Abstract
Natural killer (NK) cells, like B and T lymphocytes, are potent effector cells crucial for immunity to tumors and infections. These effector responses must be controlled to avoid inadvertent attack against normal self. Yet the mechanisms that guide NK cell tolerance differ from those guiding T and B cell tolerance. Here we discuss how NK cells are licensed by self-MHC class I through their inhibitory receptors, resulting in the functional competence to be triggered through their activation receptors. We discuss newly published data with respect to issues related to licensing, thereby providing a framework for unifying concepts on NK cell education.
Natural killer (NK) cell receptors: activation and inhibition
NK cells are endowed with potent effector mechanisms such as cytotoxicity and cytokine production. In contrast to other lymphocytes, NK cells do not express rearranged, antigen-specific receptors and NK effector function is instead dictated by integration of signals received through germ-line-encoded receptors that can recognize ligands on their cellular targets [1]. Functionally, NK cell receptors are classified as activation or inhibitory [2] (Fig 1). Activation receptors, such as Ly49H in mouse, as well as the natural cytotoxicity receptor, NKp46, in both mice and humans, stimulate NK cell effector functions upon recognition of ligands expressed on virus-infected or transformed cells. Self-MHC Ia and Ib-specific NK cell activation receptors might also recognize ligands constitutively expressed on healthy cells, such as H2Dd and Qa1 by Ly49D and NKG2C–CD94, respectively [1]. These receptors generally do not have identifiable signaling motifs in their cytoplasmic domains, but instead have charged transmembrane residues for association with other transmembrane proteins, such as CD3ζ, FcεRIγ, or DAP12, which contain cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs). These proteins help stabilize activation receptor expression and transduce activation signals. NK cells also express other stimulatory receptors, such as CD27 and CD96 (Tactile), which recognize constitutively expressed ligands, CD70 and CD155 (PVR), respectively [1]. Signaling through these receptors is ITAM-independent.
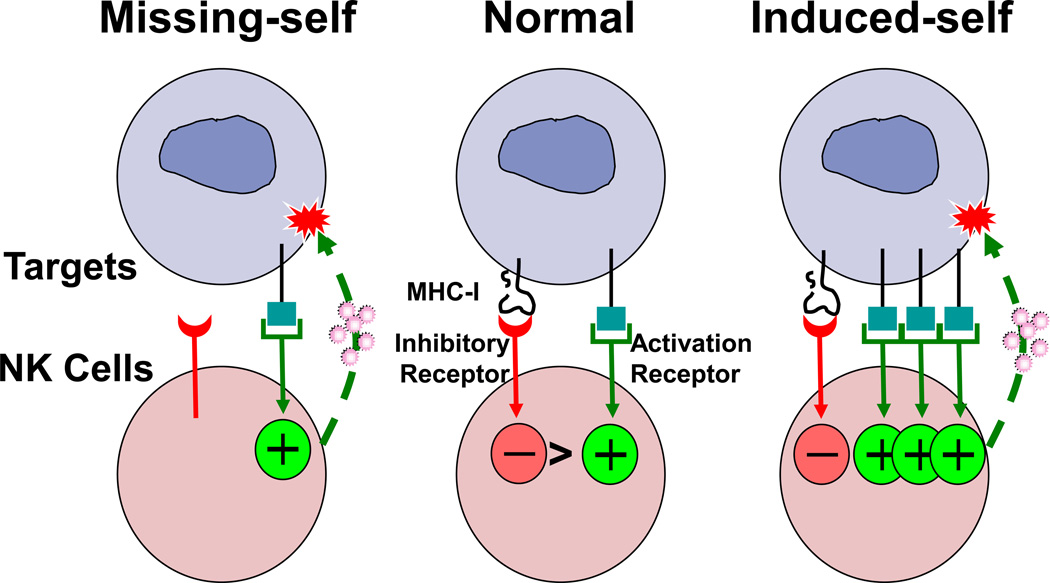
Figure 1. Effector responses of NK cells are regulated by inhibitory and activation receptors.
NK cells express receptors specific for ligands expressed on targets. Under normal circumstances, inhibitory receptors (in red) for MHC class I deliver signals that dominate over stimulation through activation receptors (in green) (middle pair of cells). When MHC class I is down-regulated, as in “missing-self,” activation receptors stimulate NK cell cytolysis of a target via exocytosis of granules (left). When a target is “stressed,” ligands for the NKG2D activation receptor are induced, as in “induced-self,” permitting NK cell activation by overcoming MHC class I-dependent inhibition (right). Depicted here is granule exocytosis against a target. NK cell responses also include cytokine production, regulated in the same way by signaling through a combination of activation and inhibitory receptors (not shown).
Inhibitory receptors are specific for MHC class Ia and Ib molecules, and other, non-MHC ligands [3], such as E-cadherin bound by KLRG1 [4,5]. In the mouse, the MHC-specific inhibitory receptors belong to the lectin-like homodimeric Ly49 family, whereas human NK cells predominantly express monomeric HLA-specific inhibitory receptors belonging to the Ig-superfamily of proteins and are known as the killer immunoglobulin-like receptors (KIRs) [6]. In addition to these topologically distinct receptors specific for MHC Ia molecules, both mice and humans express the inhibitory NKG2A–CD94 heterodimer, which binds the MHC class Ib molecule, Qa-1 and HLA-E in mouse and human, respectively. In general, the inhibitory receptors signal via immunoreceptor-based tyrosine inhibitory motifs (ITIMs) in their cytoplasmic tails, which contain tyrosine motifs that are phosphorylated following inhibitory receptor cross-linking, allowing recruitment of phosphatases.
Target stimulation of an NK cell is dependent on the integration of signals received through these receptors (Fig 1). In general, if both types of receptors are engaged, the inhibitory signals predominate and NK cells are prevented from killing and producing cytokines. If MHC class I is down-regulated on target cells i.e., “missing-self”, then the NK cell may become activated if an activation receptor is also engaged. On the other hand, certain processes, such as viral infection or genotoxic damage [7], can upregulate ligands for the NKG2D activation receptor, i.e., “induced-self”, which may overcome normal steady-state inhibitory receptor dominance and allow NK cell activation [8]. NK cells simultaneously express several different isoforms of both activation and inhibitory receptors, complicating the study of how these signaling pathways integrate in an individual NK cell during an effector response.
Because NK cells express several activation receptors that are potentially specific for self-molecules, they require mechanisms to prevent inadvertent activation against normal tissues, processes referred to as “tolerance to self”. Although the functions and specificities of individual activation and inhibitory NK receptors have been well-defined in the context of effector response, their combined role in establishing both functional capacity and self-tolerance, which together is referred to as NK cell “education”, is less well defined (Fig 2). There is general consensus that MHC-dependent NK cell education occurs through a self-MHC-specific receptor, a process termed “licensing” [9]. However, how this is achieved and the relative contributions of inhibitory versus activation receptors are still being debated. Here, we consider current models for NK cell education, examine when during the NK cell life cycle education occurs, and the potential plasticity for NK cell responsiveness. We seek to clarify existing terminology (see Glossary) and to provide a unified framework for understanding the role of MHC class I molecules and their receptors in the context of NK cell education.
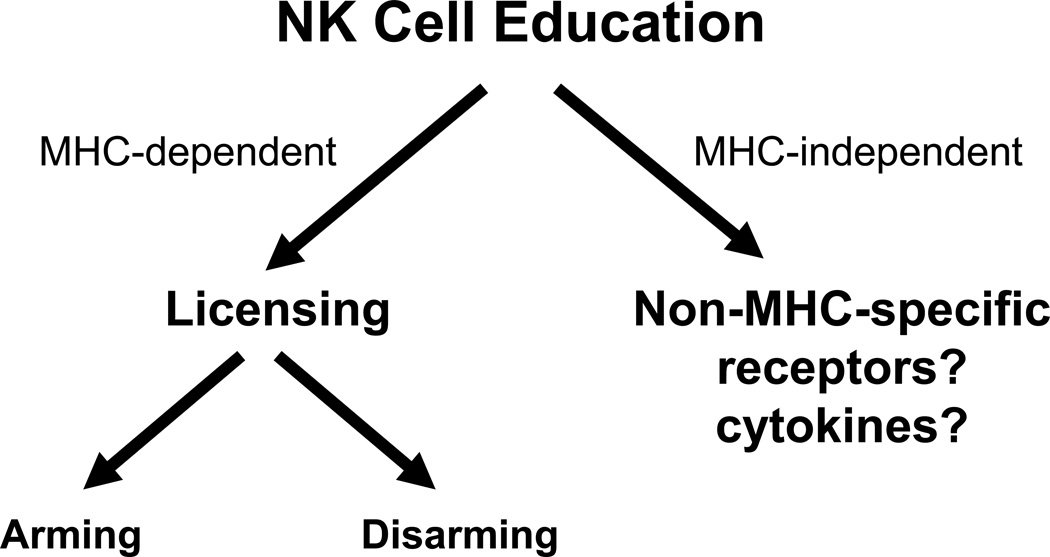
Figure 2. Terminology of NK cell education.
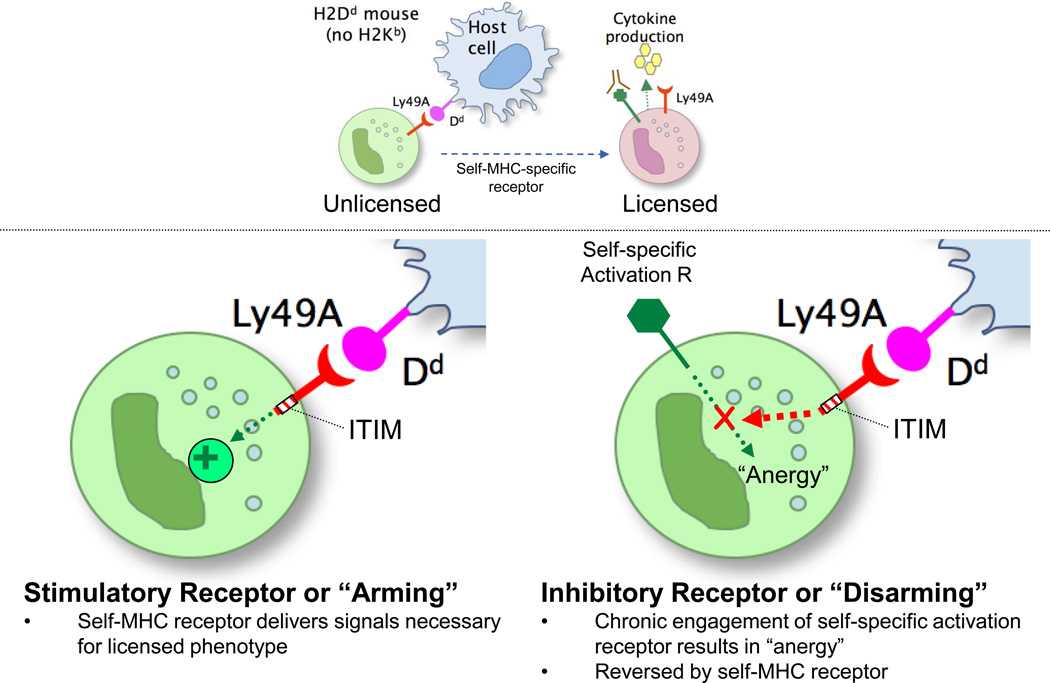
NK cell education (acquisition of effector function) can be divided into two major processes: MHC-independent and MHC-independent. MHC-dependent education, otherwise known as licensing, could be due to two major possible mechanisms, arming or disarming. The arming hypothesis proposes that the self-MHC-specific receptor confers the signals necessary to confer the licensed phenotype whereas the disarming hypothesis suggests the presence of a self-specific activation receptor. If unperturbed, this activation receptor results in chronic stimulation and an anergic-like, hypofunctional state. The self-MHC-specific MHC receptor would then modulate the action of the activation receptor and lead to a licensed NK cell. MHC-independent education could arise from non-MHC-specific receptors or cytokine stimulation.
Licensing: MHC-dependent NK cell education
NK cells are regulated by the host MHC class I environment. In retrospect, this was first noted in the description of “hybrid resistance,” a phenomenon that defies predictions based on the classic laws of solid tissue transplantation (see Glossary) [10]. In hybrid resistance, F1 hybrid mice reject bone marrow (BM) grafts from either inbred parent but not from an F1 hybrid. Rejection is mediated by host NK cells, which are regulated by the host MHC class I environment [11,12]. Studies of β2-microglobulin (β2m)-deficient mice, which lack MHC I molecules, are reminiscent of hybrid resistance, because NK cells in MHC-sufficient mice reject β2m-deficient BM whereas NK cells in β2m112 deficient mice do not [13].
Although these studies helped confirm the missing-self concept [14], they were puzzling because a prediction of missing-self is that NK cells in MHC-deficient hosts should be uninhibited and cause autoreactivity. However, MHC-deficient hosts, both mouse and human, display no overt NK cell hyperactivity [13,15,16]. Instead, when freshly isolated, the NK cells are defective in killing MHC-deficient targets, and do not reject β2m-deficient BM [16,17]. Thus, MHC class I environment regulates NK cell function. The basis for MHC-dependent NK cell education was enigmatic for quite some time. The data suggested that self-tolerance occurred because each NK cell expressed at least one self-specific inhibitory receptor [18]. However, NK cells that do not express any of the known self-specific inhibitory receptors have been detected in MHC-sufficient hosts [19]. These cells were hypofunctional, despite the apparent absence of MHC-specific receptor engagement. Thus, an NK cell inhibitory receptor repertoire specific for MHC haplotype [20] does not apparently explain NK cell hypofunction in MHC class I-deficient hosts.
Experiments on a per cell basis show that when activation receptors are cross-linked, NK cells with a self-MHC-specific Ly49 inhibitory receptor are good producers of IFNγ but their counterparts without such receptors are not. This indicates the existence of both licensed and unlicensed tolerant NK cells (Fig. 3). Licensed NK cells are functionally competent with regard to their activation receptors and are tolerant because they have an inhibitory receptor for self-MHC. Unlicensed NK cells do not have a self-MHC-specific inhibitory receptor and are tolerant because they are not functionally competent. The intact cytoplasmic domain of the inhibitory receptor, and specifically the ITIM, is required for licensing [21]. There is probably a minimal role for MHC class Ib molecules in MHC-dependent education because NK cells in β2m-deficient mice are functionally similar to NK cells from mice deficient in MHC class Ia heavy chains (H2KbDb -double deficient) [21]. This has been confirmed by recent studies in CD94-deficient mice containing NK cells, in which the NKG2A–CD94 receptor specific for Qa1 is absent [22]. Thus, it appears that MHC-dependent education, or licensing, primarily involves self-MHC-specific receptors for MHC class Ia molecules.
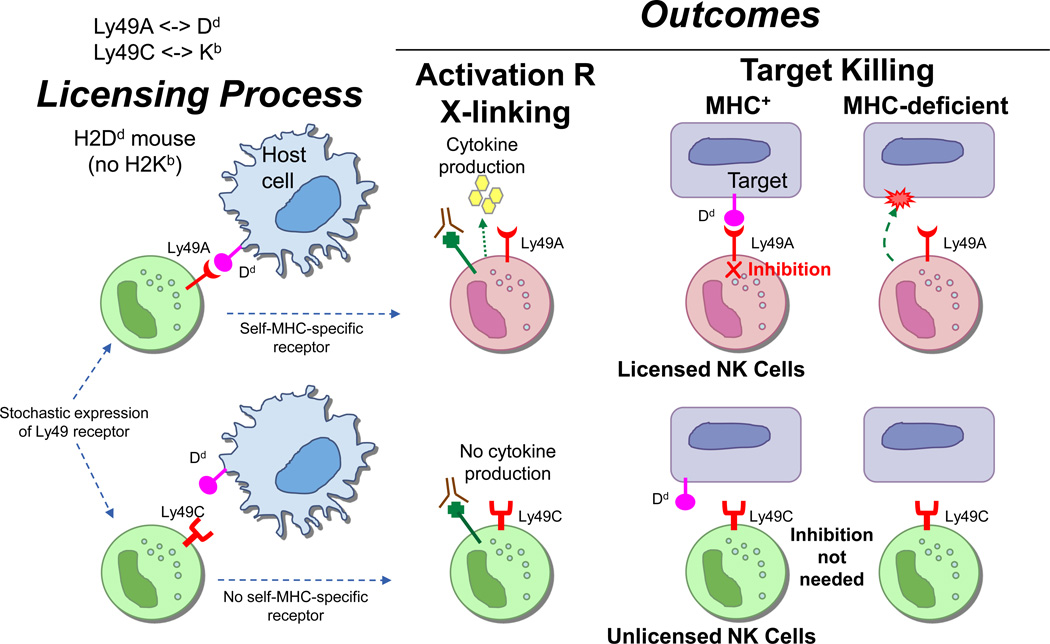
Figure 3. Licensing results in two types of self-tolerant NK cells with respect to self-MHC.
Depicted is the situation in a mouse expressing only H2Dd which, for the sake of simplicity in this diagram, can only be recognized by Ly49A and not by Ly49C. Due to stochastic (probabilistic) [74] expression of Ly49 receptors, only cells expressing Ly49A will engage H2Dd as self-MHC, resulting in licensed NK cells. When their activation receptors are cross-linked, they readily produce cytokines. They are also capable of attacking targets lacking self-MHC and are tolerant to self because they are inhibited by self-MHC, through the same receptor (Ly49A) that conferred licensing. By contrast, NK cells not expressing Ly49A are not licensed by self-MHC, even though they express another Ly49 receptor such as Ly49C. They poorly produce cytokines when their activation receptors are cross-linked, and they are tolerant to self because they cannot be activated by targets, even those lacking self-MHC. In this context, inhibition by MHC I is not needed to achieve tolerance. To avoid clutter, activation receptors that trigger killing of susceptible targets are not shown. Adapted from [31].
Studies of human NK cells support the concept that a self-MHC-specific inhibitory receptor confers functional competence of activation receptors [23,24]. NK cells expressing a KIR specific for self-HLA display more robust cytokine production than self-KIR-negative NK cells. KIR and HLA Tg mice also demonstrate similar effects [25]. These data provide additional evidence indicating that Ly49s and KIRs represent receptors that are related by convergent evolution [26,27].
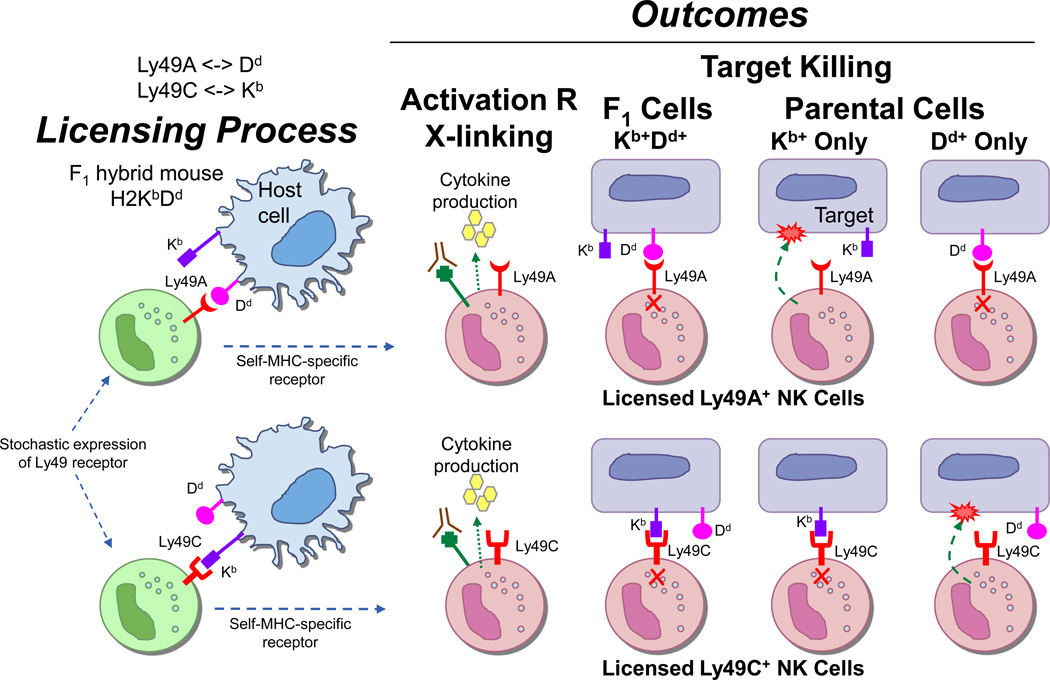
The licensing hypothesis is compatible with hybrid resistance because each NK cell is separately licensed by different MHC alleles, depending on receptor expression (Fig. 4). Because the F1 hybrid expresses both parental alleles, NK cells expressing a receptor specific for parent A’s MHC would be licensed by parent A alleles but not B. Conversely NK cells may be licensed by parent B but not parent A alleles. BM transplanted from parent A into the F1 hybrid, would be attacked by NK cells licensed by parent B alleles but not parent A alleles, and vice-versa. Thus, licensing predicts that the NK cell pool can discriminate between normal targets expressing the full complement of MHC alleles and pathologic cells lacking expression of one or more MHC ligands.
Figure 4. Hybrid resistance is compatible with licensing.
Depicted is the situation in a F1 hybrid mouse expressing H2Kb and H2Dd which, for the sake of simplicity in this diagram, can only be recognized by Ly49C and Ly49A, respectively. Both Ly49A+ and Ly49C+ NK cells are licensed by host MHC class I alleles. However, since they are separately licensed, they manifest different reactivities against parental targets lacking one or the other MHC class I allele, as illustrated. To avoid clutter, activation receptors that trigger killing of susceptible targets are not shown.
Many signals integrate to control NK cell activation; engagement of activation receptors may also contribute to BM rejection. Indeed, BM cells from certain mouse strains express NKG2D ligands, and blocking NKG2D can result in BM engraftment, even when expression of the full complement of MHC I alleles is absent [28]. In F1 hybrid mice, the parental donor MHC class I haplotype dictates the extent of NKG2D-mediated rejection [29], presumably via “missing-self” and licensing effects. While not all strains of mice seem to express NKG2D ligands on their BM, BM may express unidentified activating ligands that similarly collaborate with MHC effects for host NK cell-mediated rejection.
Mechanisms of licensing: Arming versus disarming?
Several mechanisms have been proposed to account for how NK cells become licensed through self-MHC-specific receptors. Best-known models are “arming” and “disarming”, proposed by Raulet and colleagues [30] (Figs 2, 5). In the arming model, the self-specific inhibitory receptor provides all of the signals required for licensing to occur, more akin to a stimulatory receptor [31]. Although it might seem difficult to reconcile transmission of a “positive” signal through the ITIM, a domain required for licensing [21] yet traditionally described to initiate inhibitory signaling cascades, receptors can signal different processes, depending on context. For example, the TCR is generally conceived to be an activation receptor [32], but under some circumstances it can deliver apoptotic signals, confer positive or negative selection, result in anergy, etc. MHC-specific receptors involved in licensing were first described as “inhibitory” in the context of an effector response against targets, but they might deliver qualitatively different signals in tolerance induction. Indeed, inhibitory receptor signaling itself seems to be more complicated than just recruitment of tyrosine phosphatases [33].
Figure 5. Arming versus disarming models.
Depicted are the two major mechanisms, arming and disarming, that have been proposed to explain how the self-MHC-specific receptor confers licensing upon contact with self-MHC.
The disarming theory proposes that in the absence of self-MHC class I recognition, NK cells are hyporesponsive as a consequence of stimulation by a putative self-ligand for an undefined self-specific activation receptor [30] (Fig 5). Unopposed signaling through activation receptors would account for the hyporesponsive phenotype in NK cells obtained from an MHC class I-deficient environment. Indirect evidence for a self-specific activation receptor comes from studies indicating that MHC-deficient cells are susceptible to killing by NK cells from WT mice, suggesting that the MHC-deficient cells constitutively express a ligand for an unknown activation receptor [17].
The disarming model, particularly with regard to a self-specific activation receptor, is complicated by several factors. The specificity and function of several NK cell activation receptors are controversial. For example, the Ly49D activation receptor has specificity for H2Dd in functional studies [34], but physical binding studies do not corroborate this specificity and H2Dd can also bind inhibitory receptors such as Ly49A [35]. For other receptors, such as the 2B4 receptor, there is functional ambiguity that may be context dependent [36]. Other receptors, such as CD2, are expressed on other immune cells, which may complicate analysis of their NK cell-specific function in general immune responses. Finally, ligands for some activating receptors, such as NKG2D, are known to be stress-induced, making the study of endogenous self-specific activation receptor-ligand interactions challenging.
Transgenic (Tg) mouse models, in which NK cell activating ligands are constitutively and continuously expressed, support the concept that chronic engagement of an activation receptor on NK cells can lead to an anergic-like state. In mice Tg for NKG2D ligands, apparently all NK cells (because all NK cells express NKG2D) become hypofunctional [37,38]. NK cells from these mice also show defects in cytotoxicity against MHC I-deficient target cells (31), suggesting defects in multiple signaling pathways. Similar findings were reported in mice expressing m157, a virally-encoded ligand for Ly49H where the capacity of NK cells to be stimulated through Ly49H was reduced [39,40]. This tolerance is dependent on the ITAM-signaling chain DAP12 but not DAP10 [41] which also can associate with Ly49H [42,43]. Additionally, Ly49H+ NK cells from these mice had defective responses to Ly49H-independent stimuli, including cytokines, and other activating receptors [39,40]. In each of these models the stimulated activation receptor was downregulated, but this alone cannot account for the hyporesponsive phenotype, as multiple NK cell receptors were impacted. Human NK cells expressing the activation receptor KIR2DS1 demonstrate hyporesponsiveness when derived from donors bearing its ligand, HLA-C2, supporting the findings in mice [44]. Thus, in the disarming hypothesis, the idea that hypofunction or anergy induced via chronic stimulation through an activation receptor, is supported by Tg models and human studies.
The phenotype of NK cells from the activation ligand Tg mice, however, differed from normally licensed NK cells. Notably, in m157 Tg mice, the Ly49H+ NK cells were generally hypofunctional as compared to Ly49H+ NK cells from WT mice [40], even when they expressed a self-MHC-specific receptor. Specifically, in m157 Tg mice on the H2b background, Ly49C+H+ NK cells did not show better function than Ly49C−H+ NK cells following activation receptor cross-linking, even though Ly49C licenses NK cells due to recognition of H2Kb. As engagement of a self-MHC-specific receptor did not reverse the anergic phenotype, disarming might not best describe the mechanism by which NK cells become licensed.
These tests of the disarming hypothesis, however, have their limitations. First, it is possible that the ectopically expressed ligands were present on different tissues than the ligands for the theoretical self-specific activation receptor. Second, “anergy” itself could be subject to a balance between activation and “inhibitory” receptors. As such, the amount of ectopically expressed ligand (and the corresponding stimulation through the activation receptor) may be too high to be overcome by the self-MHC-specific inhibitory receptors. Also, the affinities of the relevant ligands may be significantly different from the affinities of the enforced self-specific activation receptor-ligand interactions. Finally, the putative, naturally occurring self-specific activation receptor may have other properties that differ from the receptors tested. Until a clearly defined, putative self-specific activation receptor has been better tested, licensing via disarming remains a possible mechanism.
When does licensing occur …and with whom?
There are two sites, termed site 1 and site 2, on MHC class I that potentially contact Ly49 receptors [45]. Site-directed mutagenesis indicated that site 2 is relevant for interaction in effector inhibition [46,47]. It is located below the peptide-binding domain and consists of contact residues in α1, α2, and α3 of the MHC class I heavy chain, and species-specific determinants present in mouse but not human β2m [46–49]. H2Dd mutant Tg mice indicate that site 2 is required for Ly49A-dependent licensing [50]. This was corroborated by swapping human for mouse β2m (human β2m Tg on mouse β2m-deficient background) in which licensing that is dependent on Ly49A and Ly49C requires site 2 in their respective ligands, H2Dd and H2Kb. Thus, Ly49-dependent licensing and effector inhibition involve the same recognition site on MHC class I ligands.
Licensing was first thought to occur during NK cell development in the BM [21] because: 1) Tolerance develops in immature cells in other lymphocytes; 2) NK cell development occurs in the BM in a series of stages [51]; Ly49 receptors are first expressed at an intermediate stage of development that is followed by constitutive proliferation; and 3) If an NK cell expresses a self-specific Ly49, the amount of proliferation is higher than when the NK cell expresses the same receptor but in a host without a ligand for the given Ly49 [21].
Recent studies that involved adoptive transfer of MHC class I-deficient splenic NK cells to WT hosts [52,53] showed that, after transfer, donor NK cells gained the capacity to be triggered through their activation receptors. Furthermore, donor NK cells that expressed an inhibitory receptor capable of interacting with a host MHC class I molecule showed greater functional capacity [52]. Additionally, when the pairings of inhibitory receptors and MHC class I were restricted, such that only one inhibitory receptor, Ly49C, could interact with host MHC class I, the increase in functional capacity was limited to only donor Ly49C+ NK cells, indicating that the expression of an inhibitory receptor for host MHC class I is necessary for the gain in function. Taken together, the adoptive transfer data support the notion that NK cell licensing is not strictly a developmental process that occurs in the BM, because it can occur in peripheral NK cells that are generally considered to be mature.
The recent adoptive transfer studies add to the debate whether the educating signal may be delivered in trans, via interaction of an NK inhibitory receptor with MHC I on a different cell, or in cis, in which the interaction occurs on the surface of the same cell. Several experimental models provide evidence for cis interactions of these receptors in the plane of the membrane [54]. Additionally, in experiments where a mutant Ly49A receptor, with an inflexible stalk incapable of cis-binding to MHC I, was transgenically expressed, NK cells from these mice did not appear to be properly educated, though they could be inhibited through this receptor, suggesting cis interactions were necessary for NK licensing [55]. In contrast, the adoptive transfer studies using donor MHC class I-deficient splenic NK cells and WT hosts indicate that trans interactions are sufficient to fully license NK cells, as exposure of MHC I-deficient cells to an MHC I-sufficient environment imparted normal function [52,53]. While it is possible that MHC-deficient NK cells could acquire MHC class I via receptor-dependent processes or trogocytosis [56–59], results from the inverse transfer, WT NK cells to β2m-deficient hosts, imply that cis interactions are not sufficient for licensing, as these cells appeared to lose the licensed phenotype. However, a role for cis interactions in NK education in environments with normal MHC I expression cannot be excluded.
The MHC-dependent education of unlicensed, peripheral NK cells by host MHC class I, in trans, might be relevant to human therapies, particularly NK cell-mediated control of neoplasia [60–62]. Adoptive transfer of NK cells into tumor-bearing hosts may result in licensing of donor NK cells by host HLA class I [63]. These cells may be able to mount an attack against tumors which have down-regulated host HLA molecules. Similarly, donor NK cells that are already licensed in the donor may become more potent effector cells when receiving additional licensing signals through host HLA molecules and might be able to mount a more vigorous attack, as postulated by the “tuning” model.
Fine tuning of licensing?
We have discussed licensing as an all or none phenomenon, but this might be related to the original experimental systems that were designed to robustly detect the effect of various factors on education. Under the tuning or rheostat model, NK cells acquire function in a graded manner depending on receptor—self-MHC interactions [64]. NK cells with more inhibitory receptors that are capable of interacting with different self-MHC class I alleles are functionally more responsive to activation receptor stimulation than NK cells with fewer self-MHC class I contacts. Accordingly, in mice with different numbers of MHC alleles [65], NK cell function is greater in hosts expressing multiple MHC class I alleles, indicating a positive correlation between expression of MHC I alleles and education or licensing of the NK cell population. In particular, for an individual NK cell, the more inhibitory receptors capable of binding host MHC I molecules the greater the NK cell effector function [66,67]. Therefore, it appears that there is a positive correlation between the number of inhibitory receptors contacting self-MHC and increased NK cell function.
The affinity between an NK cell receptor and its MHC I ligand also seems to impact NK cell licensing [68]. Studies of MHC-congenic mice revealed that Ly49A+ NK cells were differentially licensed, depending on the MHC haplotype expressed. This effect could be rank ordered and correlated with the degree to which the given MHC haplotype inhibited killing by Ly49A+ NK cells. The extent of effector inhibition by the MHC haplotype was greater than the licensing effect, suggesting licensing is less sensitive to receptor-MHC ligand interactions than effector inhibition. This is supported by experiments using a mutant form of Ly49A, which inhibited killing, but did not to license the NK cells [55]. These data suggest a protective effect whereby MHC alleles do not educate NK cells that cannot be inhibited, else autoreactivity would ensue.
These data indicate that licensing is not an “on or off” process. Rather, it appears to vary depending on signal strength. The licensing signal varies at the organism level with respect to the number of host MHC alleles, the cellular level with respect to the number of different NK cell receptors on a given cell interacting with host MHC, and at the receptor level with respect to apparent affinity for self-MHC.
Licensing has also been studied in the context of host heterozygosity or homozygosity for MHC alleles, which provides a gene dosage effect of potentially none, one, or two alleles for an NK cell receptor [24,68]. The data from in vitro assays of NK cell education indicate that a single self-MHC class I gene (as in a heterozygote) is sufficient for the full licensing effect for a given self-specific receptor in both mice and humans. These findings are somewhat surprising given the considerations above. However, epidemiological data indicate that homozygosity at both the KIR and HLA loci are needed to see an effect, such as resolution of hepatitis C virus infection [69]. These epidemiological findings are difficult to explain in terms of effector inhibition alone which would predict more inhibition, but is consistent with enhanced licensing. If so, however, the requirement for HLA homozygosity for enhanced licensing is at odds with licensing effects on NK cells where MHC effects due to heterozygosity are similar to homozygosity. These discrepancies are as yet unresolved and it is possible that the current in vitro licensing assays are not fully sensitive to MHC heterozygosity effects, or epidemiological studies may be showing the summation of multiple MHC effects, or indeed of other receptors, such as activation receptors [44].
Less is more?
While data supporting the gain-in-function component of the tuning hypothesis grow, other data [70] seem to dispute the theory that more inhibitory interactions increase the potential for NK cell function. In the context of viral infection, NK cells expressing inhibitory receptors for self, i.e., licensed NK cells, are less protective than unlicensed NK cells. Furthermore, licensed NK cells showed less robust proliferation following infection. Additionally, injection of licensed NK cells into neonates offered less protection from MCMV infection than injection of unlicensed NK cells. It was concluded that the benefits of licensing are negligible and offset by the liabilities of a cell population with restricted function.
It is important to consider that licensing accounts for self-tolerance and steady-state function of NK cells in a non-inflammatory situation and that conditions during viral infection are different from those in the uninfected environment [21]. When the licensing hypothesis was first proposed, it was noted that cytokine stimulation appeared to “override” the MHC-dependent effects of licensing, in that unlicensed NK cells showed enhanced activation receptor function following cytokine exposure [21]. Similar findings have been reported with human NK cells [71]. It was proposed that unlicensed cells might function in viral infection [21,31,71], a setting dominated by strong inflammatory cytokine production and generalized NK cell activation [72,73]. In this setting, a strict requirement for MHC-dependent education and subsequent inhibition by self-MHC might be overruled by the host’s need to quickly eradicate an infection, allowing unrestrained NK cells to attack [70]. In this case “education” (in terms of acquisition of function) would occur via an alternative pathway that does not involve MHC (Fig 2).
Loss of function: Tuning down or anergy?
When NK cells from an MHC-sufficient mouse are transferred into an MHC-deficient host they become hyporesponsive. These data suggest that licensing is reversible, and imply that a licensed NK cell in a WT mouse must continually sample the MHC class I environment to maintain its licensed or educated state [53]. In such a scenario, NK cell education is a dynamic process that occurs during the entire lifetime of the NK cell, rather than as a single event such as with positive selection of T cells.
Loss of MHC class I contact with a previously engaged inhibitory receptor can also lead to NK cell activation as opposed to a decrease in function. Cells that lack MHC class I expression are eliminated when injected into WT mice, as a result of host NK cell activation. Such data imply stimulation through an as yet unknown activation receptor on WT NK cells for a ligand expressed on MHC I-deficient cells, suggesting that transfer of WT NK cells into an MHC-deficient environment could lead to chronic stimulation and resultant anergy, as seen in mice ectopically expressing activation receptor ligands as already discussed [37–40]. Thus, the loss of WT NK cell function in an MHC-deficient environment could be due to loss of the education or licensing effect or due to anergy from chronic stimulation, among other possibilities. Current data do not permit discrimination among these possibilities.
Concluding remarks
Licensing, the education of NK cells by self-MHC, provides a unifying hypothesis that extends the missing-self hypothesis and also helps explain hybrid resistance. Recent data from a number of laboratories indicate that licensing effects vary in relation to the strength of the licensing signal. Moreover, MHC-dependent licensing effects on NK cell function can be bypassed depending on the context, such as viral infection.
While much progress has been made in understanding the role of MHC-specific receptors in NK cell education, there remain many unanswered questions. Primarily, the exact molecular mechanisms dictating licensing, including the identities of intracellular signaling molecules, as well as their targets, remain unknown. Additionally, the identification of a marker for licensed NK cells, other than a functional assay, would greatly aid research into the longitudinal aspects of their function. Much remains to be discovered for full understanding of the processes underlying NK cell education.
Acknowledgements
The authors thank members of the Yokoyama laboratory who have contributed to understanding NK cell function and differentiation over the years. Work in the Yokoyama laboratory is supported by grants from the NIH (AI33903, AI51345, AI57160) and the Howard Hughes Medical Institute.
Glossary
- Missing-self
Hypothesis to explain why NK cells are better able to attack cells lacking MHC class I molecules. NK cells survey tissues for normal levels of MHC class I molecules that are generally ubiquitously expressed. In the presence of normal target cell expression of MHC class I, NK cells do not attack, but NK cells can detect when MHC class I is down-regulated, such as in viral infection. NK cells can then attack MHC class I-deficient cells.
- Hybrid resistance
A phenomenon by which F1 hybrid mice reject parental bone marrow grafts, defying the classic laws of solid tissue transplantation. Described nearly 50 years ago, it is now recognized that hybrid resistance is mediated by host NK cells in an MHC-dependent manner.
- NK cell education
A broader definition of host effects on NK cell function which could which could include MHC-dependent (licensing) or MHC-independent effects.
- Licensing
MHC-dependent process that results in enhanced capacity of NK cell to be activated when stimulated through an activation receptor. Licensing is mediated by a self-MHC class I-specific inhibitory receptor through its ITIM and thus constitutes a second function for the MHC class I-specific receptors that were first described in terms of inhibiting effector functions, such as target killing.
- Arming
Hypothesis to explain the licensing effect. Postulates that self-MHC class I-specific receptor delivers signals necessary for licensed phenotype. Conceptually, the simplest model but it is difficult to reconcile with the known role of ITIM in effector inhibition.
- Disarming
Second hypothesis to explain the licensing effect. Proposes the existence of a self-specific activation receptor that, if unperturbed, leads to “anergic state” where NK cells are hyporesponsive. The role of self-MHC class I-specific receptors is to modulate this anergy. Conceptually, this can be reconciled with the traditional role of MHC class I-specific receptor in effector inhibition but it is more complicated than arming and requires identification of the self-specific activation receptor normally causing anergy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yokoyama WM. Natural killer cells. In: Paul WE, editor. Fundamental Immunology. 6th edn. Chapter16. Lippincott-Raven; 2008. pp. 483–517. [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5(5):363–374. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 4.Grundemann C, et al. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176(3):1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- 5.Ito M, et al. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203(2):289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasser S, et al. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudkowicz G, Stimpfling JH. Hybrid Resistance to Parental Marrow Grafts: Association with the K Region of H-2. Science. 1964;144:1339–1340. doi: 10.1126/science.144.3624.1339. [DOI] [PubMed] [Google Scholar]
- 11.Yu YYL, et al. Murine natural killer cells and marrow graft rejection. Annual Review of Immunology. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- 12.Öhlén C, et al. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989;246:666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- 13.Bix M, et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 14.Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunology Today. 1990;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 15.Hoglund P, et al. Recognition of beta 2-microglobulin- negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci (USA) 1991;88:10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa H, et al. Tolerance of NK and LAK activity for HLA class I deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I. Hum Immunol. 1999;60(1):32–40. doi: 10.1016/s0198-8859(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 17.Liao NS, et al. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 18.Valiante NM, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7(6):739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Held W, et al. The NK cell receptor repertoire: formation, adaptation and exploitation. Curr Opin Immunol. 2003;15(2):233–237. doi: 10.1016/s0952-7915(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 22.Orr MT, et al. Development and function of CD94-deficient natural killer cells. PLoS ONE. 2010;5(12):e15184. doi: 10.1371/journal.pone.0015184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105(8):3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sola C, et al. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc Natl Acad Sci U S A. 2009;106(31):12879–12884. doi: 10.1073/pnas.0901653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumperz JE, Parham P. The enigma of the natural killer cell. Nature. 1995;378(6554):245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- 27.Kelley J, et al. Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet. 2005;1(2):129–139. doi: 10.1371/journal.pgen.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogasawara K, et al. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6(9):938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beilke JN, et al. The requirement for NKG2D in NK cell-mediated rejection of parental bone marrow grafts is determined by MHC class I expressed by the graft recipient. Blood. 2010;116(24):5208–5216. doi: 10.1182/blood-2010-05-285031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity. 2006;24(3):249–257. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Murphy K, et al. Janeway's Immunobiology. Garland Science; 2008. [Google Scholar]
- 33.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adapter CRK. Immunity. 2008;29(4):578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura MC, et al. Mouse Ly-49D recognizes H-2Dd and activates natural killer cell cytotoxicity. J Exp Med. 1999;189(3):493–500. doi: 10.1084/jem.189.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta IK, et al. A "chimeric" C57L-derived Ly49 inhibitory receptor resembling the Ly49D activation receptor. Cell Immunol. 2001;209(1):29–41. doi: 10.1006/cimm.2001.1786. [DOI] [PubMed] [Google Scholar]
- 36.Eissmann P, et al. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105(12):4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- 37.Wiemann K, et al. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175(2):720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6(9):928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 39.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205(8):1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205(8):1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolanos FD, Tripathy SK. Activation receptor-induced tolerance of mature natural killer cells in vivo requires signaling through the receptor and is reversible. J Immuno. 2011 doi: 10.4049/jimmunol.1003046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orr MT, et al. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206(4):807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tassi I, et al. DAP10 associates with Ly49 receptors but contributes minimally to their expression and function in vivo. Eur J Immunol. 2009;39(4):1129–1135. doi: 10.1002/eji.200838972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fauriat C, et al. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115(6):1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 45.Tormo J, et al. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 1999;402(6762):623–631. doi: 10.1038/45170. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto N, et al. The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand. J Exp Med. 2001;193(2):147–158. doi: 10.1084/jem.193.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, et al. Binding of the natural killer cell inhibitory receptor Ly49A to its major histocompatibility complex class I ligand. Crucial contacts include both H-2Dd and beta 2-microglobulin. Journal of Biological Chemistry. 2002;277(2):1433–1442. doi: 10.1074/jbc.M110316200. [DOI] [PubMed] [Google Scholar]
- 48.Michaelsson J, et al. MHC Class I Recognition by NK Receptors in the Ly49 Family Is Strongly Influenced by the beta(2)-Microglobulin Subunit. J Immunol. 2001;166(12):7327–7334. doi: 10.4049/jimmunol.166.12.7327. [DOI] [PubMed] [Google Scholar]
- 49.Mitsuki M, et al. A species-specific determinant on beta2-microglobulin required for Ly49A recognition of its MHC class I ligand. Int Immunol. 2004;16(2):197–204. doi: 10.1093/intimm/dxh017. [DOI] [PubMed] [Google Scholar]
- 50.Choi T, et al. Ly49-dependent NK cell licensing and effector inhibition involve the same interaction site on MHC ligands. J Immunol. 2011;186(7):3911–3917. doi: 10.4049/jimmunol.1004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nature Immunol. 2002;3(6):523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 52.Elliott JM, et al. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207(10):2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joncker NT, et al. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207(10):2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8(4):269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalifour A, et al. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30(3):337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Carlin LM, et al. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194(10):1507–1517. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sjostrom A, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory ly49 receptors. J Exp Med. 2001;194(10):1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmer J, et al. H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: Implications for NK cell function. J Exp Med. 2001;194(10):1531–1539. doi: 10.1084/jem.194.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang QJ, et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS ONE. 2008;3(8):e3097. doi: 10.1371/journal.pone.0003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruggeri L, et al. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–218. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 61.Miller JS, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu KC, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller JS, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 64.Brodin P, et al. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30(4):143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Johansson S, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201(7):1145–1155. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brodin P, et al. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113(11):2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 67.Joncker NT, et al. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182(8):4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jonsson AH, et al. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J Immunol. 2010;184(7):3424–3432. doi: 10.4049/jimmunol.0904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 70.Orr MT, et al. 'Unlicensed' natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11(4):321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Juelke K, et al. Education of hyporesponsive NK cells by cytokines. Eur J Immunol. 2009;39(9):2548–2555. doi: 10.1002/eji.200939307. [DOI] [PubMed] [Google Scholar]
- 72.Wang LL, et al. Inducible expression of the gp49B inhibitory receptor on NK cells. J Immunol. 2000;164(10):5215–5220. doi: 10.4049/jimmunol.164.10.5215. [DOI] [PubMed] [Google Scholar]
- 73.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2(10):951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 74.Saleh A, et al. Identification of probabilistic transcriptional switches in the Ly49 gene cluster: a eukaryotic mechanism for selective gene activation. Immunity. 2004;21(1):55–66. doi: 10.1016/j.immuni.2004.06.005. [DOI] [PubMed] [Google Scholar]