Abstract
Purpose
To compare the efficacy of vitrification and conventional freezing of whole ovaries.
Methods
Ovaries obtained from 5-year-old female bovines were cryopreserved by conventional freezing, rapid freezing and vitrification. The ovarian cortical strips were cryopreserved by conventional freezing. Follicular viability was assessed using the trypan blue test; the percentage of morphologically normal primordial follicles, hormones concentrations in the culture supernatants, and lactate dehyrogenase levels were measured.
Results
The efficacy of cryopreservation of whole ovaries by vitrification was higher than those by conventional freezing and rapid freezing. Conventional freezing of ovarian cortical strips was more effective than cryopreservation of whole ovaries by conventional freezing, rapid freezing, and vitrification.
Conclusions
Vitrification seems to be more suitable than conventional freezing for cryopreservation of whole ovaries. However, further studies are required to improve the efficacy of vitrifying whole ovaries.
Keywords: Conventional freezing, Cryopreservation, Rapid freezing, Vitrification, Whole ovaries
Introduction
Advances in cancer therapy have enabled long-term remission and even cure of many cancers. Because germ cells are particularly susceptible to cytotoxic treatments (especially alkylating agents and radiation), ovarian failure and infertility are common complications that can have an impact on the quality of life for young survivors of cancer [1, 2]. Surgical removal of ovarian tissue, followed by cryostorage and later autotransplantation, offers the possibility to circumvent gonadotoxic injury.
The retransplanted ovarian cortical tissues typically start to show signs of spontaneous estradiol production after 2–8 months, but cessation of the function is seen in most cases within 6–9 months after retransplantation [3–8]. The limited amount of tissue in combination with the extended ischemic period may explain the short lifespan of the graft. In a study of sheep ovarian tissue, it was found that 60–70% of follicles were lost after transplantation but only 7% of the loss was dependent on the cryopreservation procedure itself [9]. Thus, the major loss seems to occur during the warm ischemic period, probably extending over several days until neovascularization has restored the blood flow to the grafted tissue.
Cryopreservation of a whole ovary followed by retransplantation with vascular anastomosis has been suggested as a method to decrease ischemic injury and thereby to increase survival time and gain long-term ovarian cyclicity [10–12]. Successful cryopreservation and autotransplantation of whole ovaries have been achieved in a number of experimental animal species [13–16]. Despite these encouraging results, however, dramatic depletion of follicular density was observed in transplanted frozen-thawed grafts. Imhof et al. reported massive follicular depletion with less than an 8% follicular survival rate [17]. Other authors reported only 6% of viable follicles and the depletion of the entire follicular population after frozen ovarian grafts [18]. Taken together, these studies of whole ovary cryopreservation in sheep illustrate the difficulties of this method.
Cryopreserving a large-sized intact ovary is problematic because of the difficulty of adequate diffusion of cryoprotective agents into large tissue masses. Another challenge of freezing whole ovaries is related to heat transfer in such large-sized organ [12]. Imperfect freezing technologies for bulky organs can be a big barrier of whole ovary transplantation. The protocol of cryopreserving whole ovaries should be optimized before the procedure can be of clinical significance.
There are 2 main methods of cryopreserving biologic tissue: either classic “slow” freezing or vitrification by rapid cooling. Slow cooling uses an optimal cooling rate specific to a given cell to avoid producing intracellular ice crystals. Vitrification is an alternative approach to cryostorage. At a sufficiently low temperature, a highly concentrated aqueous cryoprotectant solution becomes so viscose that it solidifies without the formation of ice crystals. In 1985, Rall and Fahy reported the first ice–free cryopreservation of mouse embryos by vitrification [19]. Until now, vitrification has been adopted to cryopreserve embryos, oocytes and ovarian tissue. Studies that compare the effectiveness of vitrification and conventional freezing of oocytes, embryos and ovarian tissue have been performed [20–23]. However, which method is more suitable for cryopreservation of a complex and heterogeneous system such as an intact ovary: vitrification or conventional freezing? There is no consensus on this question. The aim of this study was therefore to compare the effectiveness of vitrification with conventional freezing of whole ovaries.
Materials and methods
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated.
This study was approved by the Animal Research Ethical Committee of the Shandong University. We collected ovaries with ovarian pedicle from on average 5-year-old bovines at the Shandong Hypor Liuhe Breeding Farm. All the selected ovaries were in follicular phase and the ovarian vessels were cut so as to be as long as possible. Ovaries were perfused with heparinized (100 IU ml−1) Ringer,s solution immediately after removal from bovines, and ovaries and vessels were transported to the laboratory at 10°C to minimize ischemia.
Twenty-five ovaries were randomly assigned to control groups and experimental groups. The experimental groups included: group A, group B, group C, and group D.
Freezing/thawing or warming procedures
Slow-freezing of ovarian cortical tissue (group A)
The procedure was carried out according to the protocol described by Gosden and colleagues [24]. Cortical strips from ovaries were cut into 1 × 1 × 1 mm slices free of fat and mesentery. The ovarian strips were placed into 2-mL cryotubes (Corning Coaster Corporation, Cambridge, MA, USA) filled with a cryoprotective mixture. The cryoprotective mixture contained modified phosphate-buffered saline (DPBS) medium, 1.5 mmol/L dimethyl sulfoxide (DMSO) and 15% heated-inactivated fetal calf serum (FCS) (Sijiqing Co., Hangzhou, China). The cryotubes were cooled in a programmable freezer (Kryo 10, Series III; Planer, Sunbury-on-Thames, UK) with the following program: (1) cooled from 0°C to 8°C at −2/min; (2) seeded manually by touching the cryotubes with forceps prechilled in liquid nitrogen; (3) cooled to −40°C at −0.3°C/min; (4) cooled to −150°C at −30°C/min, and (5) transferred to liquid nitrogen (−196°C) immediately for storage.
Thawing of ovarian cortical tissue One week later, the cryotubes were removed from the dewar and held at room temperature for 2 min before plunging in a water bath at 37°C for another 2 min with gentle shaking. The contents of the cryotubes were quickly emptied into culture dishes (Becton Dickinson, NY, USA) with Leibovitz L-15 medium and washed 3 times with fresh medium to remove cryoprotectant before further processing.
Slow-freezing of whole ovaries (group B)
1. Ovarian Perfusion. A cannule (18 GA 1.77, 1.3 × 35 mm, Venflon) was inserted into the vessel and secured with sutures. The cannule was connected with the perfusion hiose (Braun, Melsungen, Germany) to a peristaltic STC-521 Seringe pump (Terufusion, Tokyo, Japan). The perfusion rate was set at 2.5 mL/min. 2. Controlled-rate freezing. The procedure was carried out according to the protocol described by Martinez-Madrid and colleagues [12]. The ovaries were perfused via the ovarian artery with the cryoprotective mixture for 15 min at 4°C. The cryoprotective mixture contained DPBS medium, 10% (v/v) DMSO, and 15% FCS. After perfusion, the 30 ml cryogenic vial (Scientific Laboratory supplies, Nottingham, UK) containing the ovary was placed in a 5100 Cryo 1°C freezing Container (nalgen, VWR, Belgium) precooled at 4°C, and deposited in a −80°C freezer. This confers a cooling rate of −1°C/min. After 24 h in the −80°C freezer, the cryovial containing the ovary was transferred to liquid nitrogen. 3. Thawing of ovaries. One week later, the vials were removed from the dewar and held at room temperature for 2 min before plunging in a water bath at 37°C for another 2 min with gentle shaking. To remove the cryoprotectant, the ovaries were bathed and perfused in 3 steps of 10 min each at room temperature with a reversed sucrose concentration gradient (0.25 M, 0.5 M and 1.0 M sucrose) in Leibovitz L-15 medium to prevent osmotic injuries. The perfusion rate was 2.5 ml/min.
Rapid freezing of whole ovaries (group C)
The ovaries were perfused via the ovarian artery with the cryoprotective mixture. The perfusion rate was set at 2.5 mL/min. The cryoprotective mixture contained DPBS medium, 15% (v/v) DMSO, 15% (v/v) propylene glycol (PROH) and 15% FCS. To ensure osmotic balance, the cryoprotective mixture was introduced in 2 steps of increasing concentration: 50% for 10 min at 4°C, and 100% for 10 min at 4°C. After perfusion, the ovaries were transferred into liquid-nitrogen-proof ethyl vinyl acetate cryobags (Corning Coaster Corporation, Cambridge, MA, USA) containing the cryoprotective mixture. The ovaries were vitrified by plunging the bags into liquid nitrogen.
Thawing of ovaries The procedure was identical to that of group B.
Vitrification protocol for whole ovaries (group D)
The ovaries were perfused via the ovarian artery with the cryoprotective mixture. The perfusion rate was set at 2.5 mL/min. The cryoprotective mixture contained DPBS medium, 25% (v/v) DMSO, 25% (v/v) PROH and 15% FCS. To ensure osmotic balance, the cryoprotective mixture was introduced in two steps of increasing concentration: 50% for 15 min at 4°C, and 100% for 15 min at 4°C. After perfusion, the ovaries were transferred into liquid-nitrogen-proof ethyl vinyl acetate cryobags containing the cryoprotective mixture. The ovaries were vitrified by plunging the bags into liquid nitrogen.
Warming of ovaries The procedure was identical to that of group B.
Evaluation of ovarian follicular viability in the ovarian tissue
The cryopreserved thawed ovarian tissue was evaluated for follicular viability in term of plasma membrane function and structural integrity by trypan blue exclusion test. Ovarian fragments were thinly sectioned in Leibovitz L-15 medium supplemented with 1 mg/mL (200 IU/ml) type 1 collagenase, incubated at 37°C for 2 h and pipetted every 30 min. Collagenase activity was inhibited by 50% FCS. The suspension was filtered through a 70-μm nylon filter (Becton Dickinson Labware, Frankline Lakes, NJ, USA) and centrifuged at 400 g for 5 min. The precipitate was diluted with 50 μl of Leibovitz L-15 medium and kept in a water bath at 37°C. Twenty μl of 0.4% trypan blue was added to the suspension containing the follicles, deposited on a glass slide and examined under inverted microscope. For each group, hundred follicles were examinated. The percentages of viable follicles were determined by calculating the percentage of unstained cells.
Histological examination
Ovaries were fixed in Bouin,s solution for light microscopic evaluation. Serial 5-um sections were prepared; every 10th section of each ovary was mounted on a glass slide, and stained with hematoxylin and eosin.
Follicular morphology was examined by microscope (magnification, ×400). For each ovary, 100 primordial follicles were counted in sections where the oocyte nucleus was visible, and their morphology was recorded. Normal follicles had a complete layer of flattened pregranulosa cells, oocytes with cytoplasm, and a normal nucleus. Abnormal follicles were classified as follows: pyknotic nucleus, and nucleus and cytoplasm damage combined.
Culture of frozen ovarian tissue
An in vitro culture system was used as described by Scott and colleagues [25]. The strips from the thawed ovaries were immersed in the base medium, cut into small pieces (1 × 1 × 1 mm), and placed into 24-well culture dishes (Corning, USA). Millicell culture plate inserts (Millipore, Sundbyberg, Sweden) coated with 100 μL pre-diluted Martrigel extracellular matrix (Becton Dickinson, Sttokholm, Sweden) were put into each well to support the growth of the ovarian tissues. Every insert contained 2 pieces of ovarian tissue. The culture medium comprised α-MEM with 5% HAS, 1% ITS (10 μg/mL insulin, 5.5 μg/mL transferrin and 7.6 ng/mL sodium selenite), 0.5 IU/ml human menopausal gonadotropin (hMG, Livzon, China), 50 μg/ml vitamin C, 0.47 mmol/L pyruvate acid, 2 mmol/L L-glutamine, 100 IU/ml penicillin, and 100 μg/mL streptomycin. Initially, 150 μL culture medium was added inside the insert and 550 μL outside it. The tissues were cultured in a humidified incubator at 37°C with 5.5% CO2 for 14 days. Every other day, 400 μL of the culture medium outside the inserts was replaced by fresh medium.
Hormones assays
At 14th day after culture, the spent medium was collected and stored at −80°C for later analysis. The levels of 17-β estradiol (minimum detection limit: 5.0 pg/mL) and progesterone (minimum detection limit: 0.1 pg/mL) were measured using a heterogeneous competitive magnetic separation immunoassay (LRW, Shenzhen, Guangdong, China).
Lactate dehyrogenase (LDH) assay
Cytotoxicity of vitrification solution was assessed using lactate dehyrogenase (LDH) assay. Ovarian tissues were cut into 2 mm3 blocks under a dissecting microscope and put into dishes filled with virtification solution containing DPBS medium, 25% (v/v) DMSO, 25% (v/v) PROH and 15% FCS. Control group: exposure to PBS medium at 4°C for 10 min. Group I: exposure to vitrification solution at 4°C for 10 min. Group II: exposure to vitrification solution at 4°C for 20 min. Group III: exposure to vitrification solution at 4°C for 30 min. Group IV: exposure to vitrification solution at 4°C for 40 min. Group V: exposure to vitrification solution at 4°C for 50 min. Tissues were retrieved, washed briefly in medium and placed individual microtitre wells containing 150 μL of sterile PBS for overnight incubation at 37°C. The LDH levels in surrounding PBS were measured with a UV assay on a BM/Hitachi 717 apparatus at 37°C (Boehringer Mannheim, East Sussex, UK).
Statistical analysis
Follicle viability and the percentage of morphologically normal primordial follicles were compared using X2 analysis. The hormone levels of culture supernatant and LDH levels were compared by analysis of variance (ANOVA). Values were considered significant when P < 0.05. SAS version 8.1 software (SAS Institute, Cary, NC, USA) was used for all statistical analysis.
Results
Ovarian follicle viability
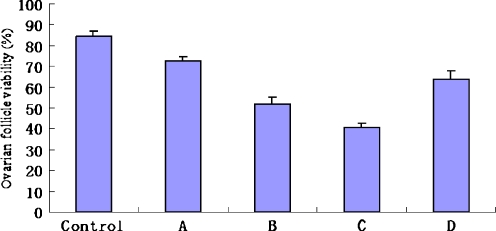
Figures 1, 2 and 3 showed the ovarian follicle viability in each group. The follicle viability in the fresh control group (84.5% ± 2.6%) was significantly higher than those in all experimental groups (P < 0.05). The follicle viability in group A (72.5% ± 2.1%) was the highest in all experimental groups (P < 0.05). The follicle viability in group D (63.7% ± 4.3%) was significantly higher than those in group B (51.9% ± 3.5%) and group C (40.5% ± 2.3%) (P < 0.05), and the follicle viability in group B was significantly higher than those in group C (P < 0.05).
Fig. 1.
Ovarian follicle viability in each group
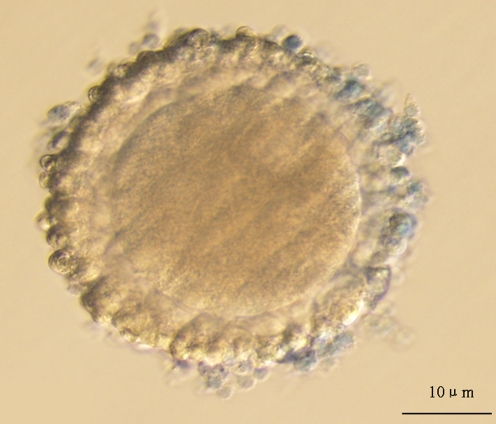
Fig. 2.
The oocyte was considered as live oocyte after trypan blue staining
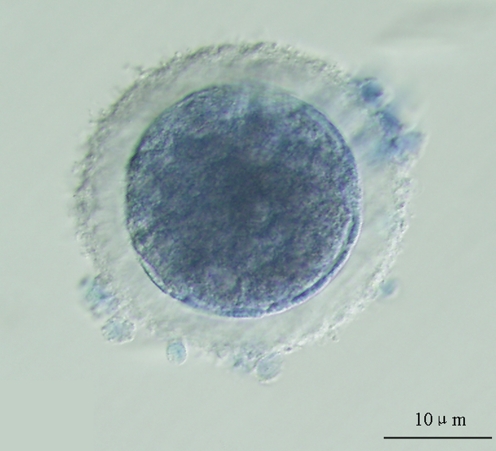
Fig. 3.
The oocyte stained blue was considered as dead oocyte
Histological examination of primordial follicles
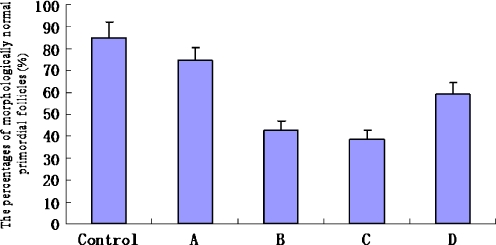
Figures 4, 5 and 6 showed the percentage of morphologically normal primordial follicles in each group. The percentage of morphologically normal primordial follicles in fresh control group (85.2% ± 7.1%) was significantly higher than those in all experimental groups (P < 0.05). The percentage of morphologically normal primordial follicles in group A (74.5% ± 5.7%) was the highest in all experimental groups (P < 0.05). The percentage of morphologically normal primordial follicles in group D (59.3% ± 5.1%) was significantly higher than those in group B (42.9% ± 3.9%) and group C (38.6 ± 4.1%) (P < 0.05). However, differences between the group B and group C were not significant (P > 0.05).
Fig. 4.
The percentage of morphologically normal primordial follicles in each group
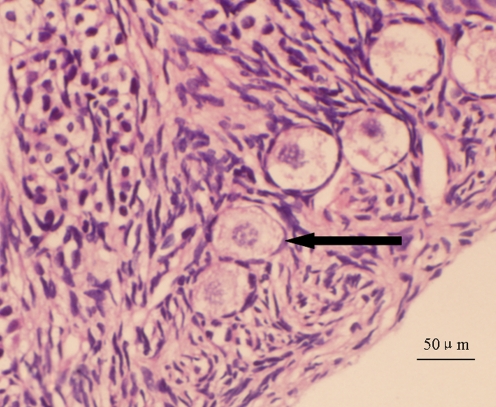
Fig. 5.
Morphologically normal primordial follicle: follicle spherical in shape with even distribution of pregranulosa cells, spherical oocyte, and a normal nucleus (←)
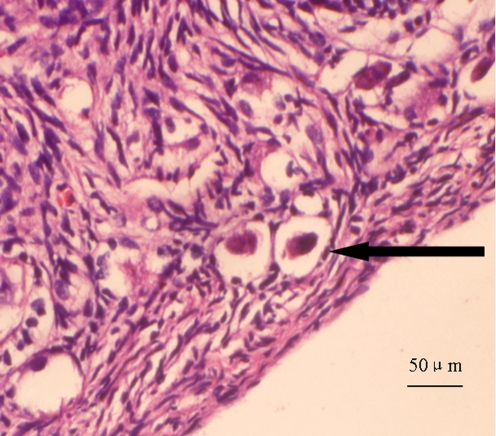
Fig. 6.
Morphologically abnormal primordial follicle showing shrinkage of oocyte and pyknotic nucleus (←)
Hormone assays
Table 1 showed 17-β estradiol and progesterone concentration in the culture supernatants in each group. 17-β estradiol and progesterone concentration in the culture supernatants in fresh control group (395 pg/mL and 4.21 ng/mL) were significantly higher than those in all experimental groups respectively (P < 0.05). 17-β estradiol and progesterone concentration in the culture supernatants in group A (339 pg/mL and 3.61 ng/mL) were the highest in all experimental groups. Estradiol 17-β and progesterone concentration in the culture supernatants in group D (295 pg/mL and 3.29 ng/mL) were significantly higher than those in group B (263 pg/mL and 2.52 ng/mL) and group C (251 pg/mL and 2.45 ng/mL) respectively (P < 0.05). However, the differences in 17-β estradiol and progesterone concentrations in the culture supernatants between the group B and group C were not significant (P > 0.05).
Table 1.
Concentration of 17-β estradiol and progesterone of frozen-thawed ovarian pieces cultured in vitro
| The hormone concentration in the culture supernatants in each group. | ||
|---|---|---|
| Group | Estradiol 17-β (pg/mL) | Progesterone (ng/mL) |
| Control group | 395 | 4.21 |
| Group A | 339 | 3.61 |
| Group B | 263 | 2.52 |
| Group C | 251 | 2.45 |
| Group D | 295 | 3.29 |
Lactate dehyrogenase (LDH) assay
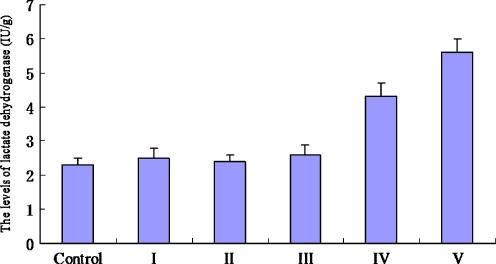
Figure 7 showed the levels of LDH released from ovarian tissues in each group. There were no significant differences in the levels of LDH among control group (2.3 ± 0.2 IU/g), group I (2.5 ± 0.3 IU/g), group II (2.4 ± 0.2 IU/g), and group III (2.6 ± 0.2 IU/g) (P > 0.05). However, the levels of LDH in group IV (4.3 ± 0.4 IU/g) and group V (5.6 ± 0.4 IU/g) were significantly higher than that in control group (P < 0.05).
Fig. 7.
The levels of lactate dehydrogenase in each group
Discussion
Cryopreserving pieces of ovarian cortex prior to treatment is one strategy for preserving fecundity [3]. However, earlier work revealed that almost 50% of primordial follicles were lost as a result of initial ischemia rather than as a result of the freeze-thaw process itself [9, 26]. Tissue ischemia remains a problem because the process of revascularization can take 2–7 days to complete [24, 27]. In theory, cryopreservation of an intact ovary in vitro, followed by autologous transplantation using microvascular anastomosis, could achieve immediate blood supply in vivo to maximize graft survival.
Although whole ovary cryopreservation followed by successful autotransplantation by vascular anastomosis has been performed in experimental animals [18], it is still a highly experimental procedure with low success rates. Onions et al. [28] found that cryoinjury causes follicular death as well as irreversible damage to the vascular system of the ovary. Damaged ovarian vasculatures can induce thromboembolism after transplantation which leads to severe tissue ischemia and follicular loss.
Cryopreservation of an entire organ with its vascular pedicle is much more complicated than ovarian cortical strips or suspended cells (oocyte) or group of cells (embryo). The major technical problems of whole ovary freezing is the larger volume of tissue that causes slow penetration of the cryoprotectant and unsteady heat transfer. In particular, the capillary vessels are sensitive to the freezing and thawing procedure [29]. Several technical aspects of this procedure should be optimized in research on experimental animals.
Cryopreservation as a process can be divided into 2 methods: conventinal freezing and vitrification. Studies that compare the effectiveness of vitrification and conventional freezing for oocytes, embryos and ovarian tissue have been published. On the basis of a study on cryopreservation of over 16,000 human embryos, Kuwayama et al. recently reported that vitrification compared with slow cooling resulted in high survival rates at all stages of embryonic development. The pregnancy rate per transfer of 4-cell vitrified embryos was 27%. However, there was no difference in pregnancy and live birth rates between the 2 cryopreservation methods [20]. It was reported that the cleavage and blastocyst formation rates of vitrified oocytes were significantly higher than those of slow-frozen oocytes. Compared with slow-frozen oocytes, vitrified oocytes were more likely to maintain normal meiotic spindles and chromosome alignment. However, the incidence of aneuploidy was similar [21]. Isachenko et al. compared the effectiveness of conventional freezing and vitrification of human ovarian tissue. The results revealed that conventional freezing was more promising than vitrification in cryopreservation of human ovarian tissue, because of the higher developmental potential [22]. Contrary to these finding, a study by Keros et al. reported that vitrification maintained the morphological integrity of the ovarian stroma better than slow-programmed freezing [23].
With regard to cryopreservation of whole ovaries, which method is preferable: slow-freezing or vitrification? The data on it is limited; therefore we carried out a comparative investigation of slow-freezing and vitrification of whole ovaries. In this study, we choose bovine as animal models. The reasons were: (i) bovine ovaries are comparable in size with human ovaries; (ii) only 1 or 2 follicles mature each cycle as the maturation of multiple follicles greatly influences the ovarian volume and therefore the outcome of the freezing process (multiple follicles mature each cycle in pig); and (iii) the bovine has a monthly cycle [30].
Histological examination is important tool to evaluate the protocol of ovary cryopreservation. Follicle morphology is usually analyzed through several criteria: (i) eosinophilia and vacuolations are the most commonly used criteria for oocyte cytoplasm; (ii) contraction and clumping of nucleus chromatin material are usually considered signs of atresia [31–33]. However, in our study, we noticed that: (i) oocyte cytoplasm staining frequently varied from one slice to another, independent of any follicular damage; (ii) oocyte cytoplasm vacuolations were also observed on fresh ovarian tissue. Previous studies also reported that the vacuolization of cytoplasm in oocyte appeared in both the fresh and the frozen-thawed groups analyzed by HE stain [34, 35]. It seems that this phenomenon happened in the fixation and the stain procedures. As a consequence, we have chosen to evaluate oocyte morphology considering only the chromatin material aspect. Since the trypan blue test provides a means to estimate the follicular viability after cryopreservation, it has been used as a tool to assess the efficiency of the cryopreservation procedure [36–38]. In addition to the results of the follicular viability test and histological examinations, the hormonal activity of thawed and cultured tissue is a criterion for the effectiveness of the cryopreservation protocol. The steroidogenic activity of ovarian tissue is known to be an indicator of tissue viability [22]. The skill of surgeon affected the ovarian transplantation with vascular anastomosis. Therefore, in this study, the efficacy of cryopreservation was analyzed in vitro experiment without transplantation.
In this study, follicle viability, the percentage of morphologically normal primordial follicles and hormone levels in group D (vitrification protocol) were significantly higher than those in group C (rapid freezing). Vitrification is a process that involves a physical process by which a highly concentrated solution of cryoprotectants solidifies during cooling without formation of ice crystals. Briefly, the vitreous transition temperature (Tg) is defined as the temperature below which a solid phase without ice crystals can be obtained. The critical cooling rate (Vccr) is the lowest rate that allows a given solution to vitrify. Vitrification is successful if cooling reaches Tg at a speed higher than the Vccr of the cryoprotectant solution. The Vccr decreases with increasing cryoprotectant concentration [37–39]. A big barrier of organ cryopreservation is the restriction of cooling and heating rates to those that are permitted by the dimensions and geometry of the organ. Therefore, vitrification of whole ovaries requires high concentrations of cryoprotectants to reduce the critical cooling rate and make all the solutions solidified. It can explain why vitrification protocol is more effective than rapid freezing (the concentration of cryoprotectant is low). Highly concentrated cryoprotective medium and lengthy perfusion period are key factors for successful vitrification of whole ovaries. As long as the cooling rate is higher than Vccr, all the aqueous solutions can be induced in an amorphous, so-called vitreous solid phase; any ice crystal formation can be avoided in the ovarian component, particularly in the inner cell and vasculatures. Thus, vitrification should be more suitable than conventional freezing for cryopreserving larger-volume and heterogeneous system such as an intact ovary. In this study, follicle viability, the percentage of morphologically normal primordial follicles and hormone levels in group D (vitrification) were significantly higher than those in group B (conventional freezing). The results support this hypothesis. Nevertheless, high concentrations of cryoprotectants could put cells to the risk of excessive toxicity. In the present study, the release of the intracellular enzyme LDH was used to assay cell damage caused by cytotoxicity of vitrification solution. The results showed that the level of LDH released from ovarian tissue exposure to vitrification solution containing 25% (v/v) DMSO and 25% (v/v) PROH at 4°C for 30 min was not significantly higher than that in control group, so it suggest this vitrification solution is appropriate for vitrifying ovaries.
Cryopreservation of whole ovaries using the slow-freezing protocol has been performed in humans by Martinez-Madrid et al. [40] and Bedaiwy et al. [41], showing high follicular viability, normal histological architecture and no evidence of damage to the vessels after this procedure. However, different from the above results, our study showed that the efficacy of slow-freezing of whole ovaries was lower than slow-cooling ovarian cortical strips. Slow-freezing uses an optimal cooling rate specific to a given cell so as to make the cells gradually dehydrated. However, it is a challenge to control the heat conductivity to achieve an even distribution of heating and cooling energy in the whole organ during slow-freezing process. This may be the reason of insufficiency of slow-freezing whole ovaries.
Transplantation of whole ovaries by vascular anastomosis has been considered as a promising strategy to restore fertility in cancer patients, and vitrification seems to be more suitable than conventional freezing for preserving the whole organ. However, the efficacy of the procedure should be improved before any further clinical applications take place.
Acknowledgements
Appreciation is extended to Dr Bin Li, Ph.D., for assistance in statistics analysis and Dr Yu Wang Ph.D. from department of pathology for supporting this work.
Conflict of interest statement The authors declare that there are no conflicts of interest.
Footnotes
Supported by: 1. Postdoctoral Foundation for Innovation Project of Shandong Province, China (Grant No: 200903074). 2. Shandong Major Scientific Innovation Foundation (2006GG1102021).
Caspsule
Vitrification seems to be more suitable than conventional freezing for cryopreservation of whole ovaries.
References
- 1.Apperley JF, Reddy N. Mechanism and management of treatment-related gonadal failure in recipients of high dose chemoradiotherapy. Blood Rev. 1995;9:93–116. doi: 10.1016/S0268-960X(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 2.Baker TG. Radiosensitivity of mammalian oocytes with particular reference to the human female. Am J Obstet Gynecol. 1971;110:746–61. doi: 10.1016/0002-9378(71)90271-7. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K, Karlikay G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 4.Oktay K, Buyuk E. Fertility preservation in women undergoing cancer treatment. Lancet. 2004;363:1830. doi: 10.1016/S0140-6736(04)16322-8. [DOI] [PubMed] [Google Scholar]
- 5.Radford JA, Lieberman BA, Brison DR, Smith AR, Critchlow JD, Russell SA, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin’s lymphoma. Lancet. 2001;357:1172–5. doi: 10.1016/S0140-6736(00)04335-X. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Dolmas M, Demylle D. Live birth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 7.Meirow D, Levron J, Eldar-Geva T. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–46. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- 9.Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology. 1999;140:462–71. doi: 10.1210/en.140.1.462. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Chen H, Yin H, Kim SS, Lin Tan S, Gosden RG. Fertility after intact ovary transplantation. Nature. 2002;415:385. doi: 10.1038/415385a. [DOI] [PubMed] [Google Scholar]
- 11.Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Charies B, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79:594–602. doi: 10.1016/S0015-0282(02)04842-2. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Madrid B, Dolmans MM, Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–4. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Revel A, Elami A, Bor A, Yavin S, Natan Y, Arav A. Whole sheep ovary cryopreservation and transplantation. Fertil Steril. 2004;82:1714–5. doi: 10.1016/j.fertnstert.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Migishima F, Suzuki-Migishima R, Song SY, Kuramochi T, Azuma S, Nishijima M, et al. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod. 2003;68:881–7. doi: 10.1095/biolreprod.102.007948. [DOI] [PubMed] [Google Scholar]
- 15.Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischemia and genotype. Hum Reprod. 2003;18:1165–72. doi: 10.1093/humrep/deg236. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Chen SG, Wu GJ, Wang J, Yu CP, Liu JY. Autologous heterotopic transplantation of intact rabbit ovary after frozen banking at −196°C. Fertil Steril. 2006;86(4 Suppl):1059–66. doi: 10.1016/j.fertnstert.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Imhof M, Bergmeiter H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85(Suppl 1):1208–15. doi: 10.1016/j.fertnstert.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Courbiere B, Caquant L, Mazoyer C, Franck M, Lornage J, Salle B. Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertil Steril. 2009;91:2697–706. doi: 10.1016/j.fertnstert.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Rall WF, Fahy GM. Ice free cryopreservation of mouse embryos at −196°C by vitrification. Nature. 1985;313:573–5. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 20.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14. doi: 10.1016/S1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 21.Huang JY, Chen HY, Tan SL, Chian RC. Effect of choline-supplemented sodium-depleted slow freezing versus vitrification on mouse oocyte meiotic spindles and chromosome abnormalities. Fertil Steril. 2007;88:1093–100. doi: 10.1016/j.fertnstert.2006.12.066. [DOI] [PubMed] [Google Scholar]
- 22.Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, an molecular biological evalution. Reproduction. 2009;138:319–27. doi: 10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- 23.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 24.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 25.Scott JE. carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online. 2004;9:287–93. doi: 10.1016/S1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- 26.Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11:1487–91. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- 27.Dissen GA, Lara HE, Frahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology. 1994;134:1146–54. doi: 10.1210/en.134.3.1146. [DOI] [PubMed] [Google Scholar]
- 28.Onions VJ, Webb R, McNeilly AS, Campbell BK. Ovarian endocrine profile and long-term vascular patency following heterotopic autotransplantation of cryopreserved whole ovine ovaries. Hum Reprod. 2009;24:2845–55. doi: 10.1093/humrep/dep274. [DOI] [PubMed] [Google Scholar]
- 29.Kim SS. Time to re-think: ovarian tissue transplantation versus whole ovary transplantation. Reprod Biomed Online. 2010;20:171–4. doi: 10.1016/j.rbmo.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Gerritse R, Beerendonk CCM, Tijink MSL, Heetkamp A, Kremer JAM, Braat DDM, et al. Optimal perfusion of an intact ovary as a prerequisite for successful ovarian cryopreservation. Hum Reprod. 2008;23:329–35. doi: 10.1093/humrep/dem384. [DOI] [PubMed] [Google Scholar]
- 31.Fabbri R, Venturoli S, D’Errico A, Iannascoli C, Gabusi E, Valeri B, et al. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89:259–66. doi: 10.1016/S0090-8258(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 32.Neto V, Buff S, Lornage J, Bottolier B, Guérin P, Joly T. Effects of different freezing parameters on the morphology and viability of preantral follicles after cryopreservation of doe rabbit ovarian tissue. Fertil Steril. 2008;89:1348–56. doi: 10.1016/j.fertnstert.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Sadeu JC, Cortvindt R, Ron-EI R, Kasterstein E, Smitz J. Morphological and ultrastructural evaluation of cultured frozen-thawed human fetal ovarian tissue. Fertil Steril. 2006;85:1130–41. doi: 10.1016/j.fertnstert.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 34.Perdrix A, Macé B, Milazzo JP, Liard-Zmuda A, Baron M, Rives N. Ovarian tissue thawing: a comparison of two conditions. Fertil Steril. 2010;93:307–10. doi: 10.1016/j.fertnstert.2009.07.967. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y. Reply: Human ovarian tissue: vitrification versus conventional freezing. Hum Reprod. 2009;24:1768–9. doi: 10.1093/humrep/dep105. [DOI] [PubMed] [Google Scholar]
- 36.Fauque P, Amor AB, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7. doi: 10.1016/j.fertnstert.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 37.Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin JF, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84(Suppl 2):1065–71. doi: 10.1016/j.fertnstert.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 38.Courbiere B, Odagescu V, Baudot A, Massardier J, Mazoyer C, Salle B, et al. Cryopreservation of the ovary by vitrification a an alternative to slow-cooling protocols. Fertil Steril. 2006;86(4 Suppl):1243–51. doi: 10.1016/j.fertnstert.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil. 2005;8:231–9. doi: 10.1080/14647270500054803. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;87:1153–65. doi: 10.1016/j.fertnstert.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Bedaiwy MA, Hussein MR, Charles B, Falcone T. Cryopreservation of intact human ovary with its vascular pedicle. Hum Reprod. 2006;21:3258–69. doi: 10.1093/humrep/del227. [DOI] [PubMed] [Google Scholar]