Abstract
The changes in signal transduction associated with the acquisition of specific cell fates remain poorly understood. We performed massive parallel assessment of kinase signatures of the radiations of the hematopoietic system, including long-term repopulating hematopoietic stem cells (LT-HSC), short-term repopulating HSC (ST-HSC), immature natural killer (iNK) cells, NK cells, B cells, T cells and myeloid cells. The LT-HSC kinome is characterised by non-canonical Wnt, Ca2+ and classical protein kinase C (PKC)-driven signalling, which is lost upon the transition to ST-HSC, whose kinome signature prominently features receptor tyrosine kinase (RTK) activation of the Ras/MAPK signalling cassette. Further differentiation to iNK maintains signalling through this cassette but simultaneously leads to activation of a PI3K/PKB/Rac signalling, which becomes the dominant trait in the kinase signature following full differentiation towards NK cells. Differentiation along the myeloid and B cell lineages is accompanied by hyperactivation of both the Ras/MAPK and PI3K/PKB/Rac signalling cassette. T cells, however, deactivate signalling and only display residual G protein-coupled pathways. Thus, differentiation along the hematopoietic lineage is associated with major remodelling of cellular kinase signature.
Introduction
In recent years substantial insight has been gained in the epigenetic, transcriptional and translational changes that accompany alterations in differentiation status and the determination of mammalian cell fate.1–10 The corresponding changes in cellular biochemistry in general, however, and specifically the relation between cell fate and cell kinome remain much less understood, it even being uncertain whether otherwise unchallenged cells display differentiation-stage specific kinome signatures. The prevailing view is that signal transduction is initiated by external cues, but that otherwise unchallenged cells display little developmental stage-specific active signal transduction.11–13 Nevertheless comprehensive characterization of these kinase activities during differentiation would likely provide substantial support for our efforts to understand the nature of stem cells and their differentiation at a molecular level.
This consideration prompted us to characterise kinase signature during differentiation along the hematopoietic lineages. Kinome analysis implements array technologies that comprehensively measure enzymatic activities present in whole cell lysates, usually employing peptide substrates.14 Arrays have been assembled that contain multiple consensus sequences for a broad range of protein kinases present in the mammalian genome, allowing detection of phosphorylation events mediated by kinases present in whole cell lysates.15 We explored a technology that measure enzymatic activity towards peptide substrates spotted on glass.16,17 Here we employ this methodology to approach questions involving the nature of stem cells and their differentiation to lineage-committed cells and we observe that different subsets in the radiations of hematopoietic cell fates are characterized by prominent changes in their cellular kinome, challenging the classical concept that basal signalling in these cells is independent of cell fate and stem cell progression.
Experimental Section
FACS purification of cell populations for kinome analysis
LT-HSC and HSC were sorted from whole BM cells prepared from forelimbs, hindlimbs and vertebral columns of C57BL6 mice as described by Christensen and Weissman and Desponts et al18,19. Myeloid, T, B and NK cells were sorted from spleens of C57BL6 mice. All animal experimentation was conducted and performed in accordance with relevant guidelines and regulations (supervised by William G. Kerr’s institutional committee on ethics of animal experimentation). NK lineage cells were identified as cells that express NK1.1, but lack CD3, Gr1 and IgM to exclude myelo-granulocytic cells, T cells and B cells, respectively. B cells were sorted based on B220 expression with exclusion of cells that express NK1.1, CD3 and Mac1 to exclude NK cells, T cells and myelo-granulocytic cells. Myeloid cells were sorted based on Mac1/CD11b expression with exclusion of cells that express NK1.1, CD3 and B220 to exclude NK cells, T cells and B cells. T cells were sorted based on CD3 expression with exclusion of cells that express NK1.1, B220 and Mac1 to exclude NK cells, B cells and myelo-granulocytic cells. Dead cells were excluded from all sorted populations based on DAPI dye uptake.
FACS analysis of inhibitor effects on hematopoietic phenotype
Anti-coagulated human peripheral blood was collected from healthy donors after informed consent. Whole blood was cultured in the absence or presence of 2 μM SB203580, 10 μM LY294002 (both from LC Laboratories, Woburn, MA), 10 μM U0126 (Cell Signaling Technology, Beverly, MA), or 25 μM PP1 (Sigma Aldrich) for 36h. After erythrocyte lysis, cells were washed with FACS buffer (PBS, 0.5% fetal calf serum, 2mM EDTA) and stained with CD3-Amcyan, CD19-APC, CD66b-FITC (BD Biosciences, Rockville, MD). Flow cytometry was performed using FACSCanto (BD Biosciences). Gating strategy was as follows: all nucleated cells were gated in G1, duplicates were subsequently excluded using FSC-H vs FSC-W and SSC-H vs SSC-W dotplots (G2 and G3). Dead cells were excluded by Dapi stain (G4). T-cells (CD3+), B-cells (CD19+) and myeloid cells (CD66b+) were determined as a percentage of G4. Changes due to treatment with inhibitors were expressed as percentage of untreated control.20,21 Mean of two independent experiments is shown.
Kinome profiling
The specificity of kinases to their substrates is dependent on a multitude of factors, like physical localisation, but a very important factor is the amino acid context of the substrate threonine, serine, or tyrosine amino acid, the amino acids surrounding the substrate amino acid conferring specificity to the kinase reacting. This characteristic of kinases is being exploited for kinome profiling using peptide arrays. In our approach, we employed the entire complement of kinase substrates described in Phosphobase (see supplementary table 8),22 which were spotted on hydrogel-coated glass (spotting procedure is described in Diks et al23) resulting in a slides that display 1,152 individual peptide kinase substrates of mixed species in duplicate. The sequence of the peptides involved as well as the source proteins from which these peptides were derived can be found in the supplementary data and in more detail on http://www.pepscanpresto.com/files/PepChip%20Kinase%20Map%20File%200103.xls). Procedures for performing kinome arrays were described in detail16,23,24. In short, after sorting cells were lysed in 50μl lysis buffer (20mM Tris-HCl pH 7.5, 150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM MgCl2, 1mM glycerophosphate, 1mM Na3VO4, 1mM NaF,1μg/ml Leupeptin, 1μg/ml Aprotinin, 1mM PMSF) and before profiling 10 ml of the peptide array incubation mix (50% glycerol, 50μM ATP, 0.05% v/v Brij-35, 0.25mg/ml BSA, [33P]-γ-ATP (1MBq)) was added to the filtered lysates and mixture loaded onto the microarray chip and allowed to phosphorylate the peptide substrates for 90 min at 37°C. The chips were washed twice with TBS 0.1% Triton X-100, twice in 2M NaCl, and twice in de-mineralized water and air-dried. The slides were then exposed to a phosphor imager plate for 72h and acquisition of the peptide array performed using a phosphor-imager (Storm™, Amersham Biosciences, Sweden). Levels of incorporated radioactivity, which correspond to the phosphorylation status were quantified by array software Scanalyze (Eisen Software) and exported to Microsoft Excel. Kinase activities in lysates from 3 individual sorts on different days were analysed in duplicate. As a control, slides incubated with 33P-α-ATP (1MBq)) were employed, which demonstrated that the signal observed represented covalent transfer of the terminal phosphor atom. Examples of kinome slides can be found in Supplementary figure 1.
For data analysis, first every peptide was given an “on” call or “off” call (Markov state analysis). To this end, first an average signal was calculated for each peptide using the three biological replicates (each consisting of two technical replicates) yielding an aggregate dataset for each the hematopoietic subsets. Subsequently, for each of the aggregate datasets, either “on” calls or “off” calls were given to each peptide substrate (Markov state analysis). In order to do this, we assumed that the subset of signals representing the 1-e−1 fraction of peptides having the lowest phosphorylation of all peptides contained pure noise and did represent meaningful phosphorylation. The distribution of this noise was fitted as a single exponent, using the amplitude-sorted row number of these substrates as the X domain of the distribution and this single exponent was assumed to describe noise for the entire dataset for this hematopoietic subset. These fits are shown for each hematopoietic subset in supplementary figure 2. Now for all data points within the subset, when the actual amplitude observed minus 1,96 the standard deviation was in excess of the value expected from distribution describing the noise, a substrate was given an “on” call. Subsequently, kinomes of the different hematopoietic subsets were contrasted. For this analysis values between different sets were compared, and when one set displayed an “on” call, whereas the other set received an “off” call and when the absolute difference in signal was at least at least 10 % of the strongest signal, a peptide was considered to represent a differential kinase activity and the result was used to construct provision signal transduction schemes representing the difference in signalling between the two datasets, analogous to the construction of provisional signal transduction schemes described earlier by van Baal et al.16 Construction of the hematopoietic core kinome was done according to Diks et al.24
Results
The hematolymphoid core kinome
The hematolymphoid system constitutes a well-characterised hierarchical system of subsequent differentiation steps. Sophisticated FACS protocols allow isolation of highly pure subpopulations. We endeavoured to characterize the kinase signatures associated with differentiation in the haematopoiesis, including those of LT-HSC, ST-HSC, B cells, T cells, myeloid cells and natural killer (NK) cells (see schema in Fig. 1). Our method for the isolation of LT-HSC and ST-HSC is based on Christensen and Weissman,25 where Flk2− and Flk2+ subsets of Kit+Sca1+Lin− BM cells are defined as LT- and ST-HSC, respectively. Other differentiated hematolymphoid cells were isolated based on the expression of cell specific receptors: B220+ for B cells, CD3+ for T cells, Mac1+ for myeloid cells and NK1.1+CD3− cells for NK cells. Three independent sorts were performed for each cell type, consisting of a minimum of 200,000 target cells from which lysates were prepared and analyzed on an individual chip with duplicate arrays. Kinome profiles were generated for each independent cell sort by incubating cell lysates on arrays exhibiting 976 different kinase substrates in the presence of 33P-γ-ATP. The arrays incorporated substantial amounts of radioactivity (see scans of representative arrays, Suppl. Fig. 1). The technical quality of the profiles was good as the average Pearson product moment was well in excess of 0.85 between both the technical and the biological replicas for all cell types analyzed. We then used Markov state analysis to make “on” or “off” calls for each kinase substrate in each cell type analyzed. An “on” call means the kinase substrate is significantly phosphorylated relative to the amount of random 33P-γ-ATP retained by that substrate. By performing such analysis (Suppl. Fig. 2), we define the total number of substrates on the array that are significantly phosphorylated (“on”) for each cell (Table 1) as well as the core kinome that is “on” in all hematolymphoid cell types (Table 2). The core kinome shared by all hematolymphoid cells, consisting of only 7 substrates, is surprisingly low and provided an initial indication for large differences in the active kinomes of different hematolymphoid cell types.
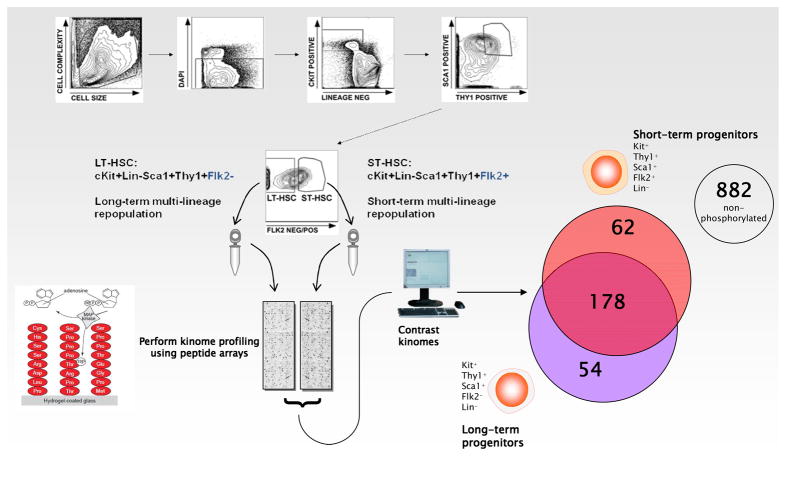
Fig. 1. Schema depicting high-speed cell sorting of LT-and ST-HSC populations and kinome assessment.
LT-HSC and ST-HSC were sorted from whole BM cells via gating on viable cells (based on DAPI dye exclusion) that are c-kit+Lin− and that are also Sca1+Thy1+ double positive with a final Flk2 gate to obtain the Flk2− and Flk2+ subsets of Sca1+Kit+Thy1+Lin− cells (LT-HSC and ST-HSC, respectively). Lysates were then incubated on PepChips in the presence of 33P-γ-ATP. Densitometry of the PepChips was performed followed by Markov state analysis for all substrates. For each combination of cell types overlapping and divergent portions were determined (see Venn diagram for LT-HSC and ST-HSC comparison).
Table 1.
The hematopoietic core kinome. Peptide substrates consistently phosphorylated by every hematopoietic nucleated cell.
| Peptide # | Peptide sequence | Source protein |
|---|---|---|
| 106 | GRTGRRNSI | Synthetic non-existing peptide |
| 148 | KIQASFRGH | Neurogranin |
| 293 | KTTASTRKV | Cystic fibrosis transmembrane conductance regulator |
| 309 | RRSRSRSRS | Lamin B receptor |
| 337 | KLRRSSSVG | Acetylcholine receptor protein delta |
| 390 | KRPSDRAKA | Myelin basic protein |
| 469 | RRFSV | Pyruvate kinase |
| 586 | KRPSARAKA | Myelin basic protein |
Table 2.
Kinase activity in hematopoietic subsets
| Cell type | Number of consistently phosphorylated peptides |
|---|---|
| Long-term progenitors | 232 |
| Short-term progenitors | 240 |
| Immature NK cells | 291 |
| Mature NK cells | 283 |
| B cells | 242 |
| Myeloid cells | 175 |
| T cells | 130 |
Kinome signatures in the hematolymphoid system are highly cell type specific
Our direct comparison of the active kinomes of LT-HSC, ST-HSC, myeloid, B cell, T cell and NK cells revealed that they are markedly different, suggesting that both progression within the stem cell compartment (Fig. 1, Fig 2A) as well as hematolymphoid lineage commitment (Fig. 3) is associated with specific signal transduction (for densitometry values for all substrates and their Markov test of significance for each cell type see Suppl. Tables 1–7). This contrasts with the classical view of signal transduction during hematopoiesis which considers untransformed cells as signalling-quiescent under steady-state unstimulated conditions with basal kinase activity only required for house-keeping and cytoskeletal reorganization.23,24,26–28 Although a number of kinase substrates that show differential phosphorylation between alternative cell fates in the hematopoietic differentiation process are associated with the regulation of metabolism or cytoskeletal organization, many kinase substrates that are part of canonical signal transduction pathways are found to be differentially phosphorylated by specific hematopoietic cell types. The most straightforward interpretation of this observation is that in contrast to the currently prevailing view29 (Irish et al, 2006), within the hematopoietic system differences in cell commitment are associated with constitutive differences in cellular signal transduction.
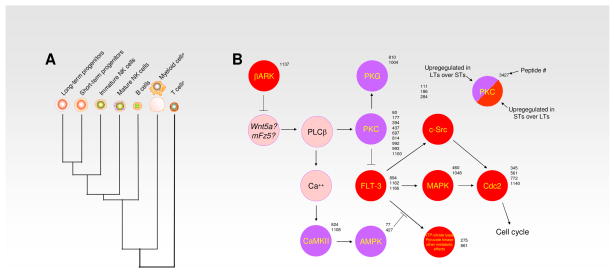
Fig. 2. Cluster analysis of the stem cell compartment and lineage committed cells of the hematolymphoid compartment.
(A) Cluster analysis of the kinomes of LT-HSC, ST-HSC, iNK, mNK, B cells, T cells and myeloid cells demonstrate their relative degree of diversity from each other as indicated here by successive radiations. (B) Signalling pathways found to be altered between LT-HSC and ST-HSC. Relative to the ST-HSC kinome, the LT-HSC kinome is characterised by non-canonical Wnt, Ca2+ and classical PKC-driven signalling, which is lost upon the transition to ST-HSC, whose kinome signature prominently features receptor RTK activation of the Ras/MAPK signalling cassette.
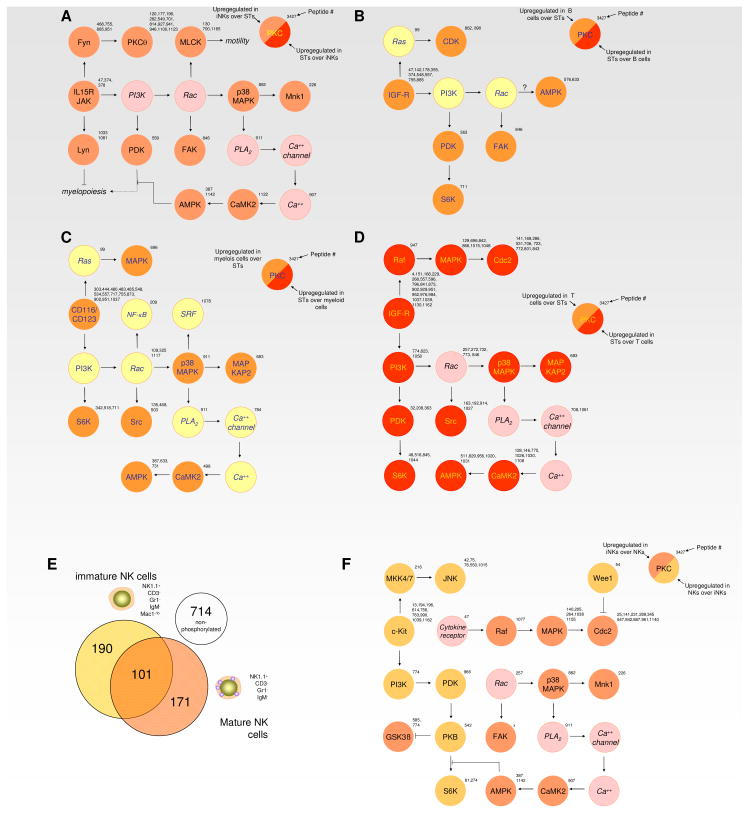
Fig. 3. Signalling variations following lineage commitment from multi-potent stem/progenitor cells in the hematolymphoid system.
Differentiation along the hematopoietic lineage is associated with major remodelling of cellular kinase signature, commitment to different lineages being associated with highly specific kinome profiles. (A) Signalling pathways found to be altered between ST-HSC and iNK cells and shows that this transition leads to activation of a PI3K/PKB/Rac driven signalling cassette. (B) Signalling pathways found to be different between ST-HSC and B cells prominently include hyperactivation of the Ras/MAPK signalling cassette as well as induction PI3K and Rac signalling in the latter cell type. (C) Signalling pathways found to be altered between ST-HSC and myeloid cells. (D) Signalling pathways found to be altered between ST-HSC and T cells, shows that T cells deactivate signalling associated with the ST-HSC kinome and only display residual G protein-coupled pathways. (E) Venn diagram showing the overlap and diversity of “on” substrates for iNK and mNK cells. (F) Signalling pathways found to be altered between iNK and mNK cells. The PI3K/PKB/Rac-driven signalling cassette becomes the dominant trait in the kinase signature following full differentiation towards NK cells
Contrasting kinomes in LT-HSC and ST-HSC
The above mentioned notion prompted us to contrast kinomes between the different cell types in the hematopoietic cell system and extract differential activation of signalling pathways from our profiles. First we compared the kinomes of LT-HSC and ST-HSC, two cell types that are phenotypically highly similar, but that differ in their potential to mediate long-term repopulation of the blood compartment. Although many of the phosphorylation events associated with cellular house keeping are shared between the two cell types, the transition from LT-HSC to ST-LSC is associated with a complete reorganisation of cellular signal transduction (Figs. 1 and 2). Whereas the LT-HSC kinome is dominated by G-protein coupled receptor-dependent activation of protein kinase C (PKC) activity and Ca2+ signalling in addition to a substantial amount of adenosine monophosphate-activated protein kinase (AMPK) activity (Fig. 2, Suppl. Table 1), in ST-HSC this LT-HSC-specific signalling is suppressed, probably via negative signalling through adrenergic receptor kinase, β1 (BARK) and thus release of a receptor tyrosine kinase (RTK) dependent cascade that drives Ras/MAP kinase signalling to produce proliferation, as evident by the induction of cyclin-dependent kinases (Fig. 2, Suppl. Table 2). The transcriptome of LT-HSC and ST-HSC has been characterized and contrasted earlier by Hüttmann et al.30 and found a substantial number of differentially-expressed transcripts (1055 unique transcripts in LT-HSC out of a total of 3112 transcripts and 1550 unique transcripts in LT-HSC out of a total of 3617 transcripts respectively), but could not explain as to how these differential expression patterns were maintained. Especially non-canonical Wnt signaling in the LT-HSC kinome may be important here. The LT-HSC profiles contain a fair amount of PKC activity, which may result from non-canonical Wnt signalling 31. Furthermore, our profiles also contain an upregulation of phosphorylation of the peptide “DPPGTESFV” in the ST-HSC kinome, which is a signature peptide in the Fz-type receptors and associated with downregulation of non-canonical Wnt signalling, and by inference upregulation of this signalling in the LT-HSC32. Furthermore, the induction of PKG (evident from peptides “SARLSAKPA” and “RKRSRAE”) in the LT-HSC kinome is hallmark of non-canonical Wnt signaling as well33. Finally, the ultimate sign of non-canonical Wnt signaling is Ca2+-dependent stimulation of phosphorylation of the peptides “THYGSLPQK” and “TRQASISGP” , both significantly more phosphorylated in the LT-HSC kinome as compared to the ST-HSC kinome34. Thus it is tempting to speculate that the LT-HSC and ST-HSC specific signalling, and especially non-canonical Wnt signalling observed in the present study plays an important role in the contrasts in the transcriptome between these two cell types described earlier by Hüttmann et al.35 Also in agreement with this earlier dataset are the changes in cell cycle activity. The Hüttmann study sees downregulation of cell cycle genes and upregulation of cell cycle inhibitors in LT-HSC over ST-HSC, whereas the current study documenents increased cell cycle kinase activity in the latter cell population. Thus in general the two datasets are in good agreement.
Specific kinomes in alternative radiations of the hematolymphoid system
Subsequently, we contrasted the kinomes of cells committed to the myeloid lineage (Fig. 3C), the B cell lineage (Fig. 3B), and the T cell lineage (Fig. 3D) to that of ST-HSC. All the kinase signatures obtained were to a high degree unique, again in contrast to the classical concept that cellular signal transduction is mainly reactive towards changes in the milieu extérieur. Thus specific cell types display specific constitutive activation of signal transduction pathways. However, the difference between the ST-HSC and myeloid kinomes (Fig. 3C) as well as the difference between the ST-HSC and B cell kinomes (Fig. 3B) almost exclusively entails gain of signalling pathways during differentiation along the hematopoietic lineages, whereas almost no activities are lost. Thus the remodelling of the kinome following differentiation of the ST-HSC consists of addition of additional signalling pathways to the kinome that presumably mediate the functional characteristics and gene expression required from these committed cells. When the kinome of myeloid committed cells is contrasted to that of ST-HSC, usually strong Rac signalling is found in myeloid-committed cells (Fig. 3C), which fits well with the high expression of this GTPase and its effectors in this lineage as well as the myelosuppression observed in transplantation and autoimmune patients treated with the Rac inhibitor azathioprine.36,37 Likewise, when B cell signalling is contrasted to ST-HSC kinome, a diversified kinase signature emerges with a prominent PKC component, whereas the T cell kinome is distinguished by a strong deactivation of the PI3 kinase pathway and signalling that is apparently mainly dependent on residual G protein-coupled signalling (Fig. 3D). We conclude that, contrasting to the classical view, massive and unique remodelling of the cellular kinome is a general trait of differentiation in the hematolymphoid system.
Specific kinomic changes upon differentiation of iNK cells to mature NK cells
Finally, to assess whether significant remodelling of the kinome occurs during development of a given hematolymphoid lineage, we contrasted the kinomes of ST-HSC, iNK cells and mature NK (mNK) cells (Fig. 3A,E,F). iNK cells represent an immature NK lineage in the process of differentiating to mature NK, with low Mac1/CD11b expression used to distinguish these cells from their mature counterparts.38,39 Interestingly, this further commitment of hematopoietic cells is not accompanied by the loss of kinase activity for specific substrates as iNK cells show activation of various signalling highways not observed in ST-HSC (Fig. 3A), in particular activation of a PI3 kinase-activated pathway, leading to activation of PDK-dependent signalling, activation of a Rac pathway, which can promote p38MAPK activity, activation of a Fyn/PKCθ-signalling cassette as well as the induction of Lyn, which may play a role in preventing myelopoiesis upon commitment to the NK-differentiation pathway. When the iNK kinome is contrasted to the mature NK kinome (Fig. 3E,F); however, activation of the Ras/MAP kinase cascade is lost and probably consequently cell cycle progression-related phosphorylation events are down regulated as well, although the induction of Wee1 which is observed in our profiles may be important here as well. Also Rac activation and its associated signalling are diminished, but activation of the PDK1/PKB pathway is even stronger as that seen in iNK cells. This remarkable dichotomy in iNK signalling versus NK signalling may be a reflection of their different qualities in the organism: unless they encounter an activating target mature NK cells are believed to be in a quiescent state during most of their life, whereas immature NK cells go through a sequential waves of proliferation often in order to expand their compartment from a small number of committed progenitors. The unique kinase signatures of iNK and NK cells further support that even under steady state conditions, different stages in cell commitment are associated with the constitutive activation of specific signalling pathways.
Functional importance of kinomic changes for hematopoietic cell physiology
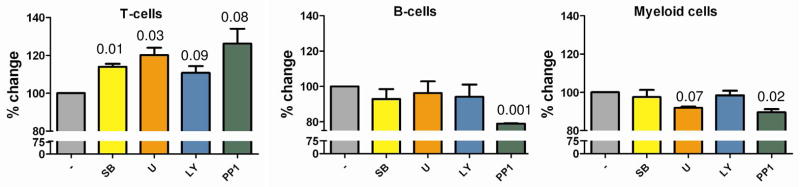
The unique kinase signatures in the different radiations of the hematopoietic system maybe interpreted as the signalling pathways involved are important for maintaining cell type. A prediction of this notion, however, is that pharmacological inhibition of such pathways would specifically interfere with survival of the radiations exhibiting constitutive activation of the pathways involved. Hence we decided to test this notion directly in a panel of healthy volunteers, whose peripheral blood was ex-vivo exposed to low concentrations of pharmacological inhibitors of the p38MAP kinase pathway (4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4- pyridyl)imidazole SB203580), the p42/p44MAP kinase pathway (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene, U0126), the PI3 kinase pathway (LY294002) and the activation of Src-like kinases (4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine, PP1). Toxicity of these compounds is low40, and selectivity of these inhibitors for their mentioned substrates was demonstrated previously 41 The results are presented in figure 4. Indeed, broadly activation of certain pathways in specific cell types is necessary for survival. For instance, in our profiles we observe strong activation of Src in the myeloid compartment, and its inhibition specifically reduces the myeloid component of ex vivo blood cultures. But also conversely, differentiation along the T cell lineage is associated with downregulation of p38MAP kinase activity and p42/p44 MAP kinase activity and pharmacological inhibition of these activities corresponds to an increased T cell component. Thus, in toto, the profiles obtained using peptide arrays correlate remarkably well with an importance of the kinase pathways involved in sustaining (or inhibiting) signalling through these pathways and hence our results strongly support a concept where a steady-state signal transduction activity profile is important for maintaining cell phenotype.
Fig. 4. Signalling variations maintain lineage commitment in the hematolymphoid system.
Anti-coagulated human peripheral blood was collected from healthy donors after informed consent. Whole blood was cultured in the absence or presence of 2 μM SB20358010, 10 μM LY294002 , 10 μM U0126 , or 25 μM PP1 for 36h. T-cells (CD3+), B-cells (CD19+) and myeloid cells (CD66b+) were determined as a percentage of G4. Changes due to treatment with inhibitors were expressed as percentage of untreated control.
Discussion
The changes in transcriptome and proteome associated with differentiation of pluripotent stem cells and their subsequent commitment to various lineages have been widely explored over the past decade.6,42–46 In particular, much analysis has been done to elucidate the molecular determinants associated with cellular commitment. Although many important gene expression changes were revealed by these analyses, there remain many discrepancies between the data sets47–50, and it is fair to say that we still have an incomplete understanding of the molecular nature of pluripotent cells and their commitment to differentiated lineages. To a certain extent this lack of insight can be attributed to the fact that many aspects of cellular behaviour are dynamically regulated and do not always lend themselves readily to technologies that determine the expression levels of gene products or the absolute level of post-translational modifications. An equally important factor in regulation is the activity of enzymes that are active in these particular signalling pathways and in particular the activity of enzymes that phosphorylate tyrosine, serine and threonine residues on proteins which determines to a large extent the behaviour of cells and their subsequent fate. These corresponding changes in cellular biochemistry in general, however, and especially the influence cell fate has on the cellular kinome remained less well understood, it even being uncertain whether otherwise unchallenged cells displayed differentiation-stage specific kinome signatures4–7.
We reasoned that comprehensive characterization of kinase activities during differentiation would likely provide substantial support for our efforts to understand the nature of stem cells and their differentiation at a molecular level. Kinome analysis implements an array technology that comprehensively measures enzymatic activities present in whole cell lysates. Arrays have been assembled that contain multiple consensus sequences for a broad range of protein kinases present in the mammalian genome, allowing detection of phosphorylation events mediated by kinases present in whole cell lysates. A comprehensive validation of this technology was achieved by evaluating the altered regulation of multiple signaling pathways in B lymphocytes from patients with systemic lupus erythematosus.51 Here we employed this technology to approach questions involving the nature of stem cells and their differentiation to lineage-committed cells.
Using peptide arrays we performed a massive parallel assessment of the kinase signature of the radiations of the hematopoietic system, including LT-HSC, ST-HSC, immature natural killer (iNK) cells, NK cells, B cells, T cells and myeloid cells. The LT-HSC kinome is characterised by non-canonical Wnt, Ca2+ and classical protein kinase C (PKC)-driven signalling, which is lost upon the transition to ST-HSC, whose kinome signature prominently features receptor tyrosine kinase (RTK) activation of the Ras/MAPK signalling cassette. Further differentiation to iNK maintains signalling through this cassette but simultaneously leads to activation of a PI3K/PKB/Rac driven signalling cassette, which becomes the dominant trait in the kinase signature following full differentiation towards NK cells. Differentiation along the myeloid and B cell lineages is accompanied by hyperactivation of the Ras/MAPK signalling cassette as well as induction PI3K and Rac signalling as well as Src signal transduction. The latter seems important, as inhibition of this pathway reduces B cell numbers. T cells, however, deactivate signalling associated with the ST-HSC kinome and only display residual G protein-coupled pathways. Thus, differentiation along the hematopoietic lineage is associated with major remodelling of cellular kinase signature, commitment to different lineages being associated with highly specific kinome profiles.
Conclusion
Our findings have important consequences with respect to the school that attributes to signal transduction in untransformed cells mainly a reactive function. The substantial constitutive kinase signalling and the highly cell-type unique profiles obtained do not fit well with this concept, but suggest rather that basal activation of specific signalling may be essential for maintaining phenotype, at least in the hematolymphoid lineage. The substantial constitutive kinase signalling and the highly cell-type unique profiles obtained do not fit well with the classical view, but suggest rather that basal activation of specific signal transduction pathways may prove essential for maintaining cell phenotype. Indeed, application of kinase inhibitors and agonists, or modulators of phosphataes that oppose these kinases, have substantial potential for modulating differentiation in the hematopoietic system and may thus in the future be useful for combating pathology. Such a hypothesis would fit well with the observations that unprovoked steady-state activation of specific pathways is associated with response to chemotherapy (maybe a specialised form of cell fate) in leukemia.52
Supplementary Material
Acknowledgments
This work was supported in part by funds from the U.S. NIH (R21 DK071872, RO1 HL72523, R01 HL085580) and the Paige Arnold Butterfly Run to WGK and regional programme of innovative actions in Groningen (IAG) and Top Institute Pharma (TI Pharma) to MPP. During the early phase of this study, WGK was The Newman Scholar of the Leukemia and Lymphoma Society of America and is currently the Murphy Family Professor of Children’s Oncology Research and Empire Scholar of the State of NY. MPP and SHD are supported by the TIP initiative of the Dutch goverment. The authors declare no competing commercial interests.
Reference List
- 1.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 2.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 3.Evans CA, Tonge R, Blinco D, Pierce A, Shaw J, Lu YN, Hamzah HG, Gray A, Downes CP, Gaskell SJ, Spooncer E, Whetton AD. Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility. Blood. 2004;103(10):3751–3759. doi: 10.1182/blood-2003-09-3294. [DOI] [PubMed] [Google Scholar]
- 4.Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. Plos Biology. 2004;2(10):1640–1651. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huttmann A, Duhrsen U, Heydarian K, Klein-Hitpass L, Boes T, Boyd AW, Li CL. Gene expression profiles in murine hematopoietic stem cells revisited: Analysis of cDNA libraries reveals high levels of translational and metabolic activities. Stem Cells. 2006;24(7):1719–1727. doi: 10.1634/stemcells.2005-0486. [DOI] [PubMed] [Google Scholar]
- 6.Bowman TV, Merchant AA, Goodell MA. Molecular profiling of hematopoietic stem cells. Methods Mol Med. 2007;134:1–16. doi: 10.1007/978-1-59745-223-6_1. [DOI] [PubMed] [Google Scholar]
- 7.Lu R, Markowetz F, Unwin RD, Leek JT, Airoldi EM, MacArthur BD, Lachmann A, Rozov R, Ma'ayan A, Boyer LA, Troyanskaya OG, Whetton AD, Lemischka IR. Systems-level dynamic analyses of fate change in murine embryonic stem cells. Nature. 2009;462(7271):358–U126. doi: 10.1038/nature08575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji H, Ehrlich LIR, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, Rossi DJ, Inlay MA, Serwold T, Karsunky H, Ho LN, Daley GQ, Weissman IL, Feinberg AP. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467(7313):338–U120. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodrich JA, Tjian R. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nature Reviews Genetics. 2010;11(8):549–558. doi: 10.1038/nrg2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji H, Ehrlich LIR, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, Rossi DJ, Inlay MA, Serwold T, Karsunky H, Ho LN, Daley GQ, Weissman IL, Feinberg AP. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467(7313):338–U120. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S. Phosphoproteomic Analysis of Human Embryonic Stem Cells. Cell Stem Cell. 2009;5(2):204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hoof D, Munoz J, Braam SR, Pinkse MWH, Linding R, Heck AJR, Mummery CL, Krijgsveld J. Phosphorylation Dynamics during Early Differentiation of Human Embryonic Stem Cells. Cell Stem Cell. 2009;5(2):214–226. doi: 10.1016/j.stem.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 13.McNeill H, Woodgett JR. When pathways collide: collaboration and connivance among signalling proteins in development. Nature Reviews Molecular Cell Biology. 2010;11(6):404–413. doi: 10.1038/nrm2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D, Sylvester JE, Parker LL, Zhou GC, Kron SJ. Peptide Reporters of Kinase Activity in Whole Cell Lysates. Biopolymers. 2010;94(4):475–486. doi: 10.1002/bip.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moritz A, Li Y, Guo AL, Villen J, Wang Y, MacNeill J, Kornhauser J, Sprott K, Zhou J, Possemato A, Ren JM, Hornbeck P, Cantley LC, Gygi SP, Rush J, Comb MJ. Akt-RSK-S6 Kinase Signaling Networks Activated by Oncogenic Receptor Tyrosine Kinases. Science Signaling. 2010;3(136) doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Baal JWPM, Diks SH, Wanders RJA, Rygiel AM, Milano F, Joore J, Bergman JJGH, Peppelenbosch MP, Krishnadath KK. Comparison of kinome profiles of Barrett's esophagus with normal squamous esophagus and normal gastric cardia. Cancer Research. 2006;66(24):11605–11612. doi: 10.1158/0008-5472.CAN-06-1370. [DOI] [PubMed] [Google Scholar]
- 17.Ritsema T, Brodmann D, Diks SH, Bos CL, Nagaraj V, Pieterse CMJ, Boller T, Wiemken A, Peppelenbosch MP. Are Small GTPases Signal Hubs in Sugar-Mediated Induction of Fructan Biosynthesis? Plos One. 2009;4(8) doi: 10.1371/journal.pone.0006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desponts C, Hazen AL, Paraiso KHT, Kerr WG. SHEP deficiency enhances HSC proliferation and survival but compromises homing and repopulation. Blood. 2006;107(11):4338–4345. doi: 10.1182/blood-2005-12-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuhler GM, Tyl MR, Olthof SG, Drayer AL, Blom N, Vellenga E. Distinct roles of the mTOR components Rictor and Raptor in MO7e megakaryocytic cells. European Journal of Haematology. 2009;83(3):235–245. doi: 10.1111/j.1600-0609.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 21.Versteeg HH, Sorensen BB, Slofstra SH, Van den Brande JHM, Stam JC, Henegouwen PMPV, Richel DJ, Petersen LC, Peppelenbosch MP. VIIa/tissue factor interaction results in a tissue factor cytoplasmic domain-independent activation of protein synthesis, p70, and p90 S6 kinase phosphorylation. Journal of Biological Chemistry. 2002;277(30):27065–27072. doi: 10.1074/jbc.M110325200. [DOI] [PubMed] [Google Scholar]
- 22.Kreegipuu A, Blom N, Brunak S. PhosphoBase, a database of phosphorylation sites: release 2.0. Nucleic Acids Research. 1999;27(1):237–239. doi: 10.1093/nar/27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diks SH, Kok K, O'Toole T, Hommes DW, van Dijken P, Joore J, Peppelenbosch MP. Kinome profiling for studying lipopolysaccharide signal transduction in human peripheral blood mononuclear cells. Journal of Biological Chemistry. 2004;279(47):49206–49213. doi: 10.1074/jbc.M405028200. [DOI] [PubMed] [Google Scholar]
- 24.Diks SH, Parikh K, van der Sijde M, Joore J, Ritsema T, Peppelenbosch MP. Evidence for a Minimal Eukaryotic Phosphoproteome? Plos One. 2007;2(8) doi: 10.1371/journal.pone.0000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud O, Gjertsen BT, Nolan GP. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118(2):217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Irish JM, Kotecha N, Nolan GP. Innovation - Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nature Reviews Cancer. 2006;6(2):146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 28.Miranda MB, Johnson DE. Signal transduction pathways that contribute to myeloid differentiation. Leukemia. 2007;21(7):1363–1377. doi: 10.1038/sj.leu.2404690. [DOI] [PubMed] [Google Scholar]
- 29.Irish JM, Kotecha N, Nolan GP. Innovation - Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nature Reviews Cancer. 2006;6(2):146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 30.Huttmann A, Duhrsen U, Heydarian K, Klein-Hitpass L, Boes T, Boyd AW, Li CL. Gene expression profiles in murine hematopoietic stem cells revisited: Analysis of cDNA libraries reveals high levels of translational and metabolic activities. Stem Cells. 2006;24(7):1719–1727. doi: 10.1634/stemcells.2005-0486. [DOI] [PubMed] [Google Scholar]
- 31.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22(5):717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Wang YF, Tan CG, Fagan RJ, Klein PS. Phosphorylation of frizzled-3. Journal of Biological Chemistry. 2006;281(17):11603–11609. doi: 10.1074/jbc.M600713200. [DOI] [PubMed] [Google Scholar]
- 33.Wang HY. WNT-Frizzled signaling via cyclic GMP. Frontiers in Bioscience. 2004;9:1043–1047. doi: 10.2741/1310. [DOI] [PubMed] [Google Scholar]
- 34.Verkaar F, Zaman GJR. A model for signaling specificity of Wnt/Frizzled combinations through co-receptor recruitment. Febs Letters. 2010;584(18):3850–3854. doi: 10.1016/j.febslet.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Huttmann A, Duhrsen U, Heydarian K, Klein-Hitpass L, Boes T, Boyd AW, Li CL. Gene expression profiles in murine hematopoietic stem cells revisited: Analysis of cDNA libraries reveals high levels of translational and metabolic activities. Stem Cells. 2006;24(7):1719–1727. doi: 10.1634/stemcells.2005-0486. [DOI] [PubMed] [Google Scholar]
- 36.Mcleod HL, Miller DR, Evans WE. Azathioprine-Induced Myelosuppression in Thiopurine Methyltransferase Deficient Heart-Transplant Recipient. Lancet. 1993;341(8853):1151. doi: 10.1016/0140-6736(93)93168-z. [DOI] [PubMed] [Google Scholar]
- 37.Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, Hildner K, Bartsch B, Holtmann M, Blumberg R, Walczak H, Iven H, Galle PR, Ahmadian MR, Neurath MF. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4(+) T lymphocytes. Journal of Clinical Investigation. 2003;111(8):1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. Journal of Immunology. 2006;176(3):1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Iizuka K, Kang HSP, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nature Immunology. 2002;3(6):523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 40.Fuhler GM, Knol GJ, Drayer AL, Vellenga E. Impaired interleukin-8-and GRO alpha-induced phosphorylation of extracellular signal-regulated kinase result in decreased migration of neutrophils from patients with myelodysplasia. Journal of Leukocyte Biology. 2005;77(2):257–266. doi: 10.1189/jlb.0504306. [DOI] [PubMed] [Google Scholar]
- 41.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans CA, Tonge R, Blinco D, Pierce A, Shaw J, Lu YN, Hamzah HG, Gray A, Downes CP, Gaskell SJ, Spooncer E, Whetton AD. Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility. Blood. 2004;103(10):3751–3759. doi: 10.1182/blood-2003-09-3294. [DOI] [PubMed] [Google Scholar]
- 43.Huttmann A, Duhrsen U, Heydarian K, Klein-Hitpass L, Boes T, Boyd AW, Li CL. Gene expression profiles in murine hematopoietic stem cells revisited: Analysis of cDNA libraries reveals high levels of translational and metabolic activities. Stem Cells. 2006;24(7):1719–1727. doi: 10.1634/stemcells.2005-0486. [DOI] [PubMed] [Google Scholar]
- 44.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 45.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 46.Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. Plos Biology. 2004;2(10):1640–1651. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huttmann A, Duhrsen U, Heydarian K, Klein-Hitpass L, Boes T, Boyd AW, Li CL. Gene expression profiles in murine hematopoietic stem cells revisited: Analysis of cDNA libraries reveals high levels of translational and metabolic activities. Stem Cells. 2006;24(7):1719–1727. doi: 10.1634/stemcells.2005-0486. [DOI] [PubMed] [Google Scholar]
- 48.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 49.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 50.Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. Plos Biology. 2004;2(10):1640–1651. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taher TE, Parikh K, Flores-Borja F, Mletzko S, Isenberg DA, Peppelenbosch MP, Mageed RA. Protein Phosphorylation and Kinome Profiling Reveal Altered Regulation of Multiple Signaling Pathways in B Lymphocytes From Patients With Systemic Lupus Erythematosus. Arthritis and Rheumatism. 2010;62(8):2412–2423. doi: 10.1002/art.27505. [DOI] [PubMed] [Google Scholar]
- 52.Kornblau SM, Minden MD, Rosen DB, Putta S, Cohen A, Covey T, Spellmeyer DC, Fantl WJ, Gayko U, Cesano A. Dynamic Single-Cell Network Profiles in Acute Myelogenous Leukemia Are Associated with Patient Response to Standard Induction Therapy. Clinical Cancer Research. 2010;16(14):3721–3733. doi: 10.1158/1078-0432.CCR-10-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.