Abstract
In an attempt to assess the effect of perfluorinated compounds (PFC) on oocytes quality and fertilization rate, we studied follicular fluid (FF) PFC levels in 18 patients undergoing IVF-ET cycles. A significant correlation (R = 0.75; P < 0.001) was observed between FF PFC levels and fertilization rate. Moreover, patients with FF PFC contamination had significantly lower fertilization rate (p < 0.02) and number of embryos transferred (p < 0.02), compared to the PFC negative group.
Keywords: Infertility, IVF, PFC contamination, Follicular fluid
Introduction
The increased exposure to environmental and industrial contaminants have been postulated to be responsible to the global decline in fertility [1–3]. Among these are the perfluorinated compounds (PFCs), that are produced industrially by electrochemical fluorination [4], and are world-widely used as emulsifiers in cleaning products, as inert components in pesticides, food containers, non-stick frypans (commercially named “Teflon”, produced from PFOA), shampoos, toothpaste, and so forth [5, 6].
Surprisingly, while many animal and human studies have suggested the associations between exposure to PFCs compounds to altered reproductive functions [7, 8], the direct effect of PFC on female fertility has been scarcely studied [9, 10]. Moreover, the biological mechanisms by which exposure to PFCs interferes with fertility are unknown. It was postulated that by interrupting the hypothalamic–pituitary–ovarian regulation, the PFCs may cause irregular menstrual cycles and delayed ovulation [10, 11].
Prompted by the aforementioned observations we conducted this pilot study aiming to examine follicular fluid (FF) PFC levels in patients undergoing IVF-ET cycles, and to assess the effect of PFC contamination on oocyte quality and fertilization rate.
Materials and methods
The study population consisted of infertile patients admitted to our IVF unit, who reached the ovum pick-up (OPU) stage. The elimination of bias in this selection, for the purposes of this study, was achieved by excluding poor responders, i.e. women in whom <5 oocytes were retrieved.
Informed consent was obtained from all participants before inclusion in the study, which was approved by the local Human Investigation Committee. The study required no modification of our routine IVF-ET treatment protocols. Routine IVF or intracytoplasmic sperm injection (ICSI) was then performed, as appropriate.
For the purpose of the study, in addition to the routine embryologic work, the clear FF was collected immediately after oocytes aspiration for PFC determinations, while oocytes quality was assessed according to previously published criteria [12]. The top quality oocytes (TQO) were defined as those with round appearance, a smooth first polar body, dispersed cytoplasmic granula and normal perivitelline space.
For toxicological analysis of the FF, an extraction was performed according to the analytical procedure described by Hansen et al. [13] and Kannan et al. [14], with some modifications by Corsolini et al. [15]. After centrifugation at 1200 × g for 15 min, the samples were homogenized with Milli-Q water. Subsequently, 0.5 M tetrabutylammonium (TBA) hydrogen sulphate solution and sodium carbonate buffer (0.25 M, pH 10) were added to each sample before extraction with methyl tert-butyl ether (MTBE). The organic and aqueous layers were separated by centrifugation, rinsed with MTBE and separated again. The solvent was evaporated under nitrogen, replaced with methanol and the extract passed through a nylon mesh filter (0.2 μm). Milli-Q water was prepared as extraction blanks.
Concentrations of PFCs were measured using high performance liquid chromatography (HPLC) with electrospray ionization (ESI) tandem mass spectrometry. Analyte separation was performed using a Finnigan Surveyor Plus HPLC System. Chromatographic separation was achieved using a Betasil© C18 column (Thermo Electron Corporation, San Jose, CA). For quantitative determination, the HPLC system was interfaced to a Finnigan LTQ linear ion trap mass spectrometer (Thermo 150 Electron Corporation, San Jose, CA) operated in negative electrospray mode. Instrumental parameters were optimized to transmit the [M-H]- ion for all the analytes. The repeatability and reproducibility were performed in triplicate and were 85% and 90% respectively.
Data on patient age and infertility-treatment-related variables were collected from the files. Number of oocytes retrieved, oocytes quality and number of embryos transferred were assessed and compared between patients with and without FF PFC contamination.
Statistical analysis was performed with paired-Student’s t-test, Chi square and correlation analysis, as appropriate. Results are presented as means ± standard deviations; p < 0.05 was considered significant.
Results
Eighteen patients with a mean age of 33.6 ± 2.6 years were evaluated. Of whom, 8 had FF PFC contamination (PFC positive group) and 8 had no PFC in their FF (PFC negative group). The embryological characteristics according to patients’ FF PFC contamination are presented in Table 1.
Table 1.
Embryological characteristics according to patients’ FF PFC contamination
| PFS- negative (n = 8) | PFC- positive (n = 8) | p- value | |
|---|---|---|---|
| Age (yrs) | 33.25 ± 3.58 | 34.0 ± 1.2 | ns |
| Number of oocytes retrieved | 7.25 ± 1.58 | 7.38 ± 2.13 | ns |
| Percent of top quality oocytes | 61.9 ± 14.4 | 54.4 ± 16.2 | ns |
| Fertilization rate | 100 ± 0 | 83.35 ± 17.8 | p < 0.02 |
| Number of embryos transferred | 3 ± 0 | 2.5 ± 0.53 | p < 0.02 |
| Pregnancy rate | 25% | 25% | ns |
| Follicular fluid PFC levels (ng) | 0 | 6.65 ± 3.54 |
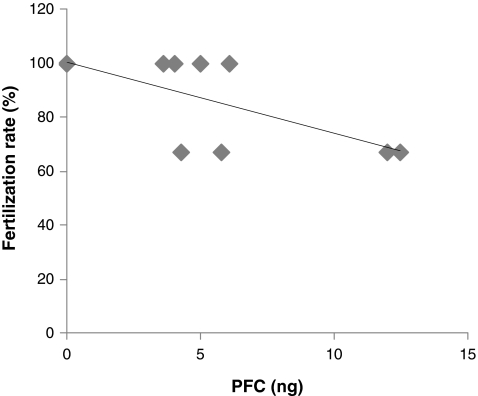
While no significant differences in patients’ age, number of oocytes retrieved or the percentage of TQO were observed between the two study groups, patients with FF PFC contamination had significantly lower fertilization rate and number of embryos transferred, compared to the PFC negative group. Moreover, while no correlation was observed between the percentage of TQO and FF PFC levels, a significant correlation (R = 0.75; P < 0.001) was observed between FF PFC levels and fertilization rate (Fig. 1).
Fig. 1.
Correlation between follicular fluid PFC levels and oocytes fertilization rate
Discussion
Human exposure to environmental and occupational chemicals has increased considerably in the past 50 years, with a concomitant increased awareness to these threats on fertility [1, 16–18]. PFCs are now ubiquitous and accumulate in humans and in the environment. While their acute toxicity is not high, their real problem is their stability and therefore their ability to persist.
Previous studies focused predominantly on compounds such as pesticides, dioxins and PCBs [19, 20], with scarce information regarding the effect of PFCs on female fertility [9, 10]. The latter, concentrate on the association between maternal PFC plasma levels and decreased parity or increased time to pregnancy, while in the present study, we offer an explanation to the aforementioned observations by demonstrating a detrimental effect of PFC on oocytes fertilization capacity with the consequent decrease in the number of embryos transferred.
The main limitation of our pilot study is its rather small sample size (16 patients), which could not demonstrate of any significant differences in oocytes quality or pregnancy rate. Moreover, in light of the Italian regulation, which limits the number of oocytes to be fertilized, we were unable to draw any conclusion on embryos quality. Furthermore, we also did not evaluate PFC contamination of the seminal plasma, and therefore could not discriminate between risks by a common (both partners) rather than a female exposure.
In conclusions, to our knowledge, in the present pilot study, we highlighted for the first time, the possibly high percentage of patients (in this cohort 50%) with increased PFC contamination in human follicular fluids, and its potential detrimental effect of oocytes fertilization capacity. The finding of PFCs in FF may imply that long-term exposure of humans may lead to reproductive impairment. Moreover, high PFCs’ levels may contribute to the otherwise reduced fertility in many young women. This preliminary study should be corroborated in larger studies, clarifying the effects of current environmental and occupational exposure to PFCs on reproductive health and the physiological mechanisms underlying these effects. The long-term effects of PFCs’ accumulation in the body are not predictable and therefore precautions should be taken to reduce or eliminate contact with these compounds.
Footnotes
Capsule
Contamination with perfluorinated compounds decreases fertilization rate and the number of embryos transferred in patients undergoing IVF-ET cycles.
References
- 1.Sharpe RM. Hormones and testis development and the possible adverse effect of environmental chemicals. Toxicol Lett. 2001;20:221–232. doi: 10.1016/S0378-4274(01)00298-3. [DOI] [PubMed] [Google Scholar]
- 2.Taskinen HK, Kyyronen P, Sallmen M, Virtanen SV, Liukkonen TA, Huida O, Lindbohm ML, Anttila A. Reduced fertility among female wood workers exposed to formaldehyde. Am J Ind Med. 1999;36:206–212. doi: 10.1002/(SICI)1097-0274(199907)36:1<206::AID-AJIM29>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1, 1-dichloro-2, 2-bis (p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005;162:523–532. doi: 10.1093/aje/kwi240. [DOI] [PubMed] [Google Scholar]
- 4.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renner R. Growing concern over perfluorinated chemicals. Environ Sci Technol. 2001;39:A154–160. doi: 10.1021/es012317k. [DOI] [PubMed] [Google Scholar]
- 6.Tittlemier S, Ryan JJ, Oostdam JV. Presence of anionic perfluorinated organic compounds in serum collected from Northern Canadian populations. Organohalogen Compound. 2004;66:4009–4014. [Google Scholar]
- 7.Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- 8.Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. Transplacental exposure of neonates to perfluorooctane sulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health. 2007;80:643–648. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 9.Fei CY, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod. 2009;24:1200–1205. doi: 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]
- 11.Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS, MohanKumar SM. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111:1485–1489. doi: 10.1289/ehp.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update. 2003;9:251–262. doi: 10.1093/humupd/dmg021. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KJ, Clemen LA, Ellefson ME, Johnson HO. Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ Sci Technol. 2001;35:766–770. doi: 10.1021/es001489z. [DOI] [PubMed] [Google Scholar]
- 14.Kannan K, Hansen SP, Franson CJ, Bowerman WW, Hansen KJ, Jones PD, Giesy JP. Perfluorooctane sulfonate in fish-eating water birds including Bald Eagles and Albatrosses. Environ Sci Technol. 2001;35:3065–3070. doi: 10.1021/es001935i. [DOI] [PubMed] [Google Scholar]
- 15.Corsolini S, Guerranti C, Perra G, Focardi S. Polybrominated diphenyl ethers, perfluorinated compounds and chlorinated pesticides in Swordfish (Xiphias glaudius) from the Mediterranean Sea. Environ Sci Technol. 2008;42:4344–4349. doi: 10.1021/es703057f. [DOI] [PubMed] [Google Scholar]
- 16.Oliva A, Spira A, Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16:1768–1776. doi: 10.1093/humrep/16.8.1768. [DOI] [PubMed] [Google Scholar]
- 17.Hauser R, Sokol R. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult male. Fertil Steril. 2008;89:e59–65. doi: 10.1016/j.fertnstert.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Mendola P, Messer LC, Rappazzo K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril. 2008;89:e81–94. doi: 10.1016/j.fertnstert.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Spanò M, Toft G, Hagmar L, Eleuteri P, Rescia M, Rignell-Hydbom A, Tyrkiel E, Zvyezday V, Bonde JP. INUENDO. Exposure to PCB and p, p’-DDE in European and Inuit populations: impact on human sperm chromatin integrity. Hum Reprod. 2005;20:3488–3499. doi: 10.1093/humrep/dei297. [DOI] [PubMed] [Google Scholar]
- 20.Giwercman AH, Rignell-Hydbom A, Toft G, Rylander L, Hagmar L, Lindh C, Pedersen HS, Ludwicki JK, Lesovoy V, Shvets M, Spanò M, Manicardi GC, Bizzaro D, Bonefeld-Jorgensen EC, Bonde JP. Reproductive hormone levels in men exposed to persistent organohalogen pollutants: a study of inuit and three European cohorts. Environ Health Perspect. 2006;114:1348–53. doi: 10.1289/ehp.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]