Abstract
Rationale
The chemokine receptor Ccr6 is a G protein-coupled receptor expressed on various types of leukocytes identified in mouse atherosclerotic lesions. Recent evidence suggests that both CCR6 and its ligand CCL20 are also present in human atheroma; however, their functional roles in atherogenesis remain undefined.
Objective
To delineate the role of Ccr6 in atherogenesis in the apolipoprotein E-deficient (ApoE−/−) mouse model of atherosclerosis.
Methods and Results
Both Ccr6 and Ccl20 are expressed in atherosclerotic aorta from ApoE−/− mice. Aortic lesion area in Ccr6−/− ApoE−/− mice was ~40% and ~30% smaller than in Ccr6+/+ApoE−/− mice at 16 and 24 weeks of age, respectively. Transplantation of bone marrow from Ccr6−/− mice into ApoE−/− mice resulted in ~40% less atherosclerotic lesion area than for bone marrow from Ccr6+/+ mice; lesions in Ccr6−/− ApoE−/− mice had 44% less macrophage content compared to lesions in Ccr6+/+ApoE−/− mice. Ccr6 was expressed on a subset of primary mouse monocytes. Accordingly, Ccl20 induced chemotaxis of primary monocytes from wild type but not Ccr6−/− mice; moreover, Ccl20 induced monocytosis in ApoE−/− mice in vivo. Consistent with this, we observed 30% fewer monocytes in circulating blood of Ccr6−/− ApoE−/− mice, mainly due to fewer CD11b+Ly6Chigh inflammatory monocytes.
Conclusions
Ccr6 promotes atherosclerosis in ApoE-deficient mice, which may be due in part to Ccr6 support of normal monocyte levels in blood, as well as direct Ccr6-dependent monocyte migration.
Keywords: atherosclerosis, inflammation, monocytes, aorta
Introduction
Atherosclerosis is a chronic inflammatory disease and the leading cause of mortality in the developed world.1 Many different inflammatory cell types have been shown to accumulate in atherosclerotic plaques, including monocytes/macrophages, dendritic cells (DCs), T cells, B cells and neutrophils.2 Of these, monocytes/macrophages are particularly important since they are recruited in the largest numbers to atherosclerotic sites, where they ingest oxidized low-density lipoprotein (oxLDL) and produce inflammatory mediators. Selective depletion of macrophages has been reported to inhibit early atherogenesis, suggesting a critical role for monocyte recruitment during atherogenesis.3
The chemokine family coordinates directional movement of leukocytes to inflammatory sites by signaling through G protein-coupled receptors.4 A subset of chemokines can induce monocyte chemotaxis, while others may selectively trigger monocyte arrest on inflamed endothelium.5 In vivo, gene targeting studies have revealed that specific chemokines (Ccl2, Ccl5 and Cx3cl1) and chemokine receptors (Ccr2, Ccr5, Cx3cr1 and Cxcr6) play non-redundant roles in promoting atherogenesis through macrophage accumulation in plaque.6–9
Ccr6 is the sole receptor for macrophage inflammatory protein-3α (Ccl20) and is expressed on various cell types that have been identified in atherosclerosis, including CD4+ T cells, CD8+ T cells, NKT cells, NK cells, B cells, DCs and neutrophils. 10, 11 With regard to monocytes, CCL20 has been shown to induce human monocyte chemotaxis in vitro.12 Ex vivo, IL-1β has been reported to stimulate synoviocytes from inflamed joints of rheumatoid arthritis patients to recruit CCR6+ human monocytes in a CCL20 dependent manner,13 and in vivo, monocytes may accumulate via Ccr6 and Ccl20 in a mouse model of dermatitis.14 In humans, both CCR6 and CCL20 have been detected in carotid plaques; however, their functional role in atherogenesis is undefined.15 Here, we address this question by using Ccr6−/− mice in the ApoE−/− mouse model of atherosclerosis.
Methods
More details of the Methods used in this study are given in the Online Data Supplement.
Ccr6−/− mice were generated as previously described.16 Ccr6−/− ApoE−/− mice were obtained by crossing ApoE−/− mice on a C57BL/6J background (Jackson Labs) with Ccr6−/− mice on a C57BL/6N background (NCI-DCT). Female littermate Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice were weaned at 6 weeks, fed a Western diet (TD88137; Harlan Teklad, Madison, WI) for an additional 10 or 18 weeks, and then sacrificed for analysis. Female mice sacrificed at 16 weeks of age were subjected to all the analyses detailed below. All mice were kept in pathogen-free conditions and animal study protocols were approved by the Animal Care and Use Committee of the NIAID, NIH.
Results
Atherogenesis is Reduced in Ccr6−/− ApoE−/− Mice
Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice had similar lesion distribution, with the highest density occurring in the lesser curvature of the aortic arch in both groups of mice (Figure 1A). However, female Ccr6−/− ApoE−/− mice had approximately 40% and 30% less total lesion area than Ccr6+/+ApoE−/− mice at age 16 and 24 weeks, respectively (Figure 1B). Atherosclerotic lesion size in the aortic root was decreased by 43% in Ccr6−/− ApoE−/− mice relative to Ccr6+/+ApoE−/− mice (Figure 1C and 1D), which was similar to the lesion area reduction in the whole aorta. Male mice were also studied and Ccr6−/− ApoE−/− mice had an ~30% reduction in total lesion area at age 19 weeks (13 weeks on Western diet, Supplemental Figure I). No significant difference was found between the two genotypes in either body weight or serum levels of total cholesterol, HDL, LDL/VLDL or triglycerides (Supplemental Table I). In addition, no differences in these lipids were observed between wild type C57BL/6 mice and Ccr6−/− mice (data not shown).
Figure 1. Ccr6 deficiency reduces atherogenesis in the ApoE−/− mouse model of atherosclerosis.
Female Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice were fed a high-fat Western diet and analyzed for atherosclerosis development in whole aorta at age 16 weeks (A, B) and 24 weeks (B) and aortic root at age 16 weeks (C, D). A, Representative photographs of Sudan IV-stained mouse aortas (red: positive staining). B, Quantification of the atherosclerotic lesions (red area) in A, shown as percentage of the whole aorta. Sixteen or seven mice were used in each group (***P<0.001 for 16 weeks and *P<0.05 for 24 weeks). C, Representative frozen aortic root sections stained with Oil Red O. D, Aortic root lesion size (mean area) was quantified by IVision software (n=8 in each group; **P=0.009). In B and D, each symbol represents data from a single mouse, and each data set is summarized as mean ± SEM.
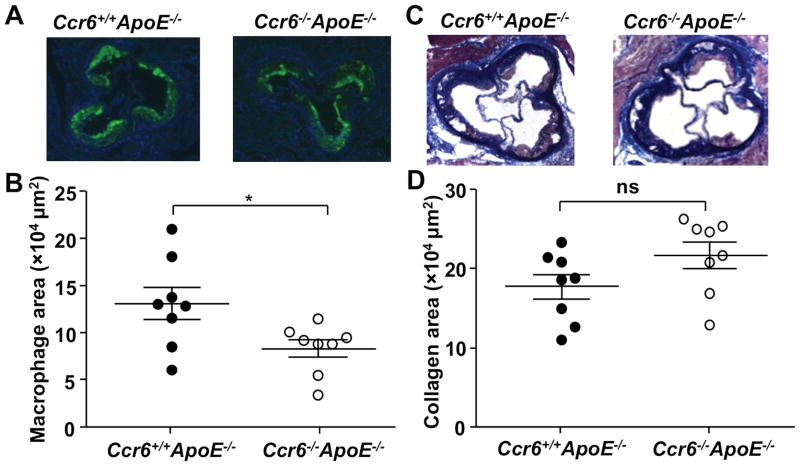
Macrophage Content in the Aortic Root Is Significantly Reduced in Ccr6−/− ApoE−/− Mice
Circulating monocytes can be recruited to atherosclerosis-prone arteries at an early stage where they may then differentiate into lipid-laden macrophages known as foam cells.2 To determine whether Ccr6 may affect monocyte recruitment and foam cell accumulation, we examined macrophage content in the aortic root by MOMA-2 staining. Compared to Ccr6+/+ApoE−/− mice, Ccr6−/− ApoE−/− mice had 44% less macrophage content in the aortic root area whereas the content of T cells or DCs was not affected (Figure 2A, 2B and data not shown). Masson’s Trichrome staining was performed to compare aortic root collagen content, which may affect the stability of atherosclerotic plaques. Although a trend toward an increase of the collagen content (blue area) in the aortic root of Ccr6−/− ApoE−/− mice was observed, it was not statistically significant (Figure 2C and 2D).
Figure 2. Ccr6 deficiency reduces macrophage accumulation in plaque in the ApoE−/− mouse model of atherosclerosis.
A, Representative photomicrographs of frozen sections from the aortic root of Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice stained with MOMA-2. B, Quantification of data in A by digital morphometry, as described in Methods (n=8 per group, *P=0.027). C, Representative photomicrographs of Masson Trichrome stained frozen aortic root sections (blue color: collagen; red color: muscles; black color: nuclei). D, Quantification of data in C by digital morphometry (n=8 per group, P=0.098). In B and D, each symbol represents data from a single mouse (female, age 16 weeks), and each data set is summarized as mean ± SEM.
Circulating Monocyte Counts Are Significantly Reduced in Ccr6−/− ApoE−/− Mice
Interpretation of the macrophage deficit that we observed in the aorta of Ccr6−/− ApoE−/− mice relative to Ccr6+/+ApoE−/− mice must take into account the monocyte level in the blood of the two strains. Therefore, we next quantified the numbers and percentages of monocytes and other leukocyte subsets in the blood by complete blood count (CBC). We found that circulating monocyte counts were reduced by 30% in Ccr6−/− ApoE−/− mice compared to Ccr6+/+ApoE−/− mice (Figure 3A); whereas no significant difference was observed in the number of total white blood cells, peripheral blood lymphocytes, neutrophils, eosinophils and basophils between Ccr6+/+ApoE−/− mice and Ccr6−/− ApoE−/− mice or between wild type C57BL/6 mice and Ccr6−/− mice (Supplemental Figure II). Notably, Ccr6 deficiency did not cause monocytopenia in C57Bl/6 mice, only in mice lacking ApoE who were fed a high-fat Western diet (Supplemental Figure II). The monocyte percentage of the total peripheral blood leukocytes was also significantly reduced in Ccr6−/− ApoE−/− mice (Figure 3B). Since there are two major mouse monocyte subsets, the CD11b+Ly6Chigh and CD11b+Ly6Clow monocytes,17 we next compared the absolute number and percentage of these cell subsets in both blood and bone marrow using FACS analysis. There was a marked reduction of blood CD11b+Ly6Chigh monocytes in Ccr6−/− ApoE−/− mice compared to Ccr6+/+ApoE−/− mice, and at the same time we found a significant increase of CD11b+Ly6Chigh monocytes in the bone marrow of Ccr6−/− ApoE−/− mice (Figure 3C). Ccr6 deficiency did not affect the differentiation or the apoptosis of bone marrow derived monocytes (Supplemental Figure III and data not shown) and this difference appeared to be specific for peripheral blood and bone marrow, since the absolute number and percentage of splenic CD11b+Ly6Chigh monocytes was similar between Ccr6+/+ApoE−/− mice and Ccr6−/− ApoE−/− mice (data not shown). In contrast, there was no significant difference for the distribution of CD11b+Ly6Clow monocytes (gated on Ly6G negative leukocytes) in the blood and bone marrow of Ccr6+/+ApoE−/− mice and Ccr6−/− ApoE−/− mice (Figure 3D), indicating that the monocytopenia in Ccr6−/− ApoE−/− mice was mainly caused by reduction of the CD11b+Ly6Chigh cell subset. In addition, we found that bone marrow transplantation of Ccr6−/− cells into irradiated ApoE−/− mice resulted in significantly less atherosclerotic lesion area in the aorta than when Ccr6+/+ bone marrow cells were transplanted (Figure 3E). Body weight and serum levels of total cholesterol, HDL, LDL/VLDL were similar for the two transplanted groups of mice (data not shown). Thus, the Ccr6+ cells affecting atherogenesis in this model appear to be bone marrow-derived.
Figure 3. Ccr6 deficiency significantly reduces circulating inflammatory monocytes in the ApoE−/− mouse model of atherosclerosis.
Absolute numbers (A) and percentages (B) of total circulating blood monocytes in Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice (female, age 16 weeks) were determined by an automatic hematology analyzer, as described in Methods (n=9 in each group; *P=0.011 in A and **P=0.006 in B). C, Percentages of CD11b+Ly6Chigh monocytes in the blood and bone marrow of Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice were determined by FACS analysis (n=5 in each group; *P=0.038 for blood and *P=0.025 for bone marrow). D, Percentages of CD11b+Ly6Clow monocytes in the blood and bone marrow of Ccr6+/+ApoE−/− and Ccr6−/− ApoE−/− mice were determined by FACS analysis (n=5 in each group; P=0.17 for blood and P=0.45 for bone marrow). E, Quantification of the atherosclerotic lesions in lethally irradiated ApoE−/− mice after either Ccr6+/+ or Ccr6−/− bone marrow transplantation, shown as percentage of the whole aorta. Ten mice were used in each group (*P=0.017). In A, B and E, each symbol represents data from a single mouse, and each data set is summarized as mean ± SEM.
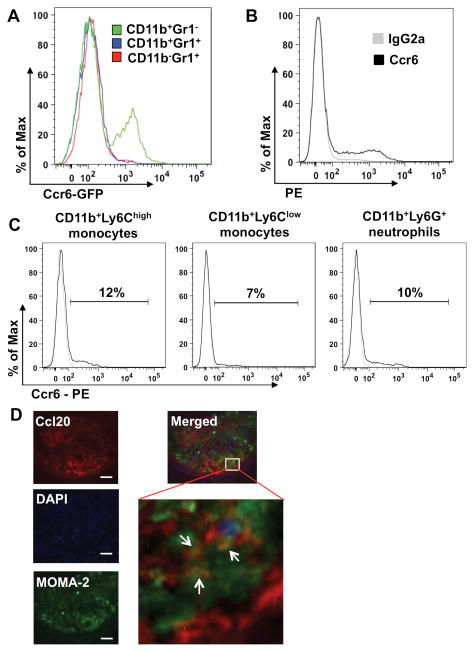
Ccr6 Is Expressed on Primary Mouse Monocytes and Ccl20 Is Expressed on Lesional Macrophages
Ccr6 has been shown to be expressed on many cell types, including T cells, B cells, NK cells, NKT cells, immature DCs and neutrophils,10, 11 and it may also be expressed on monocytes,12, 14, 18 which we examined in greater detail by FACS analysis. First, we checked the expression of Ccr6 on CD11b+Gr1− cells and CD11b+Gr1+ cells by using C57BL/6 mice and Ccr6 EGFP knock-in mice.19 We found that a subset of splenic CD11b+Gr1− cells is Ccr6+ while CD11b+Gr1+ cells are Ccr6− (Figure 4A, 4B and Supplemental Figure IV A). Since Gr1 antibody may interact with both Ly6C and Ly6G, we then used specific antibodies against CD11b, Ly6C and Ly6G to further differentiate inflammatory monocytes (CD11b+Ly6Chigh), resident monocytes (CD11b+Ly6Clow) and neutrophils (CD11b+Ly6G+), as reported previously.7, 20 About 12% of spleen CD11b+Ly6Chigh inflammatory monocytes (11.80 ± 3.10%, n=6, ApoE−/− mice) stained positive for Ccr6 and Ccr6 was also found on a subset of spleen CD11b+Ly6Clow monocytes and CD11b+Ly6Ghi neutrophils in both Ccr6+/+ApoE−/− mice and C57BL/6 mice (Figure 4C, Supplemental Figure IV B, C and data not shown). In addition, Ccr6 was found on a small subset of blood monocytes and bone marrow-derived macrophages (BMDM) in Ccr6 EGFP knock-in mice, C57BL/6 mice and Ccr6+/+ApoE−/− mice, whereas no Ccr6+ cells could be detected in monocytes from Ccr6−/− ApoE−/− mice (Supplemental Figure IV D and data not shown). FACS analysis of spleen cells from Ccr6+ Cx3cr1+GFP mice showed that ~20% CD11b+Ly6Chigh cells are Cx3cr1+, while CD11b+Ly6Clow cells are almost all Cx3cr1− (Data not shown). Both Ccr6 and Ccl20 were found to be expressed in the whole aorta of Ccr6+/+ApoE−/− mice as measured by real-time PCR (Supplemental Figure V). Expression was confirmed at the protein level by immunofluorescence, and Ccl20 was found to be co-localized with macrophages and smooth muscle cells/endothelial cells in atherosclerotic lesions (Figure 4D, Supplemental Figure VI and data not shown), which is consistent with previous reports.11 We also attempted to stain Ccr6 in these sections, but all commercially available antibodies showed high non-specific reactivity when tested on Ccr6−/− tissue.
Figure 4. Ccr6 is expressed on mouse primary monocytes and Ccl20 is expressed on lesional macrophages.
A, Leukocytes from the spleen of Ccr6 EGFP knock-in mice were stained with antibodies to CD11b and Gr1 and Ccr6 expression on CD11b+Gr1−, CD11b+Gr1+, CD11b− Gr1+ cells was examined by GFP expression. B, Representative flow cytometry staining for Ccr6 on splenic CD11b+Gr1− cells from C57BL/6 mice. C, FACS analysis of Ccr6 expression on CD11b+Ly6Chigh monocytes, CD11b+Ly6Clow monocytes and CD11b+Ly6G+ neutrophils. Leukocytes from the spleen of ApoE−/− mice were stained with specific antibodies against CD11b, Ly6C and Ly6G. The percentage of the total gated population is indicated above the bracket. D, Representative immunofluorescence photomicrographs of aortic root sections from Ccr6+/+ApoE−/− mice stained with antibodies to Ccl20, MOMA-2 (macrophages), DNA (DAPI) and corresponding secondary antibodies (left panel, bar = 50 μm). Right panel is a merged picture of the three stainings (top: 200×, insert: 400×; arrows indicate the overlapping of Ccl20 and MOMA-2 staining).
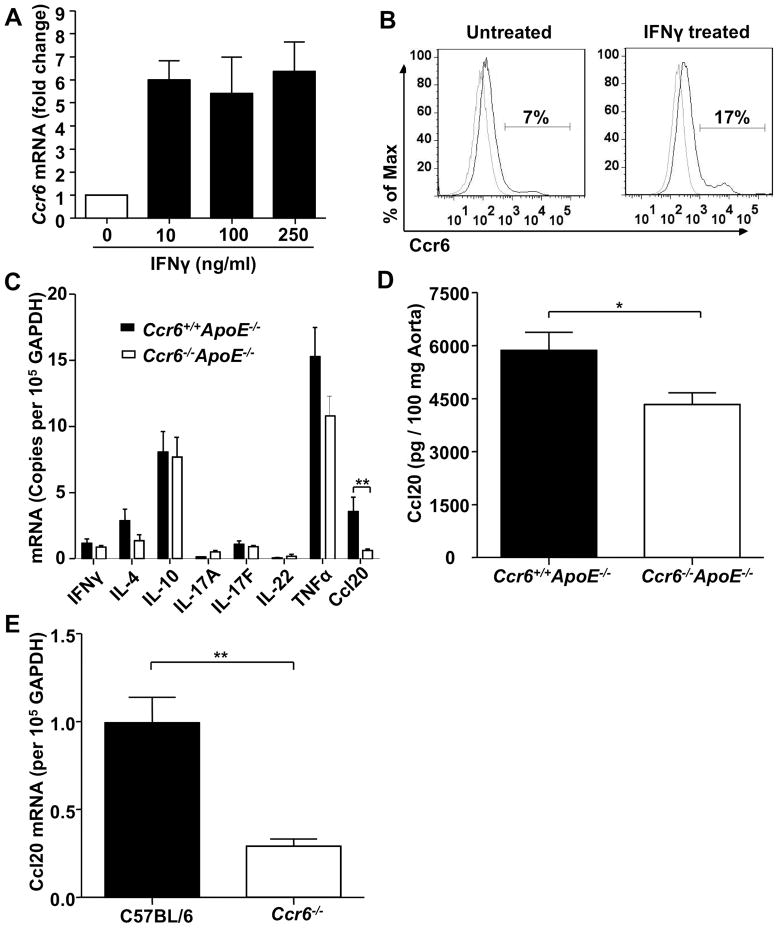
Ccr6 Expression on Monocyte/Macrophages Is Up-regulated by IFNγ and Ccl20 Expression Is Significantly Reduced in Ccr6 Deficient Mice
We next studied how the pro-inflammatory cytokines IFNγ, TNFα, IL-1β and IL-6, which have all been implicated in atherogenesis,2 may regulate Ccr6 expression. For this, we used RAW 264.7 cells, a mouse monocyte/macrophage cell line. As with primary monocytes (Figure 4), we found that RAW 264.7 cells constitutively expressed Ccr6 on the cell surface, and IFNγ induced a 3-fold and 6-fold increase of Ccr6 mRNA expression on purified primary mouse monocytes and RAW 264.7 cells, respectively (Figure 5A and Supplemental Figure VII), which was further confirmed by surface expression analysis (Figure 5B), while the other cytokines (TNFα, IL-1β, IL-6) had no effect on Ccr6 expression in either cell type (data not shown). To further characterize differences between Ccr6+/+ApoE−/− mice and Ccr6−/− ApoE−/− mice that might contribute to the difference in lesion progression, we measured RNA expression in whole aorta for other chemokines and chemokine receptors (Ccl1-25, Ccr1-10, Cxcl1-16, Cxcr1-7, Cx3cl1, Cx3cr1) as well as several hallmark inflammatory cytokines, e.g. IFNγ (Th1), IL-4 (Th2), IL-10 (Treg), IL-17A, IL-17F, IL-22 (Th17) and TNFα. No significant difference was observed for any of the cytokines/chemokines tested except for Ccl20, which showed an 80% mRNA reduction in the Ccr6−/− ApoE−/− mice (Figure 5C, Supplemental Figure VIII and data not shown). The level of Ccl20 protein in the aorta of Ccr6−/− ApoE−/− mice was also significantly reduced as measured by ELISA (Figure 5D), but the magnitude of the effect was less than for Ccl20 mRNA. In addition, Ccr6−/− mice showed 70% less Ccl20 mRNA than C57BL/6 mice in the whole aorta (Figure 5E). We also measured the serum levels of Ccl20, but no significant difference was found between Ccr6+/+ApoE−/− mice and Ccr6−/− ApoE−/− mice or between wild type C57BL/6 mice and Ccr6−/− mice (data not shown).
Figure 5. Ccr6 is constitutively expressed on mouse RAW 264.7 monocyte/macrophages and Ccr6 deficiency significantly reduces Ccl20 expression in the aortas of ApoE−/− mice and C57BL/6 mice (female, 16 weeks).
A and B, IFNγ induced production of Ccr6 mRNA (A) and protein (B) in RAW 264.7 cells. C, Real-time PCR analysis of inflammatory cytokine levels in mouse aortas (n=6 in each group; **P=0.004). D, ELISA analysis of Ccl20 protein level in mouse whole aorta (n=4 in each group; *P=0.038). E, qPCR analysis of Ccl20 expression in aortas from wild type C57BL/6 mice and Ccr6−/− mice (n=3 mice in each group; **P=0.009). In panel B, percentages of positive cells were enumerated inside the gate. All samples were normalized to GAPDH since Ccr6 knockout did not affect GAPDH expression in ApoE−/− mice (data not shown). Data are summarized as mean ± SEM.
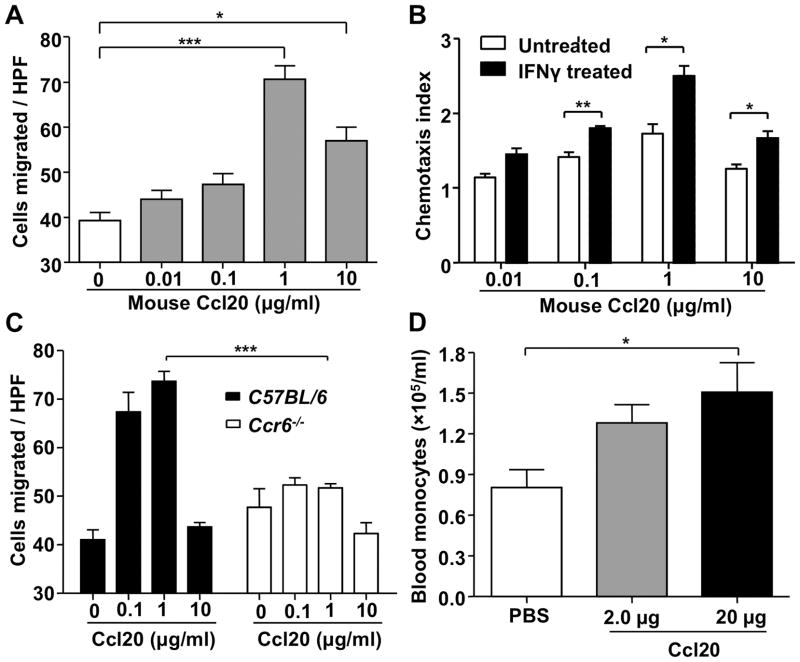
Ccr6 Is Functional on Mouse Monocytes and Ccl20 Induces Blood Monocytosis in vivo
Consistent with the expression of Ccr6 on RAW 264.7 cells and primary monocytes, we found that RAW 264.7 cells were chemotactically responsive to Ccl20 in a dose-dependent manner and Ccl20-induced chemotaxis of these cells was increased by 50% after Ccr6 up-regulation by IFNγ stimulation (Figure 6A and B). In addition, Ccl20 could induce the chemotaxis of purified splenic monocytes from wild type but not Ccr6−/− mice, indicating that Ccl20 signaling on this cell type involves Ccr6 (Figure 6C). Consistent with this, in vivo we found that tail vein injection of Ccl20 was able to induce peripheral blood monocytosis in ApoE−/− mice (Figure 6D) and this reflected a selective egress of the CD11b+Ly6Chigh monocyte subset (Data not shown). The number of circulating neutrophils was also increased at the same time point, but the increase was not significant (Supplemental Figure IX A). Since spleen has recently been identified as a reservoir of undifferentiated monocytes,21 we also tested whether Ccl20 treatment affects spleen monocyte content. We found that the total number of splenic monocytes was reduced after i.v. injection of Ccl20 but the reduction was not significant (Supplemental Figure IX B).
Figure 6. Ccl20 induces chemotaxis and mobilization of mouse monocytes in vitro, ex vivo and in vivo.
A, Ccl20 induced chemotaxis of RAW 264.7 cells (HPF: high power field, 400×; ***P<0.001: 1 μg/ml vs. 0 μg/ml; *P=0.012: 10 μg/ml vs. 0 μg/ml). B, IFNγ stimulation increased chemotactic responsiveness of RAW 264.7 cells to Ccl20 (**P=0.006: 0.1 μg/ml; *P=0.014: 1 μg/ml; *P=0.019: 10 μg/ml). Chemotaxis index was calculated by comparing the number of cells migrating towardCcl20 and the number of cells migrating toward control media. C, Ccl20 induced chemotaxis of purified splenic monocytes from wild type but not Ccr6−/− mice (HPF: high power field, 400×; n=5 in each group, ***P<0.0001). D, Ccl20 injection by tail vein induced monocytosis in ApoE−/− mice (female, 9 weeks old). Blood monocyte levels were quantified 15 hours post injection (n=3–4 in each group, *P=0.033: 20 μg Ccl20 vs. PBS). Each experiment was repeated two or three times and representative data are shown as mean ± SEM.
Discussion
In the present study we have demonstrated that genetic deficiency of Ccr6 significantly reduces lesion development in the ApoE−/− mouse model of atherosclerosis. The reduction was apparent throughout the aorta and the aortic root, was large at both of two time points measured over 6 months of age, and was not attributable to changes in cholesterol or triglyceride levels. At the cellular level, protection was associated with a major reduction in macrophage accumulation in plaque in Ccr6−/− ApoE−/− mice compared to Ccr6+/+ApoE−/− mice. Transplantation studies indicated that protection was mediated by a Ccr6+ bone marrow-derived cell(s).
Consistent with a direct effect of monocyte/macrophage Ccr6 signaling on macrophage accumulation in the vessel wall in the model, we found that 1) both Ccr6 and its ligand Ccl20 were expressed in atherosclerotic aorta in this model; 2) Ccr6 is expressed on a subset of primary mouse monocytes and the mouse monocyte/macrophage cell line RAW 264.7; 3) Ccr6 mediates chemotactic responses of both primary mouse monocytes and RAW 264.7 cells to Ccl20 in vitro; 4) Ccr6−/− ApoE−/− mice were monocytopenic compared to Ccr6+/+ApoE−/− mice; and 5) Ccl20 could induce monocytosis when injected into Ccr6+/+ApoE−/− mice. Taken together, we propose that the mechanism by which Ccr6 deficiency protects against atherogenesis in this model involves Ccr6-dependent monocyte trafficking into the vessel wall due to reduced monocyte levels in the blood and/or reduced migration capacity into atherosclerotic lesions.
The magnitude of protection in the absence of Ccr6 (40%) was comparable to what has been reported previously for Ccr2−/− (36%),6 Cx3cr1−/− (28%)7 and Ccr5−/− (50%)8 mice in the same ApoE−/− mouse model that we used. Importantly, Ccr6 deficiency in ApoE−/− mice did not affect the expression of other chemokine receptors (e.g. Ccr2, Cx3cr1, Ccr5) or chemokines in the atherosclerotic aorta (Supplemental Figure VIII), suggesting that these receptors may have non-redundant roles in atherogenesis. This could reflect action at different stages of atherogenesis or on different subsets in the monocyte migration process. For example, although all of these receptors are known to mediate monocyte/macrophage migration, Cx3cr1 may be more important as an adhesion receptor fostering interactions of foam cells with each other and with smooth muscle cells once they migrate into the vessel wall.5 The fact that Ccr6 deficiency results in a level of protection similar to these other monocyte/macrophage receptors may at first seem surprising since it is expressed on only a small subset of monocytes. As a potential mechanism, Ccr6 appears to operate at two steps important in macrophage accumulation: control of blood monocyte levels and direct recruitment of the cells into vessel wall. Dual action could greatly amplify the overall effect on pathogenesis beyond what might be expected based simply on the frequency of Ccr6+ cells. Additional work will be needed to define temporal and spatial expression of these ligands and receptors in the model to gain further insight into different mechanisms of monocyte recruitment. It is important to note that all of these receptors (Ccr2, Ccr5, Cx3cr1 and Ccr6) are also expressed on other leukocyte subsets represented in lesions in the model, which could also be contributing to pathogenesis through their recruitment and function. In particular, Ccr6 is expressed on neutrophils, dendritic cells, NKT cells, B cells and subsets of CD4+ T lymphocytes, and it is known that depletion of any of these cell types results in reduced atherogenesis in the model.2, 10, 22 However, the major leukocyte subtype by far present in atherosclerotic lesions is the foam cell, derived from blood monocytes.2
We found that Ccr6 deletion in ApoE−/− mice not only significantly reduced the percentage of monocytes among peripheral blood leukocytes but also the total absolute monocyte counts in the blood, while other cell subsets were unaffected (Figure 3). Monocyte levels did not appear to depend on Ccr6 in ApoE+/+ mice, which were maintained under the same conditions as ApoE−/− mice (Supplemental Figure II). The monocytopenia in Ccr6−/− ApoE−/− mice was caused by a significant reduction of Ly6Chigh inflammatory monocytes in the blood. At the same time, there was a significant increase of Ly6Chigh inflammatory monocytes in the bone marrow of Ccr6−/− ApoE−/− mice, indicating that Ccr6 likely affects the bone marrow egress of these cells. This is reminiscent of a previously identified role of Ccr2 in controlling monocyte mobilization from the bone marrow.23 It has been reported that Ccr6−/− mice did not exhibit gross abnormalities in any major organ but they have increased numbers of T cells in the intestinal mucosa.24, 25 Also, it has been shown that Ccr6−/− mice have impaired development of M cells and underdeveloped Peyer’s patches with a two-fold decrease of total leukocytes in the intestinal mucosa,26 However, this is the first report of Ccr6 regulation of blood monocytosis. Monocytosis is an independent predictor of subclinical carotid atherosclerosis and is a predictor of atherosclerotic plaque progression in acute myocardial infarction.27, 28 In the mouse model of atherosclerosis it has also been reported that monocytes accumulate continuously in the aorta during atheroma formation.29 In particular, total blood Ly6Chigh monocytes increase dramatically in hypercholesterolemic ApoE−/− mice fed a high-fat diet compared with wild type mice,29 which is consistent with our findings in the present study (Supplemental Figure II). We found that both Ly6Chigh and Ly6Clow monocytes express low levels of Ccr6 but only Ly6Chigh monocytes were dysregulated in Ccr6−/− ApoE−/− mice, which may reflect the different characteristics of these two subsets, e.g. Ly6Chigh monocytes have been shown to preferentially adhere to activated endothelium and accumulate in atherosclerotic plaques when compared with Ly6Clow monocytes.29 Thus, we propose that the significant reduction of circulating monocyte counts in Ccr6−/− ApoE−/− mice may directly reduce the monocytes available for recruitment to atherosclerotic sites, as an explanation for reduced macrophage content and atherosclerotic lesion size in these mice. Future studies comparing effects of transferring Ccr6+ versus Ccr6− monocytes or effects resulting from monocyte-specific deletion of Ccr6 will be needed to further refine this conclusion.
We found that Ccr6−/− ApoE−/− mice had an 80% reduction of Ccl20 expression in the aorta compared with Ccr6+/+ApoE−/− mice, whereas the systemic serum level of Ccl20 in these two groups was similar (Figure 5). This suggests that Ccr6 and Ccl20 may form a local positive feedback loop in the vessel wall. A CCL20/CCR6 positive feedback loop has been previously described for Th17 cells, which are both CCR6 positive and able to produce CCL20.30 We found very few Th17 cells in atheroma, but they could contribute to macrophage accumulation. Previous studies showed that IL-17A may reduce, increase or have no effect on atherosclerosis development31–33 and recently Madhur et al. reported that IL-17A deficiency does not alter plaque burden in ApoE−/− mice,34 indicating that more studies will be needed to clarify the role of IL-17 and Th17 cells in atherogenesis.
Our data on Ccr6 and the previously reported results connecting other chemokines and chemokine receptors to outcome in ApoE−/− mice are consistent with the inflammation theory of atherogenesis. In this regard, Ccr6 and Ccl20 have been linked to multiple other mouse models of chronic inflammatory disease, including psoriasis, inflammatory bowel disease, rheumatoid arthritis and experimental autoimmune encephalomyelitis.16, 35, 36, 37 Recently, a triallelic dinucleotide polymorphism of CCR6 was correlated with the expression level of CCR6 and was associated with susceptibility to rheumatoid arthritis, Grave’s disease and Crohn’s disease;38 however, its association with atherosclerosis has not been defined yet.
In conclusion, Ccr6 deficiency in ApoE−/− mice causes significant reduction of circulating blood monocytes and reduces progression of atherosclerosis. Ccr6−/− ApoE−/− mice had markedly less Ccl20 in the aorta, suggesting a local positive feedback loop. Considering the atherogenic effect of Ccr6 in this mouse model, Ccr6 should be further considered in the molecular pathogenesis and therapeutic targeting of this disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Ccr6 is a G protein-coupled receptor expressed on T cells, B cells, and DCs
CCR6, and its sole ligand, CCL20 have been detected in human carotid plaques
Selective depletion of monocytes/macrophages inhibit early atherogenesis
What New Information Does This Article Contribute?
Ccr6 deficiency in ApoE−/− mice reduced atherogenesis and macrophage content in aortic roots
Ccr6 deficiency decreased circulating inflammatory monocytes and increased bone marrow inflammatory monocytes in ApoE−/− mice
Ccr6 functions on a subset of mouse monocytes
CCR6 is a chemokine receptor that plays a pathogenic role in several autoimmune diseases. It has been detected in human atherosclerotic plaques, but its functional role in atherogenesis is still unknown. We determined that genetic deletion of Ccr6 in ApoE−/− mice significantly reduced atherosclerosis development and macrophage accumulation in aortas. Transplantation studies suggested that Ccr6 expresion in+ bone marrow-derived cell(s) caused this phenotype. Ccr6 deficiency significantly reduced the numbers of circulating blood monocytes in ApoE−/− mice, that were associated with increased inflammatory monocytes in the bone marrow, suggesting that Ccr6 may control egress of monocytes from the bone marrow into the blood. Also, we showed that Ccr6 is expressed on a small subset of mouse monocytes and is functional, since Ccl20 induced migration of mouse monocytes both in vitro and in vivo. Our findings provide the first in vivo evidence that Ccr6 is a mediator of atherogenesis. The data suggest that the mechanism may involve direct recruitment of inflammatory monocyte to the vessel wall and/or regulation of monocyte egress from bone marrow to blood, among other possibilities. These preclinical results support further study of Ccr6 as a potential target for therapeutic development in atherosclerosis.
Acknowledgments
We thank Dr. Ifor R. Williams and Dr. Donald Cook for providing the Ccr6 EGFP knock-in mice and thank Dr. Owen M. Schwartz and Dr. Steven Becker for technical assistance with the Leica AF6000 LX microscope.
Sources of Funding
This research was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Non-standard Abbreviations and Acronyms
- ApoE
apolipoprotein E
- BMDM
bone marrow-derived macrophages
- DC
dendritic cell
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- oxLDL
oxidized low-density lipoprotein
- TNF
tumor necrosis factor
- VLDL
very-low-density lipoprotein
Footnotes
Disclosures
None.
References
- 1.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 5.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 6.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 8.Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, Mach F, Weber C. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- 9.Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. 2007;116:1801–1811. doi: 10.1161/CIRCULATIONAHA.106.678474. [DOI] [PubMed] [Google Scholar]
- 10.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- 11.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 12.Ruth JH, Shahrara S, Park CC, Morel JC, Kumar P, Qin S, Koch AE. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 13.Tanida S, Yoshitomi H, Nishitani K, Ishikawa M, Kitaori T, Ito H, Nakamura T. CCL20 produced in the cytokine network of rheumatoid arthritis recruits CCR6+ mononuclear cells and enhances the production of IL-6. Cytokine. 2009;47:112–118. doi: 10.1016/j.cyto.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Aït-Yahia S, Vicari A, Kaiserlian D, Dubois B. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz A, Lipfert B, Cicha I, Schubert K, Klein M, Raithel D, Daniel WG, Garlichs CD. Accumulation of immune cells and high expression of chemokines/chemokine receptors in the upstream shoulder of atherosclerotic carotid plaques. Exp Mol Pathol. 2007;82:245–255. doi: 10.1016/j.yexmp.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, Farber JM. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravindran R, Rusch L, Itano A, Jenkins MK, McSorley SJ. CCR6-dependent recruitment of blood phagocytes is necessary for rapid CD4 T cell responses to local bacterial infection. Proc Natl Acad Sci U S A. 2007;104:12075–12080. doi: 10.1073/pnas.0701363104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucharzik T, Hudson JT, 3rd, Waikel RL, Martin WD, Williams IR. CCR6 expression distinguishes mouse myeloid and lymphoid dendritic cell subsets: demonstration using a CCR6 EGFP knock-in mouse. Eur J Immunol. 2002;32:104–112. doi: 10.1002/1521-4141(200201)32:1<104::AID-IMMU104>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 21.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 25.Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutiérrez J, Torres M, Martínez-A C, Márquez G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lügering A, Floer M, Westphal S, Maaser C, Spahn TW, Schmidt MA, Domschke W, Williams IR, Kucharzik T. Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer’s patches. Am J Pathol. 2005;166:1647–1654. doi: 10.1016/S0002-9440(10)62475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35:1619–1624. doi: 10.1161/01.STR.0000130857.19423.ad. [DOI] [PubMed] [Google Scholar]
- 28.Nozawa N, Hibi K, Endo M, Sugano T, Ebina T, Kosuge M, Tsukahara K, Okuda J, Umemura S, Kimura K. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J. 2010;74:1384–1391. doi: 10.1253/circj.cj-09-0779. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taleb S, Tedgui A, Mallat Z. Interleukin-17: friend or foe in atherosclerosis? Curr Opin Lipidol. 2010;21:404–408. doi: 10.1097/MOL.0b013e32833dc7f9. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A Critical Function of Th17 Proinflammatory Cells in the Development of Atherosclerotic Plaque in Mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- 34.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of Interleukin 17 in Inflammation, Atherosclerosis, and Vascular Function in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varona R, Cadenas V, Flores J, Martinez AC, Marquez G. CCR6 has a non-redundant role in the development of inflammatory bowel disease. Eur J Immunol. 2003;33:2937–2946. doi: 10.1002/eji.200324347. [DOI] [PubMed] [Google Scholar]
- 36.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelharsdt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 38.Kochi Y, Okada Y, Suzuki A, Ikari K, Terao C, Takahashi A, Yamazaki K, Hosono N, Myouzen K, Tsunoda T, Kamatani N, Furuichi T, Ikegawa S, Ohmura K, Mimori T, Matsuda F, Iwamoto T, Momohara S, Yamanaka H, Yamada R, Kubo M, Nakamura Y, Yamamoto K. A regulatory variant in CCR6 is associated with rheumatoid arthritis susceptibility. Nat Genet. 2010;42:515–519. doi: 10.1038/ng.583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.