Abstract
In epidemiologic studies, high intake of β-cryptoxanthin has been associated with a decreased risk of lung cancer, particularly among current smokers. However, data are not available from well-controlled animal studies to examine the effects of β-cryptoxanthin on cigarette smoke-induced lung lesions, and the biological mechanisms by which β-cryptoxanthin might affect lung carcinogenesis. We evaluated the effects of β-cryptoxanthin supplementation on cigarette smoke-induced squamous metaplasia, inflammation, and changes in protein levels of pro-inflammatory cytokine [tumor necrosis factor alpha (TNFα)] and transcription factors [nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1)], as well as on smoke-induced oxidative DNA damage [8-hydroxy-2′-deoxyguanosine (8-OHdG)] in the lung tissue of ferrets. Thirty six male ferrets were assigned to cigarette smoke exposure or no exposure and to low-dose, or high-dose β-cryptoxanthin, or no dose (2 × 3 factorial design) for 3 months. β-Cryptoxanthin supplementation dose-dependently increased plasma and lung β-cryptoxanthin levels in ferrets, whereas cigarette smoke exposure lowered plasma and lung β-cryptoxanthin levels. β-Cryptoxanthin at both doses significantly decreased smoke-induced lung squamous metaplasia and inflammation. β-Cryptoxanthin also substantially reduced smoke-elevated TNFα levels in alveolar, bronchial, bronchiolar and bronchial serous/mucous gland epithelial cells and in lung macrophages. Moreover, β-cryptoxanthin decreased smoke-induced activation of NF-κB, expression of AP-1 and levels of 8-OHdG. The beneficial effects of β-cryptoxanthin were stronger for high-dose β-cryptoxanthin than for low-dose β-cryptoxanthin. Data from this study indicate that β-cryptoxanthin provides a beneficial effect against cigarette smoke-induced inflammation, oxidative DNA damage and squamous metaplasia in the lungs.
Keywords: cigarette smoke, β-cryptoxanthin, lung inflammation, squamous metaplasia
Introduction
Lung cancer is the second most common incident cancer and the leading cause of cancer death among men and women in the US (1). Smoking is a strong risk factor for lung cancer (2). Although smoking prevention and cessation are the best approaches to prevent lung cancer, more than 23% of adults in the US continue to smoke (2). Therefore, it is important to identify and study dietary factors that have the potential to prevent the development of lung cancer.
β-Cryptoxanthin, a major carotenoid that is rich in pumpkin, papayas, tangerines and tangerine juice, sweet red peppers, oranges and orange juice, carrots, plums, watermelon, corn, and peaches, holds promise to be a chemopreventive agent against lung cancer. This is suggested by the findings from large epidemiologic studies that have shown a reduced risk of developing lung cancer to be associated with higher intakes or blood levels of β-cryptoxanthin (3, 4). In the pooled analysis using the primary data from seven large prospective cohorts in North America and Europe with 3,155 incident cases of lung cancer diagnosed among 399,765 participants, β-cryptoxanthin intake was associated with a significant 24% reduction in risk of developing lung cancer comparing the highest with the lowest quintile of intake, but there were no significant associations for intakes of other carotenoids including lycopene, α-carotene, β-carotene, and lutein and zeaxanthin (3). This protective effect of β-cryptoxanthin was strongest among current smokers and independent of intakes of other nutrients found in fruits and vegetables such as vitamin C, folate, and other carotenoids, and independent of the use of multivitamin supplements (3). In a meta-analysis, the inverse association between β-cryptoxanthin intake and lung cancer was strongest among intakes of specific carotenoids examined, the summary relative risks (RRs) [95% confidence intervals (CIs)] were 0.80 (0.72, 0.89) for β-cryptoxanthin, 0.86 (0.77, 0.97) for lycopene, 0.89 (0.79, 1.00) for α-carotene, 0.89 (0.79, 1.00) for lutein and zeaxanthin, and 0.92 (0.83, 1.01) for β-carotene (4). For serum carotenoids, the summary RRs (95% CIs) were 0.71 (0.51, 0.98) for lycopene, 0.82 (0.40, 1.68) for β-cryptoxanthin, 0.84 (0.66, 1.07) for β-carotene, 0.89 (0.59, 1.33) for α-carotene and 0.95 (0.67, 1.36) for lutein and zeaxanthin (4). However, the possibility that the results from observational studies on β-cryptoxanthin and lung cancer risk might be confounded by other compounds found in foods cannot be completely excluded. Thus, data from intervention studies are needed.
Cigarette smoke can lead to lung inflammation (5), which has been suggested to play an important role in lung carcinogenesis (5, 6). Pro-inflammatory cytokines are secreted in response to inflammatory stimuli in the lung with subsequently elevated reactive oxygen and/or nitrogen species (ROS/RNS), which result in oxidative DNA damage with potentially mutagenic consequences and tumor initiation (5). The binding of pro-inflammatory cytokines to their receptors triggers the mitogen-activated protein kinase (MAPK) pathway that leads to the activation of two pivotal transcription factors, nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1) (7-11). NF-κB and AP-1 activate the expression of numerous genes, including those important for inflammation, cell proliferation and apoptosis (7-11). We hypothesize that β-cryptoxanthin prevents lung inflammation and carcinogenesis through suppressing NF-κB and AP-1 mediated responses. However, data are not available from well-controlled animal studies to evaluate the effects of β-cryptoxanthin supplementation on lung inflammation and carcinogenesis caused by cigarette smoke exposure. Such information, including biologic activities and appropriate dosage of β-cryptoxanthin, is necessary to obtain before thinking of investing in β-cryptoxanthin human intervention trials.
In the present study, we evaluated the chemopreventive effects of β-cryptoxanthin supplementation in two doses that are relevant to human intakes on cigarette smoke-induced lung carcinogenesis in ferrets by assessing lung pathological changes (inflammation and squamous metaplasia). We also investigated potential biological mechanisms by which β-cryptoxanthin could affect lung carcinogenesis by measuring lung tissue levels of molecular markers, including key pro-inflammatory cytokine of TNFα, transcription factors of NF-κB and AP-1 (c-Jun and c-Fos), and oxidative DNA damage marker of 8-hydroxy-2′-deoxyguanosine (8-OHdG).
Materials and Methods
Animals and study groups
Male adult ferrets (1.0 - 1.2 kg) from Marshall Farms (North Rose, NY) were fed a semi-purified ferret diet containing no β-cryptoxanthin (Research Diets, Inc., New Brunswick, NJ) and water ad libitum. Thirty six male ferrets were randomly assigned to six groups (n = 6 in each group) for 3 months as follows: 1) control; 2) smoke exposed; 3) low-dose β-cryptoxanthin (7.5 μg/kg body weight per day); 4) high-dose β-cryptoxanthin (37.5 μg/kg body weight per day); 5) smoke-exposed plus low-dose β-cryptoxanthin; 6) smoke-exposed plus high-dose β-cryptoxanthin. The ferret body weights were recorded weekly and there were no differences in body weight among treatment groups throughout the experiment. After the experimental period, all ferrets were terminally exsanguinated under deep isoflurane anesthesia. Tissues and plasma were collected and stored at −80°C until analyzed. The Animal Care and Use Committee at the Human Nutrition Research Center on Aging at Tufts University approved this study.
β-Cryptoxanthin supplementation
β-Cryptoxanthin (CaroteNature, Lupsingen, Switzerland) was dissolved into 1 ml corn oil and fed orally to ferrets every morning for 3 months. The chemical purity of this commercial β-cryptoxanthin was examined in our laboratory using the high performance liquid chromatography (HPLC) with more than 99% of purity. Ferrets in the control group were fed the semi-purified diet plus 1 ml corn oil only. The average American intake of β-cryptoxanthin was 104 μg/day (12) (~1.5 μg/kg body weight per day in a 70 kg person), and such dose was related to a reduced risk of lung cancer in the pooled analysis of seven large cohort studies (3). Because the total absorption of carotenoids by the ferret is about 5 times less than in humans (13), the β-cryptoxanthin intake in the low-dose β-cryptoxanthin group was ~7.5 μg/kg body weight per day, which is equivalent to 105 μg/day in a 70 kg person (105 μg/day / 70 kg body weight × 5 = 7.5 μg/kg body weight/day). The high-dose β-cryptoxanthin supplemented group received 37.5 μg/kg body weight per day, which is equivalent to 525 μg/day in a 70 kg person (5 times higher than the average American intake of β-cryptoxanthin of 104 μg/day (12)). In the Singapore Chinese Health Study, those who consumed approximately 550 μg/day of β-cryptoxanthin also had significantly lower risk of lung cancer (14). Thus, both the low- and high-dose β-cryptoxanthin used in this study had been shown to provide protection against the development of lung cancer in human observational studies (3, 14).
Cigarette smoke exposure
The procedure of cigarette smoke-exposure in ferrets has been published previously (15-17). Briefly, ferrets were exposed to 10-cigarettes/30 min twice in the morning and twice in the afternoon for three months. This amount of smoke-exposure in the ferret is similar to that found in humans smoking one and a half packages of cigarettes per day in terms of the concentration of urinary cotinine equivalents (~18 μg/mL urine in the ferret) (17).
Plasma and lung tissue extraction and HPLC analysis of β-cryptoxanthin
Plasma and lung tissue extraction was conducted without saponification as described previously (17). The HPLC system consisted of a Waters 2998 Photodiode Array Detector, an Alliance Waters 2695 Separations module, a Perkin Elmer guard column (C18, 3 uM, 30 × 4.6 mm), a Perkin Elmer C18 column (4.6 × 83 mm), and a HPLC column temperature controller (model 7950, column heater/chiller; Jones Chromatography, Lakewood, CO). A Waters 2998 Photodiode Array Detector was set at 450 nm for ß-cryptoxanthin. A gradient reverse-phase HPLC system was used for the analysis. The gradient procedure and solvents used have been described elsewhere (16). ß-cryptoxanthin was identified by coelution with standards and was quantified relative to the internal standard (echinenone) by determining peak areas calibrated against known amounts of standards. In all experiments, all procedures were carried out under red light to prevent photodamage to the compounds.
Histopathologic evaluation
The right upper lobe of each lung was inflated and fixed by intratracheal instillation of 10% formalin. The samples were processed and embedded in paraffin. Four μm sections were made using a Leica microtome and stained with hematoxylin (H) and eosin (E) for histopathological examination. We examined the presence or absence of lung squamous metaplasia and conducted immunohistochemistry analysis to confirm lung squamous metaplasia. The sections were blindly examined by two independent investigators by light microscopy (ZEISS Microscopy, PixeLINK USB 2.0 (PL-B623CU) Camera, PixeLINK μScope Microscopy Software). The squamous metaplasia lesions are spotty and localized. We treated an animal as “positive” if any keratinized squamous metaplasia lesion was observed (histological examination plus confirmations by immunohistochemistry with anti-keratin antibody). Otherwise the animal was considered “negative.”
The sections of lung tissue also were blindly evaluated as to lung inflammation. The degree of severity of lung inflammation was estimated by peribronchial/bronchiolar infiltrates of inflammatory cells, alveolar septal infiltrates, and perivascular infiltrates as follows: grade 0 (none), no inflammation; grade 1 (+) (minimal), occasional cuffing with inflammatory cells; grade 2 (++) (mild), a thin layer cuffing with inflammatory cells for bronchi/bronchiole and blood vessel and a few monocytes/macrophages of alveolar septal infiltrates; grade 3 (+++) (moderate), a moderate thick layer cuffing with inflammatory cells for bronchi/bronchiole and blood vessel and a moderate number of monocytes/macrophages of alveolar septal infiltrates; and grade 4 (++++) (severe), a thick layer cuffing with inflammatory cells for bronchi/bronchiole and blood vessel and a large number of monocytes/macrophages of alveolar septal infiltrates.
Immunohistochemical assays of lung molecular markers
Four μm thick serial sections from the formalin-fixed, paraffin-embedded lung tissue block were immunostained for TNFα, NF-κB (p65), AP-1 (c-Jun and c-Fos), and 8-OHdG using the avidin–biotin complex immunoperoxidase methods (Vectastain ABC-Elite; Vector, Burlingame, CA). Primary antibodies had dilutions of 1:100 for TNFα, 1:50 for p65, c-Jun, c-Fos, and 1:200 for 8-OHdG. A negative control for each tissue section was performed under identical conditions except using the nonimmune serum instead of the primary antibodies.
Antibodies against p65 and c-Fos were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against TNFα and c-Jun were purchased from Cell Signaling Technology (Beverly, MA). Antibody against 8-OHdG was purchased from Calbiochem (San Diego, CA). For each antibody, the specific working conditions such as serial dilutions were determined in preliminary experiments. Antibodies used were all cross-reacted with the ferret proteins.
Microscopic evaluation of lung molecular markers
The sections were examined under light microscopy by two independent investigators who were blinded to the treatment groups. Representative areas of each section were selected and inflammatory or epithelial cells were counted under high magnification [400×] for a total of 10 fields. For each section, the nuclear or cytoplasmic immunoreactivity was quantified based on the percentage of positive specific cells over the total specific cells examined. When the cell nuclei were stained with brown color, the cells were considered to be positive for p65, c-Jun, c-Fos, and 8-OHdG. TNFα expression was noted in the nuclei, cytoplasm, or membrane, the cells were considered to be positive if the cell was stained with a brown color above background.
Statistical analysis
Results are expressed as means ± standard deviations (SD) and significant differences among groups were compared using ANOVA overall F-test followed by Tukey's honest test for continuous variables (plasma and lung β-cryptoxanthin concentrations and lung TNFα, p65, c-Jun, c-Fos and 8-OHdG). Two-way ANOVA was used to test for interaction between smoke exposure and β-cryptoxanthin treatments in relation to plasma and lung β-cryptoxanthin concentrations and lung molecular markers. Kruskal-Wallis overall test followed by Wilcoxon rank-sum test was used to test for statistical significance among groups for ordinal variable (lung inflammation grade) and Mantel-Haenszel overall test followed by Fisher exact test was used to test for statistical significance among groups for categorical variable (occurrence of lung squamous metaplasia). SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses. All P values were two-sided at the significance level of α = 0.05 (P value ≤ 0.05).
Results
Concentrations of β-cryptoxanthin in plasma and lung tissue of ferrets
β-Cryptoxanthin supplementation significantly dose-dependently increased the concentrations of β-cryptoxanthin in both plasma and lung tissue of the ferrets (Table 1). Because the semi-purified ferret diet used in this study did not contain β-cryptoxanthin, β-cryptoxanthin was not detected in plasma and lung tissue in the control and smoke-exposed alone groups. In the groups supplemented with β-cryptoxanthin alone, β-cryptoxanthin concentrations in both plasma and lung tissue in the high-dose β-cryptoxanthin groups were significantly higher than those in the low-dose β-cryptoxanthin groups. In the groups supplemented with β-cryptoxanthin and exposed to cigarette smoke, a similar finding pattern was observed. Plasma and lung β-cryptoxanthin concentrations in the groups that were treated with both β-cryptoxanthin and cigarette smoke exposure were lower than the corresponding groups that were supplemented with β-cryptoxanthin alone: the percentages of decrease were 31% and 36% in plasma for the low-dose group and the high-dose group, respectively, and 42% and 35% in lung tissue for the low-dose group and the high-dose group, respectively (Table 1).
Table 1.
Plasma and lung tissue concentrations* of β-cryptoxanthin, the occurrence of lung squamous metaplasia, and the grade of lung inflammation by treatment groups
| Treatment group | N | Plasma (nmol/L)† |
Lung (nmol/kg)† |
No. of ferrets with lung squamous metaplasia/total number in each group‡ |
Median values of lung inflammation grade (range)§ |
|---|---|---|---|---|---|
| Control (sham exposure) | 6 | ND | ND | 0/6 b | 1 (0-1) c |
| Low-dose β-cryptoxanthin | 6 | 69±9 b | 31±5 b | 0/6 b | 0.5 (0-1) c |
| High-dose β-cryptoxanthin | 6 | 117±20 a | 63±10 a | 0/6 b | 0.5 (0-1) c |
| Smoke | 6 | ND | ND | 6/6 a | 3 (2-4) a |
| Smoke/low-dose β-cryptoxanthin | 6 | 48±6 c | 18±4 c | 2/6 a,b | 2 (1-3) b |
| Smoke/high-dose β-cryptoxanthin | 6 | 75±13 b | 41±8 b | 1/6 b | 2 (1-3) b |
For a given column, data not sharing a common superscript letter are statistically significantly different at P ≤ 0.05.
For a given column, data not sharing a common superscript letter are statistically significantly different at P ≤ 0.05.
For a given column, data not sharing a common superscript letter are statistically significantly different at P ≤ 0.05.
Values are expressed as means ± SD (ANOVA overall F-test followed by Tukey's honest test)
Tests for interaction between smoke exposure and β-cryptoxanthin treatments were significant (P < 0.001)
Fisher's exact test
Wilcoxon test
Histopathological evaluations in lung tissue of ferrets
Lung squamous metaplasia
Normal ferret lung histology and lung squamous metaplastic lesions are exemplified in Figure 1. Both low- and high-dose β-cryptoxanthin lowered cigarette smoke-induced lung squamous metaplasia in ferrets; the reduction was significant for high-dose β-cryptoxanthin (p = 0.015) and was marginally significant for low-dose β-cryptoxanthin (p = 0.06) (Table 1). Lung squamous metaplastic lesions were observed in six of the six ferrets in the smoke-exposed alone group, but only in two of the six ferrets in the low-dose β-cryptoxanthin group with smoke exposure and only in one of the six ferrets in the high-dose β-cryptoxanthin group with smoke exposure. No lung squamous metaplasia were found in the control group, the low-dose β-cryptoxanthin alone group or the high-dose β-cryptoxanthin alone group.
Figure 1.
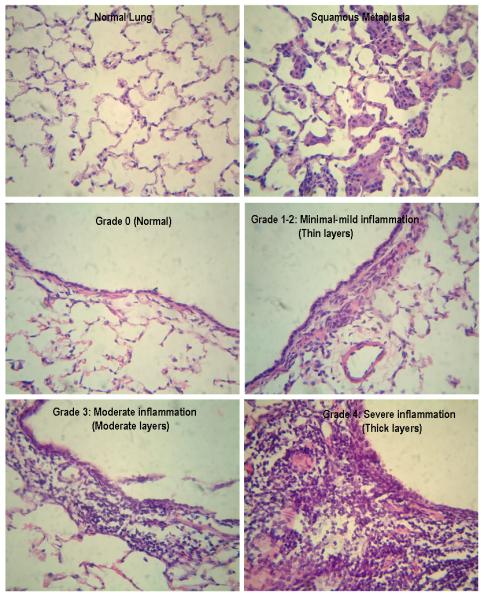
Representative squamous metaplasia and inflammation grade in lung tissue
Lung inflammation
Representative H and E staining for lung inflammation in ferrets is shown in Figure 1. Inflammation was significantly increased in lung tissue of ferrets exposed to cigarette smoke alone with the median grade of 3 (range: from 2 to 4) (Table 1). Both low- and high-dose β-cryptoxanthin significantly lowered smoke-induced inflammation with the median grade of 2 (range: from 1 to 3) in two β-cryptoxanthin groups with smoke exposure. There was no inflammation (grade 0) or only minimal inflammation (grade 1) in lung tissue of ferrets in the control group, the low-dose β-cryptoxanthin alone group, and the high-dose β-cryptoxanthin alone group with the median grades of 0.5 to 1.
Evaluations of lung molecular markers
TNFα expression
In the non-smoke-exposed groups, there was constitutive expression of TNFα in bronchial/bronchiolar epithelial cells with moderate intensity staining. There were small to moderate numbers of cells with moderate intensity of staining in alveolar and bronchial serous/mucous gland epithelial cells and in macrophages that were located in the alveoli, interstitium, and around blood vessels, bronchioles and bronchi. Cigarette smoke exposure increased TNFα expression in lung tissue (Figure 2 and Table 2). In the smoke-exposed alone group, there was strong intense staining of TNFα in all bronchial/bronchiolar epithelial cells. Strong diffuse intense staining of TNFα was also seen in alveolar epithelial cells and bronchial serous/mucous glands (both in gland epithelial cells and in the gland lumens). Large amounts of increased infiltrating macrophages that were located in the alveoli, interstitium, around blood vessels, bronchioles and bronchi were present with strong intense staining of TNFα. Treatment with both low- and high-dose β-cryptoxanthin substantially significantly reduced smoke-elevated TNFα staining in bronchial/bronchiolar and alveolar epithelial cells, bronchial serous/mucous glands, and macrophages in lung tissue; the reduction was significantly dose-dependent for alveolar epithelial cells (Figure 2 and Table 2).
Figure 2.
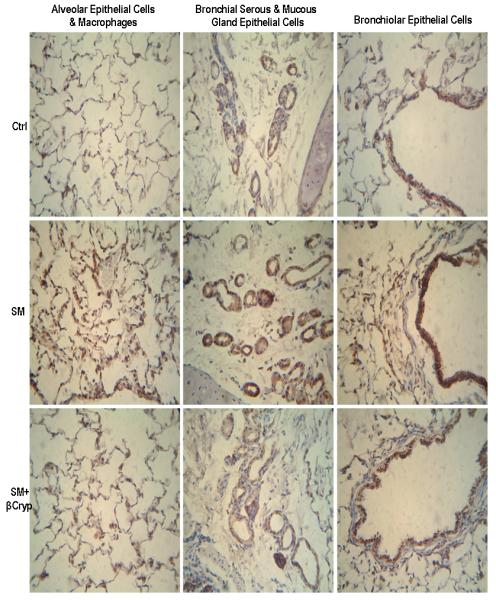
Representative immunohistochemical staining of TNFα in lung tissue. Abbreviations: Ctrl: Control group; SM: Cigarette smoke exposed group; SM+βcryp: Cigarette smoke-exposed plus β-cryptoxanthin group
Table 2.
TNFalpha expression* in lung tissue by treatment groups
| % of positive cells | ||||||
|---|---|---|---|---|---|---|
| Treatment group | Alveolar epithelial cells‡ |
Bronchiolar epithelial cells§ |
Bronchial epithelial cells§ |
Bronchial serous/mucous gland epithelial cells‡ |
% of positive macrophages‡ |
No. of total positive macrophages /mm2†‡ |
| Control | 7.2±4.3 c,d | Moderate | Moderate | 30.3±8.7 c | 16.7±5.7 c | 53.0±14.0 c |
| Low-dose β-cryptoxanthin | 5.8±3.4 d | Moderate | Moderate | 35.2±9.0 c | 15.0±6.6 c | 41.2±9.5 c |
| High-dose β-cryptoxanthin | 6.0±4.8 d | Moderate | Moderate | 29.2±10.3 c | 12.3±3.8 c | 35.2±11.6 c |
| Smoke | 85.2±14.2 a | Dark | Dark | 89.0±11.3 a | 78.8±12.1 a | 584.5±94.9 a |
| Smoke/low-dose β-cryptoxanthin | 41.5±10.3 b | Moderate to dark |
Moderate to dark |
60.2±12.6 b | 46.3±9.6 b | 314.5±79.0 b |
| Smoke/high-dose β-cryptoxanthin | 20.7±6.2 c | Moderate to dark |
Moderate to dark |
43.3±9.4 b,c | 35.3±8.1 b | 239.3±74.2 b |
Values are expressed as means ± SD (n = 6 in each group). For a given column, data not sharing a common superscript letter are statistically significantly different from each other (P ≤ 0.05, ANOVA overall F-test followed by Tukey's honest test). Ten high power (400×) fields per lung were counted for each cell type, compartment and location.
The area of a large magnification field (400× magnification) corresponded to 0.094 mm2.
Tests for interaction between smoke exposure and β-cryptoxanthin treatments were significant (P < 0.0001)
Bronchiolar/bronchial epithelial cells were all positively stained and had different degrees of brown color staining.
NF-κB (subunit p65)
In the non-smoke-exposed groups, there was constitutive cytoplasmic staining of NF-κB in bronchiolar/bronchial epithelial cells (with moderate intensity brown staining) and alveolar and bronchial serous/mucous gland epithelial cells (with light intensity brown staining) (Figure 3, panel A), and the percentages of nuclear staining of NF-κB in lung epithelial cells were small and there were no significant differences among groups (Table 3).
Figure 3.
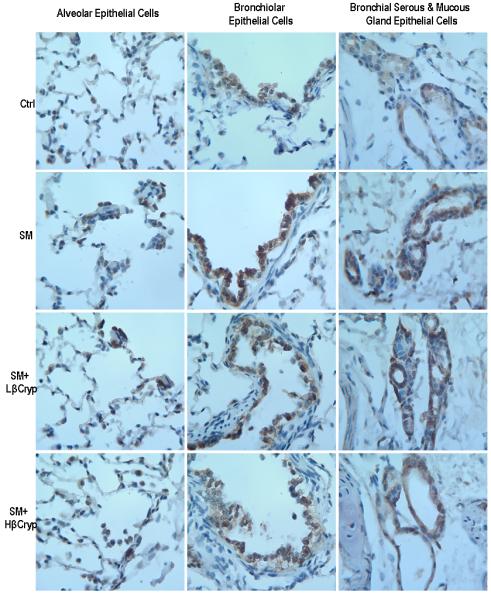
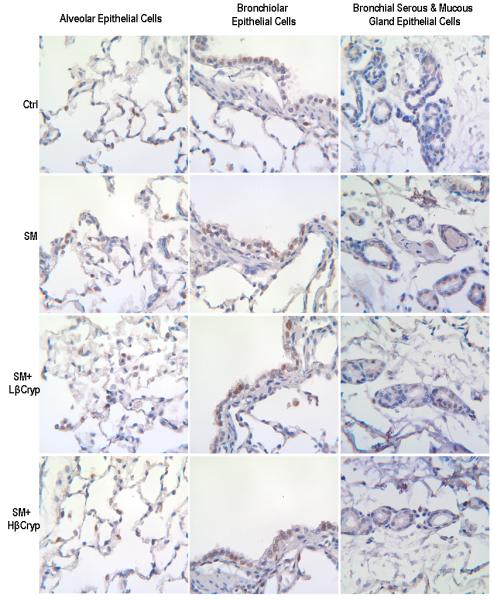
panel A. Representative immunohistochemical staining of NF-κB (subunit p65) in lung tissue. Abbreviations: Ctrl: Control group; SM: Cigarette smoke exposed group; SM+Lβcryp: Cigarette smoke-exposed plus low-dose β-cryptoxanthin group; SM+Hβcryp: Cigarette smoke-exposed plus high-dose β-cryptoxanthin group
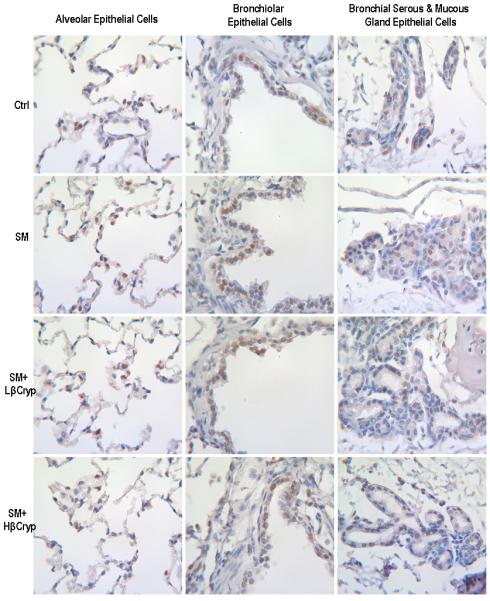
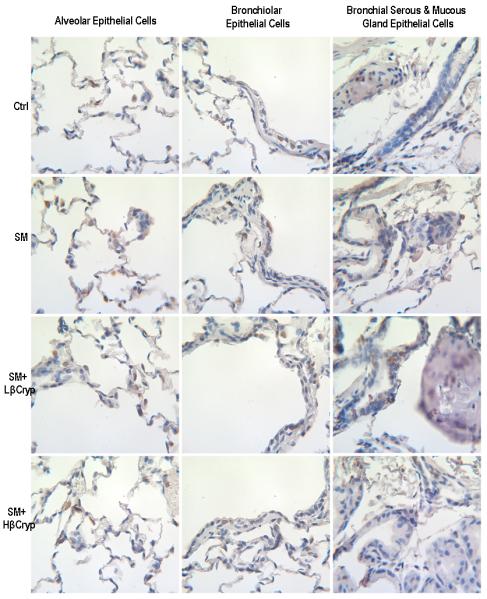
panel B. Representative immunohistochemical staining of c-Jun in lung tissue. Abbreviations: Ctrl: Control group; SM: Cigarette smoke exposed group; SM+Lβcryp: Cigarette smoke-exposed plus low-dose β-cryptoxanthin group; SM+Hβcryp: Cigarette smoke-exposed plus high-dose β-cryptoxanthin group
panel C. Representative immunohistochemical staining of c-Fos in lung tissue. Abbreviations: Ctrl: Control group; SM: Cigarette smoke exposed group; SM+Lβcryp: Cigarette smoke-exposed plus low-dose β-cryptoxanthin group; SM+Hβcryp: Cigarette smoke-exposed plus high-dose β-cryptoxanthin group
panel D. Representative immunohistochemical staining of 8-OHdG in lung tissue. Abbreviations: Ctrl: Control group; SM: Cigarette smoke exposed group; SM+Lβcryp: Cigarette smoke-exposed plus low-dose β-cryptoxanthin group; SM+Hβcryp: Cigarette smoke-exposed plus high-dose β-cryptoxanthin group
Table 3.
NF-κB (subunit p65), AP-1 (c-Jun/c-Fos), and 8-OHdG nuclear staining* in lung tissue by treatment groups
| % of positive cells | |||||
|---|---|---|---|---|---|
| Treatment group | Alveolar epithelial cells† |
Bronchiolar epithelial cells† |
Bronchial epithelial cells† |
Bronchial serous/mucous gland epithelial cells†,‡ |
% of total positive epithelial cells† |
| NF-κB | |||||
| Control | 6.67±1.55 c | 7.50±2.01 c | 6.32±1.81 d | 2.45±1.07 c,d | 7.04±1.73 c |
| Low-dose β-cryptoxanthin | 5.85±1.81 c | 8.22±2.14 c | 6.15±1.32 d | 2.62±0.64 c,d | 6.30±1.52 c |
| High-dose β-cryptoxanthin | 6.32±1.59 c | 7.83±1.97 c | 6.83±1.83 d | 2.28±0.86 d | 6.58±1.41 c |
| Smoke | 31.85±5.69 a | 43.72±7.95 a | 36.93±6.88 a | 23.87±5.07 a | 33.52±7.60 a |
| Smoke/low-dose β-cryptoxanthin | 26.93±3.96 a | 36.55±4.63 a | 28.48±3.81 b | 11.05±2.34 b | 29.67±4.71 a |
| Smoke/high-dose β-cryptoxanthin | 18.27±3.53 b | 24.68±4.77 b | 21.28±4.19 c | 6.68±1.45 c | 20.54±4.50 b |
|
| |||||
| C-Jun | |||||
| Control | 8.70±1.52 c | 11.80±2.33 c | 9.41±2.00 c | 7.73±1.14 c | 9.19±2.56 c |
| Low-dose β-cryptoxanthin | 9.08±2.39 c | 10.93±2.57 c | 9.34±1.82 c | 7.42±0.88 c | 8.62±1.96 c |
| High-dose β-cryptoxanthin | 8.51±2.40 c | 11.19±1.85 c | 8.82±2.18 c | 8.08±1.43 c | 8.34±2.39 c |
| Smoke | 23.50±3.62 a | 32.22±5.27 a | 27.47±6.42 a | 42.76±7.40 a | 25.61±4.58 a |
| Smoke/low-dose β-cryptoxanthin | 21.67±3.41 a | 30.87±4.65 a | 25.38±4.79 a, b | 35.98±5.97 a | 23.08±4.19 a |
| Smoke/high-dose β-cryptoxanthin | 16.63±2.26 b | 21.55±3.13 b | 18.73±3.46 b | 23.87±4.71 b | 17.20±2.84 b |
|
| |||||
| C-Fos | |||||
| Control | 7.35±1.31 b | 7.88±2.03 b | 6.23±1.22 b | 6.11±1.12c | 7.52±1.79 b |
| Low-dose β-cryptoxanthin | 6.72±1.55 b | 8.07±1.69 b | 6.45±1.74 b | 5.89±1.53 c | 6.63±1.60 b |
| High-dose β-cryptoxanthin | 6.92±1.61 b | 7.55±2.16 b | 5.98±1.4 b | 5.73±1.02 c | 7.03±2.35 b |
| Smoke | 16.82±2.93 a | 20.70±4.17 a | 15.02±3.10 a | 31.53±5.89 a | 17.06±3.70 a |
| Smoke/low-dose β-cryptoxanthin | 16.12±2.70 a | 19.09±3.92 a | 12.94±2.15 a | 28.40±4.72 a | 16.46±3.06 a |
| Smoke/high-dose β-cryptoxanthin | 14.97±2.41 a | 17.68±3.37 a | 12.67±2.18 a | 19.10±3.20 b | 15.37±2.63 a |
|
| |||||
| 8-OHdG | |||||
| Control | 0.45±0.13 c | 0.59±0.28 c | 0.38±0.11 c | 0.24±0.09 c | 0.67±0.20 c |
| Low-dose β-cryptoxanthin | 0.42±0.12 c | 0.48±0.20 c | 0.37±0.10 c | 0.26±0.08 c | 0.51±0.17 c |
| High-dose β-cryptoxanthin | 0.39±0.17 c | 0.46±0.12 c | 0.33±0.08 c | 0.29±0.10 c | 0.43±0.19 c |
| Smoke | 5.95±1.08 a | 6.61±1.44 a | 5.65±1.25 a | 2.27±0.50 a | 6.28±1.33 a |
| Smoke/low-dose β-cryptoxanthin | 4.83±1.12 a | 6.02±1.28 a | 4.75±1.08 a | 1.91±0.42 a | 5.57±1.19 a |
| Smoke/high-dose β-cryptoxanthin | 3.23±0.96 b | 3.35±0.63 b | 2.66±0.59 b | 1.25±0.31 b | 3.66±0.64 b |
Values are expressed as means ± SD (n = 6 in each group). For a given column, data not sharing a common superscript letter are statistically significantly different from each other (P ≤ 0.05, ANOVA overall F-test followed by Tukey's honest test). Ten high power (400×) fields per lung were counted for each compartment and location.
Tests for interaction between smoke exposure and β-cryptoxanthin treatments were significant for NF-κB (subunit p65), c-Jun and 8-OHdG (P < 0.05)
Tests for interaction between smoke exposure and β-cryptoxanthin treatments were significant for c-Fos (P = 0.0005)
Cigarette smoke exposure increased nuclear staining of NF-κB (translocation and activation of NF-κB) in lung tissue. In the smoke-exposed alone group, the percentages of epithelial cells showing nuclear staining of NF-κB were substantially higher than the control group (by 3.8 fold for alveolar, 4.8 fold for bronchiolar, 4.6 fold for bronchial, 8.7 fold for bronchial serous/mucous gland and 3.8 fold for total epithelial cells) (Table 3). Treatment with both low- and high-dose β-cryptoxanthin reduced smoke-elevated NF-κB nuclear staining in alveolar, bronchiolar, bronchial, and bronchial serous/mucous glands epithelial cells; the reduction was significantly dose-dependent in bronchial and bronchial serous/mucous glands epithelial cells, but was significant only for the high-dose β-cryptoxanthin group in alveolar and bronchiolar epithelial cells (Figure 3, panel A and Table 3). Additionally, the intensity of cytoplasmic staining of NF-κB in alveolar, bronchiolar, bronchial, and bronchial serous/mucous glands epithelial cells was also stronger in the smoke-exposed alone group, and treatment with β-cryptoxanthin substantially reduced the intensity of cytoplasmic staining of smoke-induced NF-κB in alveolar, bronchiolar, bronchial, and bronchial serous/mucous glands epithelial cells.
AP-1 (c-Jun/c-Fos)
c-Jun nuclear expression was significantly increased in the group exposed to cigarette smoke alone as compared to the control group (by 170% for alveolar, 173% for bronchiolar, 192% for bronchial, 453% for bronchial serous/mucous gland and 179% for total epithelial cells) (Table 3). Both low- and high-dose β-cryptoxanthin lowered smoke-induced c-Jun expression in alveolar, bronchiolar, bronchial, and bronchial serous/mucous glands epithelial cells, but the decrease was statistically significant only for the high-dose β-cryptoxanthin group. No difference in c-Jun expression was observed among those given low- or high-dose β-cryptoxanthin alone and in controls (Figure 3, panel B and Table 3).
c-Fos nuclear expression was also significantly increased in the group exposed to cigarette smoke as compared to the control group (by 129% for alveolar, 163% for bronchiolar, 142% for bronchial, 416% for bronchial serous/mucous gland and 127% for total epithelial cells) (Table 3). Both low- and high-dose β-cryptoxanthin somewhat lowered smoke-induced c-Fos expression in alveolar, bronchiolar, bronchial, and bronchial serous/mucous glands epithelial cells, but the reduction was significant only for high-dose β-cryptoxanthin in bronchial serous/mucous glands epithelial cells. There was also no difference in c-Fos expression among those supplemented with either low- or high-dose β-cryptoxanthin alone or in controls (Figure 3, panel C and Table 3).
8-OHdG
The percentages of cells with nuclear staining of 8-OHdG were significantly greater in the group exposed to cigarette smoke alone than the control group (by 13 fold for alveolar, 11 fold for bronchiolar, 15 fold for bronchial, and 9 fold for bronchial serous/mucous gland or total epithelial cells) (Table 3). Both low- and high-dose β-cryptoxanthin lowered 8-OHdG staining caused by smoke exposure in alveolar, bronchiolar, bronchial, and bronchial serous/mucous glands epithelial cells. The reduction, however, was statistically significant only for the high-dose β-cryptoxanthin group. No significant difference in 8-OHdG staining was seen among the groups of low-dose β-cryptoxanthin alone, high-dose β-cryptoxanthin alone, and the control (Figure 3, panel D and Table 3).
Discussion
In the present study, β-cryptoxanthin supplementation at both doses significantly decreased cigarette smoke-induced lung inflammation and squamous metaplasia. β-Cryptoxanthin also lowered smoke-induced expression of TNFα, NF-κB and AP-1 and smoke-elevated levels of 8-OHdG in the lungs.
The finding that cigarette smoke exposure lowered plasma and lung tissue levels of β-cryptoxanthin in ferrets supplemented with β-cryptoxanthin is consistent with the data from epidemiologic studies that have shown that smokers had lower circulating levels of β-cryptoxanthin than non-smokers (18, 19). Also, we found that β-cryptoxanthin supplementation dose-dependently increased plasma and lung tissue levels of β-cryptoxanthin, which suggests that the ferret absorbs β-cryptoxanthin and β-cryptoxanthin can reach the target organ of the lung. In ferrets supplemented with the low- and high-dose β-cryptoxanthin, plasma β-cryptoxanthin levels were 69 nmol/L and 117 nmol/L, respectively. Although such levels were lower than those in the average Americans [169 nmol/L (9.35 μg/dL) (20)], the beneficial effects of β-cryptoxanthin were observed in ferrets even in the low-dose β-cryptoxanthin group.
To our knowledge, the present study in ferrets is the first animal intervention study to examine the effects of β-cryptoxanthin supplementation on cigarette smoke-induced lung inflammation and carcinogenesis and to investigate biologic mechanisms using molecular markers of lung carcinogenesis. Previously, only one study reported that mice were treated initially with a carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and then given a concentrated mandarin juice (containing 100 mg hesperidin and 3.9 mg of β-cryptoxanthin per 100 g of juice) as drinking water had a significant 26% reduction in incidence of lung tumors compared with NNK-treated animals given water only (21). Because this mouse study used juice containing hesperidin, β-cryptoxanthin and other compounds, it cannot differentiate whether the protective effect was due to hesperidin or β-cryptoxanthin or others. In addition, because of no assessment of β-cryptoxanthin in blood and tissue, it is difficult to judge whether mice absorbed any intact β-cryptoxanthin in this mouse study.
Consistent with our previous studies (15, 16), we found that cigarette smoke exposure induced lung squamous metaplasia, an early precancerous lesion in the lung tissue in ferrets. Most importantly, we provided the first in vivo intervention experimental evidence that β-cryptoxanthin at both dietary and supplemental doses significantly decreased cigarette smoke-induced lung pre-cancerous lesions. This finding is consistent with epidemiologic studies that have shown an inverse association between intakes or blood levels of β-cryptoxanthin and the risk of developing lung cancer (3, 4). The results from the present in vivo study are also supported by an in vitro study showing that β-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small cell lung cancer cells (22).
The lung is a vital organ that is specialized in facilitating uptake of oxygen and release of carbon dioxide and is thus particularly susceptible to oxidative stress and damage. Besides containing carcinogens and chemicals, cigarette smoke generates ROS/RNS and causes lung inflammation (5). Enhanced ROS/RNS production from cigarette smoke exposure may interact with DNA of epithelial cells and lead to oxidative DNA damage, impaired DNA repairs, inhibition of apoptosis, and activation of proto-oncogenes by initiating signal transduction pathways, resulting in neoplastic transformation (6, 11). β-Cryptoxanthin may function as an antioxidant, which quenches ROS/RNS such as singlet molecular oxygen and free radicals (23). In vitro data suggest that β-cryptoxanthin is a more potent singlet oxygen quencher than β-carotene (24) and stimulates the repair of oxidative DNA damage (25). ROS/RNS provokes inflammatory responses, which result in the release of key pro-inflammatory cytokines such as TNFα. TNFα binds to the TNF receptor family and subsequently triggers major intracellular signaling pathways—MAPK pathways leading to the activation of two redox-sensitive transcription factors, NF-κB and AP-1. NF-κB and AP-1 regulate the expression of more than 400 genes, including those involved in inflammation, cell proliferation, and apoptosis (26-28).
In the present study, we comprehensively evaluated the severity of lung inflammation, which was estimated by peribronchial/bronchiolar, alveolar septal and perivascular infiltrates of inflammatory cells. We observed that lung inflammation was the most severe in ferrets exposed to cigarette smoke alone, and β-cryptoxanthin at both doses substantially reduced cigarette smoke-induced lung inflammation. Using immunohistochemical approach, we were able to evaluate lung molecular markers by cell types, compartments and locations. We found that cigarette smoke exposure increased the expression of TNFα, NF-κB and AP-1 and levels of oxidative damage marker of 8-OHdG in the lungs of ferrets. Importantly, we demonstrated that β-cryptoxanthin at both doses substantially reduced cigarette smoke-elevated expression of TNFα, NF-κB and AP-1 and levels of 8-OHdG. Moreover, treatment effects varied by cell types, compartments and locations. These results suggest that β-cryptoxanthin prevents lung inflammation and squamous metaplasia caused by cigarette smoke exposure through suppressing NF-κB and AP-1 mediated responses. The findings from this study provide strong evidence that β-cryptoxanthin has anti-inflammatory and anti-oxidative DNA damage effects in the lungs. This notion is also supported by the results from epidemiologic studies that have shown an inverse correlation between high blood levels or intakes of β-cryptoxanthin and circulating levels of inflammatory markers (29-32) and oxidative damage marker of 8-OHdG (33) in cross-sectional analyses, and an inverse association between high dietary intake of β-cryptoxanthin and a reduced risk of developing inflammatory diseases such as rheumatoid arthritis (34).
β-Cryptoxanthin has provitamin A activity (35) and thus can serve as a precursor of retinoic acid (36, 37), a modulator of cell proliferation and differentiation (38). However, it is unlikely that β-cryptoxanthin inhibits lung carcinogenesis through its provitamin A activity because a pooled analysis of the primary data from seven large well-implemented cohorts in North America and Europe showed that among carotenoids with provitamin A activity (α-carotene, β-carotene, and β-cryptoxanthin) only β-cryptoxanthin was significantly associated with reduced risk of lung cancer (3). In addition, a meta-analysis of published data found that the inverse associations between both intake and serum level of β-cryptoxanthin and lung cancer risk were stronger than those for β-carotene and α-carotene (4). Moreover, in human intervention trials, supplementation with β-carotene, a precursor of vitamin A, did not reduce the risk of lung cancer among apparently healthy male physicians (39), but increased lung cancer risk among smokers or asbestos workers (40, 41). Furthermore, in our previous studies in ferrets, low-dose β-carotene [a physiological dose, 0.43 mg/kg body weight/day that is equivalent to 6 mg/day in a 70 kg person (3 times higher than the average American intake of β-carotene of 2 mg/day (12))] had no effects on smoke-induced lung carcinogenesis (16) and high-dose β-carotene [a pharmacological dose, 2.4 mg/kg body weight/day that is equivalent to 30 mg/day in a 70 kg person (15 times higher than the average American intake of β-carotene of 2 mg/day (12)), a dose that was used in the beta-carotene and retinol efficacy trial (CARET), which showed an increased risk of lung cancer (41)] had harmful effects (15, 16). These data support the notion that the beneficial effects of β-cryptoxanthin on lung carcinogenesis observed in the present study was not due to its provitamin A activity.
A lesson learned from β-carotene and lung cancer studies is that physiological doses that are relevant to human intakes would be more suitable for the potential human β-cryptoxanthin chemoprevention trials. Additionally, the average intake of β-cryptoxanthin [0.1 mg/day (or 104 μg/day)] was 20 times less than the average American intake of β-carotene (2 mg/day) (12). We thus chose both low- and high-dose β-cryptoxanthin that are relevant to human β-cryptoxanthin intakes rather than the doses of β-carotene in our prior studies to evaluate the potential beneficial effects of β-cryptoxanthin on lung inflammation and carcinogenesis for the present study. The low-dose β-cryptoxanthin used for the present study was chosen because such dose was associated with reduced risk of lung cancer in the pooled analysis of seven large cohort studies (3). The β-cryptoxanthin intake in the low-dose β-cryptoxanthin group in ferrets was ~7.5 μg/kg body weight/day, which is equivalent to 105 μg/day in a 70 kg person (the average American intake of β-cryptoxanthin of 104 μg/day (12)). We chose a high-dose β-cryptoxanthin of 37.5 μg/kg body weight/day, which is equivalent to 525 μg/day in a 70 kg person (5 times higher than the average American intake of β-cryptoxanthin of 104 μg/day (12) and within the range of intake in the Singapore Chinese Health Study (14)). In the Singapore Chinese Health Study, those in the highest quintile of β-cryptoxanthin intake (~550 μg/day) also had significantly lower risk of lung cancer (14). In the present study, while both doses significantly decreased cigarette smoke-induced lung inflammation and squamous metaplasia, the strongest effect was observed for high-dose β-cryptoxanthin. This high-dose β-cryptoxanthin could be considered for the potential future human β-cryptoxanthin intervention studies.
In conclusion, data from this well-controlled animal study indicate that β-cryptoxanthin has beneficial effects against cigarette smoke-induced lung inflammation, oxidative DNA damage and lung squamous metaplasia and that β-cryptoxanthin is a potentially effective chemopreventive agent against the development of lung cancer.
Acknowledgments
Funding/Support: Supported by the American Institute of Cancer Research grant 07A003, the Prevent Cancer Foundation grant, the NIH CA104932 grant, and the US Department of Agriculture grant 1950-51000-064S. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
Footnotes
Conflict of interest: None
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Leischow SJ, Djordjevic MV. Smoking reduction and tobacco-related cancers: the more things change, the more they stay the same. xJ Natl Cancer Inst. 2004;96:86–7. doi: 10.1093/jnci/djh030. [DOI] [PubMed] [Google Scholar]
- 3.Mannisto S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev. 2004;13:40–8. doi: 10.1158/1055-9965.epi-038-3. [DOI] [PubMed] [Google Scholar]
- 4.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–83. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 6.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 7.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 8.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 9.Peek RM Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–48. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 10.Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–43. [PubMed] [Google Scholar]
- 11.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine . National Academy Press; Washington, D.C.: 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. [PubMed] [Google Scholar]
- 13.Wang XD, Krinsky NI, Marini RP, Tang G, Yu J, Hurley R, et al. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: a model for human beta-carotene metabolism. Am J Physiol. 1992;263:G480–6. doi: 10.1152/ajpgi.1992.263.4.G480. [DOI] [PubMed] [Google Scholar]
- 14.Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2003;12:890–8. [PubMed] [Google Scholar]
- 15.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–6. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological beta-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis. 2000;21:2245–53. doi: 10.1093/carcin/21.12.2245. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63:3138–44. [PubMed] [Google Scholar]
- 18.Wei W, Kim Y, Boudreau N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am J Public Health. 2001;91:258–64. doi: 10.2105/ajph.91.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabriel HE, Liu Z, Crott JW, Choi SW, Song BC, Mason JB, et al. A comparison of carotenoids, retinoids, and tocopherols in the serum and buccal mucosa of chronic cigarette smokers versus nonsmokers. Cancer Epidemiol Biomarkers Prev. 2006;15:993–9. doi: 10.1158/1055-9965.EPI-05-0664. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine . National Academy Press; Washington, D.C.: 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. [PubMed] [Google Scholar]
- 21.Kohno H, Taima M, Sumida T, Azuma Y, Ogawa H, Tanaka T. Inhibitory effect of mandarin juice rich in beta-cryptoxanthin and hesperidin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary tumorigenesis in mice. Cancer Lett. 2001;174:141–50. doi: 10.1016/s0304-3835(01)00713-3. [DOI] [PubMed] [Google Scholar]
- 22.Lian F, Hu KQ, Russell RM, Wang XD. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int J Cancer. 2006;119:2084–9. doi: 10.1002/ijc.22111. [DOI] [PubMed] [Google Scholar]
- 23.Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7:617–35. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 24.Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, et al. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis. 1997;18:89–92. doi: 10.1093/carcin/18.1.89. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo Y, Azqueta A, Luna L, Bonilla F, Dominguez G, Collins AR. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30:308–14. doi: 10.1093/carcin/bgn270. [DOI] [PubMed] [Google Scholar]
- 26.Waetzig GH, Schreiber S. Review article: mitogen-activated protein kinases in chronic intestinal inflammation - targeting ancient pathways to treat modern diseases. Aliment Pharmacol Ther. 2003;18:17–32. doi: 10.1046/j.1365-2036.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 27.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 28.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–62. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 30.van Herpen-Broekmans WM, Klopping-Ketelaars IA, Bots ML, Kluft C, Princen H, Hendriks HF, et al. Serum carotenoids and vitamins in relation to markers of endothelial function and inflammation. Eur J Epidemiol. 2004;19:915–21. doi: 10.1007/s10654-004-5760-z. [DOI] [PubMed] [Google Scholar]
- 31.Rowley K, Walker KZ, Cohen J, Jenkins AJ, O'Neal D, Su Q, et al. Inflammation and vascular endothelial activation in an Aboriginal population: relationships to coronary disease risk factors and nutritional markers. Med J Aust. 2003;178:495–500. doi: 10.5694/j.1326-5377.2003.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 32.Kritchevsky SB, Bush AJ, Pahor M, Gross MD. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152:1065–71. doi: 10.1093/aje/152.11.1065. [DOI] [PubMed] [Google Scholar]
- 33.Thomson CA, Stendell-Hollis NR, Rock CL, Cussler EC, Flatt SW, Pierce JP. Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2008–15. doi: 10.1158/1055-9965.EPI-07-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pattison DJ, Symmons DP, Lunt M, Welch A, Bingham SA, Day NE, et al. Dietary beta-cryptoxanthin and inflammatory polyarthritis: results from a population-based prospective study. Am J Clin Nutr. 2005;82:451–5. doi: 10.1093/ajcn.82.2.451. [DOI] [PubMed] [Google Scholar]
- 35.Blomhoff R. Transport and metabolism of vitamin A. Nutr Rev. 1994;52:S13–23. doi: 10.1111/j.1753-4887.1994.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 36.Siegel EM, Craft NE, Roe DJ, Duarte-Franco E, Villa LL, Franco EL, et al. Temporal variation and identification of factors associated with endogenous retinoic acid isomers in serum from Brazilian women. Cancer Epidemiol Biomarkers Prev. 2004;13:1693–703. [PubMed] [Google Scholar]
- 37.Napoli JL, Race KR. Biogenesis of retinoic acid from beta-carotene. Differences between the metabolism of beta-carotene and retinal. J Biol Chem. 1988;263:17372–7. [PubMed] [Google Scholar]
- 38.Blomhoff R. Introduction: Overview of vitamin A metabolism and function. In: Blomhoff R, editor. Vitamin A in health and disease. Marcel Dekker, Inc.; New York: 1994. pp. 1–35. [Google Scholar]
- 39.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 40.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 41.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]


