopreventive agent administration. Using this post intervention model, we have shown that Oltipraz, another chemopreventive agent, is highly effective in reducing B[a]P-induced adenomas (28). In the current study, Licofelone administered by aspiration for 16 weeks was equally effective in reducing tumor multiplicity at 0.1 mg/kg and tumor incidence at 0.03 and 0.1 mg/kg compared to B[a]P-alone treated group. The highest dose (0.3 mg/kg) of Licofelone had no effect on either tumor multiplicity or incidence. As 0.3 mg/kg was 1/100 of MTD of Licofelone and there was no significant body weight reduction at this dose following 16 weeks of aspiration, the lack of tumor inhibition at this dose can not be attributed to any toxicity of Licofelone. However, it is possible that this dose has a discernable toxicity by an uncovered mechanism (s) which is compromising the efficacy results. In fact, in some studies on efficacy evaluation, the highest dose is found to be ineffective. For example, celecoxib as an anti-inflammatory agent , was found to be efficacious at lower doses with no activity at higher doses both clinically and in preclinical studies (35). The lack of dose at the high dose was attributed to the activation of NF-kB with resulting transcription of NF-kB-dependent toxicity genes such as TNF-α.
Analysis of mechanistic biomarkers indicated that Licofelone was effective in inhibiting the expression of two key inflammatory proteins, Cox-2 and 5-Lox, at early stage of tumor progression, 2-week time point in this study. At later stage of tumor progression (16-week time point) also, Licofelone showed a dose-dependent inhibition of Cox-2 and PCNA expression in the lung. Interestingly, inhibition of 5-Lox after 16 weeks of aspiration correlated very well with the tumor inhibition data by showing no effect at 0.3 mg/kg whereas the other two lower doses (0.03 and 0.1 mg/kg) being highly effective. Dual inhibition of Cox-2 and 5-Lox has been reported to show inhibition of various tumors such as oral, colon and lung tumors (36–38). Interestingly, another study demonstrated that 5-Lox inhibitor was more effective than Cox-2 inhibitor in suppressing tumors (37). The close correlation between 5-Lox and tumor inhibition observed in our study may indicate that the tumor inhibition effect of Licofelone was via 5-lipoxygenase pathway more than the cyclooxygenase pathway in our mouse lung tumor model. Previous studies by other investigators reported that gene (mRNA) expression of survivin decreased compared to that of untreated tumor control group by administering chemopreventive agents, budesonide (39) and targretin (40), in chemically-induced mouse lung tumor model. However, tumor inhibitory efficacy of Licofelone in our study has no mechanistic correlation with survivin expression as there was only a marginal inhibition of survivin (25%) at the highest dose of 0.3 mg/kg.
Induction of apoptosis by Licofelone has been reported in various cancer cell lines including colon (26) and prostate (25) cancer cells. This study demonstrated that Licofelone can also induce apoptosis in the animal lung tumor model by showing a dose-dependent induction of apoptosis compared to that of B[a]P alone group.
The recent finding on tumor inhibition by Licofelone is one of the good examples for a dual Cox/5-Lox inhibitor being effective in suppressing cancer cell proliferation in animal model. In fact, it has been speculated that inflammation and cancer proliferation have common mechanistic pathways (i.e., Cox and Lox pathways) and dual Cox/Lox inhibitors would be potent not only for anti-inflammation but also in cancer inhibition as well (23). Our data on Cox-2 and 5-Lox with corresponding tumor inhibition in selected doses is indicative of similar mechanism. Interestingly, the Licofelone doses that were effective for tumor inhibition (0.03 and 0.1 mg/kg) showed a direct mechanistic correlation with all the biomarkers (except for survivin) tested in this study. However, not all doses that were effective in biomarkers corresponded to tumor inhibition data as has been observed in this study, where, Licofelone at 0.3 mg/kg was ineffective while the same dose showed significant inhibition of biomarker expressions. .
Budesonide that was used as a positive control tumor inhibitor in this study has shown very potent chemoprevention efficacy against chemically-induced mouse lung tumor (31, 41). Wattenberg and colleagues (31) found that budesonide administered by inhalation inhibited tumor multiplicity (mean number of tumors per mouse) by 81% at a dose of 81 μg/L of air in aerosol or 0.072 mg/kg body weight as a calculated dose. Our study showed that budesonide inhibited tumor multiplicity by 45% at a comparable dose (0.075 mg/kg). Further modification of dosing regimen (e.g., elongated treatment period) could improve tumor inhibitory effects of agents. Similar to Licofelone, the level of inhibition by budesonide in our study was more profound in tumor incidence (63% inhibition compared to B[a]P alone group), which is one of the two primary endpoints to consider along with tumor multiplicity in the assessment of chemopreventive efficacy of agents in the A/J mouse model. Most importantly, the inhibition of tumors and the biomarkers by budesonide validated the integrity of the aspiration route as an effective route for delivering agents to lungs.
In conclusion, the tumor inhibitory effect of low dose Licofelone with very good correlation to the biomarkers makes this compound worth considering for further development, as it is ideal for long term administration. In addition, the aspiration route could be highly useful for assessing potential anticancer drug candidates in the current mouse lung tumor model mimicking former or current smokers. More importantly, the aspiration route has direct applicability in humans as the drug will be delivered by the use of an oral metered inhaler, which uses the same route as the aspiration.
Acknowledgments
Funding support: Research Contract # N01-CN-53301, DCP, NCI (SSharma).
Footnotes
Disclosure of Potential Conflict of Interest
No potential conflicts in interest were disclosed.
Contributor Information
Sheela Sharma, Email: ssharma@thehamner.org.
Jin Lee, Email: jlee@thehamner.org.
Jianliang Zhou, Email: jzhou@thehamner.org.
Vernon E. Steele, Email: steelev@mail.nih.gov.
References
- 1.Decensi A, Costa A. Recent advances in cancer chemoprevention, with emphasis on breast and colorectal cancer. Eur J Cancer. 2000;36:694–709. doi: 10.1016/s0959-8049(00)00040-x. [DOI] [PubMed] [Google Scholar]
- 2.Goodman GE. Prevention of lung cancer. Crit Rev Oncol Hematol. 2000;33:187–197. doi: 10.1016/s1040-8428(99)00074-8. [DOI] [PubMed] [Google Scholar]
- 3.Khuri FR, Lippman SM. Lung cancer chemoprevention. Semin Surg Oncol. 2000;18:100–105. doi: 10.1002/(sici)1098-2388(200003)18:2<100::aid-ssu3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Lippman SM, Spitz MR. Lung cancer chemoprevention: an integrated approach. J Clin Oncol. 2001;19:74S–82S. [PubMed] [Google Scholar]
- 5.Lippman SM, Benner SE, Hong WK. Retinoid chemoprevention studies in upper aerodigestive tract and lung carcinogenesis. Cancer Res. 1994;54:2025s–2028s. [PubMed] [Google Scholar]
- 6.Blumberg J, Block G. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutr Rev. 1994;52:242–245. doi: 10.1111/j.1753-4887.1994.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 7.Lam S, McWilliams A, LeRiche J, MacAulay C, Wattenberg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526–1531. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 8.Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001;62:1433–1438. doi: 10.1016/s0006-2952(01)00747-x. [DOI] [PubMed] [Google Scholar]
- 9.Reginster JY, Bias P, Buchner A. First clinical results of licofelone (ML3000), an inhibitor of COX-1, COX-2 and 5-LOX, for the treatment of osteoarthritis. Ann Rheum Dis. 2002;61:116–117. [Google Scholar]
- 10.Laufer S, Tries S, Augustin J, Elsasser R, Albrecht W, Guserle R, et al. Acute and chronic anti-inflammatory properties of [2,2-dimethyl-6-(4- chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid. Arzneimittelforschung. 1995;45:27–32. [PubMed] [Google Scholar]
- 11.Abraham WM, Laufer S, Tries S. The effects of ML 3000 on antigen-induced responses in sheep. Pulm Pharmacol Ther. 1997;10:167–173. doi: 10.1006/pupt.1997.0090. [DOI] [PubMed] [Google Scholar]
- 12.Rotondo S, Krauze-Brzosko K, Manarini S, Evangelista V, Cerletti C. Licofelone, an inhibitor of cyclooxygenase and 5-lipoxygenase, specifically inhibits cyclooxygenase-1-dependent platelet activation. Eur J Pharmacol. 2004;488:79–83. doi: 10.1016/j.ejphar.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Singh VP, Patil CS, Kulkarni SK. Anti-inflammatory effect of licofelone against various inflammatory challenges. Fundam Clin Pharmacol. 2006;20:65–71. doi: 10.1111/j.1472-8206.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 14.Gay RE, Neidhart M, Pataky F, Tries S, Laufer S, Gay S. Dual inhibition of 5-lipoxygenase and cyclooxygenases 1 and 2 by ML3000 reduces joint destruction in adjuvant arthritis. J Rheumatol. 2001;28:2060–2065. [PubMed] [Google Scholar]
- 15.Algate DR, Augustin J, Atterson PR, Beard DJ, Jobling CM, Laufer S, et al. General pharmacology of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid in experimental animals. Arzneimittelforschung. 1995;45:159–165. [PubMed] [Google Scholar]
- 16.Heidemann A, Tries S, Laufer S, Augustin J. Studies on the in vitro and in vivo genotoxicity of [2,2-dimethyl-6- (4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid. Arzneimittelforschung. 1995;45:486–490. [PubMed] [Google Scholar]
- 17.Wallace JL, Carter L, McKnight W, Tries S, Laufer S. ML 3000 reduces gastric prostaglandin synthesis without causing mucosal injury. Eur J Pharmacol. 1994;271:525–531. doi: 10.1016/0014-2999(94)90814-1. [DOI] [PubMed] [Google Scholar]
- 18.Fiorucci S, Distrutti E, de Lima OM, Romano M, Mencarelli A, Barbanti M, et al. Relative contribution of acetylated cyclo-oxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirin. Faseb J. 2003;17:1171–1173. doi: 10.1096/fj.02-0777fje. [DOI] [PubMed] [Google Scholar]
- 19.Buchner A, Bias P, Lammerich A. Twice the therapeutic dose of licofelone-an inhibitor of COX-1, COX-2 and 5-LOX- results in a significant lower gastrointestinal ulcer incidence than naproxen in osteoarthritis patients, when administered with or without concomitant low-dose asprin. Ann Rheum Dis. 2003;62:261. [Google Scholar]
- 20.Pavelka K, Bias P, Buchner A, Lammerich A, Schulz U. Licofelone, an inhibitor of COX-1, COX-2 and 5-LOX, is as effective as celecoxib and shows improved tolerability during 12 weeks of treatment in patients with osteoarthritis of the knee. Ann Rheum Dis. 2003;62:261. [Google Scholar]
- 21.Rainsford KD, Ying C, Smith F. Effects of 5-lipoxygenase inhibitors on interleukin production by human synovial tissues in organ culture: comparison with interleukin-1-synthesis inhibitors. J Pharm Pharmacol. 1996;48:46–52. doi: 10.1111/j.2042-7158.1996.tb05875.x. [DOI] [PubMed] [Google Scholar]
- 22.Jovanovic DV, Fernandes JC, Martel-Pelletier J, Jolicoeur FC, Reboul P, Laufer S, et al. In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: suppression of collagenase 1 and interleukin-1beta synthesis. Arthritis Rheum. 2001;44:2320–2330. doi: 10.1002/1529-0131(200110)44:10<2320::aid-art394>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Leval X, Julemont F, Delarge J, Pirotte B, Dogne JM. New trends in dual 5-LOX/COX inhibition. Curr Med Chem. 2002;9:941–962. doi: 10.2174/0929867024606713. [DOI] [PubMed] [Google Scholar]
- 24.Rao CV, Swamy MV, Choi C, Janakiram NB, Patlolla JM, Steele VE. Chemoprevention of colon carcinogenesis by licofelone, a novel dual 5-LOX/COX inhibitor, in F344 rats. Proceedings for 98th AACR Annual Meeting; Los Angeles, CA. 2007. Abstract #11. [Google Scholar]
- 25.Narayanan NK, Nargi D, Attur M, Abramson SB, Narayanan BA. Anticancer effects of licofelone (ML-3000) in prostate cancer cells. Anticancer Res. 2007;27:2393–2402. [PubMed] [Google Scholar]
- 26.Tavolari S, Bonafe M, Marini M, Ferreri C, Bartolini G, Brighenti E, et al. Licofelone, a dual COX/5-LOX inhibitor, induces apoptosis in HCA-7 colon cancer cells through the mitochondrial pathway independently from its ability to affect the arachidonic acid cascade. Carcinogenesis. 2008;29:371–380. doi: 10.1093/carcin/bgm265. [DOI] [PubMed] [Google Scholar]
- 27.Nadithe V, Rahamatalla M, Finlay WH, Mercer JR, Samuel J. Evaluation of nose-only aerosol inhalation chamber and comparison of experimental results with mathematical simulation of aerosol deposition in mouse lungs. J Pharm Sci. 2003;92:1066–1076. doi: 10.1002/jps.10379. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Gao P, Steele VE. The chemopreventive efficacy of inhaled oltipraz particulates in the B[a]P-induced A/J mouse lung adenoma model. Carcinogenesis. 2006;27:1721–1727. doi: 10.1093/carcin/bgl052. [DOI] [PubMed] [Google Scholar]
- 29.Koehler DR, Sajjan U, Chow Y-H, Martin B, Kent G, Tanswell AK, et al. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15364–15369. doi: 10.1073/pnas.2436478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao GVS, Tinkle S, Weissman D, Antonini J, Kashon M, Salmen R, et al. Efficacy of a Technique for Exposing the Mouse Lung to Particles Aspirated from the Pharynx. Journal of Toxicology and Environmental Health, Part A: Current Issues. 2003;66:1441–1452. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- 31.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–5492. [PubMed] [Google Scholar]
- 32.Pereira MA, Li Y, Gunning WT, Kramer PM, Al-Yaqoub F, Lubet RA, et al. Prevention of mouse lung tumors by budesonide and its modulation of biomarkers. Carcinogenesis. 2002;23:1185–1192. doi: 10.1093/carcin/23.7.1185. [DOI] [PubMed] [Google Scholar]
- 33.Armitage P. The chi-square test for heterogeneity of proportions after adjustment for stratification. J R Stat Soc Ser B. 1966;28:150–163. [Google Scholar]
- 34.Agresti A. Models for Binary Response Variable, Cochran-Armitage Test. Chapter IV. John Wiley and Sons; New York: 1990. Categorical Data Analysis; pp. 100–102. [Google Scholar]
- 35.Niederberger E, Tegeder I, Vetter G, Schmidtko A, Schmidt H, Euchenhofer C, et al. Celecoxib loses its anti-inflammatory efficacy at high doses through activation of NF-kappaB. Faseb J. 2001;15:1622–1624. doi: 10.1096/fj.00-0716fje. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, et al. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 37.Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 38.Steele VE, Hawk ET, Viner JL, Lubet RA. Mechanisms and applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer. Mutat Res. 2003;523–524:137–144. doi: 10.1016/s0027-5107(02)00329-9. [DOI] [PubMed] [Google Scholar]
- 39.Pereira MA, Tao L, Liu Y, Li L, Steele VE, Lubet RA. Modulation by budesonide of DNA methylation and mRNA expression in mouse lung tumors. Int J Cancer. 2006;120:1150–1153. doi: 10.1002/ijc.22468. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Alyaqoub FS, Tao L, Steele VE, Lubet RA, Pereira MA. Effect of long-and short-term treatment with targretin on DNA methylation and mRNA expression in vinyl carbarmate-induced mouse lung tumors. Fourth Ann Frontiers Cancer Prev Res. 2005:166. [Google Scholar]
- 41.Li L, Tao L, Lubet RA, Steele VE, Pereira MA. Modulation by budesonide of a CpG endonuclease in mouse lung tumors. Carcinogenesis. 2007;28:1499–1503. doi: 10.1093/carcin/bgm056. [DOI] [PubMed] [Google Scholar]