Abstract
Traditional systems of medicines need more evidence-based studies on both crude drugs and purified phytomolecules. Utilization of natural products as pharmacological tools could lead to a number of new major therapeutically active metabolites. Lead molecules are further screened for their potential in terms of quality control, safety assessments, and studies about molecular pharmacology and their related properties. Identification, and quality and safety evaluation of natural products, is a fundamental requirement of industry and other organizations dealing with natural health products (NHPs). Marker analysis, based on chemo-profiling and development of characteristic fingerprints for individual plants, could help to develop uniform standardization tools. Beside such evaluations of clinical parameters, safety profiles as well as drug–herb and herb–herb interactions are the most important parameters for assessment and promotion. With the steady growth of the NHPs, advanced analytical- and mechanism-based screening should be considered for their promotion and value addition in every way for the betterment of healthcare. Thus, there is an urgent need for the development of international co-ordination to promote and develop NHPs, including their assessment, perspectives, pharmacovigilance, and potential harmonization of regulation, quality control and clinical uses.
Keywords: Ethnopharmacology, integrated approach, Ayurveda, Indian system of medicine
INTRODUCTION
Natural products have been the source of most active ingredients in western medicines. This is widely accepted to be true when applied to drug discovery. In the ‘olden days’, before the advancement of high-throughput screening and the post-genomic era, more than 80% of drug substances were obtained from natural products or inspired by natural compounds.[1] Over 100 natural-product-derived compounds are currently undergoing clinical trials and at least 100 similar projects are in preclinical development. Most currently projected drugs are derived from leads from plants and microbial sources.[2] Earlier publications, and researchers from around the world, have pointed out that relatively little of the world's plant biodiversity has been extensively screened for bioactivity.[3,4] Hence, more extensive collections of plants based on ethnomedicine/ethnic practice, or further advances in the ability to culture microbes, could provide many novel chemicals in drug discovery.
Natural products have inspired many developments in organic chemistry leading to advances in synthetic methodologies in developing several analogues of lead compounds with therapeutical potential. Acceptance of Cinchona for the treatment of malaria in the 17th century, followed by digitalis, morphine etc, and the introduction of aspirin, brought the general public to believe in the wonders of diverse floral wealth, and that natural products including plants offer large structural diversity for pharmacological treatment of various classes of disorders [Figure 1].
Figure 1.
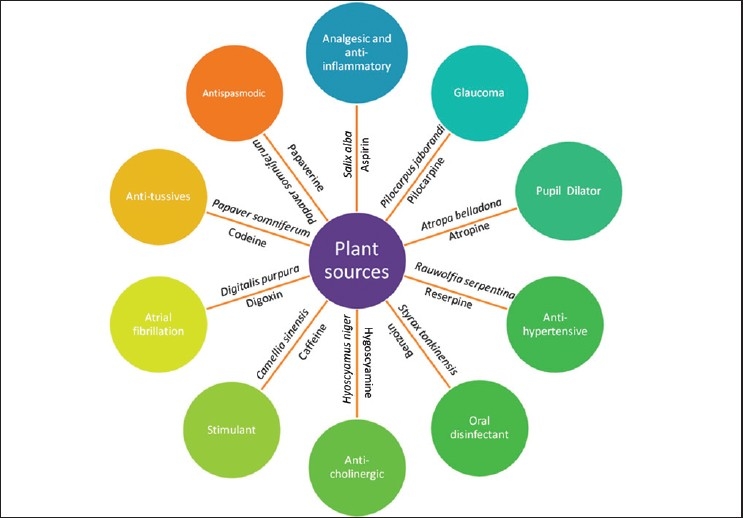
Drugs developed for various disorders from plant sources
India has an ancient heritage of traditional medicine. The Materia medica of India provides much information on ethnic folklore practices and traditional aspects of therapeutically important natural products. Indian traditional medicine is based on various systems, including Ayurveda, Siddha and Unani (ASU). With the emerging interest of the world in adopting and studying traditional systems, and in exploiting their potential from different healthcare perspectives, the Government of India has initiated several attempts to explore the possibility of evaluating these systems for their therapeutic potential as originally practiced, as well as to help generate data to put them in national healthcare programs.[5] The Ministry of Health and Family Welfare, Government of India, has undertaken various initiatives for the development and preservation of these aspects of cultural heritage [Figure 2]. The Department of AYUSH (Ayurveda, Yoga, Siddha, Unani and Homeopathy) regulates education and research in these systems. The National Medicinal Plant Board [NMPB], which deals with conservation and research issues in botanicals, is working to address issues in these areas.
Figure 2.

Various research councils of Govt. of India for promotion and development of traditional medicine
TRADITIONAL PRINCIPLE AND MODERN ELUCIDATION
The process of drug development from ethnomedicine or Ethnopharmacology typically begins with a botanist, ethnobotanist, ethnopharmacologist or plant ecologist, who collects and identifies plant(s) of interest.[6] The ethnopharmacologic approach is based on botany, chemistry and pharmacology (observation, identification, description and experimental investigation); however, other disciplines have also made vital contributions. Based on these considerations, ethnopharmacology is defined as ‘the interdisciplinary scientific exploration of biologically active agents traditionally employed or observed by man’. The objectives of ethnopharmacology are to rescue and document important cultural heritage before it is lost, and to investigate and evaluate agents employed. Thus, it plays an immense role in the evaluation of natural products, and more particularly herbal drugs from traditional and folklore resources. Field observations, and descriptions of the use and effects of traditional remedies, botanical identification and phytochemical and pharmacological studies, are all within the scope of ethnopharmacology.[7,8]
Ethnopharmacology is far more than a science of the past using outmoded approaches. It still constitutes the scientific backbone for the development of active therapeutics based on traditional medicines of various ethnic groups. It has the ultimate aim of validating traditional preparations, either through the isolation of active substances, or through various pharmacological findings.[9] The use of Ayurvedic drugs and formulations has always been an integral part of the treatment of different ailments in diverse communities across India. Research leads on Ayurvedic drugs have yielded numerous drug candidates that are now common in commercial markets. Many plants with potential therapeutic activity were first widely used as Ayurvedic medicines [Table 1].
Table 1.
Several Ayurvedic plant drugs used in TM for different ailments

The Government of India has taken effective steps to develop the quality, safety, efficacy and practice of herbal medicine along with several regulatory measures. In fact, several modern drugs used in the treatment of significant ailments have been developed from Indian medicinal plants. These include the likes of reserpine, from Rauwolfia serpentina (L.) Benth. ex Kurz, withanolide from Withania somenifera Dunal, curcumin from Curcuma longa L quinine from Cinchona officinalis L., sennoside from Cassia angustifolia, glycyrrhizin from Glycyrrhiza glabra L., and psoralen from Ruta graveolens L.[17] Further, several lead molecules have been isolated from ISM in our laboratory, such as betulinic acid from Nelumbo nucifera [immunomodulatory agent], β-Asarone from Acorus calamus L. [AChE inhibitor], mahanimbine from Murrya koenigii [AChE inhibitor] and tilianin from Semecarpus anacardium [hepatoprotective],[18] and their stated pharmacological activities confirmed.
Development of drugs from Ayurvedic plants continues, with drug companies engaged in large-scale pharmacologic screening of herbs. The ‘Sushruta-Samhita’ notes that the plant Commiphora mukul Hook was useful in the treatment of obesity and equivalent ailments. Withania somnifera (Ashwagandha) is traditionally used as an adaptogen. This is also known as ‘Indian Ginseng’ stimulating the body's immune system, and reducing inflammation. Combining the strengths of the knowledge base systems of traditional complementary and alternative medicine, such as Ayurveda, with the dramatic power of combinatorial sciences, and high throughput screening, will help in the generation of structure–activity libraries, leading to identification of active molecules. This can further be explored through clinical trials, various pharmacological studies, herbal therapeutics, pharmacokinetics and herbal pharmacovigilance. The confluence in recent years of spectacular advances in chemistry, molecular biology, genomics and chemical technology, and the related fields of spectroscopy, chromatography and crystallography, may influence several therapeutically potent lead molecules from traditional medicine.
QUALITY CONTROL IN TRADITIONAL INDIAN SYSTEMS OF MEDICINE
Quality control of traditional medicines is a critical and essential issue to be considered in assuring their safety and therapeutic efficacy, and to rationalize their use in healthcare. In almost all traditional systems of medicine, quality control has been considered from their inception by the traditional experts in medicine, the ‘Rishis’ and later by ‘Vaidyas’ and ‘Hakims’, all of whom developed medications for the sick. Today, however, changes in approach are required. The need for new chemical entities as pharmaceutical products has been explored through these plant sources. Finger printing and marker compound analyses are now increasingly used for the standardization of traditional medicinal formulations. Here, concentrations of secondary metabolites, the major constituents of herbal drugs, are studied, providing valued scientific standardization procedures. This technique not only helps in establishing the correct botanical identity, but also in regulating the chemical purity of the herbs. Of these marker compounds, some are therapeutically active, while others though not active, are present in abundance helping in their standardization;[5] for example, withanolides from Withania somnifera are therapeutically active marker compounds; on the other hand aegelin from Aegle marmelos is not therapeutically active, but its presence is already well established, so it can be used as a marker compound.
Unlike pharmaceuticals based on a single chemical entity that deal with anomalies in target cells, tissues, or organs, most herbal remedies currently lack scientific foundation and to the scientist seem to fall more into the realm of myth. In order for such remedies also to achieve sustained growth, and become acceptable to the mainstream pharmaceutical market, solid scientific evidence is needed to support their functionality claims; this applies to many botanical products.[19] Identification and quality evaluation of crude drugs is a fundamental requirement of industry and other organizations dealing with natural health products (NHP). That the plant material under examination has complex and inconsistent composition particularly for its secondary metabolite content, must also be taken into account. Therefore, analytical limits are not as precise as for a single chemical entity.[20] It is now accepted that qualitative and quantitative analysis of major bioactive chemical components (marker components) of a crude drug constitute an important and reliable part of quality control protocol, since any change in quality of the drug directly affects the constituents. Such analyses need to be developed for every aspect of single herbs and polyherbal extracts.[21] Let us take a typical example of a polyherbal formulation, ‘Triphala’, one of the oldest, and most commonly used polyherbal preparations in Indian Systems of Medicine (ISM), since ancient times. This well-known phytomedicine is a combination of Terminalia chebula, Terminalia belerica and Emblica officinalis, in equal proportions as described in the Ayurvedic Formulary of India.[22] It is prescribed by Ayurveda in first-line treatment of many aliments, as a laxative, detoxifying agent and rejuvenator and for several other purposes. A rapid, simple and accurate method with High Performance Thin Layer Chromatography (HPTLC) has been developed to standardize Triphala and its individual components using gallic acid (GA) [Table 2] in our laboratory. This method permits reliable quantification of GA with good resolution and separation of the same from other constituents of extracts of Triphala and its components [Figure 3]. The proposed methods can be used for routine quality testing, and similar methods can be developed for other herbal formulations, especially where no other accurate method of routine analysis is available as in the case of quantification of gallic acid in Triphala.[23] Quality assurance is an integral part of traditional medicine, ensuring that it delivers the required quantity of quality medicament. Today, quality assurance is the thrust area for complex traditional formulations like churnas, bhasmas, liquid orals, lehas, etc. In former days, these traditional medicinal formulations were prepared by Vaidyas in person and delivered fresh to patients based on their need (personalized medicine).Then quality assurance was not needed in the same way as now.[5]
Table 2.
Standardization of Triphala formulation

Figure 3.

HPTLC chromatogram of ‘Triphala’
INTEGRATIVE APPROACHES FOR ALTERNATIVE SYSTEM OF MEDICINE
There is a long-running debate between people, who use herbal remedies for various diseases and disorders, and those who rely on modern medicine to cure their aches and pains. Those who prefer modern medical remedies contend that herbal remedies are mostly ‘smoke and mirrors’, and that nothing can be solved by ingesting a herb. They believe that herbal remedies are mostly placebos used by people who have been falsely informed.[24] Nothing could be further from the truth.
While it is true that doctors are beginning to recommend alternative (herbal) remedies to their patients when modern drugs do not work, it is also because herbal medicines have fewer side effects, are less toxic and habit forming, in contrast to allopathic medicine, where toxic side effects may sometimes lead to death. In contrast, there is a growing evidence to show that ingredients of medicinal plants act synergistically and that suitable combinations neutralize side effects.[25]
The debate between modern medicine and herbal remedies comes down to a simple fact: every individual, regardless of education or disease, should be informed about the facts concerning their illness from the common cold up to cancer. There are benefits and drawbacks to both herbal remedies and modern medicine, and such factors should be considered on an individual basis.[26]
QUALITY ASSURANCE
Good Manufacturing Practices (GMP) for herbal drugs address every aspect, from their cultivation in the field, to their preparation in different formulations as crude powder, extracts or purified components. In this era of worldwide herbal drug revolution, there is a demand to implement GMP in the production of medicinal products from natural resources. According to these norms, not all herbal products fulfill the requirements for classification as well-established medicines. Natural drugs must (i) contain one or more herbal ingredients, (ii) not be harmful under specified conditions of application. The strong historic bond between plants and human health began to unfold in 1897 when synthetic acetyl salicylic acid (aspirin) was introduced to the world. Currently, there are about 900 licensed manufacturing units for different Indian systems of medicine producing different natural product formulations.[9]
NOVEL APPROACHES TO DRUG DELIVERY SYSTEM WITH HERBAL MEDICINE
Regarding the value addition to herbal formulation, there is a need to develop a novel drug delivery system (NDDS) for herbal drugs for several reasons. These include targeted drug delivery, reduced dose, increased solubility, enhanced absorption, reduced elimination and metabolism of the drug. A timely and targeted release improves the effectiveness of phytomolecules, broadens their application range and ensures optimal dosage, thereby improving cost-effectiveness of the product.[27]
Most herbal drugs may be considered to have therapeutic effects, but may not have them to the desired extent or at the desired dosage, or the desired target. Most have lower bioavailability due to factors like solubility problems, which cause reduced absorption, rapid metabolism and excretion. Phytosomes are advanced forms of herbal products that are better absorbed and utilized, and, as a result, produce better effects than conventional herbal extracts. Phytosomes are produced via a patented process, whereby the individual components of herbal extract are bound to phosphatidylcholine. Using this technology several phytomolecules have been targeted within novel delivery systems at our laboratory. These include curcumin,[28] quercetin,[9] hesperetin,[29] ellagic acid (EA)[30] etc., and have been proved to be more effective. However the in-vitro release study of EA from pure EA suspension, and EA-phospholipid complex at different time points has been represented. Ellagic acid exhibits notable antioxidant activity but it has certain constraints i.e. metabolized by intestinal microorganisms upon oral administration, and rapid elimination from the body meaning short plasma half-life. To overcome this limitation, a phospholipid complex of EA was prepared and evaluated in terms of pharmacokinetic profile and anti-oxidant activity. The phytosome preparation enabled 85.72% of EA to be intercalated in the phospholipid layer of the spherical structures of the complex formed. The complex has a sustained release property, and enhanced antioxidant activity, compared to free EA. A pharmacokinetic study found the complex to have higher relative bioavailability, and to act for a longer period of time [Figure 4]. The study showed that the EA phospholipid complex had better antioxidant activity at a lower dose compared to free EA, and was present in serum for a longer period of time, which may be helpful in solving the problems of rapid elimination of the molecule.[30] Based on these bioavailability and pharmacokinetic studies, several value-added formulations prepared at our laboratory have been evaluated and reported for their in vitro release, in vivo serum concentrations and therapeutic efficacy. Such experience-based practices are usually designed to prevent or cure specific diseases or symptoms in a holistic fashion, providing solutions complementary to modern medicine.
Figure 4.
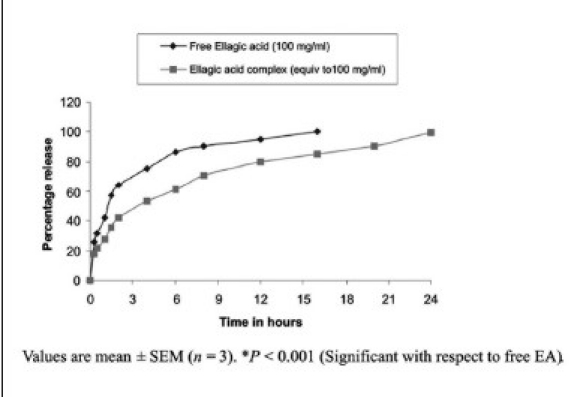
In vitro release study of EA from pure EA suspension and EA-phospholipid complex
SAFETY STUDIES
Many in the general public believe that modern drugs are dangerous foreign chemicals with side effects, while herbals are natural and safe. In fact, some herbs can also be dangerous and even cause serious diseases leading to death, if used inappropriately. The complexity of herbal drug preparations and the interpretation of bibliographic data on their safety and efficacy reflecting experience gathered during long-term use, are best addressed by involving specific expertise and experience. Further, consumers may tend to consume herbal products along with prescription medicine without their therapist's knowledge, which may lead to herb-drug interaction, via cytochrome enzymes. Cytochrome P450 (CYPs) isoenzymes are predominantly present in the liver, but are also found in the intestine, lungs, kidneys, brain etc. Several isoforms, such as CYP1A2, CYP2C9, CYP2D6 and CYP3A4 appear to be most relevant for the metabolism of clinically significant drugs. The medical literature is replete with reports suggesting that the simultaneous use of phytomolecules along with prescription medicines/OTC products may cause serious clinical adverse reactions, due to the latter's ability to alter human drug metabolism and pharmacokinetics. Manufacturer's evaluation of these supplements for toxicology, pre-clinical and clinical data is not compulsory, and is not yet subject to standard pharmaceutical criteria for safety.[31]
Various countries have produced regulatory requirements concerning safety and assessment of potential interactions between phytoconstituents and conventional medicines.[32] Regulatory agencies now require documentation of interactions between herbal drugs and CYP isoforms before licensing. Using in vitro, in vivo and in silico techniques, several phytoconstituents have been identified as inhibitors or inducers of cytochromes resulting in herb–drug interaction. Similarly, drug–drug interactions between phytoconstituents and conventional medicines have also been reported. Examples of such active compound interactions including allicin, quercetin, silymarin compounds have been published.
Herbal practitioners, researchers and manufacturers should take the initiative to make pharmacovigilance function properly in the same way as doctors reporting reactions to synthetic pharmaceuticals. They should recognize that reporting suspected adverse reactions to their herbal medicines is an act of courage, which will eventually increase rather than decrease respect for their profession. Herbal pharmacovigilance is not a negative tool but a neutral one, especially when it identifies a new serious herbal health risk. By providing evidence that certain herbal health risks are absent or negligibly small, pharmacovigilance can also be reassuring. Thus, it can also help to booster one of the main features of phytotherapeuticals, namely their relative safety when compared to conventional pharmaceuticals.[31]
SYSTEMS BIOLOGY APPROACH
Systems biology is a new field in life sciences that studies the interactions of different components (e.g., molecular pathways and regulatory networks) within an organism to elucidate relevant physiological functions and behavior.[33] The focus of this growing field is to investigate the dynamics of all genetic, regulatory and metabolic processes in a cell, and to understand the complexity of cellular networks.[34] The traditional reductionist approach to drug development may not be able to detect activity in the presence of several active components, synergy between compounds and pro-drugs. The approach is used for studying the activity of known targets as receptor-binding assays. A systems biology approach may be more effective in drug development from holistic medicine, particularly from a medicinal plant with a known evidence base.
Clinical evaluation of herbal drugs is one of the essential means of demonstrating therapeutic effects. The approach to herbal drug discovery from Indian traditional medicine consists of several steps, including identification of the plant species, to its active principles, and then synthesizing large amounts to satisfy the potentially huge demand. Varieties of challenges and issues in Indian herbal drug development have been identified, and may be overcome by international coordination and collaboration.[35]
Adoption of a systems biology approach would do much to help compete with Traditional Chinese Medicine (TCM) and modern Chinese herbal medicine. Its technological platforms, such as genomics, proteomics and metabolomics, provide powerful tools for studying the essence of TCM syndrome, and the function of herbal compound recipes [Figure 5]. Scientifically and technologically validated botanical products may be explored on a fast track using innovative approaches like reverse pharmacology and systems biology, when based on knowledge of traditional medicine. Traditional medicine is undergoing an evolutionary process as communities discover practice transforming techniques.[36] Methods for carrying out metabolic modeling by means of collecting, storing and analysing metabolomic data are considerably different. They will generally be performed by individuals or in laboratories with different skill sets, and yet will necessarily deal with the same molecules. It is therefore both essential and timely to bring together known or conditional metabolic maps of suitable organisms with metabolome measurements to provide a systems level understanding of metabolic fluxes and metabolite concentrations in these organisms, and their patterns of change under different conditions.[37,38]
Figure 5.

An overview of system biology approach in the drug discovery
CONCLUSION
Natural products have inspired many developments in drug discovery. There are many historical examples in which the natural product has not just been the medicinal product but has also helped to reveal a novel aspect of drug isolation. Because it is extremely time-consuming and expensive to create extensive collections of isolated and structurally characterized natural products, it is still attractive to screen mixtures of compounds to isolate and identify the active lead, not only from the plant extracts but also from microbes. Plants are the best combinatorial chemists still providing hidden secrets of their healing properties to relieve humanity from deadly diseases. The existing knowledge of ethnic medicines has developed several leads in healthcare and drug discovery, and also as a template for discovery. With the rapidity of modern industrialization, a lot of ethnic information used in healthcare is in danger of being lost. Hence, the time is right to develop and document traditional knowledge and medicine, and so further assist in developing suitable drugs for various ailments in the future. To develop a drug discovery from natural products, innovative approaches include use of genomics, techniques to mine previously untouched environments, and screening technologies, which, to a large extent, need to be explored through national and international collaboration and cooperation.
Acknowledgments
The authors are thankful to All India Council for Technical Education (AICTE), New Delhi for providing financial support through Research Promotion Scheme (RPS) and MODROBS project grant. Thanks are also due to AICTE for providing QIP- Fellowship to S.P.
Footnotes
Source of Support: All India Council for Technical Education (AICTE), New Delhi
Conflict of Interest: None declared.
REFERENCES
- 1.Sneader W. Drug Prototypes and Their Exploitation. UK: Wiley; 1996. [Google Scholar]
- 2.Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 3.Baker DD, Chu M, Oza U, Rajgarhia V. The value of natural products to future pharmaceutical discovery. Nat Prod Rep. 2007;24:1225–44. doi: 10.1039/b602241n. [DOI] [PubMed] [Google Scholar]
- 4.Harvey A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov Today. 2000;5:294–300. doi: 10.1016/s1359-6446(00)01511-7. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee PK. Quality Control of Herbal Drugs – An Approach to Evaluation of Botanicals (1st edn) New Delhi, India: Business Horizons; 2002. [Google Scholar]
- 6.King S. Medicines that changed the world. Pac Discovery. 1992;45:23. [Google Scholar]
- 7.Cordell GA, Colvard MD. 2005. Some thoughts on the future of ethnopharmacology. J Ethnopharmacol. 2005;100:5–14. doi: 10.1016/j.jep.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Patwardhan B. Ethnopharmacology and drug discovery. J Ethnopharmacol. 2005;100:50–2. doi: 10.1016/j.jep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee PK. Exploring green resources for drug development through ethnobotany. In: Shrivastava MM, Sanghi R, editors. Chemistry for Green Environment. Narosa Publishing House: New Delhi, India; 2005. pp. 287–99. [Google Scholar]
- 10.Mukherjee PK, Mukherjee K. Evaluation of Botanical-Perspectives of Quality Safety and Efficacy. In: Prajapati ND, Prajapati T, S Jaypura, editors. In: Advances in Medicinal Plants. 1st ed. Vol. 1. Jodhpur, Rajasthan, India: Asian Medicinal Plants and Health Care Trust; 2005. pp. 87–110. [Google Scholar]
- 11.The Ayurvedic Pharmacopoeia of India. Ministry of Helath and Family Welfare, Department of ISM and H. (First Ed) 2001;III Part I. [Google Scholar]
- 12.Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–81. doi: 10.1016/S0893-133X(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AA, Prakash T, Patil T, Viswanath Swamy AH, Gouda AV, Prabhu K, et al. Hepatoprotective and antioxidant activities of flowers of Calotropis procera (Ait) R. Br. in CCl4 induced hepatic damage. Indian J Exp Biol. 2007;45:304–10. [PubMed] [Google Scholar]
- 14.Venkatesh P, Mukherjee PK, Kumar SN, Nema NK, Bandyopadhyay A, Fukui H, et al. Mast cell stabilization and antihistaminic potentials of Curculigo orchioides rhizomes. J Ethnopharmacol. 2009;126:434–6. doi: 10.1016/j.jep.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Mankani KL, Krishna V, Manjunatha BK, Vidya SM, Singh J, Manohara SD, et al. Hepatoprotective effects of the triterpenes isolated from the stem bark of Diospyros cordifolia Roxb. J Nat Rem. 2006;6:147–52. [Google Scholar]
- 16.Varrier VP, Varrier . A compendium of species. Vol. 5. Chennai, India: Orient Longman Ltd; 1996. Indian medicinal plants. [Google Scholar]
- 17.Mukherjee PK, Venkatesh M, Gantait A. Ayurveda in Modern Medicine: Development and modification of bioactivity. In: Mander LN, Liu HW, editors. Comprehensive Natural Product Chemistry-II. 1st Ed. The Netherlands: Elsevier Publications; 2009. (In Press) [Google Scholar]
- 18.Mukherjee PK, Sahoo AK, Narayanan N, Kumar NS, Ponnusankar S. Lead finding from medicinal plants with hepatoprotective potentials. Expert Opin Drug Discov. 2009;4:545–76. doi: 10.1517/17460440902911433. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZG, Ren J. Current status and future direction of Chinese herbal medicine. Trends Pharmacol Sci. 2002;23:347–8. doi: 10.1016/s0165-6147(02)02051-5. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee PK, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethnopharmacol. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee PK. Evaluation of Indian traditional medicine. Drug Inform J. 2001;35:631–40. [Google Scholar]
- 22.New Delhi: 2002. The Ayurvedic Formulary of India Part II, Department of Indian System of Medicine and Homoeopathy. [Google Scholar]
- 23.Mukherjee PK, Rai S, Bhattacharya S, Wahile A, Saha BP. Marker analysis of polyherbal formulation, Triphala- A well known Indian traditional medicine. Indian J Trad Know. 2008;7:379–83. [Google Scholar]
- 24.Gilani AH, Rahman AU. Trends in ethnopharmacolgy. J Ethnopharmacol. 2005;100:43–9. doi: 10.1016/j.jep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Thompson S. 2006. Available from: http://www.associatedcontent.com/article/48174/herbal_remedies_v_modern_medicine.html. Herbal remedies v modern medicine.
- 26.Mukherjee PK. Exploring botanicals in Indian systems of medicine-regulatory perspectives. Clin Res Regul Aff. 2003;20:249–64. [Google Scholar]
- 27.Liu JR, Chen GF, Shih HN, Kuo PC. Enhanced antioxidant bioactivity of Salvia miltiorrhiza (Danshen) products prepared using nanotechnology. Phytomedicine. 2008;15:23–30. doi: 10.1016/j.phymed.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Maiti K, Mukherjee K, Murugan V, Saha BP, Mukherjee PK. Exploring the effect of Hesperetin-HSPC complex - a novel drug delivery system on the in-vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech. 2009;10:943–50. doi: 10.1208/s12249-009-9282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murugan V, Mukherjee K, Maiti K, Mukherjee PK. Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J Agric Food Chem. 2009;57:4559–65. doi: 10.1021/jf8037105. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee PK, Ponnusankar S, Badra S, Pandit S, Venkatesh M. Confluence of strategies for the development of botanicals. Pharma Rev. 2008a;12:114. [Google Scholar]
- 32.Mukherjee PK, Saha BP. Quest for GMP for the production of quality botanicals. In: Mukherjee PK, Verpoorte R, editors. In: GMP for Botanicals – Regulatory and Quality Issues on Phytomedicine. New Delhi, India: Eastern Publishers, Business Horizons Ltd; 2003. pp. 165–90. [Google Scholar]
- 33.Weckwerth Metabolomics in Systems biology. Annual Review in Plant Biology. 2003;54:669–89. doi: 10.1146/annurev.arplant.54.031902.135014. [DOI] [PubMed] [Google Scholar]
- 34.Kitano H. Computational systems biology. Nature. 2002;420:206–10. doi: 10.1038/nature01254. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee PK, Rai S, Kumar V, Mukherjee K, Hyland PJ, Hider RC. Plants of Indian origin in drug discovery. Expert Opin Drug Discov. 2007;2:633–57. doi: 10.1517/17460441.2.5.633. [DOI] [PubMed] [Google Scholar]
- 36.Patwardhan B, Ashok DB, Chorghade VM. Ayurveda and natural products drug discovery. Curr Sci. 2004;86:6. [Google Scholar]
- 37.Kell DB. Systems biology, metabolic modelling and metabolomics in drug discovery and development. Drug Discov Today. 2006;11:1085–92. doi: 10.1016/j.drudis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Yang LP. Application of systems biology method in the research of traditional Chinese medicine. Chin J Integr Med. 2008;6:454–7. doi: 10.3736/jcim20080504. [DOI] [PubMed] [Google Scholar]


