Abstract
Purpose
To investigate the effects of male aging on semen quality, DNA fragmentation and chromosomal abnormalities in the spermatozoa of infertile patients and fertile men.
Methods
Semen samples of 140 infertile patients (24–76 years) and 50 men with proven fertility (25–65 years) were analyzed according to WHO guidelines. DNA fragmentation was detected by TUNEL assay, while aneuploidy was assessed by FISH.
Results
In the patient group, semen volume and vitality of spermatozoa decreased significantly with age, while sperm concentration showed a statistically significant increase with age. DNA fragmentation as well as disomy of sex chromosomes and disomy 8 did not show a statistically significant change with age. However, the diploidy rate was significantly increased with patient’s age. In the control group, conventional semen parameters as well as DNA fragmentation and chromosomal abnormalities did not show a statistically significant with age.
Conclusion
Increased age in infertile men is associated with an increase in sperm concentration and diploidy, as well as a decline in semen volume and sperm vitality. However motility, morphology and DNA fragmentation are not affected by male age.
Keywords: Aneuploidy, DNA fragmentation, Male infertility, Paternal age, Semen parameters
Introduction
While female fertility ends at the entrance into menopause around the age of 50, men generally do not experience an unavoidable and clear-cut cessation of reproductive capacity. The effects of male aging on semen quality, DNA fragmentation and chromosomal abnormalities in infertile patients and fertile donors were reported since 1970 but the results are conflicting.
Conventional semen analysis, including ejaculate volume, sperm concentration, motility, and morphology determined according to World Health Organisation (WHO) criteria, are the first step in the evaluation of male infertility. Among the basic semen parameters studied, semen volume, percentage of motile spermatozoa, and the percentage of normal morphology were more consistently reported to decrease with age. However, no consistent data confirm that sperm concentration also decline with advancing years [1, 2]. Semen analysis provides limited prognostic information on fertility, and recent studies indicated that the integrity of sperm DNA might be a more accurate and precise predictor of fertility [3] an intact DNA is necessary for the correct transmission of genetic materiel to the next generation. High levels of sperm DNA damage have been reported to affect fertility potential, increase the risk of recurrent miscarriages, decrease the chances of a successful implantation, and possibly lead to negative effects on the health of offspring [4, 5]. Sperm DNA integrity as assessed by terminal desoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) [6], sperm chromatin structure assay (SCSA) [7, 8], and Comet assay [9], were also shown to be compromised with advancing age. However, this notion was not supported by all studies [10–12], and the results varied according the technique used for the detection of DNA damage.
An important issue in the evaluation of age related alteration of male fertility is the frequency of hereditary disease in offspring. Whereas it is well established that women >35 years of age bear a higher risk of conceiving genetically abnormal offsprings [13, 14], a correlation with paternal age is still at issue. For instance, Sloter et al., have shown that advancing male age is associated with a gradual and significant increase in the risk of fathering children with various chromosomal defects on a total of 320 unselected patients consulting their IVF and Urological Center [15]. In contrast, Luetjens et al. [16] did not find a significantly higher risk of producing chromosomally abnormal offsprings for men of advanced age. These conflicting results can be explained by the heterogeneity of the selection subjects, the age groups and the methods of analysis. The study settings and populations are heterogeneous, with more than half based on infertility clinic or assisted conception populations, while the others used volunteers recruited from advertisements or sperm banks. In addition, men at older ages (e.g. >50 years) were under-represented in many of these clinical studies, which limited statistical power and prevented the determination of the shape of the relationship between age and semen quality and sperm DNA damage. Moreover, only few studies were controlled for abstinence time.
In order to give more accurate results, we have designed a prospective study to investigate the effects of male ageing on semen quality, DNA fragmentation and chromosomal abnormalities in the spermatozoa of infertile patients and fertile donors. Semen samples were controlled for abstinence time and older men are included in the present study.
Materials and methods
Patients
A total of 140 men consulting for infertility evaluation at our laboratory of Cytogenetic and Reproductive Biology, Farhat Hached University Teaching Hospital, Sousse (Tunisia) were included in this study. Furthermore, 50 healthy donors, with proven fertility, were also included in the study in order to evaluate possible differences between fertile and infertile men. All the patients and donors had no history of chemotherapy, radiotherapy, or chronic illness.
This protocol was approved by the local ethics committee and all patients and controls had previously given informed consent for the study.
Semen analysis
Semen samples were collected by masturbation in to sterile cups following 3 days of sexual abstinence and semen analysis was performed after semen liquefaction for 30 min at room temperature. Basic semen parameters (volume, vitality, concentration, and total motility) were assessed according to the World Health Organization guidelines [17]. Sperm concentration was determined with an improved Neubauer Hemacytometer® counting chamber. Sperm morphology was evaluated using the Diff-Quick staining method. At least 100 spermatozoa per patient were examined at a magnificent of X 100 according to the David classification [18].
Semen preparation for FISH analysis and TUNEL assay
An aliquot of the fresh semen was washed twice in Phosphate Buffered Saline (PBS, pH 7.4) and centrifuged at 400 g for 5 min. The sediment was then fixed in methanol/acetic acid (3: 1) for at least 30 min at 4°C. The fixed specimens were smeared on slides and stored at –20°C until further processing.
Measurement of DNA fragmentation
The presence of apoptosis-related DNA strand breaks in spermatozoa was evaluated by the terminal desoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) assay, using the ApopTag® Apoptosis Detection Kits (QBiogene, Paris, France) in controls and patients. For cell permeabilisation, slides were incubated in phosphate buffer saline (PBS) with a solution of 1% Triton X100 (Sigma). The procedure was carried out according to the manufacturer’s instructions. Briefly, the specimens were washed twice in PBS 1X, equilibrated with the equilibration buffer at room temperature for 10 s and incubated in a dark moist chamber at 37°C, for 1 h, with the Terminal Desoxynucleotidyl Transferase (TdT) solution in order to allow DNA elongation. After stopping the enzyme reaction, the slides were washed twice in PBS and the DNA elongation was revealed by incubation of the cells with anti-digoxigenin antibody coupled to peroxidase, during 30 min in a dark moist chamber. The peroxidase was revealed with DiAminoBenzidine (DAB). Slides were then counterstained with Harris’ haematoxylin (RAL, Martillac, France) and finally mounted using Faramount mounting (Dako, Carpinteria, CA, USA).
Controls were also included in every experiment: for the negative control TdT was omitted and we have include proteinase K digestion to control for nonspecific incorporation of nucleotides or for nonspecific binding of enzyme-conjugate, whereas positive controls were generated by incubating the sperm cells for 10 min at room temperature with DNase I.
Slides were observed under a microscope (Zeiss, Oberkochen, Germany) equipped with a 100 magnification lens. Spermatozoa with fragmented DNA had brown-colored nuclei, whereas the other cells were blue-gray (counter coloration with Harris’s haematoxylin). On each slide, approximately 500 cells were counted, and the percentage of spermatozoa with fragmented DNA (DFI) was calculated.
Aneuploidy analysis
Fluorescence in situ hybridization, which employs sequence-specific DNA probes incorporated with fluorescently labeled nucleotides, was carried out on each patient and control, using alpha centromeric probes for chromosomes 8, X and Y.
Sperm head decondensation
In order to render the sperm chromatin accessible to DNA probes, slides were incubated in NaOH 1N, at room temperature for 2 min. The slides were distilled water washed. Then, they were dehydrated through an ethanol series (70–90–100%) and air-dried.
DNA probes
The probe mixture for triple FISH consisted of a repetitive DNA sequence of centromeric probes for chromosome X (pDMX1) labeled fluorescein isothiocyanate (FITC), for chromosome Y (pLAY5.5) labeled Rhodamine and for chromosome 8 (pZ8.4) labeled FITC and Rhodamine. The probes were provided by the University of Bari (Bari, Italy).
The use of an autosomal probe, in addition to X and Y probes, allowed the distinction between disomy and diploidy.
Hybridization procedure
Slides were incubated in a denaturation solution of 70% formamide, 20X standard saline citrate (SSC) (pH 5,3) and distilled water at 72°C for 2 min. Slides were snap-cooled in 70% ethanol at –20°C for 2 min and then dehydrated through an ethanol series (90–100%) at room temperature. The probes, precipitated and denatured at 72°C for 8 min, were applied directly to the slides which were then covered with a cover slip and sealed with rubber cement. Slides were hybridized for 2 h in a dark humidified container at 37°C. Finally slides were washed in 1× SSC, counterstained with 4′,6-diamidino-2-pheneylindole (DAPI) and stored in the dark at 4°C prior to carrying out microscopic observation.
Scoring criteria
The slides were observed using an Axioplan epifluorescence microscope (Leica, Wetzlar, Germany) with the appropriate set of filters: single band DAPI, FITC, and Rhodamine. For each probe a minimum of 500 spermatozoa were counted per patient. Only intact spermatozoa bearing a similar degree of decondensation and clear hybridization signals were scored; disrupted or overlapping spermatozoa were excluded from analysis.
Statistical analysis
Statistical analysis was performed using SPSS.13 (SPSS, Chicago, IL, USA). All variables were initially tested in order to determine variance homogeneity and data normality. Data are represented as Mean±Standard deviation (SD). Group comparisons were made using student’s t-test. Pearson’s correlation was performed to examine the relationship between paternal age and standard semen parameters, DNA fragmentation and aneuploidy rate. All hypothesis testing were two sided with a probability value of 0.05 deemed as significant.
Results
Characteristics of study population
A total of 140 semen samples from infertile patients were analysed regarding the semen parameters, DNA fragmentation, and aneuploidy. Subject’s ages ranged from 24 to 76 years. The mean age was 37.65 years.
56.83% of the subjects were between 30 and 40 years old; 7.91 % were <30 years and 35.25 % were >40 years old. The results of our study were allocated to four age groups:
Younger than 30 years (27.09 years in average; n = 11)
30–39 years (34.61 years in average; n = 79)
40–49 years (42.61 years in average; n = 40)
≥50 years (54.50 years in average; n = 10)
For control group, subjects ranged in age from 25 to 65 years with a mean of 37.34 years. Also they were distributed into four groups:
Younger than 30 years (27.12 years in average; n = 10)
30–39 years (35.04 years in average; n = 16)
40-49 years (43.50 years in average; n = 14)
≥50 years (56.50 years in average; n = 10)
Effects of age on semen parameters
The results of the basic semen parameters according to male age were presented in Table 1. For infertile patients, we show that semen volume, vitality, total motility, and percentage of normal morphology decline with advancing age. Only sperm concentration increases with advancing age. However no statistical significance was demonstrable between any of the age groups of the patients (p > 0.05). In addition total sperm count did not significantly differ with age group (p > 0.05).
Table 1.
Descriptive statistics and comparisons of conventional semen parameters between the four age subgroups in both fertile and infertile men
| Age group (Years) | Volume (ml) | Concentration (X 106/ml) | Total motility (%) | Abnormal forms (%) | Necrozoospermia (%) |
|---|---|---|---|---|---|
| Fertile men | |||||
| 20–29 (n = 10) | 2.83 ± 0.92 | 95.75 ± 22.93 | 53.75 ± 4.43 | 58.38 ± 9.56 | 22.28 ± 05.70 |
| 30–39 (n = 16) | 2.87 ± 0.53 | 93.07 ± 29.46 | 51.33 ± 6.81 | 55.78 ± 7.26 | 25.06 ± 3.30 |
| 40–49 (n = 14) | 2.48 ± 0.31 | 100 ± 46.20 | 48.12 ± 5.12 | 57.66 ± 7.39 | 21.36 ± 2.80 |
| 50–70 (n = 10) | 2.06 ± 0.11 | 99.12 ± 31.34 | 47.56 ± 3.53 | 58.12 ± 10.94 | 24.35 ± 3.67 |
| Infertile men | |||||
| 20–29 (n = 11) | 3.26 ± 1.63 | 62.09 ± 40.92 | 26.36 ± 17.04 | 74.18 ± 13.33 | 32.82 ± 25.90 |
| 30–39 (n = 79) | 3.31 ± 1.58 | 76.07 ± 59.86 | 29.00 ± 15.84 | 79.22 ± 17.26 | 31.06 ± 19.00 |
| 40–49 (n = 40) | 2.08 ± 1.31 | 97.97 ± 86.13 | 25.54 ± 14.29 | 81.66 ± 17.39 | 36.76 ± 21.56 |
| 50–70 (n = 10) | 2.46 ± 1.11 | 98.12 ± 61.34 | 22.00 ± 17.43 | 85.12 ± 10.94 | 52.62 ± 30.38 |
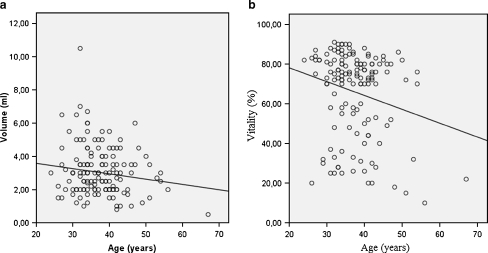
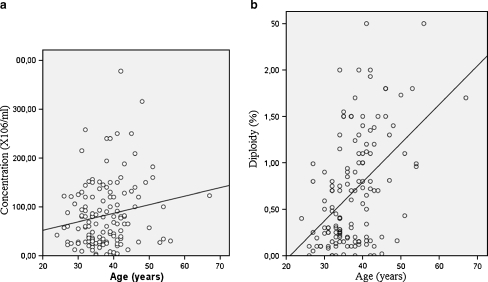
Using Pearson’s correlation test, we didn’t found a statistically significant relationship between age, and percent motility (r = −0.088; p = 0.305), percent normal morphology (r = 0.026; p = 0.765), and total sperm count (r = 0.025; p = 0.768). However a significantly inverse relationship was shown between patient’s age, semen volume (r = −0.183; p = 0.032), and vitality (r = −0.219; p = 0.01) (Fig. 1). Sperm concentration was significantly and positively associated with male age (r = 0.196; p = 0.021) (Fig. 2a).
Fig. 1.
Scatter graph illustrating the negative associations between age and semen volume (a; r = −0.183; p = 0.032) as well as age and vitality of spermatozoa (b; r = −0.219; p = 0.01)
Fig. 2.
Scatter graph illustrating the positive associations between age and sperm concentration (a; r = 0.196; p = 0.021) as well as age and diploidy rate (b;r = 0.544; p = 0.01)
For the controls, semen volume, sperm concentration, total sperm count, morphology and progressive motility did not differ significantly between the four age groups. In addition, these conventional semen parameters did not show a statistically significant correlation with age (p > 0.05).
Effects of age on DNA fragmentation
The TUNEL assay results stratified by male age were presented in Table 2.
Table 2.
Descriptive statistics and comparisons of DNA fragmentation and chromosomal abnormalities between the four age subgroups in both fertile and infertile men
| Age group | DFI (%) | Disomy X (%) | Disomy Y (%) | Disomy XY (%) | Disomy 8 (%) | Diploidy (%) |
|---|---|---|---|---|---|---|
| Fertile men | ||||||
| 20–29 (n = 10) | 10.50 ± 1.41 | 0.10 ± 0.01 | 0.33 ± 0.11 | 0.48 ± 0.03 | 0.13 ± 0.05 | 0.09 ± 0.06 |
| 30–39 (n = 16) | 10.37 ± 4.39 | 0.15 ± 0.03 | 0.39 ± 0.12 | 0.54 ± 0.19 | 0.16 ± 0.06 | 0.14 ± 0.09 |
| 40–49 (n = 14) | 9.93 ± 4.07 | 0.17 ± 0.04 | 0.35 ± 0.10 | 0.52 ± 0.16 | 0.17 ± 0.10 | 0.12 ± 0.06 |
| 50–70 (n = 10) | 9.00 ± 5.65 | 0.14 ± 0.06 | 0.46 ± 0.15 | 0.55 ± 0.21 | 0.14 ± 0.15 | 0.13 ± 0.04 |
| Infertile men | ||||||
| 20–29 (n = 11) | 26.23 ± 13.49 | 0.36 ± 0.30 | 1.08 ± 0.59 | 1.62 ± 1.02 | 0.47 ± 0.34 | 0.38 ± 0.37 |
| 30–39 (n = 79) | 27.66 ± 15.84 | 0.38 ± 0.31 | 1.09 ± 0.75 | 1.75 ± 1.07 | 0.69 ± 0.83 | 0.44 ± 0.51 |
| 40–49 (n = 40) | 30.42 ± 16.85 | 0.44 ± 0.30 | 1.37 ± 0.85 | 1.96 ± 1.16 | 0.68 ± 0.52 | 0.61 ± 0.58 |
| 50–70 (n = 10) | 31.62 ± 18.01 | 0.58 ± 0.18 | 1.22 ± 0.31 | 1.98 ± 0.55 | 0.61 ± 0.16 | 1.02 ± 0.53 |
In infertile patients there was a trend for increased levels of DNA fragmentation with advancing age but these results were not statistically significant (p > 0.05). No correlation was found between male age and level of DNA fragmentation (r = 0.08; p > 0.05).
In the controls there was no statistically significant difference between the four age groups in the percentages of cells with fragmented DNA. In addition using the Pearson rank correlation coefficient we did not find a significantly correlation (r = −0.094; p = 0.516).
Effects of age on aneuploidy rate
The results of aneuploidy frequencies for chromosomes 8, X, and Y stratified by male age are also presented in Table 2. Only sperm diploidy was significantly different among patients groups, with an obvious increase with advancing age (0.38 % in men younger than 30 years versus 1.02 % in patients 50 years and older; p < 0.01).
A Pearson correlation coefficient was employed in order to study the relationship between the patient’s age and the various abnormality frequencies. There was no correlation of male age with the disomy for chromosomes 8, X, Y, and XY (p > 0.05). However, the diploidy rate was significantly increased with patient’s age (r = 0.544; p = 0.01). This association between age and diploidy was presented in Fig. 2b.
For fertile men, there was no correlation of donor age with the frequency of X- or Y- bearing sperm, disomy for chromosomes 8, X, Y, XY, or diploidy (p > 0.05).
Discussion
Many studies have analysed age-dependant variations of sperm parameters but only few were controlled for abstinence time. In addition, the selection of subjects, the age groups (rarely include males >60 years), and the methods of analysis are heterogeneous and the results are conflicting. The particularity of the present study is that semen samples were controlled for abstinence time (after 3 days of abstinence) and older men were also included in this study. Our results found that increased age of infertile men is associated with a decrease in semen volume, a decrease in sperm vitality, and an increase in sperm concentration. Sperm motility and percent normal sperm morphology decrease with age but this decline was not statistically significant. Classical parameters of spermiograms did not change significantly in over 800 fertile men between 21 and 50 years of age. Unfortunately, elderly men were not included in that particular study [19]. However Levitas et al. detected a statistically significant and inverse relationship between semen volume, sperm quality, and patient age, but the patients had a longer period of sexual abstinence before the testing [20]. So we show that potential confounders that might explain changes with age, such as duration of abstinence and age groups, could change result totally. The methodologically superior studies suggest that in general the semen volume [21–23], the percentage of motile sperm cells [21, 23] and the percentage of the sperm cells with normal morphology [23–25] decline with age. However, no consistent data confirm that sperm concentration also decline with advancing years. Abstinence adjusted studies do not provide a uniform picture. A significant age–dependent decrease [2, 25] as well as constant values over the age range [19] or even a non significant age dependent increase with age [26] have been detected in healthy men. In infertile patient’s sperm concentration remains unalterated [22] or increases [21, 23, 26–29]. In our study we had found that sperm concentration increases with age in infertile men but remains constant in fertile men. For total sperm count we show that it did not significantly differ with age group of fertile and infertile men. Few studies have analyzed the total sperm count and this decreases with age in fertile men [2] but remains constant in infertile men [21].
Age may not only impact semen quality, but also the genetic integrity of the sperm. Sperm DNA damage has been attributed to a variety of intra- and extra-testicular factors. Probably the most important are the improper packaging and ligation during sperm maturation [30, 31], the production of reactive oxygen species by oxidative stress [32, 33] and the defective apoptosis before ejaculation [33, 34]. However the impact of age on sperm DNA fragmentation is still an unsolved question. Several techniques are currently available that assess sperm DNA damage directly or indirectly by evaluating sperm chromatin compaction. The most common tests are SCSA, COMET, and TUNEL assays [31]. Using the TUNEL assay, we did not found a significant association between the paternal age and the levels of DNA fragmentation in infertile and fertile men. Using the same technique, our findings are in accordance with the data of Colin et al. [10] and in discordance with the results of Vagnini et al. [6] and Plastira et al. [29] who reported that male aging affects the chromatin integrity of spermatozoa, but only in infertile population. Using a different assay for measuring DNA strand damage in sperm, the SCSA which measures the susceptibility of sperm DNA to in situ acid induced denaturation, Spano et al. [7] found a strong association of DNA fragmentation index with age among men 18–55 years olds. Similar trends were found by others investigators [8, 35–38]. In contrast, Schmid et al. [11] using the same methods, reported that male age only influences single strand breaks and age was not associated with sperm DNA damage under neutral conditions, which is thought to represent double strand DNA breaks. Using a modified Nicoletti assay, Winkle et al. [12] suggest that the amount of spermatozoa with fragmented DNA is not affected by male age. So we show that the results of literature varied according the technique used, but the majority of studies show a positive relationship between age and DNA damage. The limited sample size analyzed in our study, compared to other studies relating paternal age and sperm DNA damage, may explain the lack of significant association of DNA fragmentation and male age.
Initial attempts to relate paternal age and chromosomes abnormalities were only carried out when the interspecies human/hamster fertilization system became available. The results were controversial in part because there were few analyzable metaphases, and also because the oldest men studies were just 44 years of age. With the advent of FISH, it became possible to analyze a much larger number of sperm. Studies which analyzed the age–dependent alteration of aneuploidy frequency in chromosomes are severely limited due to low case numbers. So far no age effect has been found for aneuploidies in chromosomes 6, 8, 12, 13, 14 or 18 [16] and varying results for chromosomes 1, 9, 21, X, and Y. Using a triple color FISH X-Y-8, we did not find a paternal age effect on sex chromosomes disomy (XX, YY, XY) in infertile patients and in donors. Our results are in accordance with the data of Bosch et al. [39, 40] and Luetjens et al. [16], but in discordance with others studies which found an effect of age on the production of disomic sex chromosomes with varying results for XX, YY, XY [41–47].
A statistically significant tendency to a linear increase of diploidy with age was observed for our infertile men. The same result was shown by Bosch et al. [39, 40]. However Martin et al. did not support this correlation between paternal age and diploidy [43].
In conclusion our study demonstrated that increased age in infertile men is associated with an increase in sperm concentration and diploidy rate, as well as a decline in semen volume, and sperm vitality. Moreover we had found that sperm DNA fragmentation as well as disomy X, Y, XY, and 8 were not affected by infertile male age. However in the control group, conventional semen parameters in addition to DNA fragmentation and chromosomal abnormalities did not show a statistically change with age.
Footnotes
Capsule
Increased age in infertile men is associated with an increase in sperm concentration and diploidy, as well as a decline in semen volume and sperm vitality.
References
- 1.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–248. doi: 10.1016/S0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 2.Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–454. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 3.Lewis SE, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322:33–41. doi: 10.1007/s00441-005-1097-5. [DOI] [PubMed] [Google Scholar]
- 4.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, François Guerin J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 5.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, Giwercman A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–179. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 6.Vagnini L, Baruffi RL, Mauri AL, Petersen CG, Massaro FC, Pontes A, Oliveira JB, Franco JG. The effects of male age on sperm DNA damage in an infertile population. Reprod Biomed Online. 2007;15:514–519. doi: 10.1016/S1472-6483(10)60382-3. [DOI] [PubMed] [Google Scholar]
- 7.Spano M, Kolstad AH, Larsen SB, Cordelli E, Leter G, Giwercman A, Bonde JP. The applicability of the flow cytometric sperm chromatin structure assay in epidemiological studies. Hum Reprod. 1998;13:2495–2505. doi: 10.1093/humrep/13.9.2495. [DOI] [PubMed] [Google Scholar]
- 8.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS, Evenson D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci USA. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) its relationship fertilization embryo development. Hum Reprod. 2002;17:990–998. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 10.Colin A, Barroso G, Gomez-Lopez N, Duran EH, Oehninger S. The effect of age on the expression of apoptosis biomarkers in human spermatozoa. Fertil Steril 2010 (in press). [DOI] [PubMed]
- 11.Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, Anderson D, Wyrobek AJ. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 12.Winkle T, Rosenbusch B, Gagsteiger F, Paiss T, Zoller N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J Assist Reprod Genet. 2009;26:41–46. doi: 10.1007/s10815-008-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hook EB, Schreinemachers DM, Willey AM, Cross PK. Rates of mutant structural chromosome rearrangements in human foetuses: data from prenatal cytogenetic studies and associations with maternal age and parental mutagen exposure. Am J Hum Genet. 1983;35:96–109. [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolaidis P, Petersen MB. Origin and mechanisms of nondisjunction in human autosomal trisomies. Hum Reprod. 1998;13:313–319. doi: 10.1093/humrep/13.2.313. [DOI] [PubMed] [Google Scholar]
- 15.Sloter ED, Marchetti F, Eskenazi B, Weldon RH, Nath J, Cabreros D, Wyrobek AJ. Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertil Steril. 2007;87:1077–1086. doi: 10.1016/j.fertnstert.2006.08.112. [DOI] [PubMed] [Google Scholar]
- 16.Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod. 2002;17:1826–1832. doi: 10.1093/humrep/17.7.1826. [DOI] [PubMed] [Google Scholar]
- 17.World Health O. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge, UK, Published on behalf of the World Health Organization by Cambridge University Press; 1999.
- 18.David G. Editorial: sperm banks in France. Arch Fr Pediatr. 1975;5:401–404. [PubMed] [Google Scholar]
- 19.Schwartz D, Mayaux MJ, Spria A, Moscato ML, Jouannet P, Czyglik F, David G. Semen characteristics as a function of age in 833 fertile men. Fertil Steril. 1983;39:530–535. doi: 10.1016/s0015-0282(16)46946-3. [DOI] [PubMed] [Google Scholar]
- 20.Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6, 022 semen samples. Andrologia. 2007;39:45–50. doi: 10.1111/j.1439-0272.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 21.Rolf C, Behre HM, Nieschlag E. Reproductive parameters of older couples compared to younger men of infertile couples. Int J Androl. 1996;19:135–142. doi: 10.1111/j.1365-2605.1996.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 22.Rolf C, Kenkel S, Nieschlag E. Age-related disease pattern in infertile men: increasing incidence of infections in older patients. Andrologia. 2002;34:209–217. doi: 10.1046/j.1439-0272.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 23.Andolz P, Bielsa MA, Vila J. Evolution of semen quality in northeastern Spain: a study in 22 759 infertile men over a 36 year period. Hum Reprod. 1999;14:731–735. doi: 10.1093/humrep/14.3.731. [DOI] [PubMed] [Google Scholar]
- 24.Mladenovic I, Micic S, Papic N, Genbacev O, Marinkovic B. Sperm morphology and motility in different age populations. Arch Androl. 1994;32:197–205. doi: 10.3109/01485019408987786. [DOI] [PubMed] [Google Scholar]
- 25.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 26.Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analysis in 1, 283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–1014. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 27.Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. BMJ. 1996;312:471–472. doi: 10.1136/bmj.312.7029.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irvine S, Cawood E, Richardson D, MacDonald E, Aitken J. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. BMJ. 1996;312:467–471. doi: 10.1136/bmj.312.7029.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plastira K, Msaouel P, Angelopoulou R, Zanioti K, Plastiras A, Pothos A, Bolaris S, Paparisteidis N, Mantas D. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–443. doi: 10.1007/s10815-007-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sailer BL, Jost LK, Evenson DP. Mammalian sperm DNA susceptibility to in situ denaturation associated with the presence of DNA strand breaks as measured by the terminal desoxynucleotidyl transferase assay. J Androl. 1995;16:80–87. [PubMed] [Google Scholar]
- 31.Zini A, Libman J. Sperm DNA damage: clinical significance in the era of assisted reproduction. CMAJ. 2006;175:495–500. doi: 10.1503/cmaj.060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal A, Saleh RA. Utility of oxidative stress test in the male infertility clinic. Zhonghua Nan Ke Xue. 2002;8:1–9. [PubMed] [Google Scholar]
- 33.O’Brien J, Zini A. Sperm DNA integrity and male infertility. Urology. 2005;65:16–22. doi: 10.1016/j.urology.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–37. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 35.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Sati L, Ovari L, Bennett D, Simon SD, Demir R, Huszar G. Double probing of human spermatozoa for persistent histones, surplus cytoplasm, apoptosis and DNA fragmentation. Reprod Biomed Online. 2008;16:570–579. doi: 10.1016/S1472-6483(10)60464-6. [DOI] [PubMed] [Google Scholar]
- 37.Moskovtsev SI, Willis J, Mullen JB. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril. 2006;85:496–499. doi: 10.1016/j.fertnstert.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 38.Moskovtsev SI, Willis J, White J, Mullen JB. Sperm DNA damage: correlation to severity of semen abnormalities. Urology. 2009;74:789–793. doi: 10.1016/j.urology.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 39.Bosch M, Rajmil O, Martinez-Pasarell O, Egozcue J, Templado C. Linear increase of diploidy in human sperm with age: a four-colour FISH study. Eur J Hum Genet. 2001;9:533–538. doi: 10.1038/sj.ejhg.5200659. [DOI] [PubMed] [Google Scholar]
- 40.Bosch M, Rajmil O, Egozcue J, Templado C. Linear increase of structural and numerical chromosome 9 abnormalities in human sperm regarding age. Eur J Hum Genet. 2003;11:754–759. doi: 10.1038/sj.ejhg.5201049. [DOI] [PubMed] [Google Scholar]
- 41.Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ. Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Gen. 1995;4:2227–2232. doi: 10.1093/hmg/4.12.2227. [DOI] [PubMed] [Google Scholar]
- 42.Robbins WA, Baulch JE, Moore DI, Weire HU, Blakey D, Wyrobek AJ. Three-probe fluorescence in situ hybridization to assess chromosome X, Y, and 8 aneuploidy in sperm of 14 men from two healthy groups: evidence for a paternal age effect on sperm aneuploidy. Reprod Fertil Dev. 1995;7:799–809. doi: 10.1071/RD9950799. [DOI] [PubMed] [Google Scholar]
- 43.Martin RH, Spriggs E, Ko E, Rademaker AW. The relationship between paternal age, sex ratios, and aneuploidy frequencies in human sperm, as assessed by multicolor FISH. Am J Hum Gen. 1995;57:1395–1399. [PMC free article] [PubMed] [Google Scholar]
- 44.Kinakin B, Rademaker A, Martin R. Paternal age effect of YY aneuploidy in human sperm, as assessed by fluorescence in situ hybridization. Cytogenet Cell Genet. 1997;78:116–119. doi: 10.1159/000134641. [DOI] [PubMed] [Google Scholar]
- 45.Asada H, Sueoka K, Hashiba T, Kuroshima M, Kobayashi N, Yoshimura Y. The effects of age and abnormal sperm count on the nondisjunction of spermatozoa. J Assist Reprod Genet. 2000;17:51–59. doi: 10.1023/A:1009454114973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guttenbach M, Kohn FM, Engel W, Schmid M. Meiotic nondisjunction of chromosomes 1, 17, 18, X, and Y in men more than 80 years of age. Biol Reprod. 2000;63:1727–1729. doi: 10.1095/biolreprod63.6.1727. [DOI] [PubMed] [Google Scholar]
- 47.Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, Wyrobek AJ. Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet. 2001;69:1046–1054. doi: 10.1086/323763. [DOI] [PMC free article] [PubMed] [Google Scholar]