Abstract
The role of adventitia-derived reactive oxygen species (ROS) in vascular disease and impaired vascular relaxation is not clear. Based on robust adventitial ROS generation and effects on MAPK involvement in vascular dysfunction, we hypothesized that adventitia-derived ROS hydrogen peroxide (H2O2) impairs vascular relaxation through activation of medial smooth muscle p38 MAPK. By using a novel in vivo model, the adventitial surface of rat carotid arteries was bathed in situ for 90 min with vehicle, angiotensin II (AngII; 500 nM), AngII+H2O2-scavenger catalase (3,000 U/ml), AngII+p38 MAPK inhibitor SB203580 (10 μM), or AngII+superoxide dismutase (SOD; 150 U/ml). After these in vivo treatments, ex vivo tone measurements on isolated vessels revealed that periadventitial application of AngII impaired both acetylcholine-induced (endothelium-dependent) and sodium nitroprusside-induced (endothelium-independent) relaxations. In vivo coincubation with catalase or SB203580 significantly improved, but SOD exacerbated AngII-induced impairment of in vitro endothelium-dependent and -independent vascular relaxations. Western blots of vascular media, separated from the adventitia, demonstrated increased medial p38 MAPK activation and decreased medial phosphatase SHP-2 activity in AngII-treated vessels. These effects were reversed by in vivo periadventitial addition of catalase. These findings provide the first evidence that adventitia-derived H2O2 participates in vascular dysfunction through p38 MAPK activation and SHP-2 inhibition. Antioxid. Redox Signal. 15, 1507–1515.
Introduction
Numerous reports demonstrate that cardiovascular disorders (CVDs) are associated with altered endothelial and vascular smooth muscle cell (VSMC) function and structural remodeling, but the underlying mechanisms are poorly understood. It is known that increased reactive oxygen species (ROS) production contributes to the pathogenesis of CVD through phenotypic changes of endothelial and VSMCs and augmentation of arterial constriction (15, 28). Over the last decade, much attention has been given to the role of endothelium- and VSMC-derived ROS in vascular pathophysiology (20), and yet the contribution of the adventitial layer to vascular-tone dysfunction has largely been ignored. To date, studies have reported that adventitial fibroblasts are a major source of ROS in a variety of CVDs including, among others, AngII-induced hypertension (6), diabetes mellitus (37), atherosclerosis (5), and vascular injury (14).
Adventitial NADPH oxidase–derived ROS play a direct role in adventitial remodeling after balloon injury (27) and have been implicated in medial smooth muscle hypertrophy (33). A study from our laboratory demonstrated that targeted inhibition of adventitial NADPH oxidase, followed by attenuated ROS production, substantially reduced AngII-induced medial hypertrophy and accumulation of 4-hydroxynonenal in the media (16). These findings were consistent with the paracrine role of adventitial ROS, most likely the stable and tissue-permeant ROS H2O2, on medial growth. However, none of these studies examined whether adventitia-derived ROS can exert a paracrine effect on vascular tone.
Increasing evidence suggests that mitogen-activated protein kinase (MAPK) pathways are activated by inflammatory cytokines and oxidative stress and involved in various CVDs, such as hypertension and atherosclerosis (12, 35). Among the MAPKs, a potential target of adventitial ROS-mediated paracrine signaling is the redox-sensitive p38 MAPK. p38 MAPK was shown to be activated in vascular cells by a variety of stimulants, including H2O2 (31), and to play an important role in endothelial dysfunction (13, 34). Importantly, protein tyrosine phosphatases (PTPs) play a major role in limiting MAPK activity by specific dephosphorylation of phosphotyrosine-regulatory residues (26). One such PTP is SHP-2, the inhibition of which was recently linked in vitro to increased ROS generation in vascular smooth muscle cells from hypertensive rats (30).
In the present study, we tested the hypothesis that adventitia-derived H2O2 mediates impairment of vascular relaxation through paracrine activation and inactivation of medial p38 MAPK and SHP-2, respectively.
Materials and Methods
Animals and perivascular treatment of carotid arteries with angiotensin II in situ
Male Sprague–Dawley rats (250–300 g; Charles River, Ann Arbor, MI) were anesthetized with pentobarbital (50 mg/kg, IP; Ovation Pharmaceuticals, Deerfield, IL). Common carotid arteries (CCAs) were exposed in situ, and periarterial incubation “wells” were created by inserting a suture around the omohyoid muscle and back through the submandibular gland and skin. The sternothyroid muscle was cut, and the tissue surrounding the carotid was gently spread while carefully avoiding damage to the carotid and nearby vessels, thereby creating an incubatory reservoir surrounding each carotid artery. The procedure was continued until approximately 8–10 mm of the carotid was exposed.
One CCA from each rat was treated perivascularly with vehicle (0.9% sodium chloride solution; n = 15), and the contralateral CCA with AngII (500 nM; n = 7, Sigma-Aldrich, St. Louis, MO) for 90 min, with a solution change each 15 min, ascertaining no cross-flow or spillage of contents between spaces surrounding each artery. In other experiments, carotid arteries were treated with AngII + catalase (3,000 U/ml; n = 6, Sigma-Aldrich); AngII + superoxide dismutase (SOD, 150 U/ml; n = 6, Sigma-Aldrich); or AngII + the p38 MAPK inhibitor SB203580 (10 μM, n = 9; Santa Cruz Biotechnology, Santa Cruz, CA) in the same manner. After the treatment period, rats were killed by cutting the vena cava. The carotid arteries were rapidly removed, rinsed, and placed in ice-cold PSS buffer (in mM: NaCl 130, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, NaHCO3 14.9, glucose 5.5, CaCl2 1.6, and EDTA 0.026). All protocols were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital and are consistent with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
Vessel myography
Carotids were cleared of adherent adipose tissue, with care not to damage the adventitia, and cut into rings approximately 3–4 mm in length. Rings were placed on myograph stirrups (Danish Myo Technology, Atlanta, GA) in 5 ml PSS buffer maintained at 37°C, pH 7.4, gassed with 95% O2 and 5% CO2, and brought to an optimal resting tension of 1,000 mg by increasing tone by 100 mg every 10 sec. Rings were allowed to stabilize for 1 h, replacing the PSS solution with a fresh one every 20 min. Viability of the vessels was ascertained by a contractile response to potassium chloride (100 mM KCl in PSS solution, KPSS) for 30 min (until contractions reached plateau). Rings were then washed 3 times with PSS and allowed to stabilize to baseline. Phenylephrine (Phe; Sigma-Aldrich) concentration–response curves (10−8 to 10−6 M) were generated by measuring contraction plateaus at each concentration. After they reached a stable plateau phase, a cumulative concentration-dependent response for acetylcholine (ACh, 10−8 to 10−6 M) was induced. Carotid arterial rings were washed 3 times with PSS and allowed to stabilize for 10 min, followed by an additional wash and a 10-min stabilization period. Endothelium-independent vasorelaxation was induced by sodium nitroprusside (SNP, 10−8 to 10−6 M) in a similar manner.
Western blot
Carotid arteries treated as detailed above were removed. CCAs were cut longitudinally and denuded of the endothelium by gentle rubbing with the blunt edge of a forceps. After immobilizing the tissue, we gently peeled the smooth muscle medial layer from the adventitia and placed it in cold cell-lysis buffer containing phosphatase and protease inhibitors (Cell Signaling Technology, Danvers, MA). The media was homogenized on ice for 1 min and sonicated on ice 3 times for 10 sec (Heat Systems sonicator; cycle time, 1 sec, % duty cycle 40). Samples were then centrifuged at 9,000 g for 30 sec, the supernatant collected, and protein concentrations determined with Bradford protein assay. Homogenates were loaded on SDS-PAGE gels and subjected to Western blot with monoclonal antibody to phosphorylated p38 MAPK [Cell Signaling, phospho-p38 MAPK (Thr180/Tyr182) (3D7) rabbit mAb 1:1,000], total p38 MAPK antibody (Cell Signaling; rabbit anti-p38 MAPK 1:1,000), phosphorylated SHP-2 antibody [Cell Signaling; rabbit anti-phospho-SHP-2 (Tyr542) 1:1,000], or GAPDH antibody (Millipore, Billerica, MA; mouse anti-GAPDH 1:1,000). Densitometric analysis was performed by using Density version 1.2.1, and data are expressed as a ratio of phospho- to total p38 MAPK and phospho-SHP-2 to GAPDH.
Statistical analysis
Vasodilator responses are expressed as a percentage of Phe-induced preconstriction. All results are expressed as mean ± SEM. Comparison between relaxation curves was assessed with two-way ANOVA. Comparisons between individual concentrations across relaxation curves were assessed with one-way ANOVA. Comparison between normalized optical-density values for Western blots was assessed with the Student t test. A value of p < 0.05 was considered to be statistically significant.
Results
Perivascular treatment with AngII did not alter phenylephrine-induced constriction of common carotid arteries
To assess whether acute perivascular AngII application affected the development of carotid artery active tone, phenylephrine (Phe)-concentration–response curves were determined. Phe (10−8–10−6 M) induced a concentration-dependent constriction of vehicle- and AngII-treated common carotid arteries. Periadventitial incubation of carotid arteries with AngII did not affect either the sensitivity or the maximal response to Phe-induced vasoconstriction. Maximal Phe-induced (10−6 M) contractions were not significantly different: 91.7 ± 4.6% and 95.6 ± 3.5% for vehicle- and AngII-treated arteries, respectively. In this regard, treatments with catalase (3,000 U/ml) + AngII or the p38 inhibitor SB203580 (p38i, 10 μM) + AngII were not significantly different from AngII treatment alone. Maximal Phe-induced (10−6 M) contractions were 101.2 ± 1.6% and 98.5 ± 3.6% for AngII + catalase and AngII + p38i-treated arteries, respectively.
Effect of periarterial application of AngII on vascular relaxation
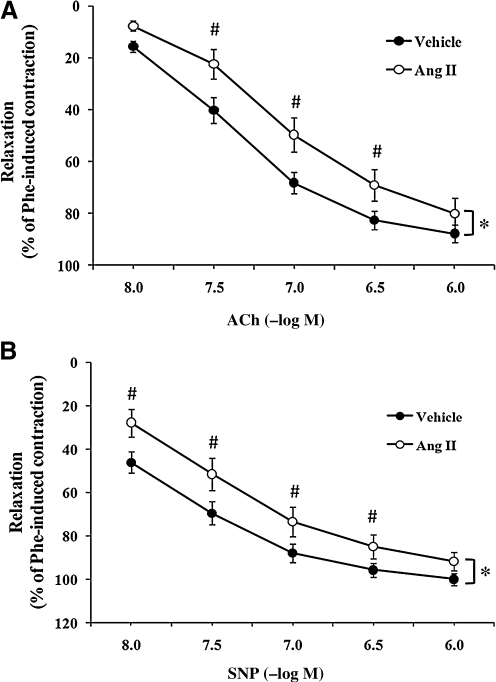
Endothelium-dependent relaxation was assessed by examining a cumulative concentration-dependent response to acetylcholine (ACh, 10−8 to 10−6 M) (Fig. 1A). Periadventitial treatment with AngII significantly decreased endothelium-dependent ex vivo relaxation to Ach, as compared with vehicle-treated vessels, as evidenced by a rightward shift in the relaxation curve (ANOVA, p < 0.05). Point differences were observed at concentrations of ACh up to 3 × 10−7 M, with the most prominent differences in relaxation observed at 10−7 M ACh (68.3 ± 4.1% vs. 49.7 ± 6.6% for vehicle- vs. AngII-treated arteries, respectively; p < 0.05).
FIG. 1.
Periadventitial angiotensin II (AngII) treatment induced impairment of endothelium-dependent and endothelium-independent carotid artery relaxation. The perivascular layer of rat carotid arteries was incubated in situ for 90 min with vehicle (0.9% sodium chloride solution; n = 15) or AngII (500 nM; n = 7). Isolated carotid arteries were preconstricted with phenylephrine (Phe, 10−6 M). (A) Concentration–response curves for acetylcholine (Ach)-induced endothelium-dependent relaxation of isolated carotid arteries. (B) Concentration–response curves for sodium nitroprusside (SNP)-induced endothelium-independent relaxation of isolated carotid arteries. Data represent mean ± SEM. *p < 0.05 indicates significant differences in relaxation between vehicle and AngII groups. #p < 0.05 indicates significant differences at individual concentrations between vehicle and AngII.
Endothelium-independent relaxation also was assessed under the same conditions by evaluating for differences in sodium nitroprusside (SNP; 10−8–10−6 M) responses (Fig. 1B). As with endothelium-dependent relaxations, endothelium-independent relaxation was significantly impaired after AngII treatment, as compared with vehicle (p < 0.05). Point differences were observed at concentrations of SNP up to 10−6 M, again with the most prominent differences in relaxation observed at 10−7 M (87.9 ± 4.3% vs. 73.5 ± 6.7% for vehicle vs. AngII-treated arteries, respectively; p < 0.05).
Effect of periarterial application of catalase on impaired vascular relaxation
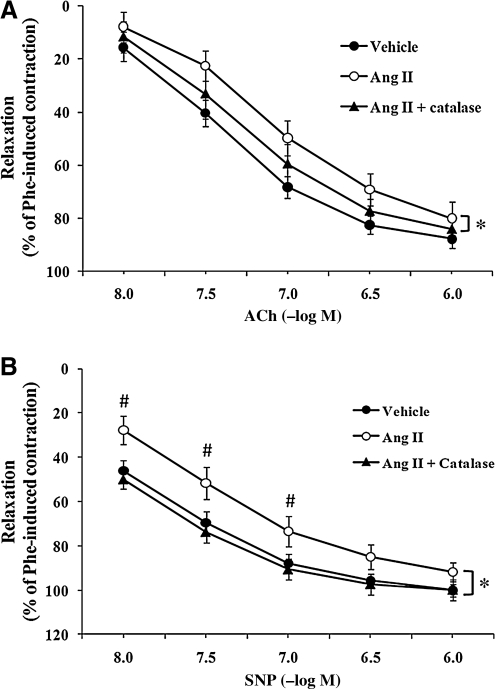
To investigate whether adventitia-derived H2O2 could be responsible for the impairment of relaxation caused by AngII, the H2O2 scavenger catalase (3,000 U/ml) was added to the perivascular incubation wells (in situ) in the presence of AngII. As shown in Fig. 2A, catalase caused a significant improvement in relaxation of AngII-treated arteries, as evidenced by a leftward shift (p < 0.05). Maximal differences in relaxation response were observed at ACh concentration of 3 × 10−8 M (22.5 ± 5.6% vs. 33.0 ± 9.3% for AngII- vs. AngII + catalase-treated arteries, respectively).
FIG. 2.
Perivascular incubation of catalase improved AngII-induced impairment of vascular relaxation. The perivascular layer of rat carotid arteries was incubated in situ for 90 min with vehicle (n = 15), AngII (500 nM; n = 7) or AngII + catalase (3,000 U/ml; n = 6). Isolated carotid arteries were preconstricted with phenylephrine (10−6 M). (A) Concentration–response curves for ACh-induced endothelium-dependent relaxation of isolated carotid arteries. (B) Concentration–response curves for SNP-induced endothelium-independent relaxation of isolated carotid arteries. Data represent mean ± SEM. *p < 0.05 indicates significant differences in relaxation between AngII and AngII + catalase groups. #p < 0.05 indicates significant differences at individual concentrations between AngII and AngII + catalase.
Likewise, SNP-induced endothelium-independent relaxations displayed a significant improvement when carotid arteries were coincubated with AngII + catalase as compared with AngII treatment alone. As illustrated in Fig. 2B, catalase produced a complete reversal of the impairment caused by AngII (p < 0.05, comparing AngII and AngII + catalase groups); point differences were also observed at SNP concentrations up to 10−7 M. The maximal difference between the two groups was achieved at an SNP concentration of 3 × 10−8 M (51.6 ± 7.3% vs. 73.7 ± 4.8%, respectively; p < 0.05).
Effect of periarterial application of SOD on impaired vascular relaxation
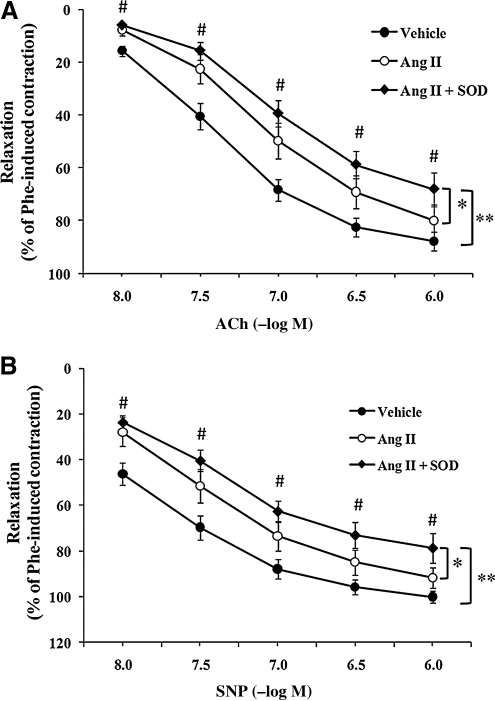
To examine whether superoxide anion (O2-) was also involved in AngII-induced impairment of vascular relaxation, CCAs were treated in situ with SOD (150 U/ml). Coincubation of arteries with AngII and SOD did not improve AngII-induced impairment of ACh-induced relaxation. Somewhat unexpectedly, SOD application resulted in further impairment of vascular relaxation. As shown in Fig. 3A, concentration–response curves to ACh in AngII + SOD- versus AngII-treated arteries were significantly worsened (p < 0.05), as were differences between AngII + SOD versus vehicle (p < 0.05). Significant differences at individual concentrations between AngII + SOD versus vehicle were observed at all ACh concentrations (p < 0.05). Maximal differences were observed at 10−7 M (39.4 ± 5.1% and 68.3 ± 4.1%, AngII + SOD- vs. vehicle-treated arteries, respectively, p < 0.05).
FIG. 3.
Superoxide dismutase (SOD) did not improve AngII-induced endothelium-dependent and endothelium-independent relaxation. The perivascular layer of rat carotid arteries was incubated in situ for 90 min with vehicle (n = 15), AngII (500 nM; n = 7) or AngII + SOD (150 U/ml; n = 6). Isolated carotid arteries were preconstricted with phenylephrine (10−6 M). (A) Concentration–response curves for ACh-induced endothelium-dependent relaxation of isolated carotid arteries. (B) Concentration–response curves for SNP-induced endothelium-independent relaxation in isolated carotid arteries. Data represent mean ± SEM. *p < 0.05 indicates a significant difference in relaxation between AngII and AngII + SOD groups. **p < 0.05 indicates a significant difference in relaxation between vehicle and AngII + SOD groups. #p < 0.05 indicates significant differences at individual concentrations between vehicle and AngII + SOD.
Likewise, coincubation with SOD worsened AngII-induced impairment of endothelium-independent relaxation (AngII + SOD vs. AngII or vehicle; p < 0.05; Fig. 3B). Differences at individual concentrations of SNP between AngII + SOD versus vehicle were significant (p < 0.05). Maximal differences were observed at SNP concentration of 3 × 10−8 M (40.4 ± 4.6% vs. 69.6 ± 5.3%, AngII + SOD- vs. vehicle-treated arteries, respectively; p < 0.05).
Effect of p38 MAPK inhibitor on impairment of vascular relaxation
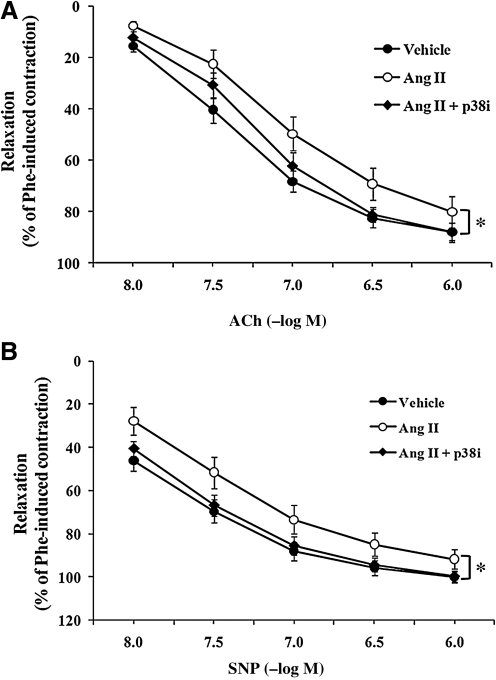
To investigate whether p38 MAPK inhibition could ameliorate the impairment of vascular relaxation, vessels were incubated in situ with the p38 inhibitor SB203580 (p38i, 10 μM). Perivascular co-incubation of AngII + SB203580 significantly improved AngII-induced impairment of endothelium-dependent vasodilatation compared with AngII treatment alone (p < 0.05; Fig. 4A); the difference appeared most pronounced at ACh 3 × 10−7 M (69.1 ± 6.1% vs. 81.3 ± 2.8%, AngII- vs. AngII + p38i-treated arteries, respectively).
FIG. 4.
The p38 MAPK inhibitor SB203580 improved AngII-induced impairment of endothelium-dependent and -independent relaxation. The perivascular layer of rat carotid arteries was incubated in situ for 90 min with vehicle (n = 15), AngII (500 nM; n = 7), or AngII + SB203580 (p38i, 10 μM; n = 9). Isolated carotid arteries were preconstricted with phenylephrine (10−6 M). (A) Concentration–response curves for ACh-induced endothelium-dependent relaxation in isolated carotid arteries. (B) Concentration–response curves for SNP-induced endothelium-independent relaxation in isolated carotid arteries. Data represent mean ± SEM. *p < 0.05 indicates significant differences in relaxation between AngII and AngII + p38i groups.
Similarly, SNP-induced endothelium-independent relaxation was also improved significantly after periadventitial incubation with AngII + p38i, as compared with AngII alone (p < 0.05; Fig. 4B). SNP-induced (3 × 10−8 M) relaxation was 51.6 ± 7.3% and 66.7 ± 4.9% for AngII- vs. AngII + p38i–treated arteries, respectively.
Effect of adventitial H2O2 on medial smooth muscle cell p38 MAPK and SHP-2
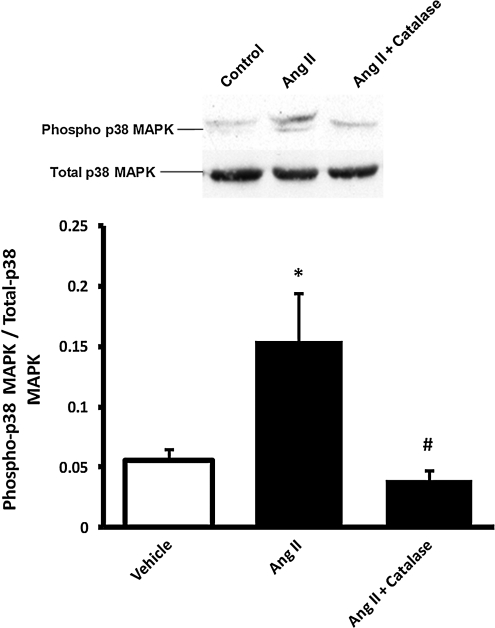
To further examine the role of p38 MAPK in adventitial H2O2-dependent impairment of vascular relaxation, the medial smooth muscle layer was dissected from vessels as described, and homogenates were evaluated for p38 MAPK activation. As shown in Fig. 5, Western blots showed increased activation of medial smooth muscle p38 MAPK, as demonstrated by a higher ratio of phospho- to total p38 MAPK, after perivascular treatment of vessels with AngII, as compared with vehicle controls. Periadventitial treatment with catalase (AngII + catalase) completely abolished AngII-induced activation of medial p38 MAPK.
FIG. 5.
Perivascular AngII treatment induced medial p38 MAPK phosphorylation that was inhibited by co-treatment with catalase. The perivascular layer of rat carotid arteries was incubated in situ for 90 min with vehicle (n = 4), AngII (500 nM; n = 4), or AngII + catalase (3,000 U/ml; n = 4). Medial homogenates were subjected to Western blot with phospho- or total p38 MAPK antibody. Bar graphs represent averaged optical-density data expressed as a ratio of phospho- to total p38 MAPK. *p < 0.05 indicates a significant difference between vehicle and AngII groups. #p < 0.05 indicates a significant difference between AngII and AngII + catalase.
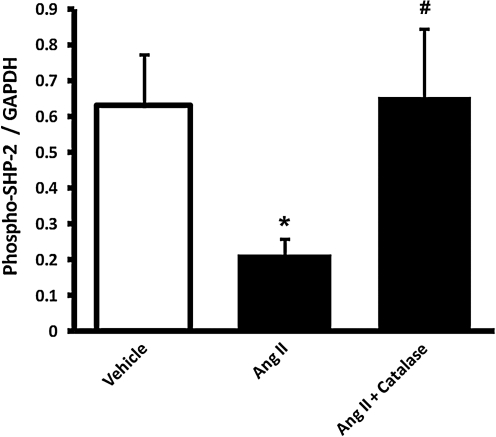
To examine whether an important phosphatase regulating p38 MAPK (SHP-2) could modulate this response, SHP-2 phosphorylation (activation) was compared among the groups. Figure 6 shows that periarterial AngII markedly inhibited medial SHP-2 activity, as demonstrated by decreased phosphorylation of Tyr542. Coincubation with catalase completely reversed this inhibition.
FIG. 6.
Perivascular AngII treatment induced medial SHP-2 dephosphorylation that was reversed by co-treatment with catalase. The perivascular layer of rat carotid arteries was incubated in situ for 90 min with vehicle (n = 4), AngII (500 nM; n = 4), or AngII + catalase (3,000 U/ml; n = 4). Medial homogenates were subjected to Western blot with phospho-SHP-2 (SHP-2-[Tyr542]) or GAPDH antibody. Bar graphs represent averaged optical-density data expressed as a ratio of phospho-SHP2 to GAPDH. *p < 0.05 indicates a significant difference between vehicle and AngII groups. #p < 0.05 indicates a significant difference between AngII and AngII + catalase.
Discussion
The findings of this study demonstrate for the first time that perivascular AngII-induced adventitial release of H2O2 leads to vascular dysfunction through activation of p38 MAPK and inhibition of SHP-2 in the media. Our results demonstrated that ACh-induced endothelium-dependent as well as SNP-induced endothelium-independent relaxations were impaired after periadventitial AngII treatment. Perivascular incubation of carotid arteries with catalase or the p38 MAPK inhibitor SB203580 significantly improved AngII-induced impairment of vascular relaxation, whereas adventitial SOD delivery worsened AngII-induced vascular dysfunction. Furthermore, adventitial exposure to AngII increased phosphorylation of p38 MAPK and decreased phosphorylation of the protein tyrosine phosphatase SHP-2 in the media, indicating activation and inhibition of these important signaling agents, respectively. Importantly, these effects were reversed by co-application with catalase to the adventitia. Taken together, these data are consistent with a significant paracrine signaling role of adventitia-derived H2O2 in vascular-tone dysfunction, suggesting for the first time that (a) H2O2 derived from the adventitia impairs vascular relaxation; and (b) impairment is mediated through remote activation of medial p38 MAPK pathway. Furthermore, our results suggest that (c) inactivation of medial SHP-2 is involved in this process.
Previous reports from our laboratory show that AngII-induced ROS production in the adventitia exceeds that of other segments of the vessel wall (7, 16, 32). In the present study, AngII solution was applied in situ to the outside of the carotid arteries with the intent of increasing ROS production, predominantly from the adventitia versus other segments of the vessel. Previous findings have demonstrated that SOD applied to the adventitial layer was incapable of crossing the external elastic lamina and penetrating from the adventitia to the media (23). Although we did not verify whether applied catalase was restricted to the adventitia in the current study, the anatomic structure of the intact vessel wall and experimental conditions favored catalase exerting its scavenging effect mainly in the adventitia. That is, because catalase is a larger protein than SOD and was applied to the outside of the intact carotid artery, it is expected that the former did not cross the external elastic lamina. Finally, it is plausible that the effect of adventitially applied catalase may have, in part, been mediated through H2O2 diffusion from the outer media to the adventitia, where its concentration would be lower because of the localized presence of catalase. However, all matters considered, we expect that the effect of periadventitially applied catalase was mediated predominantly via decreasing adventitial ROS where H2O2 levels are expected to have been highest as a result of a targeted delivery of AngII.
It is also important to note that short-term periadventitial application of AngII in the current experimental model induced a relatively modest impairment of vascular function (Fig. 1A and B), consistent with the effect of AngII being acute and mediated primarily by the adventitia. Our expectations are that in the current model, the contribution of other segments of the vessel wall to the impairment of vascular relaxation was likely minimal. In studies in which the entire blood vessel was exposed to AngII, impairments in relaxation were considerably more profound (22). It must be emphasized, therefore, that the current studies were designed to focus on the potential role of adventitial ROS on vascular relaxation. Nonetheless, the relative contribution of the different layers of the carotid artery was not tested in this study, and thus, it is impossible to affirm such conclusions here.
Our results suggest that the key ROS responsible for the development of vascular dysfunction on exposure of the adventitia to AngII is H2O2 and not O2-. This novel contention is supported by our findings that (a) catalase, which scavenges H2O2, inhibited the effect of AngII (Fig. 2); and (b) treatment with SOD, which converts O2- to H2O2, enhanced AngII-induced impairment of vascular relaxation (Fig. 3). These findings are consistent with the fact that H2O2 is relatively stable and freely diffusible within and between cells, rendering it the most likely ROS to mediate paracrine signaling across the vessel wall. Another study by Gao et al. (10) supports a paracrine effect of perivascular H2O2 derived from adipose tissue; however, in that study, H2O2 was purported to suppress constriction. The differences related to vascular tone between those studies and our findings are not clear, yet could be related to species and tissue differences. Our previous study in mice showed that in the mouse abdominal aorta, periarterial O2- was involved in impairment of vascular relaxation (23). Furthermore, a previous report by Didion et al. (8) showed that AngII applied periarterially to rabbit cerebral vessels worsened endothelium-dependent but not -independent relaxations. In contrast, our findings demonstrate that periarterial application of AngII to rat carotid artery impairs both endothelium-dependent and -independent relaxation of the carotid artery and to a similar degree. Taken together, we expect that these differences arise from the distinct effects of H2O2 versus O2- on vascular tone. Whereas O2- is known to favor scavenging of endothelium-derived nitric oxide (NO), H2O2 might be expected to perturb signaling pathways downstream of NO.
With regard to the vasoactivity of H2O2, multiple seminal studies demonstrated its ability to cause relaxation of blood vessels through activation of guanylate cyclase (18, 19). Conversely, we and others have shown that under some conditions, blood vessels can constrict in response to H2O2 (1, 2). Those studies corroborate a number of other articles favoring a constrictor effect of H2O2. For example, H2O2 was shown to lead to activation of voltage-dependent Ca2+ channels and increased intracellular Ca2+, leading to vasoconstriction (9, 29, 36). More pertinent to the current findings, other studies demonstrated that H2O2-induced vasoconstriction via activation of protein kinase C, p38 MAPK, and ERK1/2 and increased production of cyclooxygenase products, mechanisms that led to downstream modulation of myosin light-chain kinase (3, 17). Inquiries into these mechanisms upstream and downstream of p38 MAPK are currently the topic of intensive investigation in the laboratory and are outside the scope of the current study. For a thorough review of the potential mediators in this pathway, please refer to Ardanaz et al. (3).
It is important to point out that the effect of H2O2, per se, was not examined in the current study. That is, we did not apply H2O2 to a vessel with either passive or active tone but rather observed its ability to influence relaxation responses to ACh and SNP. Moreover, this was tested indirectly by examining the effect of its scavenger catalase. This may be considered a limitation of the study; however, these studies are unique from the perspective of investigating the influence of ambient, endogenous H2O2 in the adventitia on relaxation responses. Clearly, application of catalase ameliorated AngII-induced impairment of relaxation. The data suggest that H2O2 derived from the adventitia initiates a series of events that predispose the media to impaired relaxation. The findings also suggest that H2O2 is released from the vascular adventitia and can diffuse into the media unless catalase is introduced. Another possibility is that adventitial H2O2 is responsible for the release of a secondary factor that inhibits relaxation in a paracrine manner.
Finally, our findings suggest that two important mediators of this response are p38 MAPK and SHP2 phosphatase. The role of medial p38 MAPK in AngII-induced impairment of vascular function was demonstrated in two ways. By using the p38 MAPK inhibitor SB203580, we were able to reverse AngII-induced impairment of endothelium-dependent and -independent relaxations. Second, we found that medial p38 MAPK was activated (increased phosphorylation) by AngII applied to the adventitia, and this effect was abolished by the addition of catalase. This observation is consistent with AngII-induced release of H2O2 from the vascular adventitia permeating the vascular media and activating p38 MAPK and promoting impaired relaxation.
Further supporting a role for MAPK pathways in this response, our results show inhibition of the PTP SHP-2 in the media by perivascular AngII and its reversal by catalase. PTPs regulate signaling pathways by dephosphorylating tyrosine residues, thereby inactivating protein kinases, including p38 MAPK (25). PTPs contain redox-regulated thiol-containing cysteine residues, which when oxidized render the phosphatase inactive (11, 24). A critical redox-regulated tyrosine residue in SHP-2 is Tyr542, whose dephosphorylation leads to its inactivation. Thus, we were also interested in the possibility that adventitial H2O2 induced dephosphorylation/inactivation of SHP-2 at Tyr542. Our data demonstrate that medial SHP-2 inactivation occurs in response to periadventitial application of AngII, and this was reversed by catalase, implicating adventitia-derived H2O2 in this process. Our results suggest that inactivation of medial SHP-2 could play a permissive role in adventitial H2O2-mediated vascular dysfunction, allowing greater accumulation of phosphorylated p38 MAPK. Further studies are necessary to test a more direct role of SHP-2 in this response, by the use of either inhibitors or loss-of-function techniques.
Interestingly, evidence suggests that NADPH oxidase is activated by p38 MAPK in fMLP-stimulated neutrophils (4) and endothelial cells subjected to hyperoxia (21). Thus, it is tempting to speculate that a similar mechanism exists in adventitial fibroblasts, and that activation of adventitial p38 MAPK leads to further increases in adventitial H2O2 production and even greater medial p38 MAPK activation.
Taken together, the results of our current study demonstrate for the first time that perivascular application of AngII impairs vascular relaxation in a mechanism that involves release of adventitial H2O2, activation of medial p38 MAPK, and reduction of medial SHP-2 activation. The results suggest that adventitia-derived H2O2 plays an important paracrine role in the development of vascular-tone dysfunction by influencing key kinase and phosphatase activities. Adventitial ROS are expected to participate in a far more complex interaction with the media and endothelium in promoting vascular dysfunction.
Abbreviations Used
- Ach
acetylcholine
- AngII
angiotensin II
- CVD
cardiovascular disorder
- H2O2
hydrogen peroxide
- KCl
potassium chloride
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- O2−
superoxide
- Phe
phenylephrine
- PTP
protein tyrosine phosphatase
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- VSMC
vascular smooth muscle cell
Acknowledgments
This work was supported by National Institutes of Health grant HL079207 and HL55425. We thank Dr. Francis Miller and Sheila Frizzell for their critical review of the manuscript. We also thank Andrew Althouse for his assistance in the statistical analyses of our results. PJP is an Established Investigator of the American Heart Association. GC is a recipient of an American Heart Association Postdoctoral Fellowship.
Author Disclosure Statement
The authors have no competing financial interest in relation to the work described.
References
- 1.Ardanaz N. Beierwaltes WH. Pagano PJ. Comparison of H2O2-induced vasoconstriction in the abdominal aorta and mesenteric artery of the mouse. Vascul Pharmacol. 2007;47:288–294. doi: 10.1016/j.vph.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Ardanaz N. Beierwaltes WH. Pagano PJ. Distinct hydrogen peroxide-induced constriction in multiple mouse arteries: potential influence of vascular polarization. Pharmacol Rep. 2008;60:61–67. [PubMed] [Google Scholar]
- 3.Ardanaz N. Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med. 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 4.Azuma Y. Kosaka K. Kashimata M. Phospholipase D-dependent and -independent p38MAPK activation pathways are required for superoxide production and chemotactic induction, respectively, in rat neutrophils stimulated by fMLP. Eur J Pharmacol. 2007;568:260–268. doi: 10.1016/j.ejphar.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Azumi H. Inoue N. Takeshita S. Rikitake Y. Kawashima S. Hayashi Y. Itoh H. Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 6.Cifuentes ME. Rey FE. Carretero OA. Pagano PJ. Upregulation of p67phox and gp91phox in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol. 2000;279:H2234–H2240. doi: 10.1152/ajpheart.2000.279.5.H2234. [DOI] [PubMed] [Google Scholar]
- 7.Csanyi G. Taylor WR. Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didion SP. Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–2042. doi: 10.1161/01.STR.0000081225.46324.AA. [DOI] [PubMed] [Google Scholar]
- 9.Favero TG. Zable AC. Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 10.Gao YJ. Lu C. Su LY. Sharma AM. Lee RMKW. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendriks WJ. Stoker AW. Protein tyrosine phosphatases: sequences and beyond. FEBS J. 2008;275:815. doi: 10.1111/j.1742-4658.2008.06248.x. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y. Dietrich H. Metzler B. Wick G. Xu Q. Hyperexpression and activation of extracellular signal-regulated kinases (ERK1/2) in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 2000;20:18–26. doi: 10.1161/01.atv.20.1.18. [DOI] [PubMed] [Google Scholar]
- 13.Ju H. Behm DJ. Nerurkar S. Eybye ME. Haimbach RE. Olzinski AR. Douglas SA. Willette RN. p38 MAPK inhibitors ameliorate target organ damage in hypertension, Part 1: p38 MAPK-dependent endothelial dysfunction and hypertension. J Pharmacol Exp Ther. 2003;307:932–938. doi: 10.1124/jpet.103.057422. [DOI] [PubMed] [Google Scholar]
- 14.Kawai J. Ando K. Tojo A. Shimosawa T. Takahashi K. Onozato ML. Yamasaki M. Ogita T. Nakaoka T. Fujita T. Endogenous adrenomedullin protects against vascular response to injury in mice. Circulation. 2004;109:1147–1153. doi: 10.1161/01.CIR.0000117231.40057.6D. [DOI] [PubMed] [Google Scholar]
- 15.Lee MY. Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J. Ormsby A. Oja-Tebbe N. Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 17.Lucchesi PA. Belmadani S. Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertension. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 18.Mohazzab-H KM. Agarwal R. Wolin MS. Influence of glutathione peroxidase on coronary artery responses to alterations in Po2 and H2O2. Am J Physiol. 1999;276:H235–H241. doi: 10.1152/ajpheart.1999.276.1.H235. [DOI] [PubMed] [Google Scholar]
- 19.Mohazzab HK. Kaminski PM. Fayngersh RP. Wolin MS. Oxygen-elicited responses in calf coronary arteries: role of H2O2 production via NADH-derived superoxide. Am J Physiol. 1996;270:H1044–H1053. doi: 10.1152/ajpheart.1996.270.3.H1044. [DOI] [PubMed] [Google Scholar]
- 20.Munzel T. Hink U. Heitzer T. Meinertz T. Role for NADPH/NADH oxidase in the modulation of vascular tone. Ann N Y Acad Sci. 1999;874:386–400. doi: 10.1111/j.1749-6632.1999.tb09253.x. [DOI] [PubMed] [Google Scholar]
- 21.Parinandi NL. Kleinberg MA. Usatyuk PV. Cummings RJ. Pennathur A. Cardounel AJ. Zweier JL. Garcia JG. Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan S. Kurz S. Münzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rey FE. Li XC. Carretero OA. Garvin JL. Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91phox. Circulation. 2002;106:2497–2502. doi: 10.1161/01.cir.0000038108.71560.70. [DOI] [PubMed] [Google Scholar]
- 24.Ross SH. Lindsay Y. Safrany ST. Lorenzo O. Villa F. Toth R. Clague MJ. Downes CP. Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19:1521–1530. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Ruhul Amin AR. Oo ML. Senga T. Suzuki N. Feng GS. Hamaguchi M. SH2 domain containing protein tyrosine phosphatase 2 regulates concanavalin A-dependent secretion and activation of matrix metalloproteinase 2 via the extracellular signal-regulated kinase and p38 pathways. Cancer Res. 2003;63:6334–6339. [PubMed] [Google Scholar]
- 26.Saxena M. Mustelin T. Extracellular signals and scores of phosphatases: all roads lead to MAP kinase. Semin Immunol. 2000;12:387–396. doi: 10.1006/smim.2000.0219. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y. Niculescu R. Wang D. Patel S. Davenpeck KL. Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2001;21:739–745. doi: 10.1161/01.atv.21.5.739. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y. So KF. Man RY. Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions in femoral arteries of rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;152:1033–1041. doi: 10.1038/sj.bjp.0707439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabet F. Savoia C. Schiffrin EL. Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Tabet F. Schiffrin EL. Callera GE. He Y. Yao G. Ostman A. Kappert K. Tonks NK. Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res. 2008;103:149–158. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 31.Ushio-Fukai M. Alexander RW. Akers M. Griendling KK. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II: role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 32.Wang HD. Hope S. Du Y. Quinn MT. Cayatte A. Pagano PJ. Cohen RA. Paracrine role of adventitial superoxide anion in mediating spontaneous tone of the isolated rat aorta in angiotensin II-induced hypertension. Hypertension. 1999;33:1225–1232. doi: 10.1161/01.hyp.33.5.1225. [DOI] [PubMed] [Google Scholar]
- 33.Wang HD. Xu S. Johns DG. Du Y. Quinn MT. Cayatte AJ. Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 34.Widder J. Behr T. Fraccarollo D. Hu K. Galuppo P. Tas P. Angermann CE. Ertl G. Bauersachs J. Vascular endothelial dysfunction and superoxide anion production in heart failure are p38 MAP kinase-dependent. Cardiovasc Res. 2004;63:161–167. doi: 10.1016/j.cardiores.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Willette RN. Eybye ME. Olzinski AR. Behm DJ. Aiyar N. Maniscalco K. Bentley RG. Coatney RW. Zhao S. Westfall TD, et al. Differential effects of p38 mitogen-activated protein kinase and cyclooxygenase 2 inhibitors in a model of cardiovascular disease. J Pharmacol Exp Ther. 2009;330:964–970. doi: 10.1124/jpet.109.154443. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z. Zheng T. Zhang A. Altura BT. Altura BM. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur J Pharmacol. 1998;344:169–181. doi: 10.1016/s0014-2999(97)01576-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L. Zalewski A. Liu Y. Mazurek T. Cowan S. Martin JL. Hofmann SM. Vlassara H. Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]