Abstract
The tumor suppressor p53 protects organisms from most types of cancer through multiple mechanisms. The p53 gene encodes a stress-activated transcriptional factor that transcriptionally regulates a large set of genes with versatile functions. These p53-activated genes mitigate consequences of stress regulating cell viability, growth, proliferation, repair, and metabolism. Recently, we described a novel antioxidant function of p53, which is important for its tumor suppressor activity. Among the many antioxidant genes activated by p53, Sestrins (Sesns) are critical for suppression of reactive oxygen species (ROS) and protection from oxidative stress, transformation, and genomic instability. Sestrins can regulate ROS through their direct effect on antioxidant peroxiredoxin proteins and through the AMP-activated protein kinase-target of rapamycin signaling pathway. The AMP-activated protein kinase-target of rapamycin axis is critical for regulation of metabolism and autophagy, two processes associated with ROS production, and deregulation of this pathway increases vulnerability of the organism to stress, aging, and age-related diseases, including cancer. Recently, we have shown that inactivation of Sestrin in fly causes accumulation of age-associated damage. Hence, Sestrins can link p53 with aging and age-related diseases. Antioxid. Redox Signal. 15, 1679–1690.
Introduction
Recently, we celebrated a 30-year anniversary of identification of the p53 tumor suppressor, the gene prominent for its role in suppression of carcinogenesis (73). The significance of the p53 being called “guardian of the genome” (69) is supported by the fact that it is found mutated in >50% of human cancers, and the p53-regulated pathway is inactivated in most cancers (73). The p53 gene encodes a transcription factor activated by numerous stress insults such as DNA damage, hypoxia, nutrient deprivation, and oxidants (120). As a result, activated p53 transcriptionally regulates expression of a number of genes with diverse function. The protein products of these genes help to adapt to stress conditions through numerous mechanisms involved in maintenance of cell homeostasis and genomic stability, and prevention of propagation of the potentially detrimental cells (120) (Fig. 1). For a long time p53 was recognized as the factor that works in a restrictive manner to determine cell fate through the induction of cell cycle arrest, senescence, and cell death. However, more evidence has accumulated acknowledging the importance of p53 in prevention of cancer and some other age-associated diseases through mechanisms of good maintenance involved in the regulation of reactive oxygen species (ROS), cell signaling, and metabolism under the normal and low stress conditions (119).
FIG. 1.
Regulation of intracellular processes by p53. Tumor suppressor p53 activated by different stress stimuli regulates expression of two sets of genes depending on the nature, level, and durability of the stress. One set of genes involved in regulation of cell cycle, DNA repair, metabolism, and autophagy are activated early and responsible for support of cell viability and integrity, whereas the set of pro-oxidant and proapoptotic genes are activated by sustained and more severe stress.
p53 and Stress Response
The tumor suppressor p53 belongs to a family of proteins conserved in evolution from Caenorhabditis elegans to mammals. Only one member of the family is found in the invertebrate genome, whereas mammalian genomes contain three members of the family: p53, p63, and p73 (86). The major functions of the members of the family are different, and whereas p63 and p73 play critical roles in developmental and homeostatic control, p53 is a bona fide stress responsive gene involved in the regulation of stress response and protection from detrimental consequences of stress (86). The best evidence for this special role of p53 comes from a knockout study demonstrating that p53-deficient mice develop normally but die by 6 months of age from cancer and/or inflammation (27). Mice with a single copy of p53 live longer than p53 knockouts but are also highly susceptible to carcinogenesis (46). This phenotype recapitulates the similar phenomenon in humans suffering from Li-Fraumeni syndrome, a rare autosomal dominant hereditary disorder characterized by high susceptibility to carcinogenesis (77, 107). But why is p53 so important for our long and productive lifespan and how does it work?
Our normal everyday life is affected by many stress insults. Some of them come from our environment such as exposure to UV light, irradiation, hypoxia, starvation, temperature extremes, and poisons, consumed with food and water. Microorganisms, viruses, and injuries can induce inflammatory stress response resulting in clearing from invaders followed by tissue repair. Finally, some stressors are generated by our everyday metabolism. For example, ROS are produced as byproducts of mitochondrial oxidation and through some enzymatic reactions by xanthine oxidase, lipoxygenase, nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase, and the uncoupling of nitric oxide synthase (118). At low concentrations ROS are important for regulation of cell signaling, but their accumulation can induce oxidative stress characterized by damage of different organelles and macromolecules (5, 37). To prevent such a scenario, systems of strict control of reduction and oxidation (redox) state by numerous mechanisms have been developed by nature. The mechanism of ROS decomposition involves enzymes such as superoxide dismutases, catalases, glutathione-dependent peroxidases (GPX), and thioredoxin-dependent peroxidases (peroxiredoxins [Prx]) (5). Moreover, ROS are controlled by some nonenzymatic antioxidants, including glutathione, lipoic acid, tocopherols, ascorbic acid, and some others (5, 35, 93) (Fig. 2).
FIG. 2.
Regulation of intracellular ROS. ROS, the signaling molecules and source of oxidative stress in the cell, are produced through exogenous stimuli exposure (H2O2, UV light, and ionizing radiation), as by-products of mitochondrial metabolism, and by several enzymes. In normal cells ROS accumulation is tightly controlled by antioxidant enzymes and some nonenzymatic systems. ROS, reactive oxygen species.
The vast majority of stress insults activate p53 at the level of protein stability (73). Under normal conditions p53 has a very short life due to permanent degradation by the ubiquitin system (73). The ubiquitin system requires three types of enzymes: E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligase. The last one targets specific protein substrates for degradation by the proteasome. p53 stability is dependent on the E3 ubiquitin ligase murine double minute 2 (MDM2), which interacts with p53 through its N-terminal part (44). Moreover the MDM2 gene is a p53-inducible gene establishing a negative feedback loop for p53 regulation (44). p53 stability is regulated through phosphorylation of its N-terminus and disruption of the p53-MDM2 interaction and a number of p53 kinases activated by stress have been identified (44, 73). The best characterized system is activation of p53 by DNA damage through activation of ATM/ATR and their downstream Chk1/Chk2 kinase cascades and phosphorylation of p53 on Ser15 and Ser20 (12) (Fig. 3).
FIG. 3.
Regulation of p53 by oxidative stress. p53 is activated by oxidative stress through phosphorylation by kinases that are activated by ROS, such as ATM and AMPK. AMPK, AMP-activated protein kinase; ATM, ataxia telangiectasia mutated.
Induced p53 activates expression of a number of genes through interaction with the p53-responsive element containing two copies of consensus sequences 5′-RRRCWWGYYY-3 (R, purine; Y, pyrimidine), located in regulatory regions such as promoters, introns, and long-distant sites of the p53-inducible genes (120). It is predicted that transcription of 2583 human genes and 1713 mouse orthologs (around 5%–8% of mammalian genes) might be regulated by p53 (47). Moreover, global mapping of p53 binding sites using analysis of real binding of p53 with its target genes using chromatin precipitation with the paired-end ditag (PET) sequencing strategy allowed to identify 542 binding loci in the human genome (123). Among the characterized genes, there are many genes known to be involved in regulation of cell cycle, cell death, DNA repair, antioxidant defense, and metabolism (119). Evidently, more and more genes assigned for new function will be characterized in the future and tell us more about the p53 functions. We can expect that many components of the p53-regulated network are intertwined and involved in protection from stress and age-associated diseases, including cancer.
p53 and ROS Regulation
Multiple stresses, including oxidative, metabolic, and genotoxic, cause accumulation of ROS (42). ROS accumulation leads to p53 activation and transactivation of various p53 targets (98). The mechanism of p53 activation by ROS is not well established, but might be undertaken through phosphorylation by activated redox-dependent kinases. The potential candidates, activated directly by oxidative stress and involved in p53 phosphorylation, are ATM (1), LKB1, AMP-activated protein kinase (AMPK) (1, 56), and c-Jun N-terminal kinase (19, 38, 60) (Fig. 3). Alternative activation of p53 by ROS can be mediated by oxidative DNA damage and operated through mechanisms of DNA damage response (83). ROS can also damage the macromolecules and organelles, leading to support p53 activation (36).
The outcome of p53 activation depends on the strength and duration of the stress (98, 121). Under severe and long-lasting stress conditions, p53 induces cell death or, alternatively, permanent cell cycle arrest, to prevent propagation of cells with damaged DNA (119). DNA damage can cause mutagenesis and genomic instability, important features of carcinogenesis (72). The proapoptotic activity of p53 requires induction of apoptotic genes such as PUMA and Bax, which are activated in a p53-dependent manner in response to severe stress to induce cell death (98). Under the same conditions p53 activates expression of the genes involved in generation of ROS, such as the quinone oxidoreductase homolog gene TP53I3 (also known as PIG3), and it was proposed that they contribute to degradation of mitochondrial content and support proapoptotic activity of p53 (94, 98). Accordingly, we have shown that ROS accumulation associated with hyperactivation of p53 requires intact mitochondrial function and does not occur in the ρ0 cells that lack mitochondrial DNA and are defective for ROS-generating electron transport chain (Fig. 4) (98).
FIG. 4.
Regulation of ROS by p53. Under low stress conditions p53 regulates expression of antioxidant genes, which protect from oxidative stress. On the contrary, severe and sustainable stress activates pro-oxidant genes, which facilitate cell death.
p53-dependent cell death in response to severe stress requires induction of pro-oxidant genes to facilitate apoptotic processes (94), but under normal physiological conditions, the organism still faces low-stress insults. For instance, endogenous ROS produced as by-products of metabolism modify ∼20,000 bases of DNA per day in a single cell (4). p53 responds to stress conditions, but cell death or senescence is not always the most favorable scenario of p53 activation, and under low-stress conditions additional ROS production is not a desirable outcome, which contradicts the function of p53 as the guardian of genome. Accordingly, we have found that in conditions where p53 does not induce cell death such as under low doses of H2O2 or UV or in the ρ0 cells, p53 suppresses ROS production (98). The importance of p53 in ROS regulation under physiological conditions is supported by the data that p53-deficient cells or cells where p53 activity was compromised by MDM2, dominant-negative mutant of p53, or HPV-E6 protein have higher levels of ROS than cells with a normal status of p53 (98). These observations were supported by another group, which demonstrated that levels of p53 correlate with the resistance of HPV-positive cervical carcinoma cell lines to oxidative stress induced by hydrogen peroxide and knockdown of p53 by shRNA significantly sensitizes the cells to this oxidants (26).
We have shown that p53-deficient cells have increased levels of DNA oxidation and mutagenesis (98). Moreover, p53-deficient A549 tumor xenografts grow faster in nude mice than in a p53-proficient control. Supplementation with antioxidant N-acetyl cysteine (NAC) retards the growth of p53-deficient xenografts but does not affect growth of p53-proficient counterparts (98). The regulation of ROS by p53 is critical for its tumor suppressor activity. As previously described, p53-knockout mice die from cancers, mostly lymphomas (98). p53-knockout mice supplemented with the antioxidant NAC lived much longer and only 1 of 25 (4%) mice taken into the experiment developed lymphomas, whereas 90% of the p53-null mice not supplemented with antioxidants die from cancer by the same time (98). Genomic instability is an important hallmark of cancer (72) and p53 is the major controller of genomic stability (69), the function important for its tumor suppressor activity. Accordingly, the p53-negative lung fibroblasts isolated from mice maintained on a regular diet exhibited a much higher rate of karyotypic abnormalities and aneuploidy than the counterparts from mice supplemented with an NAC (98).
The antioxidant function of p53 depends on its role in transcriptional activation of antioxidant genes (98). The list of the p53-activated antioxidant genes includes the enzymes directly involved in ROS decomposition such as glutathione peroxidase 1 (GPX1) (52, 109), catalase (89), and manganese superoxide dismutase (52). p53 can also suppress ROS accumulation through other targets, including those involved in redox reaction, cell signaling, metabolism, and mitochondrial functions (121). The list of p53-inducible antioxidant proteins includes aldehyde dehydrogenase 4 (ALDH4), a mitochondrial-matrix nicotinamide adenine dinucleotide (NAD+)-dependent enzyme catalyzing the second step of the proline degradation; tumor protein 53-induced glycolysis and apoptosis regulator (TIGAR), the inhibitor of glycolysis lowering the fructose-2,6-bisphosphate levels (6); tumor protein 53-induced nuclear protein 1 (TP53NIP1), the protein involved in p53 phosphorylation (20); and glutaminase 2 (GLT2), a mitochondrial protein catalyzing the hydrolysis from glutamine to glutamate (51) (Fig. 4). The alternative mechanism of ROS regulation by p53 is activation of macroautophagy. Macroautophagy (later referred as autophagy) is a catabolic process where organelles and portions of cytoplasm are sequestered in double-membrane vesicles and followed to fusion with lysosomes for degradation (85). Autophagy controls cell integrity by removing malfunctioning organelles responsible for ROS generation and oxidative stress (7). Accordingly, autophagy is considered to be the mechanism of protection against genomic instability and carcinogenesis (67). p53 activates autophagy through transactivation of several proauthophagic genes such as damage-regulated autophagy modulator (24), ISG20L1 (29), or through inhibition of the target of rapamycin (TOR) pathway (see below) (34).
Sestrins
Interest in the role of p53 in antioxidant responses was kindled by identification of a novel Sestrin (Sesn) gene family involved in ROS regulation (17). In mammals, the Sesn family is composed of three members, Sesn1, Sesn2, and Sesn3 (18, 92, 117), with only one Sesn gene in Drosophila melanogaster and C. elegans (71). The first member of the family, Sesn1 or p53-activated gene 26 (PA26), was identified through screening of novel genes activated by p53 in tetracycline-regulated system (14). Sesn1 is activated by genotoxic stress in a p53-dependent manner (117). We have isolated the second member of the family Sesn2 or hypoxia-inducible gene 95 (Hi95) by microarray-based analysis of novel genes activated by prolonged hypoxia (18). Similar to Sesn1, Sesn2 is a stress responsive gene and both genes are activated by genotoxic and oxidative stress in a p53-dependent manner, whereas hypoxia can also induce Sesn2 through a p53-independent mechanism (18, 117).
Additionally, it was demonstrated that all three members of the Sesn family are activated by the transcription factor forkhead transcription factor (FoxO3A) and FoxO1, members of the FoxO gene family (88, 113). The FoxO genes encode transcriptional factors belonging to forkhead family and include four members at mammals: FoxO1, FoxO3A, FoxO4, and FoxO6, and only one gene at Drosophila (99). Similar to p53, FoxO proteins are activated by stress and regulate expression of the genes involved in regulation of cell cycle, metabolism, DNA repair, and antioxidant defense, protecting cells under stress, but some of the FoxO targets are proapototic genes responsible for inducing cell death under certain conditions (99). They also play a critical role in regulation of longevity (99). Transcription activity of FoxOs is negatively regulated by insulin and growth factors through phosphorylation by AKT and redistribution of protein from the nucleus to the cytoplasm (99). Among the members of the Sestrin family, Sesn3 has been shown the highest degree of activation by FoxO (88). The Drosophila genome contains only one Sestrin gene, dSesn, which is transcriptionally regulated by p53 and FoxO providing further evidence that Sestrin regulation is highly conserved in evolution (71).
The paradigm that the Sesn family encodes antioxidant proteins emerged from detailed analysis of primary and secondary structure of the proteins using PSI-BLAST and 3D-PSSM programs (3, 62). Indeed, we found sequence and structural similarity of N-terminal portion of the Sesns and a family of antioxidant bacterial proteins, which includes Mycobacterium tuberculosis AhpD protein (17). The homology spans at least five N-terminal α-helices of the conserved region of Sestrins and the C-terminal α-helical portion of AhpD (17). AhpD is a component of alkyl-hydroperoxide reductase participating in defense against ROS and reactive nitrogen species produced by host immune cells (13). The protein is responsible for regeneration of AhpC, a member of a conserved family of thiol-specific peroxidases called Prxs (13). Two critical cysteines (Cys) in the catalytic domain of AhpD are required for enzymatic activity of the protein (13), and only one of them is conserved between AhpD and Sesns, suggesting a different mechanism of action (17).
The mammalian Prx gene family is composed of six members, which encode proteins that occupy different compartments of the cell and regulate redox balance and cell signaling (37). The catalytic cycle passes through oxidation of catalytic Cys to Cys-SOH group, and formation of a disulfide bridge with resolving Cys and regeneration of Cys-SH groups with thioredoxin. Unlike the bacterial protein, mammalian Prx acquires sensitivity to oxidative inactivation of catalytical Cys during oxidative burst through the formation of Cys-SO2H group (37) (Fig. 5). Overoxidation of the catalytic Cys of the Prxs temporarily switch off an antioxidant firewall and allow transduction of the signaling pathways regulated by ROS (36, 37, 80). However, after signaling has been completed, the firewall should be restored. Two unrelated protein family Sesns and Sulfiredoxin take a part in regeneration of overoxidized forms of Prxs and are involved in regulation of redox balance in the cell (8, 17) (Fig. 5). Sesns play a role in regeneration of Prxs in many cell types, including different cancer cell lines and macrophages (17, 30). Moreover, stimulation of neuronal synaptic activity through the NMDA receptor involves overoxidation and reduction of Prxs in Sesn2/Srxn-dependent manner (90).
FIG. 5.
Prx cycle. During enzymatic cycles of Prxs involved in peroxide decomposition, catalytic cysteine is oxidized to SOH group and then resolved by another Cys-SH through the formation of a S-S bridge. Under oxidative burst, SOH groups can be overoxidized to SO2H, which is reduced by sulfiredoxins and Sestrins. Cys, cysteines; Prx, peroxiredoxin; Sesn, Sestrin.
Both Sesn1 and Sesn2 are critical for negative ROS regulation in different cancer cell lines and primary human and mouse fibroblasts in low stress conditions and under oxidative stress (17, 124). ROS accumulation and oxidative stress affect cell proliferation and viability. Silencing of either Sesn1 or Sesn2 in human fibroblasts significantly inhibit cell proliferation and accelerate cell senescence triggered by ROS accumulation (17). Cell death is accompanied by ROS production, which plays a potential role in amplification and completion of the process (60, 94). H2O2 treatment of Sesn2-silenced human cancer cells or Sesn2-deficient mouse lung fibroblasts induces higher levels of cell death, whereas the cells overexpressing Sesn1 or Sesn2 are more resistant to oxidative stress and hypoxia (17, 18)
Cancer cells are characterized by increased ROS production and permanent oxidative stress (5, 93). The consequences of ROS accumulation in cancer cells might be stimulation of cellular proliferation, promotion of mutagenesis, and genomic instability (5, 93). Accordingly, transformation of cells by the RAS oncogene causes accumulation of large amounts of ROS and treatment with NAC suppresses the transformed phenotype and inhibits proliferation of transformed cells (2, 5, 55). The mechanism underlying ROS production in response to Ras is not well characterized, but it was demonstrated recently that Sesns participate in this process (66). Activated forms of H-Ras and N-Ras negatively regulate expression of Sesn1 and Sesn3 genes and the inhibition of Sesn expression causes an oxidative burst, leading to increased levels DNA oxidation and chromosomal instability (66). Treatment of Ras-transformed cells with NAC or ectopic expression of Sesn1 and Sesn3 prevents accumulation of ROS and oxidative DNA damages (66). Strikingly, p53 determines the effect of Ras on ROS production, whereas elevation of ROS in response to Ras induction is transient in p53-positive cells, ROS levels stay increased permanently in p53-negative cells (66). So, inactivation of p53 might be a desirable outcome for transformed cells, allowing them to maintain high levels of ROS important for their proliferation. p53 is not the only regulator of Sesn expression in response to Ras. FoxO proteins play an important role in regulation of Sesns, and two Ras-activated proteins, AKT and ERK, negatively regulate FoxO through phosphorylation and degradation (88, 126). Accordingly, the levels of Sesn3 mRNA were significantly increased in the AKT-deficient fibroblasts as compared to AKT-positive counterparts (88). Thus, Ras potentially inhibits expression of Sesns through negative regulation of FoxO.
The opposite effect of tumor suppressors and oncogenes in regulation of Sesns indicates their potential role in tumor suppression. Tumor suppressor mechanisms operate on different stages of carcinogenesis, including transformation and tumor progression. To study the potential impact of Sesns on suppression of transformation, we examined the efficiency of transformation of Sesn2-deficient primary fibroblasts and their Sesn2-proficient counterparts in a colony-formation assay. Sesn2 knockout cells are more susceptible to Ras + E1A-induced transformation than their wild-type counterparts (16). Strikingly, the effect was reverted by treatment with antioxidant NAC, implying the importance of ROS regulation in suppression of transformation by Sesns (AVB, unpublished data). To assess the impact of Sesns in the regulation of tumor growth, we silenced either Sesn1 or Sesn2 in lung carcinoma A549 cells and examined the growth of A549 tumor xenografts in nude mice. Growth of Sesn1- and Sesn2-deficient tumors was significantly accelerated, and the effect of Sesn knockdown was attenuated by supplementation of the experimental mice with NAC (98).
p53 and TOR
The impact of p53 in redox control is not entirely confined by regulation of antioxidant or pro-oxidant genes, but might also constrain some signaling pathways associated with intrinsic ROS production. Mitochondria are the major source of ROS and pathways that affect mitochondria integrity and function and play critical roles in ROS regulation (102). Aging and age-associated diseases are characterized by ROS accumulation and mitochondrial dysfunction (102). The TOR kinase, a highly conserved protein belonging to the phosphatidylinositole kinase-related kinase (PIKK) subfamily (125), is the critical controller of mitochondrial function and aging, which is tightly linked to redox regulation (102). Activation of TOR through different mechanisms leads to accumulation of ROS, and rapamycin (a specific inhibitor of TOR) suppresses stimulatory effects of TOR on ROS production (21, 39, 65, 114). ROS can regulate TOR in both a positive and negative manner dependent on cell systems and treatment conditions (1, 10).
TOR forms two distinct protein complexes: TOR complex 1 (TORC1), which is rapamycin sensitive, and TOR complex 2 (TORC2), which is rapamycin insensitive (125) (Fig. 6). In mammals, both complexes share mammalian TOR (mTOR), mLST8, and Deptor subunits, but differ in other subunits that determine the specificity in function and regulation of these two complexes. TORC1 contains Raptor and PRAS40 and is responsible for regulation of translation, cell growth, proliferation, autophagy, ribosomal biogenesis, lipid biosyntesis, and metabolism (70, 125), whereas mTORC2 contains Rictor and mSIN1, and is involved in regulation of actin cytoskeleton and cell spreading (70). TORC1 and TORC2 also have different substrate specificity. TORC1 directly phosphorylates two proteins involved in regulation of cell translation: p70S6K and 4E-binding protein (4E-BP) (125). P70S6K phosphorylates S6 ribosomal protein potently regulating translation through this protein. Phosphorylation of 4E-BP by TORC1 relieves the inhibitory effect of 4E-BP on eIF-4E, an important factor for initiation of translation (125). Activated p70S6K also has inhibitory effects on TORC1 through phosphorylation and subsequent degradation of the insulin receptor substrate 1 (IRS1) protein, an upstream TORC1 regulator, providing a negative feedback loop (104). Two other TORC1 targets ATG13 and ULK1 are involved in the regulation of autophagy (49, 58). The AKT kinase and AKT-related serum- and glucocorticoid-induced protein kinase 1 (SGK1) were identified as substrates for TORC2, which make TORC2 a potential regulator of metabolism and cell viability (70).
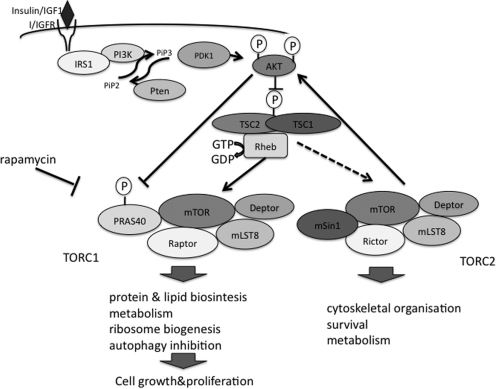
FIG. 6.
Regulation of the mTOR signaling pathway by insulin and growth factors. Insulin/IGF1 activates PI3K through the recruitment and phosphorylation of IRS1. PI3K generates PIP3 [PtdIns(3,4,5)P3] from PIP2 [PtdIns(4,5)P2] through the recruitment of PDK1 to the cytoplasmic membrane, which stimulates AKT phosphorylation by this kinase. Another AKT kinase is TORC2, regulated through a yet to be defined mechanism. AKT phosphorylates and inhibits GAP activity of TSC2, leading to Rheb inhibition and mTORC1 suppression. IGF1, insulin-like growth factor 1; IRS1, insulin receptor substrate 1; mTOR, mammalian target of rapamycin; PDK, phosphoinositide-dependent protein kinase; PI3K, phosphatidylinositol-3-kinase; PIP, phosphatidylinositol phosphate; Rheb, Ras homolog enriched in brain; TSC2, tuberoses scleroses complex 2.
These two TOR-containing complexes are regulated through different mechanisms (127). The mechanisms of TORC2 regulation are yet to be defined, and TORC1 is regulated through integrated signals from growth factors, nutrient, and stress. The critical component of TORC1 regulation is a GTPase called Ras homolog enriched in brain (Rheb), which is found in a complex with mTOR and, loaded with GTP, activates TORC1 through a poorly established mechanism. Upstream regulation of Rheb and the nodal point of regulation of the Rheb-TORC1 axis by many intracellular signaling pathways is the tuberoses scleroses complex (TSC) composed of TSC1 and TSC2 proteins. The TSC2 subunit of the complex is the GTP activating protein for Rheb, whereas TSC1 is important for stabilization of the TSC2 protein, presumably inhibiting its degradation by HERC1 E3 ubiquitin ligase (22).
Activity of the TSC1:TSC2 complex is regulated through phosphorylation by numerous upstream kinases such as AKT, ERK, RSK, AMPK, and GSK3 (70) in response to stimulation with growth factors, mitogens, and stress insults. The insulin/insulin-like growth factor 1 (IGF1) pathway is an evolutionary conserved system of regulation cell growth metabolism and aging, which exerts many of its functions through stimulation of TORC1 in a TSC1:TSC2-dependent manner. Stimulation of insulin/IGF1 receptor with insulin/IGF1 leads to phosphorylation and recruitment of IRS1 proteins and activation of phosphoinositide-3-kinase (PI3K), which in turn activates the members of the AKT family (AKT1, AKT2, and AKT3) through production of phosphatdylinositol-3,4,5-triphosphates (PtdIns(3,4,5)P3) (79). PtdIns(3,4,5)P3 induce translocation of AKT through its pleckstrin homology domain (PHD) to the cytoplasmic membrane where AKT is phosphorylated and activated by upstream phosphatydil phosphoinositide-dependent kinases PDK1 on Thr308 and PDK2 on Ser473. It was recently shown that PDK2 is TORC2 (101). PI3K-dependent AKT activation is negatively regulated by phosphatase and tensin homolog (PTEN), a lipid phosphatase counteracting the effect of PI3K and converting PtdIns(3,4,5)P3 back to PtdIns(4,5)P2 (79). Activated AKT phosphorylates multiple sites on TSC2 and inhibits its activity (54), relieving the negative effect of the TSC1:TSC2 complex on Rheb. Akt can also modulate activity of TORC1 through direct phosphorylation and dissociation of TORC1 inhibitor RASP40 (70) (Fig. 6).
Although the insulin/IGF1 pathway is critical for maintaining activity of TORC1, many stress insults, including nutrient deficiency, hypoxia, oxidative stress, and DNA damage, counteract effects of insulin and growth factors and inhibit TORC1 through activation of TSC2 (53). Many stress stimuli activate AMPK, which plays a critical role in inhibition of TORC1 through phosphorylation of TSC2 and Raptor (53, 105). AMPK is composed of three subunits, AMPKα, AMPKβ, and AMPKγ. The mammalian genome encodes two AMPKα subunits (AMPKα1 and AMPKα2), two AMPKβ subunits (AMPKβ1 and AMPKβ2), and three AMPKγ subunits (AMPKγ1, AMPKγ2, and AMPKγ) (105, 122). AMPK activity is positively regulated through phosphorylation of catalitical α-subunit on Thr172 by several upstream kinases such as LKB1, Ca/calmodulin-dependent kinases α and β, and TGF-β-activated kinase 1 (122). LKB1 is the major AMPK kinase ubiquitously expressed in different cell types that plays a critical role in activation of AMPK under low energy conditions in response to AMP (105). Activity of AMPK is also regulated through protein–protein interaction with some proteins such as kinase suppressor of Ras 2, which might work as scaffolding protein for AMPK (23).
p53 induces phosphorylation of the AMPKα subunit, providing a link between stress and TORC1 inhibition (16, 28, 34). It was demonstrated by several laboratories that DNA-damaging insults suppress translation (11, 48, 111) and induce autophagy (34), two well-established TORC1-regulated processes (96, 111, 125). We have shown that p53 regulates AMPK-TORC1 axis through induction of Sesn1 and Sesn2 genes (16). Being previously characterized as antioxidant proteins, Sesns were considered to be suppressors of ROS-dependent pathways, but we found that Sesns inhibit TORC1 through a redox-independent mechanism in a TSC2- and AMPK-dependent manner (16, 71). Ectopic expression of Sesn1 and Sesn2 induces phosphorylation of AMPKα subunit and stimulates phosphorylation of TSC2 by AMPK (16) (Fig. 7). We observed that Sesns form complexes with AMPK, TSC1, and TSC2 (16). Moreover, AMPK, TSC1, and TSC2 were coprecipitated with Sesn2, implying that Sesns can activate AMPK and stimulate TSC2 phosphorylation through protein–protein interactions working as scaffolding proteins (16), which can potentiate phosphorylation of the AMPKα subunit by upstream AMPK kinase and/or de-phosphorylation by upstream phosphatase. Inhibition of TORC1 through Sesns might cooperate with other mechanisms of TORC1 regulation by p53. It was demonstrated that p53 also activate negative modulators of TORC1 such as TSC2, AMPKβ, PTEN, and IGF1-binding protein 3 (IGF-BP3) (15, 32). Also, Polo-like kinase 2 (PLK2) kinase has been described recently as a p53 target, which interacts with the TSC1:TSC2 complex and inhibit TORC1 activity (82).
FIG. 7.
Regulation of mTOR pathway by Sestrins. Sestrins, induced by many stress insults through p53, interact with the TSC1:TSC2 complex and activate AMPK. This results in TSC2 phosphorylation and stimulation of TSC2 GAP activity, followed by inhibition of TORC1 and TORC1-dependent processes.
Regulation of AMPK in response to p53 activation provides another mechanism of suppression of ROS accumulation by Sesns. As shown in many studies, AMPK plays important roles in antioxidant defense (68, 74, 91, 106) and protects cells from oxidative stress induced by fatty acids (64). Mitochondria are the major source of ROS in cell and regulation of mitochondrial function by AMPK is important for the antioxidant activity of this kinase (106). Regulation of mitochondrial function and ROS production by AMPK potentially involves inhibition of mTOR, which modulates mitochondrial function and ROS through two mechanisms. First, mTOR enhances mitochondrial respiration (25, 97, 103). Second, mTOR interacts with mitochondrial proteins involved in regulation of mitochondrial functions such as Bcl-xl and VDAC1 (97), which can potentiate oxidative phosphorylation (OXPHOS) through the regulation of substrate permeability (97). Moreover, mTOR stimulates mitochondrial biogenesis (25). ROS are generated as by-products of mitochondrial respiration through leakage of electrons from the OXPHOS chain. Stimulation of OXPHOS by mTOR can cause ROS accumulation (35). Another mechanism of control of ROS production by the AMPK-TORC1 pathway involves activation of autophagy (34, 128). Malfunctioned mitochondria and peroxisomes produce excess ROS, and autophagy can be responsible for turnover of defective organelles protecting the cell from oxidative stress (36, 63, 128). In agreement with its role in regulation of the AMPK-TORC1 pathway, Sesn2 is a positive regulator of autophagy (AVB, unpublished observation), and is involved in activation of autophagy in response to nutrient depletion, rapamycin, lithium, and thapsigargin (76). p53 also induces autophagy through transcriptional activation of damage-regulated autophagy modulator, a lysosomal protein that supports autophagy (24). Cytoplasmic p53 inhibit autophagy (110), providing a negative feedback loop for this process.
p53, Sesns, and Aging
Our response to stress determines our lifespan expectancy (100). Many exogenous stress insults cause increased ROS production inside the cell through damage of mitochondria and some other organelles (50, 78, 116). Aging and different age-associated syndromes, including cancer, diabetes, neurodegenerative diseases, muscle dystrophy, and chronic inflammation, are characteristics of oxidative stress (36), and it was proposed by Harman in 1956 that ROS is a driving force of aging (43). In spite of the evidence for and against this theory (10), the detrimental role of ROS in many age-associated diseases is widely accepted (5, 31, 35). ROS production is tightly linked with the activity of TOR, a well-characterized aging regulator involved in many age-related pathologies (10, 102). Inhibition of TORC1 by the specific TORC1 inhibitor rapamycin extends lifespan in yeast, worms, flies, and mice (45, 57, 59, 61, 108). Inhibition of insulin/IGF1 pathway and caloric restriction, two major mechanisms of lifespan extension in different organisms, are linked to the suppression of TORC1 activity, considering the central role of TORC1 in regulation of aging by many factors (87).
p53 is a critical regulator of lifespan and its inactivation causes early death from cancer (27). To study the role of p53 in aging, several mouse models were applied with much controversy. Some mouse strains with increased p53 activity due to expression of hypermorphic p53 allele, temperature-sensitive p53 mutant, or a splice form of p53 resulted in accelerating aging but increased resistance to cancer (75, 115). In other mouse models with an extra copy of p53, or hypomorphic Mdm2 gene mutation, normal lifespan was not affected, but cancer incidence was decreased (41, 84). Finally, recent work from the Serrano lab has shown that a genetically engineered mouse strain containing an extra copy of the p53 and ARF genes (super-Arf/super-p53 or s-Arf/p53 mice) is characterized by decreased levels of age-associated damage and cancer resistance (82). Altogether, this indicates that the rigor of control of p53 status and potential contribution from the other pathways may determine the outcome of p53 activation, which might have a positive or negative impact on aging.
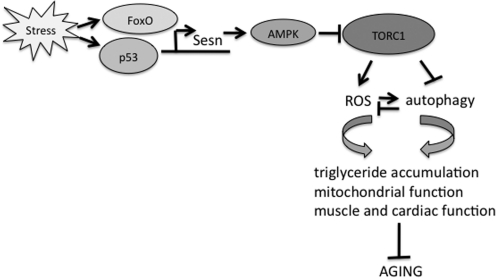
s-Arf/p53 mice have decreased levels of oxidative stress as compared to control and show increased expression of Sesn1 and Sesn2 genes (82). As mentioned before Sesn1 and Sesn2 genes are involved in the control of ROS accumulation and protection against oxidative stress, but also in regulation of the AMPK-TORC1 pathway. Not properly controlled TORC1 can accelerate aging through increased rate of metabolism, translation, and negative regulation of autophagy (9, 10, 100). Accordingly, inhibition of autophagy shortens lifespan and causes muscle atrophy and neurodegeneration, two age-associated phenomenon (40, 81, 100). The levels of autophagic proteolysis decrease with age, weakening stress responses and providing favorable soil for age-linked diseases (100). The function of the autophagy regulator p53 and activation of p53-dependent genes declines with age, potentially affecting autophagy (33). Sesns, regulated positively by p53 (18, 117) and negatively by insulin-AKT pathway (88), might mediate effects of these pathway on regulation of aging through control of ROS and TORC1 activity (112). Accordingly, deletion the only Sesn gene (dSesn) in Drosophila accelerates age-related pathologies such as triglyceride accumulation, mitochondrial dysfunction, muscle degeneration, and cardiac malfunction in many aspects phenocoping the effect of inactivation of an important autophagy regulator ATG1 (71) (Fig. 8). All age-related phenotypes of dSesn-deficient flies were prevented by treatment with AMPK activators or TORC1 inhibitor rapamycin (71). The muscles of dSesn null flies also exhibit elevation of ROS and accumulation of ubiquitinated protein aggregates, two signatures of impaired autophagy potentially contributing to the aging phenotype (71). To examine whether Sesn contribute to aging phenotype and oxidative stress through its intrinsic enzymatic activity or indirectly through the AMPK-TORC1 pathway we reconstituted dSesn null flies with the redox-deficient S125C mutant (16). This mutant is proficient in regulation of the AMPK-TORC1 pathway and is able to rescue many of the age accelerating phenotypes of dSesn null flies, such as triglyceride accumulation and progressive muscle degeneration (71). These data support the idea that regulation of the AMPK-TORC1 axis and its link with redox balance is the predominant mechanism utilized by Sesns to protect against aging and aging-related pathologies at Drosophila. Nonetheless, we cannot rule out the possibility that Sesns have AMPK and TORC1-independent mechanisms of regulation of aging in vertebrates. Accordingly, reconstitution of Sesn null mice with the redox-deficient mutant will shed light on the role of intrinsic antioxidant activity of Sesns on aging at mammals.
FIG. 8.
Role Sestrins in aging. Sestrins activated by various stresses in a p53- or forkhead transcription factor-dependent manner regulate mTOR signaling, resulting in protection from aging-related dysfunctions.
Cancer is a disease of aging and similar mechanisms might provide protection in both cases. An elevated oxidative status of aging cells can contribute to carcinogenesis facilitating transformation, genomic instability, invasiveness, and angiogenesis (5). In accordance, some mouse strains with deficiency of antioxidant enzymes are cancer prone (95). The antioxidant function of p53 seems to be important for its antiaging and tumor suppressor function, and Sestrins might be effectors of both processes. Decline of p53 function with age can repress Sesn1 and Sesn2 expression, weakening protection from oxidative stress and elimination of age-associated damages (33).
Concluding Remarks
The last years have provided more and more data that the major tumor suppressor p53 controls cancer not only through elimination of premalignant cells, but also through regulation of stress response, mitochondrial function, and cell signaling. Among them, regulation of ROS and TORC1 are two closely related processes, which potentiate aging and age-related diseases, including cancer. The novel p53-regulated antioxidant Sesn gene family involved in control of the AMPK-TORC1 axis and mitochondrial function might be a first line of defense against accumulation of detrimental damages, which potentiate aging and fuel age-associated diseases. The mouse models with deficiency in members of the Sesn family will allow us to better understand the impact of Sesns in stress response, aging, and age-related disorders.
Abbreviations Used
- 4E-BP
4E-binding protein
- ALDH4
aldehyde dehydrogenase 4 gene
- AMPK
AMP-activated protein kinase
- ATM
ataxia telangiectasia mutated
- Cys
Cystein
- FoxO
forkhead transcription factor
- GPX1
gluthatione peroxidase 1
- Hi95
hypoxia-inducible gene 95
- IGF1
insulin-like growth factor 1
- IGF-BP3
IGF1-binding protein 3
- IRS
insulin receptor substrate
- Mdm2
murine double minute 2
- MnSOD
manganese superoxide dismutase
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-L-cysteine
- NAD(P)H
nicotinamide adenine dinucleotide phosphate
- OXPHOS
oxidative phosphorylation
- PA26
p53-activated protein 26
- PDK
phosphoinositide-dependent protein kinase
- PI3K
phosphatidylinositol-3-kinase
- PIP
phosphatidylinositol phosphate
- PLK2
Polo-like kinase 2
- Prx
peroxiredoxin
- PTEN
phosphatase and tensin homolog
- Redox
reduction and oxidation
- Rheb
Ras homolog enriched in brain
- ROS
reactive oxygen species
- S6K
S6 kinase
- Sesn
Sestrin
- SGK
serum- and glucocorticoid-induced protein kinase 1
- SOD
superoxide dismutase
- TIGAR
tumor protein 53-induced glycolysis and apoptosis regulator
- TP53INP1
tumor protein 53-inducible nuclear protein 1
- TOR
target of rapamycin
- TORC1
TOR complex 1
- TORC2
TOR complex 2
- TSC
tuberoses scleroses complex protein
Acknowledgment
This work was supported by grant from NIH NIDDK pathway to independence award (K99/R00) Grant#1K99DK082080.
References
- 1.Alexander A. Cai SL. Kim J. Nanez A. Sahin M. MacLean KH. Inoki K. Guan KL. Shen J. Person MD. Kusewitt D. Mills GB. Kastan MB. Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrova AY. Kopnin PB. Vasiliev JM. Kopnin BP. ROS up-regulation mediates Ras-induced changes of cell morphology and motility. Exp Cell Res. 2006;312:2066–2073. doi: 10.1016/j.yexcr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Altschul SF. Madden TL. Schaffer AA. Zhang J. Zhang Z. Miller W. Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman KB. Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 5.Behrend L. Henderson G. Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 6.Bensaad K. Tsuruta A. Selak MA. Vidal MN. Nakano K. Bartrons R. Gottlieb E. Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Bergamini E. Autophagy: a cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Mol Aspects Med. 2006;27:403–410. doi: 10.1016/j.mam.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Biteau B. Labarre J. Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 9.Bjedov I. Toivonen JM. Kerr F. Slack C. Jacobson J. Foley A. Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- 11.Braunstein S. Badura ML. Xi Q. Formenti SC. Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29:5645–5656. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown CJ. Lain S. Verma CS. Fersht AR. Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 13.Bryk R. Lima CD. Erdjument-Bromage H. Tempst P. Nathan C. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science. 2002;295:1073–1077. doi: 10.1126/science.1067798. [DOI] [PubMed] [Google Scholar]
- 14.Buckbinder L. Talbott R. Seizinger BR. Kley N. Gene regulation by temperature-sensitive p53 mutants: identification of p53 response genes. Proc Natl Acad Sci U S A. 1994;91:10640–10644. doi: 10.1073/pnas.91.22.10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckbinder L. Talbott R. Velasco-Miguel S. Takenaka I. Faha B. Seizinger BR. Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 16.Budanov AV. Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budanov AV. Sablina AA. Feinstein E. Koonin EV. Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 18.Budanov AV. Shoshani T. Faerman A. Zelin E. Kamer I. Kalinski H. Gorodin S. Fishman A. Chajut A. Einat P. Skaliter R. Gudkov AV. Chumakov PM. Feinstein E. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 19.Buschmann T. Potapova O. Bar-Shira A. Ivanov VN. Fuchs SY. Henderson S. Fried VA. Minamoto T. Alarcon-Vargas D. Pincus MR. Gaarde WA. Holbrook NJ. Shiloh Y. Ronai Z. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol Cell Biol. 2001;21:2743–2754. doi: 10.1128/MCB.21.8.2743-2754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cano CE. Gommeaux J. Pietri S. Culcasi M. Garcia S. Seux M. Barelier S. Vasseur S. Spoto RP. Pebusque MJ. Dusetti NJ. Iovanna JL. Carrier A. Tumor protein 53-induced nuclear protein 1 is a major mediator of p53 antioxidant function. Cancer Res. 2009;69:219–226. doi: 10.1158/0008-5472.CAN-08-2320. [DOI] [PubMed] [Google Scholar]
- 21.Chen C. Liu Y. Liu R. Ikenoue T. Guan KL. Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong-Kopera H. Inoki K. Li Y. Zhu T. Garcia-Gonzalo FR. Rosa JL. Guan KL. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J Biol Chem. 2006;281:8313–8316. doi: 10.1074/jbc.C500451200. [DOI] [PubMed] [Google Scholar]
- 23.Costanzo-Garvey DL. Pfluger PT. Dougherty MK. Stock JL. Boehm M. Chaika O. Fernandez MR. Fisher K. Kortum RL. Hong EG. Jun JY. Ko HJ. Schreiner A. Volle DJ. Treece T. Swift AL. Winer M. Chen D. Wu M. Leon LR. Shaw AS. McNeish J. Kim JK. Morrison DK. Tschop MH. Lewis RE. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10:366–378. doi: 10.1016/j.cmet.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crighton D. Wilkinson S. O'Prey J. Syed N. Smith P. Harrison PR. Gasco M. Garrone O. Crook T. Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham JT. Rodgers JT. Arlow DH. Vazquez F. Mootha VK. Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 26.Ding B. Chi SG. Kim SH. Kang S. Cho JH. Kim DS. Cho NH. Role of p53 in antioxidant defense of HPV-positive cervical carcinoma cells following H2O2 exposure. J Cell Sci. 2007;120:2284–2294. doi: 10.1242/jcs.002345. [DOI] [PubMed] [Google Scholar]
- 27.Donehower LA. Harvey M. Slagle BL. McArthur MJ. Montgomery CA., Jr. Butel JS. Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 28.Drakos E. Atsaves V. Li J. Leventaki V. Andreeff M. Medeiros LJ. Rassidakis GZ. Stabilization and activation of p53 downregulates mTOR signaling through AMPK in mantle cell lymphoma. Leukemia. 2009;23:784–790. doi: 10.1038/leu.2008.348. [DOI] [PubMed] [Google Scholar]
- 29.Eby KG. Rosenbluth JM. Mays DJ. Marshall CB. Barton CE. Sinha S. Johnson KN. Tang L. Pietenpol JA. ISG20L1 is a p53 family target gene that modulates genotoxic stress-induced autophagy. Mol Cancer. 9:95. doi: 10.1186/1476-4598-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essler S. Dehne N. Brune B. Role of sestrin2 in peroxide signaling in macrophages. FEBS Lett. 2009;583:3531–3535. doi: 10.1016/j.febslet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Farooqui T. Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130:203–215. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Feng Z. Hu W. de Stanchina E. Teresky AK. Jin S. Lowe S. Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z. Hu W. Teresky AK. Hernando E. Cordon-Cardo C. Levine AJ. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci U S A. 2007;104:16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Z. Zhang H. Levine AJ. Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 36.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 37.Fourquet S. Huang ME. D'Autreaux B. Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid Redox Signal. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs SY. Adler V. Pincus MR. Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci U S A. 1998;95:10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimoto A. Akifusa S. Kamio N. Hirofuji T. Nonaka K. Yamashita Y. Involvement of mTOR in globular adiponectin-induced generation of reactive oxygen species. Free Radic Res. 2010;44:128–134. doi: 10.3109/10715760903348328. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Arencibia M. Hochfeld WE. Toh PP. Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol. 2010;21:691–698. doi: 10.1016/j.semcdb.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Cao I. Garcia-Cao M. Martin-Caballero J. Criado LM. Klatt P. Flores JM. Weill JC. Blasco MA. Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gius D. Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 43.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 44.Harris SL. Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 45.Harrison DE. Strong R. Sharp ZD. Nelson JF. Astle CM. Flurkey K. Nadon NL. Wilkinson JE. Frenkel K. Carter CS. Pahor M. Javors MA. Fernandez E. Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey M. McArthur MJ. Montgomery CA., Jr. Butel JS. Bradley A. Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993;5:225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- 47.Hoh J. Jin S. Parrado T. Edington J. Levine AJ. Ott J. The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci U S A. 2002;99:8467–8472. doi: 10.1073/pnas.132268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horton LE. Bushell M. Barth-Baus D. Tilleray VJ. Clemens MJ. Hensold JO. p53 activation results in rapid dephosphorylation of the eIF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase and inhibition of translation initiation. Oncogene. 2002;21:5325–5334. doi: 10.1038/sj.onc.1205662. [DOI] [PubMed] [Google Scholar]
- 49.Hosokawa N. Hara T. Kaizuka T. Kishi C. Takamura A. Miura Y. Iemura S. Natsume T. Takehana K. Yamada N. Guan JL. Oshiro N. Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoye AT. Davoren JE. Wipf P. Fink MP. Kagan VE. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 51.Hu W. Zhang C. Wu R. Sun Y. Levine A. Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A. 2010;107:7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussain SP. Amstad P. He P. Robles A. Lupold S. Kaneko I. Ichimiya M. Sengupta S. Mechanic L. Okamura S. Hofseth LJ. Moake M. Nagashima M. Forrester KS. Harris CC. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 53.Inoki K. Guan KL. Tuberous sclerosis complex, implication from a rare genetic disease to common cancer treatment. Hum Mol Genet. 2009;18:R94–R100. doi: 10.1093/hmg/ddp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoki K. Li Y. Zhu T. Wu J. Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 55.Irani K. Xia Y. Zweier JL. Sollott SJ. Der CJ. Fearon ER. Sundaresan M. Finkel T. Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 56.Jones RG. Plas DR. Kubek S. Buzzai M. Mu J. Xu Y. Birnbaum MJ. Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 57.Juarez MT. Twigg RW. Timmermans MC. Specification of adaxial cell fate during maize leaf development. Development. 2004;131:4533–4544. doi: 10.1242/dev.01328. [DOI] [PubMed] [Google Scholar]
- 58.Jung CH. Jun CB. Ro SH. Kim YM. Otto NM. Cao J. Kundu M. Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaeberlein M. Powers RW., 3rd Steffen KK. Westman EA. Hu D. Dang N. Kerr EO. Kirkland KT. Fields S. Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 60.Kamata H. Honda S. Maeda S. Chang L. Hirata H. Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 61.Kapahi P. Zid BM. Harper T. Koslover D. Sapin V. Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelley LA. MacCallum RM. Sternberg MJ. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J Mol Biol. 2000;299:499–520. doi: 10.1006/jmbi.2000.3741. [DOI] [PubMed] [Google Scholar]
- 63.Kim I. Rodriguez-Enriquez S. Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JE. Kim YW. Lee IK. Kim JY. Kang YJ. Park SY. AMP-activated protein kinase activation by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) inhibits palmitate-induced endothelial cell apoptosis through reactive oxygen species suppression. J Pharmacol Sci. 2008;106:394–403. doi: 10.1254/jphs.fp0071857. [DOI] [PubMed] [Google Scholar]
- 65.Kim JH. Chu SC. Gramlich JL. Pride YB. Babendreier E. Chauhan D. Salgia R. Podar K. Griffin JD. Sattler M. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 66.Kopnin PB. Agapova LS. Kopnin BP. Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroemer G. White E. Autophagy for the avoidance of degenerative, inflammatory, infectious, and neoplastic disease. Curr Opin Cell Biol. 2010;22:121–123. doi: 10.1016/j.ceb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon YN. Shin SM. Cho IJ. Kim SG. Oxidized metabolites of oltipraz exert cytoprotective effects against arachidonic acid through AMP-activated protein kinase-dependent cellular antioxidant effect and mitochondrial protection. Drug Metab Dispos. 2009;37:1187–1197. doi: 10.1124/dmd.108.025908. [DOI] [PubMed] [Google Scholar]
- 69.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 70.Laplante M. Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JH. Budanov AV. Park EJ. Birse R. Kim TE. Perkins GA. Ocorr K. Ellisman MH. Bodmer R. Bier E. Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lengauer C. Kinzler KW. Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 73.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 74.Li XN. Song J. Zhang L. LeMaire SA. Hou X. Zhang C. Coselli JS. Chen L. Wang XL. Zhang Y. Shen YH. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maier B. Gluba W. Bernier B. Turner T. Mohammad K. Guise T. Sutherland A. Thorner M. Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maiuri MC. Malik SA. Morselli E. Kepp O. Criollo A. Mouchel PL. Carnuccio R. Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- 77.Malkin D. Li FP. Strong LC. Fraumeni JF., Jr. Nelson CE. Kim DH. Kassel J. Gryka MA. Bischoff FZ. Tainsky MA, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 78.Mandelker L. Introduction to oxidative stress and mitochondrial dysfunction. Vet Clin North Am Small Anim Pract. 2008;38:1–30. doi: 10.1016/j.cvsm.2007.10.005. v, [DOI] [PubMed] [Google Scholar]
- 79.Manning BD. Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martindale JL. Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 81.Masiero E. Agatea L. Mammucari C. Blaauw B. Loro E. Komatsu M. Metzger D. Reggiani C. Schiaffino S. Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 82.Matthew EM. Hart LS. Astrinidis A. Navaraj A. Dolloff NG. Dicker DT. Henske EP. El-Deiry WS. The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions. Cell Cycle. 2009;8:4168–4175. doi: 10.4161/cc.8.24.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 84.Mendrysa SM. O'Leary KA. McElwee MK. Michalowski J. Eisenman RN. Powell DA. Perry ME. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20:16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizushima N. Yoshimori T. Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murray-Zmijewski F. Lane DP. Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 87.Narasimhan SD. Yen K. Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657–R666. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nogueira V. Park Y. Chen CC. Xu PZ. Chen ML. Tonic I. Unterman T. Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Connor JC. Wallace DM. O'Brien CJ. Cotter TG. A novel antioxidant function for the tumor-suppressor gene p53 in the retinal ganglion cell. Invest Ophthalmol Vis Sci. 2008;49:4237–4244. doi: 10.1167/iovs.08-1963. [DOI] [PubMed] [Google Scholar]
- 90.Papadia S. Soriano FX. Leveille F. Martel MA. Dakin KA. Hansen HH. Kaindl A. Sifringer M. Fowler J. Stefovska V. McKenzie G. Craigon M. Corriveau R. Ghazal P. Horsburgh K. Yankner BA. Wyllie DJ. Ikonomidou C. Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park IJ. Lee YK. Hwang JT. Kwon DY. Ha J. Park OJ. Green tea catechin controls apoptosis in colon cancer cells by attenuation of H2O2-stimulated COX-2 expression via the AMPK signaling pathway at low-dose H2O2. Ann N Y Acad Sci. 2009;1171:538–544. doi: 10.1111/j.1749-6632.2009.04698.x. [DOI] [PubMed] [Google Scholar]
- 92.Peeters H. Debeer P. Bairoch A. Wilquet V. Huysmans C. Parthoens E. Fryns JP. Gewillig M. Nakamura Y. Niikawa N. Van de Ven W. Devriendt K. PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse. Hum Genet. 2003;112:573–580. doi: 10.1007/s00439-003-0917-5. [DOI] [PubMed] [Google Scholar]
- 93.Pelicano H. Carney D. Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Polyak K. Xia Y. Zweier JL. Kinzler KW. Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 95.Pouyet L. Carrier A. Mutant mouse models of oxidative stress. Transgenic Res. 2010;19:155–164. doi: 10.1007/s11248-009-9308-6. [DOI] [PubMed] [Google Scholar]
- 96.Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst) 2004;3:927–934. doi: 10.1016/j.dnarep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Ramanathan A. Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci U S A. 2009;106:22229–22232. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sablina AA. Budanov AV. Ilyinskaya GV. Agapova LS. Kravchenko JE. Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salih DA. Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salminen A. Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 101.Sarbassov DD. Guertin DA. Ali SM. Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 102.Schieke SM. Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387:1357–1361. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- 103.Schieke SM. Phillips D. McCoy JP., Jr. Aponte AM. Shen RF. Balaban RS. Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 104.Shah OJ. Hunter T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol. 2006;26:6425–6434. doi: 10.1128/MCB.01254-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin SM. Cho IJ. Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 107.Srivastava S. Zou ZQ. Pirollo K. Blattner W. Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348:747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- 108.Sudarsanam S. Johnson DE. Functional consequences of mTOR inhibition. Curr Opin Drug Discov Devel. 2010;13:31–40. [PubMed] [Google Scholar]
- 109.Tan M. Li S. Swaroop M. Guan K. Oberley LW. Sun Y. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem. 1999;274:12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- 110.Tasdemir E. Maiuri MC. Galluzzi L. Vitale I. Djavaheri-Mergny M. D'Amelio M. Criollo A. Morselli E. Zhu C. Harper F. Nannmark U. Samara C. Pinton P. Vicencio JM. Carnuccio R. Moll UM. Madeo F. Paterlini-Brechot P. Rizzuto R. Szabadkai G. Pierron G. Blomgren K. Tavernarakis N. Codogno P. Cecconi F. Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tee AR. Proud CG. DNA-damaging agents cause inactivation of translational regulators linked to mTOR signalling. Oncogene. 2000;19:3021–3031. doi: 10.1038/sj.onc.1203622. [DOI] [PubMed] [Google Scholar]
- 112.Topisirovic I. Sonenberg N. Cell biology. Burn out or fade away? Science. 2010;327:1210–1211. doi: 10.1126/science.1187497. [DOI] [PubMed] [Google Scholar]
- 113.Tran H. Brunet A. Grenier JM. Datta SR. Fornace AJ., Jr. DiStefano PS. Chiang LW. Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 114.Tunon MJ. Sanchez-Campos S. Gutierrez B. Culebras JM. Gonzalez-Gallego J. Effects of FK506 and rapamycin on generation of reactive oxygen species, nitric oxide production and nuclear factor kappa B activation in rat hepatocytes. Biochem Pharmacol. 2003;66:439–445. doi: 10.1016/s0006-2952(03)00288-0. [DOI] [PubMed] [Google Scholar]
- 115.Tyner SD. Venkatachalam S. Choi J. Jones S. Ghebranious N. Igelmann H. Lu X. Soron G. Cooper B. Brayton C. Hee Park S. Thompson T. Karsenty G. Bradley A. Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 116.Valerie K. Yacoub A. Hagan MP. Curiel DT. Fisher PB. Grant S. Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 117.Velasco-Miguel S. Buckbinder L. Jean P. Gelbert L. Talbott R. Laidlaw J. Seizinger B. Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- 118.Victor VM. Apostolova N. Herance R. Hernandez-Mijares A. Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: mitochondria-targeted antioxidants as potential therapy. Curr Med Chem. 2009;16:4654–4667. doi: 10.2174/092986709789878265. [DOI] [PubMed] [Google Scholar]
- 119.Vousden KH. Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 120.Vousden KH. Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 121.Vousden KH. Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 122.Wang W. Guan KL. AMP-activated protein kinase and cancer. Acta Physiol (Oxf) 2009;196:55–63. doi: 10.1111/j.1748-1716.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 123.Wei CL. Wu Q. Vega VB. Chiu KP. Ng P. Zhang T. Shahab A. Yong HC. Fu Y. Weng Z. Liu J. Zhao XD. Chew JL. Lee YL. Kuznetsov VA. Sung WK. Miller LD. Lim B. Liu ET. Yu Q. Ng HH. Ruan Y. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 124.Wempe F. De-Zolt S. Koli K. Bangsow T. Parajuli N. Dumitrascu R. Sterner-Kock A. Weissmann N. Keski-Oja J. von Melchner H. Inactivation of sestrin 2 induces TGF-{beta} signaling and partially rescues pulmonary emphysema in a mouse model of COPD. Dis Model Mech. 2010;3:246–253. doi: 10.1242/dmm.004234. [DOI] [PubMed] [Google Scholar]
- 125.Wullschleger S. Loewith R. Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 126.Yang JY. Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang Q. Inoki K. Kim E. Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yen WL. Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]