Abstract
Purpose
This study aimed to assess: (1) oral symptoms of patients treated for oral or oropharyngeal cancer; (2) how patients rank the burden of oral symptoms; (3) the impact of the tumor, the treatment, and oral symptoms on functional outcome.
Methods
Eighty-nine patients treated for oral or oropharyngeal cancer were asked about their oral symptoms related to mouth opening, dental status, oral sensory function, tongue mobility, salivary function, and pain. They were asked to rank these oral symptoms according to the degree of burden experienced. The Mandibular Function Impairment Questionnaire (MFIQ) was used to assess functional outcome. In a multivariate linear regression analyses, variables related to MFIQ scores (p ≤ 0.10) were entered as predictors with MFIQ score as the outcome.
Results
Lack of saliva (52%), restricted mouth opening (48%), and restricted tongue mobility (46%) were the most frequently reported oral symptoms. Lack of saliva was most frequently (32%) ranked as the most burdensome oral symptom.
For radiated patients, an inability to wear a dental prosthesis, a T3 or T4 stage, and a higher age were predictive of MFIQ scores. For non-radiated patients, a restricted mouth opening, an inability to wear a dental prosthesis, restricted tongue mobility, and surgery of the mandible were predictive of MFIQ scores.
Conclusions
Lack of saliva was not only the most frequently reported oral symptom after treatment for oral or oropharyngeal cancer, but also the most burdensome. Functional outcome is strongly influenced by an inability to wear a dental prosthesis in both radiated and non-radiated patients.
Keywords: Functional outcome, Treatment related adverse events, Oral and oropharyngeal cancer, Oral symptoms, Radiotherapy, Mandibular function impairment questionnaire
Introduction
After oral and oropharyngeal cancer treatment, patients may report several oral symptoms, such as a restricted mouth opening, lack of saliva, an inability to wear a dental prosthesis or lack of retention of the prosthesis, loss of oral sensory function, and restricted tongue mobility [1–3]. These oral symptoms can have a negative influence on functional outcome.
Functional outcome after treatment for oral or oropharyngeal cancer is related to tumor site, tumor size, and the type of treatment received [4–8]. A study in patients treated for cancer of the base of the tongue, found that surgery which included the mandible (mandibulectomy or mandibulotomy) reduced functional outcome significantly more than surgery which did not include the mandible [5]. In that study, functional outcome was assessed by eating, speech, and diet (eating in public and normalcy of diet). Furthermore, reconstruction with free-tissue transfer results in a significantly worse functional outcome when compared to direct reconstruction techniques [5, 6, 8]. Finally, a higher T stage (T3 or T4) and a larger resection size are also associated with a poorer functional outcome [4–6, 8].
In addition to tumor and treatment characteristics, oral symptoms may also impede functional outcome. A restricted mouth opening affects mandibular function, including chewing, eating, and swallowing, and may also impede oral hygiene, dental treatment, and oncological follow-up [9]. Lack of saliva, resulting from radiation-induced damage to the salivary glands or from removal of a salivary gland, impedes consolidation of a food bolus and functional outcome significantly [10, 11]. Lack of retention and pain may inhibit biting and chewing in edentulous or partially dentate patients wearing a dental prosthesis [10]. Clinically, pain in the mouth can also impede functional outcome.
To study oral symptoms related to oral and oropharyngeal cancer, and their association with functional outcome, three aims were formulated for this study. The first was to assess oral symptoms of patients treated for oral or oropharyngeal cancer. Before treatment of oral or oropharyngeal cancer, most patients rank being cured as the most important outcome, followed by living as long as possible and having no pain. Only a few patients rank normal swallowing, normal taste, and normal salivation as important [12, 13]. It is currently unclear which oral symptoms are most burdensome to patients after treatment of oral and oropharyngeal cancer. The second aim was therefore to assess how patients rank the burden of their oral symptoms. Finally, oral or oropharyngeal cancer and the consequences of treatment can influence mandibular functioning (Fig. 1). However, it is unclear which factors have the largest impact on functional outcome. The third aim was to analyze the impact of the tumor, cancer treatment, and oral symptoms on functional outcome.
Fig. 1.
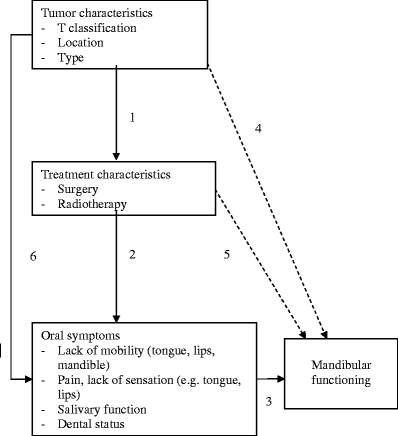
Clinical model of factors influencing mandibular functioning. Within this study, a clinical model of factors influencing mandibular functioning was hypothesized and analyzed: (1) Tumor characteristics determine treatment modalities (extent of surgery, dose of radiotherapy, etc.). (2) Besides anti-tumor effects, treatments also induce adverse effects, resulting in oral symptoms. (3) These oral symptoms may result in restrictions in mandibular functioning. (4, 5) However, it is possible that some tumor characteristics or treatment characteristics influence mandibular functioning directly without actually resulting in specific oral symptoms. (6) Finally, tumor characteristics may induce oral symptoms directly
Patients and methods
Patients
Patients aged ≥18 years, who were treated for oral or oropharyngeal cancer at the department of Oral and Maxillofacial Surgery, Division Oncology, University Medical Center Groningen, the Netherlands, were invited to participate in this cross-sectional study. Patients were informed about the study by means of a letter sent 1 week prior to their regular follow-up appointment. During this appointment, the physician further informed the patient and invited them to participate. Assessment was performed after patients signed a written informed consent.
Included in the study were patients who had completed their treatment for oral or oropharyngeal cancer at least 6 months before study assessment. Treatment consisted of surgery or a combination of surgery and radiotherapy. Excluded were patients who did not have sufficient understanding of Dutch to be interviewed or patients who were physically or mentally not fit enough to participate. Information regarding the type and localization of the tumor, TN classification, and type of treatment (surgery, radiotherapy) was retrieved from the medical records.
Oral symptoms
Assessment of oral symptoms was performed by one observer (PMH), who asked whether the patient:
experienced a restricted mouth opening (yes/no)
was able to wear a dental prosthesis (in case of an edentulous mandible or maxilla, or a partially dentate mandible or maxilla; yes/no)
experienced lack of retention of the dental prosthesis (yes/no)
experienced a loss of sensory function of the tongue (yes/no), lips (yes/no), or elsewhere in the mouth (yes/no)
experienced a restricted tongue mobility (yes/no), or lip mobility (yes/no)
experienced lack of saliva (yes/no)
experienced an excess of saliva (yes/no)
experienced pain in the mouth (yes/no)
experienced other symptoms, and if so, what kind of symptoms.
These questions were the result of a consensus between two experts (RPO, oral maxillofacial prosthetist, and JLNR, oral maxillofacial surgeon oncologist). These experts were asked to list the most frequently reported oral symptoms of patients being treated for oral and oropharyngeal cancer. Additionally, the patient was asked to rank the burden of their oral symptoms. The three most burdensome oral symptoms were recorded.
Functional outcome
Functional outcome was assessed by the Mandibular Function Impairment Questionnaire (MFIQ). This questionnaire consists of 11 items assessing perceived difficulties in mandibular function during social activities, speaking, taking a large bite, chewing hard food, chewing soft food, work and/or daily activities, drinking, laughing, chewing resistant food, yawning, and kissing. Additionally, six items assess perceived difficulties in mandibular function when eating a hard cookie, eating meat, eating a raw carrot, eating French bread, eating peanuts/almonds, and eating an apple. Eating includes taking a bite, chewing, and swallowing. Possible answers were: 0, no difficulty; 1, a little difficulty; 2, quite a bit of difficulty; 3, much difficulty; and 4, very much difficulty or impossible without help. The scores are added to give a sum score (range 0–68). A higher score indicates more perceived mandibular function impairments and a MFIQ score of ‘0’ indicates no impairment in mandibular functioning. Internal consistency of the questionnaire ranges between 0.80 and 0.95 [14]. The outcome of the questionnaire is independent of the method applied, whether by interview or filled out by the patient (r = 0.95) [14]. The MFIQ has previously been used to assess mandibular function after treatment of a chronic closed lock, subacute non-specific temporomandibular disorders, a painful disc displacement, and to determine a functional cutoff point for trismus [15–18].
Statistical analysis
Statistical analysis was performed using SPSS 16.0 for Windows software (SPPS Inc., Chicago, IL, USA). Statistical analysis included univariate analyses and multivariate linear regression analyses.
In the univariate analyses, associations between MFIQ scores and possible predictors were analyzed by means of independent samples t test and Pearson's correlation coefficient (r).
Possible predictors included age (years), gender (male/female), dental status (dentate/edentulous), T stage (T1/T2 versus T3/T4), radiotherapy (yes, no), surgery of the mandible (yes, no), interval between last oncology treatment and time of assessment (years), and oral symptoms: lack of saliva, restricted mouth opening, reduced tongue mobility, lack of retention of the prosthesis, reduced sensation of the lips, inability to wear a prosthesis, reduced sensation of the tongue, restricted mobility of the lips, reduced sensation elsewhere in mouth, pain in the mouth, excessive saliva, and swallowing problems (yes, no).
In the multivariate linear regression analysis, MFIQ score was used as the outcome variable. Variables related to MFIQ score in the univariate analyses (p ≤ 0.10) were entered as predictors (stepwise backward, entry criterion p ≤ 0.05, removal criterion p > 0.10). Interaction effects between the predictor variables were explored.
Results
One hundred and one patients were asked to participate. Twelve patients did not fulfill the inclusion criteria or refused to participate in the study, giving 89 patients (88%) to participate in the study. Patient characteristics, tumor type, tumor localization, and treatment received are summarized in Table 1. Median interval (inter quartile range) between the last oncology treatment and the time of assessment was 1.7 years (0.9 to 4.1 years). The mean MFIQ score was 24.3 (SD 16.9). MFIQ score of radiated patients (mean 28.9, SD 14.9) was significantly higher than that of non-radiated patients (mean 16.7, SD 17.6, p = 0.001). Most patients (76%) were treated for a squamous cell carcinoma, which was most frequently located in the tongue (36%). Sixty-three percent of the patients were treated with radiotherapy.
Table 1.
Characteristics of patients, tumor type, tumor localization, and tumor treatment
| Variables | |||
|---|---|---|---|
| Age (years) interval between last oncology treatment and assessment (years) | Median (inter quartile range) 1.7 (0.9 to 4.1) | Mean (SD) 61.0 (14.0) | |
| MFIQ score (scoring range 0 to 68) | 24.3 (16.9) | ||
| % | n | ||
| Gender | |||
| Male | 57 | 51 | |
| Female | 43 | 38 | |
| Tumor type | |||
| Squamous cell carcinoma | 76 | 68 | |
| Salivary gland tumor | 18 | 16 | |
| Other | 6 | 5 | |
| Tumor localizationa | |||
| Tongue | 36 | 32 | |
| Alveolar process of the mandible | 24 | 21 | |
| Floor of mouth | 19 | 17 | |
| Alveolar process of the maxilla | 11 | 10 | |
| Salivary gland | 11 | 10 | |
| Soft palate | 11 | 10 | |
| Lip | 10 | 9 | |
| Pharyngeal arch | 8 | 7 | |
| Cheek | 7 | 6 | |
| Base of the tongue | 7 | 6 | |
| Tonsil | 5 | 4 | |
| Lateral and dorsal wall of the oropharynx | 2 | 2 | |
| Buccogingival vault of the maxilla | 1 | 1 | |
| Buccogingival vault of the mandible | 1 | 1 | |
| Otherb | 1 | 1 | |
| Radiotherapy | |||
| Yes | 63 | 56 | |
| No | 37 | 33 | |
| Surgery of the mandible | |||
| Yes | 28 | 25 | |
| No | 72 | 64 | |
a N = 137. In 67% of the patients, the tumor was located on one site. In the other patients, the tumor extended over several regions. Therefore, the total number of localizations exceeded the total number of patients
bNasopharynx
Twenty patients (22%) did not wear their dental prosthesis during the assessment, of which eight patients did not wear their upper dentures and 12 patients did not wear their lower dentures. Five patients wore neither their upper or lower dentures (Table 2).
Table 2.
Dental status
| Dental status | Mandible dentate | Mandible partially dentate | Mandible edentulous | Total |
|---|---|---|---|---|
| Maxilla dentate | 13 | 5 | – | 18 |
| Maxilla partially dentate | 5 | 9 | 3 | 17 |
| Maxilla edentulous | – | 8 | 46 | 54 |
| Total | 18 | 22 | 49 | 89 |
The TN classification could be found in the medical records for 75% of the patients (Table 3). Patients with a missing TN classification were treated further in the past (mean 4.4, SD 4.2 years) than patients with a recorded TN classification (mean 2.8, SD 3.9 years, p = 0.138). Patients with a missing TN classification were also more likely to have been previously treated in another medical center.
Table 3.
TN classification on the basis of the pathology report
| Status | T1 | T2 | T3 | T4 | Total |
|---|---|---|---|---|---|
| N0 | 20 | 13 | 5 | 8 | 46 |
| N1 | 2 | 1 | 2 | 1 | 6 |
| N2 | 1 | – | – | – | 1 |
| N2b | 1 | 4 | 1 | 6 | 12 |
| N2c | – | – | – | 1 | 1 |
| N3 | – | 1 | – | – | 1 |
| Total | 24 | 19 | 8 | 16 | 67 |
TN classification was present in the medical records of 67 patients (75%)
Lack of saliva was the most frequently reported oral symptom (52%), followed by a restricted mouth opening (48%) and restricted tongue mobility (46%). Lack of saliva was ranked as the most burdensome oral symptom by 32% of patients. Restricted tongue mobility and restricted mouth opening were ranked equally burdensome by 14% of patients (Table 4).
Table 4.
Oral symptoms reported by 89 patients treated for oral or oropharyngeal cancer and ranking of symptoms according to their perceived burden
| Oral symptoms | Percent | n | Most burdening symptom (n = 88); % | Second most burdening symptom (n = 72); % | Third most burdening symptom (n = 56); % |
|---|---|---|---|---|---|
| Lack of saliva | 52 | 46 | 32 | 11 | 5 |
| Restricted mouth opening | 48 | 42 | 14 | 14 | 18 |
| Reduced tongue mobility | 46 | 41 | 14 | 24 | 13 |
| Lack of retention of the prosthesis | 39 | 34 | 9 | 10 | 5 |
| Reduced sensation of the lips | 30 | 27 | 6 | 11 | 7 |
| Inability to wear a prosthesis | 28 | 25 | 9 | 7 | 9 |
| Reduced sensation of the tongue | 27 | 24 | 7 | 8 | 13 |
| Restricted mobility of the lips | 25 | 22 | 3 | 6 | 9 |
| Reduced sensation elsewhere in the mouth | 23 | 20 | 5 | 4 | 9 |
| Pain in the mouth | 17 | 15 | 2 | 4 | 5 |
| Excess of saliva | 6 | 5 | – | – | – |
| Swallowing problems | 6 | 5 | – | – | – |
Some patients reported only the most burdensome oral symptom (n = 88) whereas others also reported the second or third most burdensome oral symptoms
A significant interaction between radiotherapy and restricted mouth opening was found to predict MFIQ scores. Therefore, the relationship between predictive variables and MFIQ scores were analyzed separately for radiated and non-radiated patients. In radiated patients, age, gender, restricted mouth opening, an inability to wear a dental prosthesis, surgery of the mandible, being fully edentulous, and T stage were significantly related to MFIQ scores (p ≤ 0.10). In non-radiated patients, a restricted mouth opening, restricted tongue mobility, reduced sensation elsewhere in mouth (other than tongue or lip), restricted lip mobility, reduced tongue sensation, an inability to wear a dental prosthesis, surgery of the mandible, and being fully edentulous were significantly related to MFIQ scores (p ≤ 0.10). For radiated patients, an inability to wear a dental prosthesis, T stage (T3/T4), and older age were predictive of higher MFIQ scores. For non-radiated patients, restricted mouth opening, an inability to wear a dental prosthesis, restricted tongue mobility, and surgery of the mandible were predictive of higher MFIQ scores (Table 5).
Table 5.
Results of multivariate linear regression analyses to predict the score on the MFIQ
| MFIQ score (scale range 0–68) | β | SE | 95% CI β | Significance of β |
|---|---|---|---|---|
| Radiated patients | ||||
| Not being able to wear a dental prosthesisa | 10.5 | 4.2 | 2.1 to 18.9 | p = 0.016 |
| T stage | 6.9 | 4.2 | −1.5 to 15.3 | p = 0.103 |
| Agec | 0.5 | 0.1 | 0.2 to 0.8 | p < 0.001 |
| Constant | −7.8 | 8.5 | −24.9 to 9.4 | p = 0.366 |
| r² = 0.45 | ||||
| Non-radiated patients | ||||
| Restricted mouth openinga | 22.9 | 3.9 | 14.9 to 30.9 | p < 0.001 |
| Not being able to wear a dental prosthesisa | 14.3 | 4.9 | 4.4. to 24.3 | p = 0.006 |
| Restricted tongue mobility a | 13.0 | 3.8 | 5.2 to 20.9 | p = 0.002 |
| Surgery of the mandiblea | 12.8 | 6.3 | −0.1 to 25.7 | p = 0.052 |
| Constant | 1.5 | 2.5 | −3.7 to 6.7 | p = 0.564 |
| r² = 0.72 | ||||
β Regression coefficient, SE standard error of β, 95% CI β 95% confidence interval of the regression coefficient, r² explained variance of the regression model
aYes = 1, no = 0
bT3/T4 = 1, T1/T2 = 0
cPer year
Discussion
The current study demonstrated that lack of saliva was not only the most frequently reported oral symptom after treatment for oral or oropharyngeal cancer, but also the most burdensome, with almost one third of the patients ranking it highest. This finding has been reported previously [19]. Radiotherapy causes damage to the salivary glands, resulting in reduced volume and altered composition of saliva [3]. The consistency of saliva changes from thin to thick, with a reduced pH and buffering capacity [3, 19]. In addition to dryness of the mouth and thirst, lack of saliva may cause an accumulation of mucus, a burning sensation, taste disturbances, difficulties in oral functioning, and problems wearing dentures [3].
A restricted mouth opening and restricted tongue mobility were reported by almost half of the patients. These oral symptoms were reported among the three most burdensome. Both symptoms are known to occur frequently after treatment for oral or oropharyngeal cancer [20, 21]. Despite the fact that lack of saliva was most frequently mentioned and was ranked most burdensome for many patients, it was not predictive for functional outcome. Generally, lack of saliva is perceived as very inconvenient, however, patients may compensate by using artificial saliva or by drinking during meals [4, 22]. Most patients prefer water as a lubricant [3]. It might also be possible that the influence of lack of saliva on the regression analyses was obscured by low statistical power.
A mean MFIQ score of 24.3 was found, indicating that averagely patients experience a substantial amount of mandibular impairment after treatment of oral and oropharyngeal cancer. The finding that radiated patients had a poorer functional outcome than non-radiated patients has been described previously [23]. However, for radiated patients and non-radiated patients the MFIQ scores were predicted by different variables. The only common variable in the regression analyses an inability to wear dental prosthesis. In non-radiated patients the impact on functional outcome was slightly larger (β = 14.3) than in radiated patients (β = 10.5). Edentulous or partially dentate patients may not be able to wear a dental prosthesis because of lack of retention resulting from anatomical changes post surgery. Pain may also prevent patients from wearing dentures, resulting in problems with biting and chewing food [1]. Patients who are fitted with a dental prosthesis are known to have a better functional outcome than those who are not fitted with prosthesis, based on List's Performance Status Scale [10].
In the current study, a higher T stage was associated with poorer functional outcome. As this relationship was found only in radiated patients, the T stage may have acted as a confounding variable. Patients with larger tumors (T3, T4) generally undergo combined treatment, this treatment results in greater tissue damage and scar formation further impeding mandibular function.
In radiated patients, an older age predicted poorer mandibular function. This relationship might be explained by normal aging processes and additional comorbidity, known to be more frequent as age increases. Comorbidity is negatively associated with swallowing function after surgical treatment for advanced oral or oropharyngeal cancer [23]. A restricted mouth opening was expected to be a strong predictor of poor mandibular function in radiated patients. However, a restricted mouth opening appeared to have the largest effect in non-radiated patients [24]. Nevertheless, restricted mouth opening, as well as restricted tongue mobility, is well known for negatively affecting oral functioning [21, 25].
It is clear that an inability to wear a dental prosthesis greatly impedes mandibular functioning. Therefore, providing patients, both radiated and non-radiated, with a dental prosthesis is an important measure to reduce mandibular impairment. Clinically, the dental prosthesis should have an optimal retention, although retention was not a significant factor in the regression analysis. Additionally, for non-radiated patients, treatment of restricted mouth opening and restricted tongue mobility may reduce mandibular impairment.
Rehabilitation of patients after treatment for oral or oropharyngeal cancer should focus on preserving mouth opening and tongue mobility. However, preservation of mouth opening after radiation therapy is difficult. The average reduction in mouth opening ranges from 18% to 32%, with the greatest decrease occurring between 1 and 9 months after radiotherapy [26, 27]. The currently frequently applied intensity-modulated radiotherapy reduces mouth opening less than conventional radiotherapy [28]. Once mouth opening has decreased, it is difficult to achieve improvement. The effects of exercises on a restricted mouth opening after oral or oropharyngeal cancer are limited with in a mean increase of 5 to 6 mm [29]. TheraBite® treatment seems to be effective [30]. Restricted tongue mobility may be improved by speech therapy, including range of motion exercises [31–33].
Strengths of this study include the use of a standardized, valid, and reliable questionnaire, and the assessment of multiple symptoms related to oral and oropharyngeal cancer. Limitations of the current study include the relatively small sample size which hampered statistical analysis, the cross-sectional study design, and the substantial number of missing data regarding T stage in the medical records.
More insight into the development of oral symptoms is needed to determine which should be prevented in order to maintain mandibular function and achieve optimal functional outcome.
Conclusions
Lack of saliva is not only the most frequently reported oral symptom after treatment for oral or oropharyngeal cancer, but also the most burdensome. Functional outcome is strongly influenced by an inability to wear a dental prosthesis in both radiated and non-radiated patients.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26:796–807. doi: 10.1002/hed.20045. [DOI] [PubMed] [Google Scholar]
- 2.Finlay PM, Dawson F, Robertson AG, Soutar DS. An evaluation of functional outcome after surgery and radiotherapy for intraoral cancer. Br J Oral Maxillofac Surg. 1992;30:14–17. doi: 10.1016/0266-4356(92)90130-B. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Jansma J, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinovic VS. Quality of life after surgical excision followed by radiotherapy for cancer of the tongue and floor of the mouth: evaluation of 78 patients. J Craniomaxillofac Surg. 1999;27:192–197. doi: 10.1016/s1010-5182(99)80050-x. [DOI] [PubMed] [Google Scholar]
- 5.Malone JP, Stephens JA, Grecula JC, Rhoades CA, Ghaheri BA, Schuller DE. Disease control, survival, and functional outcome after multimodal treatment for advanced-stage tongue base cancer. Head Neck. 2004;26:561–572. doi: 10.1002/hed.20012. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SN, Lowe D, Brown JS, Vaughan ED. The University of Washington head and neck cancer measure as a predictor of outcome following primary surgery for oral cancer. Head Neck. 1999;21:394–401. doi: 10.1002/(SICI)1097-0347(199908)21:5<394::AID-HED3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SN, Lowe D, Fisher SE, Brown JS, Vaughan ED. Health-related quality of life and clinical function after primary surgery for oral cancer. Br J Oral Maxillofac Surg. 2002;40:11–18. doi: 10.1054/bjom.2001.0706. [DOI] [PubMed] [Google Scholar]
- 8.Zuydam AC, Lowe D, Brown JS, Vaughan ED, Rogers SN. Predictors of speech and swallowing function following primary surgery for oral and oropharyngeal cancer. Clin Otolaryngol. 2005;30:428–437. doi: 10.1111/j.1365-2273.2005.01061.x. [DOI] [PubMed] [Google Scholar]
- 9.Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health-related quality of life in patients attending a Maxillofacial Oncology clinic. Oral Oncol. 2008;44(5):430–8. doi: 10.1016/j.oraloncology.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Teoh KH, Patel S, Hwang F, Huryn JM, Verbel D, Zlotolow IM. Prosthetic intervention in the era of microvascular reconstruction of the mandible–a retrospective analysis of functional outcome. Int J Prosthodont. 2005;18:42–54. [PubMed] [Google Scholar]
- 11.Logemann JA, Smith CH, Pauloski BR, Rademaker AW, Lazarus CL, Colangelo LA, Mittal B, MacCracken E, Gaziano J, Stachowiak L, Newman LA. Effects of xerostomia on perception and performance of swallow function. Head Neck. 2001;23:317–321. doi: 10.1002/hed.1037. [DOI] [PubMed] [Google Scholar]
- 12.List MA, Stracks J, Colangelo L, Butler P, Ganzenko N, Lundy D, Sullivan P, Haraf D, Kies M, Goodwin W, Vokes EE. How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol. 2000;18:877–884. doi: 10.1200/JCO.2000.18.4.877. [DOI] [PubMed] [Google Scholar]
- 13.List MA, Rutherford JL, Stracks J, Pauloski BR, Logemann JA, Lundy D, Sullivan P, Goodwin W, Kies M, Vokes EE. Prioritizing treatment outcomes: head and neck cancer patients versus nonpatients. Head Neck. 2004;26:163–170. doi: 10.1002/hed.10367. [DOI] [PubMed] [Google Scholar]
- 14.Stegenga B, de Bont LGM, de Leeuw R, Boering G. Assessment of mandibular function impairment associated with temporomandibular joint osteoarthrosis and internal derangement. J Orofac Pain. 1993;7:183–195. [PubMed] [Google Scholar]
- 15.Dijkstra PU, Huisman PM, Roodenburg JLN. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35:337–342. doi: 10.1016/j.ijom.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Fricton JR, Look JO, Schiffman E, Swift J. Long-term study of temporomandibular joint surgery with alloplastic implants compared with nonimplant surgery and nonsurgical rehabilitation for painful temporomandibular joint disc displacement. J Oral Maxillofac Surg. 2002;60:1400–1411. doi: 10.1053/joms.2002.36091. [DOI] [PubMed] [Google Scholar]
- 17.Pereira LJ, Steenks MH, de Wijer A, Speksnijder CM, van der Bilt A. Masticatory function in subacute TMD patients before and after treatment. J Oral Rehabil. 2009;36:391–402. doi: 10.1111/j.1365-2842.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- 18.Politi M, Sembronio S, Robiony M, Costa F, Toro C, Undt G. High condylectomy and disc repositioning compared to arthroscopic lysis, lavage, and capsular stretch for the treatment of chronic closed lock of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:27–33. doi: 10.1016/j.tripleo.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Bogaert W. Efficacy of the BioXtra dry mouth care system in the treatment of radiotherapy-induced xerostomia. Support Care Cancer. 2007;15:1429–1436. doi: 10.1007/s00520-006-0210-y. [DOI] [PubMed] [Google Scholar]
- 20.Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT (2010) A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer [DOI] [PubMed]
- 21.Pauloski BR. Rehabilitation of dysphagia following head and neck cancer. Phys Med Rehabil Clin N Am. 2008;19:889–928. doi: 10.1016/j.pmr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson JC, Fox PC. Salivary gland dysfunction. Clin Geriatr Med. 1992;8:499–511. [PubMed] [Google Scholar]
- 23.Kreeft AM, van der Molen L, Hilgers FJ, Balm AJ. Speech and swallowing after surgical treatment of advanced oral and oropharyngeal carcinoma: a systematic review of the literature. Eur Arch Otorhinolaryngol. 2009;266:1687–1698. doi: 10.1007/s00405-009-1089-2. [DOI] [PubMed] [Google Scholar]
- 24.Scott B, D'Souza J, Perinparajah N, Lowe D, Rogers SN (2010) Longitudinal evaluation of restricted mouth opening (trismus) in patients following primary surgery for oral and oropharyngeal squamous cell carcinoma. Br J Oral Maxillofac Surg (in press) [DOI] [PubMed]
- 25.Van Cann EM, Dom M, Koole R, Merkx MA, Stoelinga PJ. Health related quality of life after mandibular resection for oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2005;41:687–693. doi: 10.1016/j.oraloncology.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:365–373. doi: 10.1016/S1079-2104(99)70044-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang CJ, Huang EY, Hsu HC, Chen HC, Fang FM, Hsiung CY. The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope. 2005;115:1458–1460. doi: 10.1097/01.mlg.0000171019.80351.46. [DOI] [PubMed] [Google Scholar]
- 28.Hsiung CY, Huang EY, Ting HM, Huang HY. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: the reduction of radiation-induced trismus. Br J Radiol. 2008;81:809–814. doi: 10.1259/bjr/17942449. [DOI] [PubMed] [Google Scholar]
- 29.Dijkstra PU, Sterken MW, Pater R, Spijkervet FKL, Roodenburg JLN. Exercise therapy for trismus in head and neck cancer. Oral Oncol. 2006;43:389–394. doi: 10.1016/j.oraloncology.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Buchbinder D, Currivan RB, Kaplan AJ, Urken ML. Mobilization regimens for the prevention of jaw hypomobility in the radiated patient: a comparison of three techniques. J Oral Maxillofac Surg. 1993;51:863–867. doi: 10.1016/S0278-2391(10)80104-1. [DOI] [PubMed] [Google Scholar]
- 31.Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Effects of postural change on aspiration in head and neck surgical patients. Otolaryngol Head Neck Surg. 1994;110:222–227. doi: 10.1177/019459989411000212. [DOI] [PubMed] [Google Scholar]
- 32.Logemann JA, Pauloski BR, Rademaker AW, Colangelo L. Speech and swallowing rehabilitation for head and neck cancer patients. Oncology (Williston Park) 1997;11:651–656. [PubMed] [Google Scholar]
- 33.Rasley A, Logemann JA, Kahrilas PJ, Rademaker AW, Pauloski BR, Dodds WJ. Prevention of barium aspiration during videofluoroscopic swalling studies: value of change in posture. Am J Radiol. 1993;160:1005–1009. doi: 10.2214/ajr.160.5.8470567. [DOI] [PubMed] [Google Scholar]


