Abstract
Excessive reactive oxygen species Revised abstract, especially superoxide anion (O2•−), play important roles in the pathogenesis of many cardiovascular diseases, including hypertension and atherosclerosis. Superoxide dismutases (SODs) are the major antioxidant defense systems against O2•−, which consist of three isoforms of SOD in mammals: the cytoplasmic Cu/ZnSOD (SOD1), the mitochondrial MnSOD (SOD2), and the extracellular Cu/ZnSOD (SOD3), all of which require catalytic metal (Cu or Mn) for their activation. Recent evidence suggests that in each subcellular location, SODs catalyze the conversion of O2•− H2O2, which may participate in cell signaling. In addition, SODs play a critical role in inhibiting oxidative inactivation of nitric oxide, thereby preventing peroxynitrite formation and endothelial and mitochondrial dysfunction. The importance of each SOD isoform is further illustrated by studies from the use of genetically altered mice and viral-mediated gene transfer. Given the essential role of SODs in cardiovascular disease, the concept of antioxidant therapies, that is, reinforcement of endogenous antioxidant defenses to more effectively protect against oxidative stress, is of substantial interest. However, the clinical evidence remains controversial. In this review, we will update the role of each SOD in vascular biologies, physiologies, and pathophysiologies such as atherosclerosis, hypertension, and angiogenesis. Because of the importance of metal cofactors in the activity of SODs, we will also discuss how each SOD obtains catalytic metal in the active sites. Finally, we will discuss the development of future SOD-dependent therapeutic strategies. Antioxid. Redox Signal. 15, 1583–1606.
Introduction
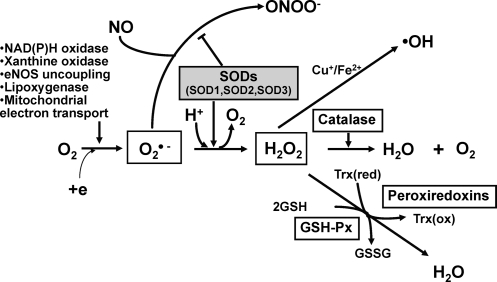
Aerobic organisms possess antioxidant defense systems that deal with reactive oxygen species (ROS) produced as a consequence of aerobic respiration and substrate oxidation. In the process of normal cellular metabolism, oxygen undergoes a series of univalent reductions, leading sequentially to the production of O2•−, hydrogen peroxide (H2O2), and H2O. Potential enzymatic source of ROS includes components of the mitochondrial electron transport chain, xanthine oxidase, the cytochrome p450 monooxygenases, lipoxygenase, nitric oxide synthase (NOS), and the NADPH oxidase (83) (Fig. 1). Superoxide anion is dismutated by superoxide dismutases (SODs) to H2O2 that is catalyzed to H2O by catalase, peroxiredoxins (Prxs), or glutathione peroxidases (GPx) (Fig. 1). Low levels of either intracellular or extracellular ROS (e.g., superoxide and H2O2) are indispensable in many biochemical processes, including intracellular signaling, defense against microorganisms, and cell function (74, 125, 223). In contrast, high dose and/or inadequate removal of ROS, especially superoxide anion, results in oxidative stress, which has been implicated in the pathogenesis of many cardiovascular diseases, including hypercholesterolemia, atherosclerosis, hypertension, diabetes, and heart failure. ROS also represent a component of the innate immune system, and they are not only involved in the respiratory burst of neutrophils, but also signal inflammatory cell chemotaxis into sites of inflammation (20).
FIG. 1.
Generation and metabolism of reactive oxygen species (ROS). Superoxide (O2•−) is produced by NADPH oxidase, xanthine oxidase, nitric oxide synthase (NOS), lipoxygenase, and mitochondrial enzymes. Superoxide is converted by superoxide dismutase (SOD) to H2O2, which, in turn, is reduced to water by catalase, glutathione peroxidases (GPx), and peroxiredoxins (Prx). In the presence of reduced transition metal (Fe2+, Cu+), H2O2 can undergo spontaneous conversion to hydroxyl radical (OH•), or related metal-associated reactive species, which is extremely reactive. Importantly, nitric oxide (NO) can be rapidly inactivated by reaction with O2•− and leading to the production of the strong oxidant peroxynitrite (ONOO−). Thus, SOD is a first line of defense against toxicity of superoxide anion radicals. The enzyme also participates in cell signaling via regulating ROS (e.g., O2•−, H2O2) and available NO.
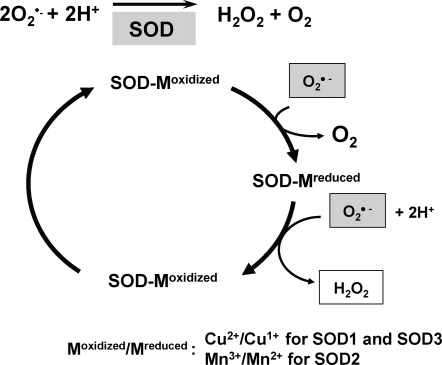
Importantly, nitric oxide (NO), which has anti-inflammatory and anticoagulant properties as well as vasodilator effect, can be rapidly inactivated by reaction with O2•− and leading to the production of the strong oxidant peroxynitrite (ONOO−). This reaction is important in common conditions leading to endothelial and mitochondrial dysfunction, including hypercholesterolemia, hypertension, diabetes, and aging, in which vascular production of O2•− is increased (83, 146). The major cellular defense against O2•− and peroxynitrite is a group of oxidoreductases known as SODs, which catalyze the dismutation of O2•− into oxygen and H2O2. In mammals, there are three isoforms of SOD (SOD1 [CuZnSOD]; SOD2 [MnSOD]; SOD3 [ecSOD]) (Table 1), and each is a product of distinct genes and distinct subcellular localization, but catalyzes the same reaction. This distinct subcellular location of these SOD isoforms is particularly important for compartmentalized redox signaling. The mechanism of dismutation of O2•− to H2O2 by SOD involves alternate reduction and reoxidation of a redox active transition metal, such as copper (Cu) and manganese (Mn) at the active site of the enzyme as shown in Figure 2 (2). This indicates that SOD activity requires a catalytic metal. Thus, this review will discuss how SODs obtain catalytic metal in the active sites and update role of each SOD in vascular diseases, such as atherosclerosis and hypertension. Regarding other mechanisms of regulation of SODs and their role in diseases, please refer to other reviews (63, 156, 243, 251).
Table 1.
Superoxide Dismutases in Vascular Tissue
| Isoform | Characteristics | Metal cofactor | Metal delivery-related protein | Location |
|---|---|---|---|---|
| SOD1 (Cu/ZnSOD) | 32 kDa, homodimer | Cu2+ (catalytic) | CCS, GSH | Cytoplasm, mitochondrial IMS, and others (nucleus, lysosomes, peroxisomes) |
| Zn2+ (stability) | Unknown | |||
| SOD2 (MnSOD) | 96 kDa, homotetramer | Mn3+ (catalytic) | Unknowna | Mitochondria matrix |
| SOD3 (ecSOD) | 135 kDa, homotetrameric secretory glycoprotein | Cu2+ (catalytic) | Atox1, ATP7A (MNK, Menkes ATPase) | Extracellular matrix, cell surface, extracellular fluids |
| Zn2+ (stability) | Unknown |
Smf2p and MTM1 in yeast.
SOD, superoxide dismutase.
FIG. 2.
Common mechanism of scavenging O2•− by SODs. Enzymatic activity of SOD involves alternate reduction and reoxidation of catalytic metal (i.e., Cu or Mn) at the active site of the enzyme. Thus, Cu or Mn will be a key modulator of SOD activity of SOD1/SOD3 or SOD2, respectively.
Basic Characteristic and Mechanism for Activation of SODs
SOD1 (cytosolic Cu/ZnSOD)
SOD1 is the major intracellular SOD (cytosolic Cu/ZnSOD) (Table 1). It exists as a 32 kDa homodimer and is mainly localized in the cytosol with a smaller fraction in the intermembrane space (IMS) of mitochondria (42, 175, 213). It has also been reported that SOD1 is also localized in nuclei, lysosomes, and peroxisomes, using immunocytochemical methods (33), and shows widespread distribution in a variety of cells (42). The enzyme is sensitive to cyanide, which helps to distinguish it from SOD2, which is relatively resistant. The human gene for SOD1 has been localized to the 21q22.1 region of chromosome 21 (136). Thus, it is responsible for Down syndrome (trisomy 21) and these patients have an extra copy of the gene and have SOD1 activity, which is 50% greater than the normal diploid population, in keeping with the gene-dosage effect. Transgenic rats containing an extra copy of the human SOD1 gene displays similar phenotype to Down syndrome, including the neurological defects and premature aging (60). Role of SOD1 in this disease remains unclear, but it is postulated that increased SOD1 activity elevates H2O2 levels, which becomes toxic (58). The most widely studied connection between SOD1 and human diseases involves the late onset neurodegenerative disease amyotrophic lateral sclerosis (ALS) (198). Over 100 mutations in the human gene SOD1 are now known to lead to some of the inherited forms of ALS, but its mechanisms remain unclear.
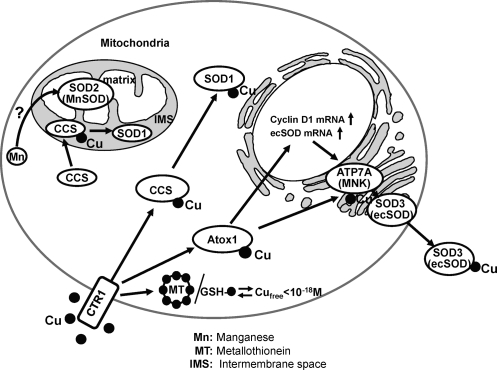
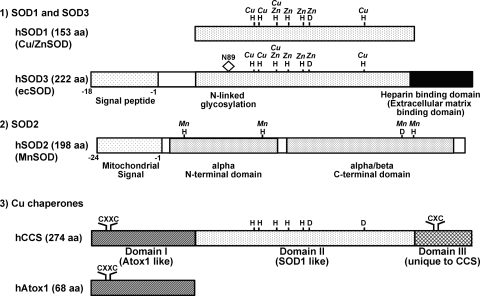
Enzymatic activity of SOD1 depends on the presence of the Cu and Zinc (Zn). Zn participates in proper protein folding and stability. The SOD activity of remetallated derivatives is proportional to the amount of Cu bound in the native Cu site (155, 194). Further, Cu is not replaceable with another metal, whereas Zn is replaceable with cobalt and Cu, and it is not necessary for enzyme activity at low pH (155, 184, 227). More importantly, the mechanism of scavenging O2•− by SOD involves alternate reduction and reoxidation of the Cu at the active site of the enzyme as shown in Figure 2. Thus, SOD1 activity requires a catalytic Cu to scavenge O2•−(155). Under physiological conditions, the level of intracellular free Cu is extraordinarily restricted (192). Thus, soluble Cu carrier proteins termed “Cu chaperones” are required to directly transfer Cu to specific cellular targets (Fig. 3). SOD1 has been shown to obtain catalytic Cu ion through interaction with the cytosolic Cu carrier protein CCS (Cu chaperone for SOD1), a Cu chaperone for SOD1, thereby increasing its activity (44). CCS activates SOD1 not only through Cu insertion, but also through disulfide bond formation. It consists of three functionally distinct protein domains (I, II, III) (172, 202) (Fig. 4). The central domain II resembles SOD1 and serves to dock CCS with SOD1. Once the CCS-SOD1 heterodimer has been formed, Cu insertion and disulfide oxidation may proceed via a CXC Cu binding motif at the C-terminal CCS domain III. The N-terminal Domain I of CCS1 including the MXCXXC Cu-binding site. Domain I is required for CCS activity, but its precise role is unclear. Although full activation of SOD1 requires CCS, there is a CCS-independent pathway, which involves GSH (27).
FIG. 3.
Metal trafficking pathways to SODs in vascular tissue. Various SOD enzymes employ catalytic metal cofactor such as copper (Cu) and manganese (Mn) to carry out the disproportion of superoxide. Under physiological conditions, the level of intracellular free Cu is extraordinarily restricted (192). Thus, once transported by Cu uptake transporter hCTR1, soluble cytosolic Cu carrier proteins termed “Cu chaperones” are required for trafficking Cu to specific Cu-containing enzymes through direct protein–protein interaction (116). Three copper chaperones have been characterized thus far: (i) uncharacterized Cu ligands and various Cu chaperones (Cox1, Cox 2, Cox 11, Cox 17, and Sco1), which deliver Cu to cytochrome c oxidase in the mitochondria (not shown); (ii) CCS (Cu chaperone for SOD1), which delivers copper to SOD1 in the cytosol and mitochondrial intermembrane space (IMS); and (iii) Atox1, which delivers copper to some of the secretory copper enzymes such as extracellular superoxide dismutase (ecSOD, SOD3) via the copper transporter ATP7A (Menkes ATPase, MNK) in the trans-Golgi network. In addition to its chaperone function, Atox1 also function as a Cu-dependent transcription factor for ecSOD and cyclin D1 (102, 103, 105). Thus, full activation of SOD3 requires both Cu chaperone function of Atox1 via ATP7A to obtain catalytic Cu as well as Cu-dependent transcription factor function for its transcriptional regulation (103, 105, 189, 190). Cytosolic concentrations of free Cu are typically maintained at exquisitely low levels (10−18 M) by metal scavenging systems, including metallothioneins (MT) and GSH (142, 192). Yeast genetic studies show that Smf2 p and Mtm1p are involved in Mn delivery to MnSOD, but role of these proteins in mammals remains unclear (44).
FIG. 4.
A schematic alignments of each SOD isoform and Cu chaperones. (1) SOD1 (CuZnSOD) and the SOD3 (ecSOD) active site domain (amino acid residues 96–193) shares about 50% homology, such that all the ligands to Cu and Zn and the arginine in the entrance to the active site in SOD1 can be identified in this domain of SOD3. The distinct region of SOD3, as compared to SOD1, includes (a) an amino-terminal signal peptide, which permits secretion from the cell; (b) an N-linked glycosylation site at Asn-89, which is useful in the separation of SOD3 from SOD1 and greatly increases the solubility of the protein; (c) C-terminal region corresponding to heparin-binding domain has a cluster of positively charged residues. This region is critical for binding to extracellular matrix, such as heparan sulfate proteoglycan. (2) SOD2 (MnSOD) protein is composed of three domains. First, N-terminal mitochondrial signal peptide directs protein synthesized in the cytoplasm to the mitochondria. Second, the active site of SOD2 contains Mn and has the N-terminal helical hairpin and C-terminal alpha/beta domain (241). They show no homology to SOD1 and SOD3, but it is similar to that of the Fe SOD, which is commonly absent from eukaryotes (21). (3) Cu chaperone CCS folds into three functionally distinct protein domains (123, 202). The N-terminal Domain I of CCS bears striking homology to Atox1, including the MXCXXC copper-binding site. The central domain of CCS (domain II) exhibits significant homology with its target of copper delivery, SOD1. Of note, this domain also share the strong homology with the central domain of SOD3, which is a catalytic site of it, such that all four of the zinc binding ligands of SOD1 and SOD3 and three of four histidine copper binding ligands are present in CCS. The C-terminal Domain III of CCS1 is quite small yet is extremely crucial for activating SOD1 in vivo (202). This peptide is highly conserved among CCS molecules from diverse species and includes an invariant CXC motif that can bind copper (202). Two identical four-helix bundles, symmetrically assembled from the N-terminal helical hairpins, form a novel tetrameric interface that stabilizes the active sites.
SOD2 (MnSOD)
SOD2 is a mitochondrial manganese (Mn) containing enzyme (MnSOD), which is composed of a 96 kDa homotetramer and localized in the mitochondrial matrix (66, 233) (Table 1). Mn at the active site of SOD2 serves to catalyze the disproportionation of O2•− to oxygen and H2O2 in a similar fashion as SOD1 and SOD3 (Cu/ZnSODs) (95) (Fig. 2). It is synthesized in the cytoplasm and directed to the mitochondria by a signal peptide (Fig. 4), where it is involved in dismutating O2•− generated by the respiratory chain of enzymes. The active site of SOD2 shows no homology to SOD1, but it is similar to that of the Fe SOD, which is commonly absent from eukaryotes (21). Both SOD2 and Fe-SOD share a common structure with the N-terminal helical hairpin and C-terminal alpha/beta domain (241). Within each subunit, both the N-terminal helical hairpin and C-terminal alpha/beta domains contribute ligands to the catalytic Mn site. Two identical four-helix bundles, symmetrically assembled from the N-terminal helical hairpins, form a novel tetrameric interface that stabilizes the active sites. Unlike SOD1, SOD2 does not exhibit product inhibition by H2O2 (10, 17). Also, SOD2 has a half-life in sera of 5–6 hr compared with 6–10 min for the SOD1 (78). The essential role of SOD2 in maintaining mitochondrial function is demonstrated by the neonatal lethality of mice with targeted disruption of the gene for SOD2. Such complete ablation of SOD2 causes dilated cardiomyopathy and neurodegeneration leading to early postnatal death (104, 133, 138).
Yeast genetic studies show that metal insertion process in SOD2 is quite different from that of SOD1, as expected from its distinct genomic and protein structure (44). In addition, it is also different from metal delivery to mitochondrial Cu-dependent enzyme cytochrome c oxidase (CCO), which involves the small ligand and various Cu binding proteins (Cox1, Cox2, Cox11, Cox17, Sco1, etc.) (195). For example, the intracellular SOD1 can obtain Cu post-translationally, by way of interaction with the CCS Cu chaperone, which also oxidizes an intrasubunit disulfide in SOD1. In contrast, metal insertion for SOD2 cannot occur post-translationally, but requires new synthesis and mitochondrial import of the SOD2 polypeptide (44). Further, in contrast to SOD1, SOD2 has modest selectivity for metal binding between Mn and Fe (159). In spite of this relatively promiscuous metal binding behavior, only the Mn form is catalytically active, reflecting a strict catalytic specificity for the metal ion. This stark contrast between low selectivity in metal binding and strict specificity for catalysis seems to be important for regulating SOD2 function (238, 239). In vivo activation of yeast SOD2 by Mn requires the Smf2p manganese transporter and Mtm1p. Smf2p provides Mn to mitochondria from intracellular vesicles, whereas Mtm1p prevents misincorporation of Fe for Mn in SOD2 (44). Role of these proteins in mammals remains unclear.
SOD3 (extracellular Cu/ZnSOD and ecSOD)
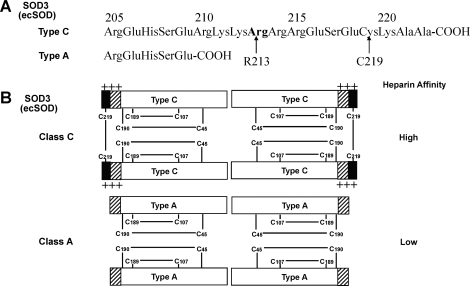
SOD3, a secretory extracellular Cu/Zn-containing SOD (ecSOD), is the major SOD in the vascular extracellular space (Table 1). In most species, SOD3 is a 135 kDa homotetramer composed of two disulfide-linked dimers (Fig. 5). The primary location of SOD3 in tissues is in the extracellular matrix and on cell surfaces with a smaller fraction in the plasma and extracellular fluids. Tissue SOD3 is thought to account for 90%–99% of the SOD3 in the body (152, 153). Tissue distribution varies among species, but in general SOD3 is expressed highly in selected tissues, such as blood vessels, the lung, kidney, uterus, and, to a lesser extent, in heart (65, 152, 178, 211). SOD3 activity is extremely sensitive to cyanide, like SOD1. In vascular tissue, SOD3 is mainly synthesized by vascular smooth muscle cells and fibroblasts (211). In injured tissue and atherosclerosis, SOD3 is also found in inflammatory cells (68, 143, 216). It is secreted and anchored to the extracellular matrix and endothelial cell surface through binding to the heparan sulfate proteoglycan (HSPGs), collagen, and fibulin-5 (67, 167, 187). Thus, despite the fact that SOD3 is predominantly made by vascular smooth muscle cells rather than endothelial cells (67, 151, 211), it binds to the extracellular matrix such as heparan sulfates on the endothelial cell surface and can be internalized by endothelial cells (36, 174). In addition to signal peptide that permits secretion from the cell, SOD3 protein is composed of three functional domains (Fig. 4) (90). First, the amino terminal residues [1–95] contain an N-linked glycosylation site at Asn-89, which is useful in the separation of SOD3 from cytosolic SOD1 and greatly increases the solubility of the protein (56). Second, the amino terminal residues [96–193] contain active site, showing about 50% homology to SOD1. Indeed, all the ligands to Cu and Zn and the arginine in the entrance to the active site in SOD1 can be identified in this domain of SOD3. Third, the amino terminal residues [194–222] contain a C-terminal region corresponding to heparin-binding domain, which has a cluster of positively charged residues and is critical for binding to heparan sulfate proteoglycan. In vivo, both circulating (type A) and tissue bound (type C) are present, with the tissue bound form being about 99% of the total SOD3 (Fig. 5). Nonproteolyzed subunits are classified as type C subunits, whereas the proteolyzed subunits are classified as type A subunits. In some humans, there is a substitution of glycine for arginine at amino acid 213 (R213G) in this heparin binding domain, which results in reduced heparan sulfate affinity and higher plasma levels (63, 67). Juul et al. demonstrated that in a prospective, population-based study of 9188 participants from The Copenhagen City Heart Study, heterozygosity for SOD3 with R213G is associated with increased risk of ischemic heart disease (110). Finally, the importance of SOD3 in the extracellular space is further stressed by the fact that 85% of mice were died within a week of induction of SOD3 ablation, which showed histological changes similar to those observed in adult respiratory distress syndrome, suggesting that SOD3 is essential for survival in the presence of ambient oxygen (75). Together with other studies using genetically altered mice and viral-mediated gene transfer, these findings indicate that SOD3 plays an important role in various oxidative stress-dependent pathophysiologies, including hypertension, heart failure, ischemia-reperfusion injury, and lung injury (63, 67, 191).
FIG. 5.
Heparin binding affinity patterns of SOD3 (ecSOD) in vivo. (A) The sequence of the heparin-binding domain is present in the full-length type C subunit and confers the heparin-binding affinity to the protein. The truncated type A subunit has no affinity to heparin. Proteolytic processing leading to the appearance of truncated ecSOD in vivo can occur both intracellularly and in the extracellular space (59). (B) The subunit compositions of the two classes of ecSOD and their relative affinities to heparin are shown. In most species, ecSOD exists as a tetramer composed of two disulfide-linked dimers (63, 67). Two of these dimers are held together noncovalently. In vivo, both circulating (class A) and tissue bound (class C) are present, with the tissue bound (class C) being approximately 99% of the total ecSOD. Class B (not shown in the figure) of ecSOD reveals partial C-terminal truncation and medium heparin affinity. Class C of ecSOD consists of all four C-terminal intact subunits (type C) that contains two disulfide bonds linking two pairs of heparin-binding domains together. In contrast, class A of ecSOD consists of all four C-terminal truncated subunits (type A) that do not contain a disulfide bond to link two heparin-binding domains. Truncation of the C-terminal region does not affect the nonconvalent protein–protein interactions stabilizing the tetramer, but affects the heparin binding properties significantly.
SOD3′s active site is very similar to that of the cytosolic Cu/Zn SOD (SOD1) (7) (Fig. 4). Using recombinant human SOD3, we demonstrated that the specific activity of SOD3 is linearly related to its Cu content, suggesting that SOD3-specific activity is Cu-dependent (105). Interestingly, the domain II in CCS, which is a copper chaperone for SOD1, shares the strong homology with the central domain of SOD3, which is a catalytic site of it, such that all four of the Zn binding ligands of SOD1 and SOD3 and three of four histidine Cu binding ligands are present in CCS1 (Fig. 4). However, it is important to note that SOD3 is a secretory protein, and thus SOD3 need to get Cu in the secretory pathways instead of cytosol, suggesting that Cu chaperone for SOD3 should be different from CCS (Fig. 3).
Cu delivery pathway to SOD3 involves Cu chaperone Antioxidant-1 (Atox1), which is different from those for SOD1 (CCS) and for mitochondrial cytochrome c oxidase [small ligand, Cox 17, and others (38, 39)] (Fig. 3). In addition to Cu chaperone function, Atox1 serves as a transcription factor to increase SOD3 transcription (103). We also identified cyclin D1 gene as another downstream target for transcription factor Atox1, which stimulates Cu-dependent cell proliferation (102). Given that SOD3 is a major Cu-dependent antioxidant enzyme that regulates extracellular levels of O2•− and that Atox1 functions as a Cu-dependent transcription factor for SOD3 and cyclin D1, it is conceivable that Atox1 serves as a sensor of intracellular Cu concentrations to regulate cell proliferation by controlling SOD3 and cyclin D1 expression.
Given that SOD3 secretes through endoplasmic reticulum-Golgi pathway where Atox1 does not exist, the cytosolic Atox1 itself is required, but not sufficient for delivering Cu to SOD3 protein to regulate its full activity. ATP7A (Menkes ATPase, MNK) has been shown to transport cytosolic Cu to the secretory pathway via Atox1. We found that vascular ATP7A is required for full activation of SOD3, but not SOD1, by transporting Cu to SOD3 via Cu-dependent interaction with SOD3. Taken together, SOD3 activity requires the Cu chaperone, Atox1, but not CCS, which delivers Cu to SOD3 via interacting with the Cu transporter ATP7A at the trans-Golgi network (TGN) in vascular cells and tissue (105, 189, 190). Unlike SOD1, it forms stable tetramers with interchain disulfides that stabilize the quaternary structure (188). However, it remains unknown whether Cu transporters are involved in disulfide formation in a similar fashion to CCS.
Biological Effects of SODs
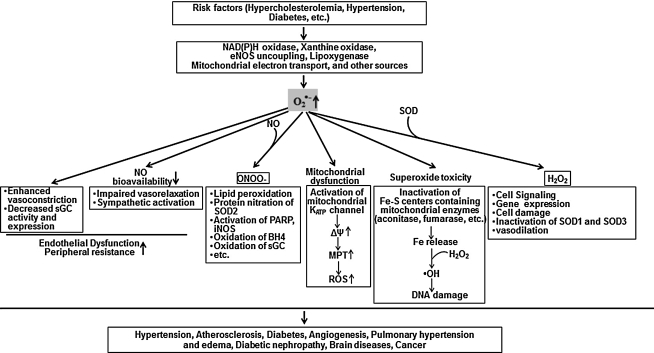
Vascular O2•− is produced normally as a byproduct of normal cellular metabolism and serves as a progenitor for a number of other ROS, including H2O2 and the ONOO− (Figs. 1 and 6). In the vessel, alterations in levels of •O2− have been shown to modulate vascular tone, gene expression, inflammation, cellular growth, signaling, and apoptosis. SODs serve as the front-line defense against ROS in living cells, catalyzing the redox disproportionation of O2•− into H2O2 and molecular oxygen. There are several consequences of this enzymatic activity (Figs. 6–8).
FIG. 6.
Role of superoxide in various physiological and pathophysiological functions. SOD has a potential impact on various biological function and pathogenesis by regulating NO signaling, ROS (O2•−, H2O2) signaling, and mitochondrial function.
FIG. 8.
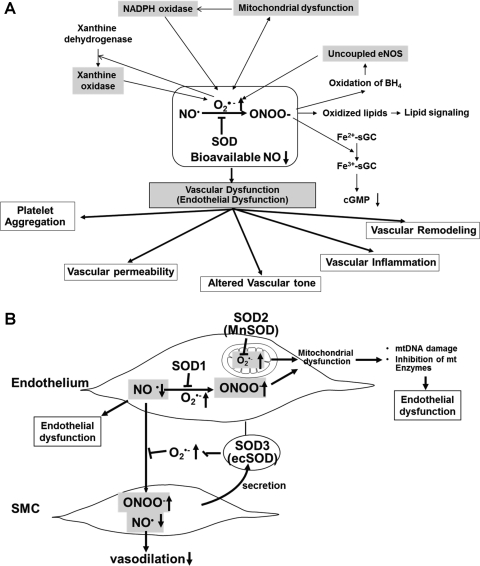
(A) Role of nitric oxide–superoxide interactions in vascular (endothelial) dysfunction in cardiovascular disease and (B) protective role of SODs in oxidative stress-dependent vascular (endothelial) dysfunction. (A) NO rapidly reacts with O2•− generated by ROS-generating enzymes, including NADPH oxidase, xanthine oxidase, and mitochondria, to form peroxynitrite anion (ONOO−), which in turn oxidizes various molecules, such as the heme of sGC, lipids, and the endothelial NOS (eNOS) cofactor BH4. This in turn induces uncoupled eNOS to promote further increase in O2•−. These consequences will be further enhanced by interaction of ROS-generating enzymes. Both O2•− and ONOO− promote mitochondrial dysfunction, thereby increasing mitochondrial ROS production. Mitochondria-derived ROS, which in turn further activates NADPH oxidase, results in increased ROS production and reduced NO bioavailability. Further, either O2•− or ONOO− can stimulate other ROS-generating enzymes, such as xanthine oxidase. The loss of bioavailable NO and formation of ONOO− can lead to vascular inflammation, vascular remodeling, altered vascular tone, enhanced vascular permeability, and increased platelet aggregation. These responses are inhibited by SODs. (B) Because of its location, SOD3 (ecSOD) plays a critical role in preventing O2•−-mediated destruction of NO• released from the endothelium at the extracellular space, whereas SOD1 preserves NO levels within the endothelium. Thus, SODs regulate endothelial function and NO mediating signaling by inhibiting O2•−-mediated inactivation of NO•, thereby increasing bioavailable NO•. Because O2•− and NO• are both radicals and contain unpaired electrons in their outer orbitals, they undergo an extremely rapid, diffusion-limited radical–radical reaction (6.7×109 M−1 s−1, three times faster than the dismutation of O2•− by SOD). This reaction leads to the formation of nitrite, nitrate, and, very importantly, the peroxynitrite anion (ONOO−), which in turn induces endothelial dysfunction, vascular inflammation, vascular remodeling, altered vascular tone, enhanced vascular permeability, and increased platelet aggregation. Both O2•− and ONOO− promote mitochondrial dysfunction, thereby increasing mitochondrial ROS production, reducing NO bioavailability, mitochondrial (mt)DNA damage, and inhibition of mitochondrial enzymes. These events result in endothelial dysfunction.
Role of SOD in redox signaling
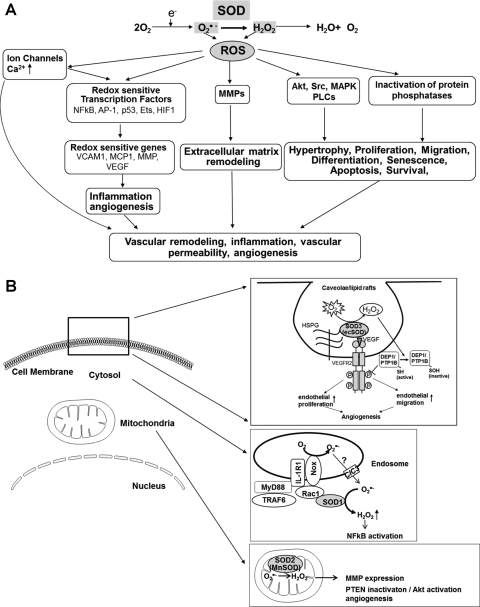
As shown in Figure 1, intracellular ROS levels are regulated by the balance between ROS-generating enzymes and antioxidant enzymes, which include SOD, catalase, GPx, and thioredoxin system. All of three SOD enzymes have various biological effects partially through H2O2, the dismutation product of O2•−, since H2O2 can function as a signaling molecule (193) (Fig. 7A). These ROS, including O2•− and H2O2, can stimulate cellular responses (hypertrophy, proliferation, migration, etc.) via oxidation of signaling molecules (Akt, Src, PLCs, MAPK, etc.) or via oxidative inactivation of protein tyrosine phosphatases (PTPs), thereby promoting tyrosine phosphorylation-mediated redox signaling or regulating protein function (193, 218, 224). ROS can also activate redox sensitive transcription factors (e.g., NFKB, AP-1, etc.) and MMPs, which contributes to vascular inflammation, angiogenesis, and extracellular matrix remodeling. Evidence reveals that the production of ROS by NADPH oxidase (Nox) complex has been implicated in signal transduction after receptor stimulation (125). NADPH oxidase consist of seven isoforms: Nox1, Nox2 (gp91phox), Nox3, Nox4, Nox5, Duox1, and Duox2. It is generally accepted that Nox complexes produce O2•− on the extracytoplasmic face of cellular membranes (224), which will produce O2•− outside the cell from the plasma membrane-bound Noxes and in the lumen of a vesicular compartment from the intracellular Nox complexes (137). It is proposed that O2•− is dismutated to H2O2, which can diffuse in part through aquaporin channels in the plasma membrane, or that O2•− is penetrated through the cell membrane anion chloride (ClC-3) channels to initiate intracellular signaling (18, 86, 157, 162). Since ROS are diffusible molecules, compartmentalized production of ROS by NADPH oxidase localized at specific subcellular compartments such as caveolae/lipid rafts and endoplasmic reticulum (ER)/endosomes (224) as well as mitochondria (147) plays an important role in activation of specific redox signaling events. Of note, SOD1 localizes in cytosol and mitochondria IMS, SOD2 localizes in mitochondria matrix, and SOD3 is anchored to the extracellular matrix via binding to heparan sulfate proteoglycans (HSPGs), collagen, or fibulin-5 (67, 167, 187, 201), as mentioned above. Thus, O2•− dismutation to H2O2 by the three isoforms of SODs may also contribute to activation of many redox signaling events, as described below (Fig. 7B).
FIG. 7.
(A) Role of SOD in redox-sensitive signaling pathways and (B) role of SODs in activation of redox signaling at specific compartments. (A) Production of O2•− and its metabolite H2O2 lead to activation of redox-sensitive kinases and potentially inactivation of specific phosphatases to modulate redox-sensitive signaling, including hypertrophy, proliferation, and migration. Activation of redox-sensitive transcription factors leads to redox-sensitive changes in expression of proinflammatory genes, such as vascular cellular adhesion molecule 1 (VCAM1), monocyte chemotactic protein 1 (MCP1), and intercellular adhesion molecule 1 (ICAM1). ROS modulate ion channels and, therefore, influence intracellular Ca2+ and K1+ concentrations. Finally, ROS can activate matrix metalloproteinases (MMPs). (B) Extracellular H2O2 generated by SOD3 (ecSOD) localized at caveolae/lipid rafts via binding to heparan sulfate proteoglycans (HSPGs) promotes VEGF receptor type2 (VEGFR2) signaling linked to angiogenesis via oxidative inactivation of protein tyrosine phosphatases (PTPs; DEP1 and PTP1B) (180); SOD1 is recruited to redox active endosomal surface where it binds to Rac1 to regulate Nox2 activity. Thus, SOD1-Nox2-mediated increase in O2•− exits endosomes through chloride channels (ClC3) and SOD1-mediated dismutation of O2•− at the endosomal surface produces the localized H2O2, thereby promoting redox activation of NF-kB (162); MnSOD (SOD2) localizes in mitochondria matrix. SOD2 (MnSOD) overexpression-induced H2O2 induces the tumor suppressor PTEN oxidation, leading to enhanced formation of phosphatidylinositol 3,4,5-triphoshate, resulting in activation of Akt and angiogenesis in vivo (40). Thus, O2•− dismutation to H2O2 by the three isoforms of SODs contributes to activation of specific redox signaling events at distinct compartments.
SOD1 and redox signaling
SOD1 is shown to be actively recruited to redox active endosomal surface after interleukin-1 stimulation (137), thereby facilitating localized production of H2O2 and activating redox signaling at endosomes. SOD1 also regulates endosomal Nox2 activity by binding to Rac1 (a coactivator of Nox2) and regulating Rac1 activity (85). Thus, SOD1-Nox2-mediated increase in O2•− exits endosomes through chloride channels and then SOD1-mediated dismutation of O2•− at the endosomal surface produces the localized H2O2 required for redox activation of NF-kB (162) (Fig. 7B). Morikawa et al. reported that SOD1-derived H2O2 acts as an endothelium-derived hyperpolarization factor (EDHF) to regulate endothelial function in vivo (160). Overexpression of human SOD1 increases VEGF mRNA and protein expression through generation of intracellular H2O2 (81). Inhibition of SOD1 by copper chelator ATN-224 in tumor and endothelial cells prevents the formation of H2O2, resulting in the protection of PTPs from H2O2-mediated oxidation. This, in turn, inhibits growth factor-mediated phosphorylation of ERK1/2 (107). These findings suggest that SOD1-derived H2O2 functions as a second messenger to regulate various signal transductions involved in inflammation, angiogenesis, and vascular function.
SOD2 and redox signaling
SOD2 overexpression induces the tumor suppressor PTEN oxidation through H2O2 increase, leading to enhanced formation of phosphatidylinositol 3,4,5-triphoshate, which results in activation of Akt and blood vessel formation in vivo (40) (Fig. 7B). H2O2 generated by SOD2 sustains long-term ERK1/2 signaling induced by nerve growth factor, leading to cell differentiation (28). Further, SOD2 converts O2•− to H2O2 in the mitochondria, activating HIF1α-O2-sensitive K+ channel pathway, resulting in the pathogenesis of pulmonary hypertension (8). Thus, overexpression of SOD2 increases angiogenesis, cell differentiation, and pulmonary hypertension via generating signaling molecule H2O2 at mitochondria rather than antioxidant function.
SOD3 and redox signaling
SOD3 is a major secreted extracellular enzyme that catalyzes the dismutation of O2•− to H2O2, and anchors to endothelial cell surface through heparin-binding domain (HBD). Evidence suggests that extracellular redox state regulates intracellular signaling (74) or tumor growth (30) by modulating plasma membrane-associated proteins. Exogenous H2O2 induces expression of both VEGF and VEGFR2 (77) and pro-angiogenic responses in ECs (226). We demonstrated that mice lacking SOD3 show impaired postischemic angiogenesis (117), which is associated with decrease in H2O2 (180) as well as enhanced O2•− production and decreased available NO (117) in ischemic muscles in hindlimb ischemia model. Mechanistically, extracellular H2O2 generated by SOD3 localized at caveolae/lipid rafts via HBD promotes VEGF receptor type2 signaling via oxidative inactivation of protein tyrosine phosphatases (PTPs) in these microdomains (180). This in turn promotes endothelial proliferation and migration, leading to angiogenesis (Fig. 7B). Further, SOD3 overexpression reduces hypoxia-induced erythropoietin gene expression presumably due to a decrease in signaling intermediate O2•− (250), whereas SOD3 knockout mice show elevation of erythropoietin gene expression induced by hypoxia (214). SOD3 gene transfer in rat hindlimb ischemia model increased glucose metabolism and cell proliferation, thereby promoting recovery of tissue injury (129). This is mediated through the activation of mitogenic Ras-ERK1/2 and PI3kinase-Akt signaling pathways, leading to increased expression of transcription factors AP1 and CRE and VEGF-A and cyclin D1 expression. These reports suggest that SOD3-derived H2O2 stimulates various redox signaling pathways to promote angiogenesis and tissue repair.
H2O2 derived from SODs may be involved in pathological states by Fenton type reaction and peroxidase activity. In combination with some transition metals like iron or Cu, H2O2 can react to form hydroxyl radical or related metal-associated reactive species, a highly reactive ROS, and thus induces cellular injury via the Fenton reaction (Fig. 2). In prooxidant pathological states such as atherosclerosis and hypertension, SOD1 and SOD3 are inactivated probably due its peroxidase activity (89, 109). In this activity, H2O2, the dismutation product of O2•−, can inactivate SOD1 through reacting with the Cu center of this SOD, thereby forming the Cu–OH radical and leading to enzyme inactivation (89). This effect can be prevented by scavenging the Cu–OH radical with small anionic antioxidants, such as urate or nitrite. These reactions appear to be occurred in several disease states, as discussed below. The role of H2O2 in SOD function may be consistent with the notion that protective effects of SOD have a dose optimum in several pathophysiological models (166).
Role in NO signaling
NO exerts numerous beneficial antiatherogenic endothelial functions by elevation of cGMP and vasorelaxation via activation of soluble guanylate cyclase (sGC) and inhibition of platelet aggregation and inflammatory response. Given that O2•− reacts with NO• at almost diffusion-limited rates, SOD regulates endothelial function and NO-mediating signaling by inhibiting oxidative inactivation of NO. The role of SOD in vascular function will be discussed later. The reaction between O2•− and NO results not only in loss of NO bioactivity but also in formation of the ONOO−, a potent oxidant, which may contribute to lipid peroxidation and membrane damage. Further, ONOO− has multiple effects (Figs. 6 and 8A), including (i) selective nitration of tyrosine residues in proteins, such as prostacyclin synthase and Mn-SOD (41, 82); (ii) activation of poly (ADP-ribose) polymerase (PARP) and expression of inducible NOS (iNOS), potentially important mediators of vascular dysfunction in disease states (176, 207); (iii) oxidation of the heme of sGC to its ferric NO-insensitive state (208); (iv) oxidation of the endothelial NOS (eNOS) cofactor BH4 (132); and (v) oxidation of the zinc–thiolate complex in eNOS (257). The latter two effects can produce eNOS “uncoupling,” a condition in which the normal flow of electrons within the enzyme is diverted such that eNOS produces O2•− rather than NO. Further, ONOO− has been shown to inactivate SOD2, but it has no or milder effect on SOD1 in vitro (5, 99). Tyrosine nitration and inactivation of SOD2 are observed in chronic rejection of human renal allografts (144), suggesting that inactivation of SOD2 by ONOO− may play a role in their model. The role of ONOO− in regulating activity of SOD1 and SOD3 in oxidative stress-dependent cardiovascular diseases, including vascular injury (135) and atherosclerosis (89), remain unclear.
Role in mitochondria function
SOD regulates mitochondria function by above function (i.e., scavenging O2•−, producing H2O2, inhibition of production of ONOO− by protecting NO) in the mitochondrial matrix (SOD2) and in the intermembrane space (SOD1). Mitochondria are one of the major sources of chronic ROS production under physiological conditions (11). ROS in mitochondria are produced by oxidative phosphorylation pathway involved in energy production. In the mitochondrial electron transport chain, Complex I and III are the primary source of O2•− production in mitochondria (163). Superoxide is released into the matrix from complex I, whereas it is released into both the matrix and intermembrane space by complex III (163). In addition to being a major site of ROS production, mitochondria are a target for ROS/RNS and compromised by severe and/or prolonged oxidative stress (256). This will represent a vicious cycle to amplify mitochondrial ROS, whereby mitochondrial ROS/RNS causes oxidative damage to mitochondrial DNA (mtDNA), which leads to further mitochondrial dysfunction and oxidant generation. Indeed, mice lacking one of electron transport chain components, such as the adenine nucleotide translocator ANT1, have increased levels of oxidative species and mtDNA damage (62). Importantly, the electron transport chain contains several NO reactive-redox metal centers (24, 61). Thus, mitochondrial NO is an important regulator of O2•− production (72, 73). Further, NO undergoes radical–radical reaction with O2•− at near diffusion-limited rates forming ONOO−, which causes mitochondrial dysfunction by irreversible nitration of proteins, inactivation of enzymes, DNA damage, and disruption of mitochondrial integrity. At physiological concentrations, NO modulates mitochondrial oxygen consumption by inhibiting cytochrome c oxidase in a reversible process (25, 37), which contributes to the regulation of respiration and exhibits pro- and antiapoptotic responses. Role of mitochondrial NO in vascular function remains unclear.
Prevention of superoxide-induced cytotoxicity
SODs inhibit O2•−-induced cytotoxicity via inactivation of iron-sulfur (Fe-S) centers containing mitochondrial enzymes, such as aconitase and fumarase. This reaction is important, since O2•−-induced inactivation of Fe-S containing enzymes results in release of iron and subsequent formation for highly toxic hydroxyl radical or related iron-associated reactive species by reacting with H2O2 (Figs. 1 and 6), which may contribute to DNA damage. Indeed, mitochondrial SOD2 knockout mice exhibit decreased activities of Fe-S centers containing enzyme aconitase and increased oxidative damage to mitochondrial DNA (240).
SOD and Vascular Function
As a result of rapid reaction of O2•− and NO to produce ONOO− as well as its membrane impermeability, O2•− has distinct effects depending on its subcellular location. Thus, in mammals, three isoforms of SODs exist in different subcellular localization, as mentioned before. The role of individual SODs in relation to endothelium under normal conditions and disease states is discussed as shown below (Fig. 8).
SOD1 and vascular function
Because of its cytosolic location, SOD1 plays an important role in endothelial function by protecting NO release from endothelium. Studies from SOD1 knockout mice exhibits increased levels of vascular O2•− and ONOO−, increased myogenic tone, augmented vasoconstrictor responses (in response to serotonin and phyenylephrine), and impaired endothelium-dependent (NO-mediated) relaxation in both large arteries and microvessels (51). Thus, SOD1 not only protects NO-mediated vasorelaxation, but also counteracts vasoconstrictor responses. Increases in vascular permeability after ischemia are greatly enhanced in SOD1-deficient mice (119). Alterations in expression of SOD1 may also impact vascular structure. For example, deficiency in SOD1 produces hypertrophy of cerebral arterioles (13). In contrast, transgenic mice overexpressing SOD1 improves vascular dysfunction in models of subarachnoid hemorrhage (112) and hypoxia with reoxygenation (139) as well as in response to ceramide (47), lipopolysaccharide (LPS) (49), and overexpression of β-amyloid precursor protein (96). Angiotensin II (Ang II)-induced expression of monocyte chemoattractant protein (MCP-1) and monocyte infiltration into the vessel wall are inhibited in SOD1 transgenic mice (100). Adenovirus-mediated gene transfer of SOD1 decreases vascular O2•− levels in atherosclerosis and diabetes (158, 249) to improve endothelial function in diabetes (249), and protects endothelial function in vascular tissues with aging (50), treated with various oxidative stimulants such as LPS (49), and Ang II (48). There is increasing evidence that H2O2 may function as an EDHF in some blood vessels. Experiments in gene-targeted mice suggest that SOD1 specifically may function as an EDHF-synthase, as it appears to be the major source of H2O2 in small mesenteric arteries (160).
SOD2 and vascular function
Various pharmacological inhibitors of mitochondrial energy metabolism significantly increase mitochondrial ROS production and impair endothelium-dependent vascular relaxation (22, 43, 53). Rotenone (which inhibits electron transport at flavin mononucleotide) abolished acetylcholine-induced, endothelium-dependent relaxation of rat and mouse carotid arteries (43) and rat and rabbit aortas (79, 196, 232). Similarly, antimycin A (which inhibits electron transport at cytochrome b–c1) and oligomycin (which inhibits mitochondrial ATP synthase) inhibit the production of endothelial NO in rabbit aorta (79). However, rotenone did not affect vascular relaxation induced by NO donors (196), which suggests that intact mitochondrial function plays an important role in the production of NO in endothelial cells. Under normal conditions and during acute oxidative stress such as hypoxia, SOD2 plays a minor role because vasomotor function is similar between WT and SOD2+/− mice (6). Indeed, basal O2•− production is rather decreased in SOD2+/− mice, which could be due to decreased oxygen consumption.
Under pathological conditions and aging, SOD2 plays an important role in regulating endothelial function. SOD2 deficiency appears to be responsible for endothelial dysfunction by increasing O2•− and causing chronic mitochondrial damage in ApoE−/− mice (173). However, O2•− scavenger tiron failed to restore endothelial function, suggesting that chronic mitochondrial damage by deficiency of SOD2 may produce irreversible changes. Aged heterozygous SOD2+/− mice showed the most pronounced phenotype such as severely impaired vasorelaxation, highest levels of mitochondrial ROS formation and mtDNA damage, suggesting that mitochondrial radical formation significantly contributes to age-dependent endothelial dysfunction (235).
SOD3 and vascular function
Vascular SOD3 is localized in high concentrations between the endothelium and the smooth muscle, where endothelium-derived NO must transverse to stimulate smooth muscle relaxation (181). Because of its extracellular location, SOD3 plays a critical role in preventing destruction of NO• released from the endothelium. Importance of regulation of NO bioavailability by SOD3 has been shown in various experimental disease models. For example, adenovirus-mediated SOD3 gene transfer has been shown to protect against vascular dysfunction with aging (26), reduce systemic vascular resistance and arterial pressure in spontaneously hypertensive rats (35), and improve endothelial dysfunction in hypertension and in heart failure models (64, 97). In addition, SOD3-deficient mice display enhanced impairment of endothelial dysfunction in several models of hypertension and aging, which can be rescued by exogenously added SOD. To support this notion, ATP7A mutant mice lacking SOD3 function with impaired Cu delivery show endothelial dysfunction in a similar phenotype as SOD3−/− mice (189).
Numerous pathophysiologies, including atherosclerosis, aging, cigarette smoking, and diabetes, are associated with a decline in the production and/or biological activity of endothelium derived NO• (118). In these conditions, the loss of NO leads to a decline in SOD3 expression, since eNOS is a positive regulator for SOD3 expression (69, 179), although there are conflicting results (210). Landmesser et al. have shown that in patients with coronary artery disease, activity of endothelium-bound SOD3, released by heparin bolus injection, is positively correlated with flow-dependent, endothelium-mediated dilation (124). It is tempting to speculate that other conditions associated with a long-term loss of NO would also decrease expression of SOD3, adversely impacting the vascular redox state.
Physical exercise has been associated with a reduction in cardiovascular morbidity and mortality (19, 57, 183). One mechanism that may underlie this beneficial effect involves an upregulation of the eNOS, increasing local production of NO• (205). Paradoxically, exercise also increases total body oxygen uptake, increasing production of ROS (106), increasing the susceptibility of plasma LDL to oxidation (206) and increasing conjugated diene formation (200). The manner in which the vasculature adapts to this oxidant stress remains unclear. Since exercise training increases SOD3 expression in eNOS-dependent manner, this SOD3 response may represent an important physiological adaptation that would counteract this increase in oxidant stress in response to exercise training. Thus, the beneficial effect of exercise training on endothelium-dependent vasodilatation observed in several studies (84, 88, 94) may not only be due to an increase in expression of eNOS, but also due to an increase in SOD3 expression, which serves to preserve ambient levels of NO by decreasing its reaction with O2•−. Importantly, unidirectional laminar shear stress dramatically increases expression of the cytosolic SOD1 in human aortic endothelial cells (98), which would further enhance available NO in response to exercise training.
SOD and Atherosclerosis
Oxidative alterations of lipoproteins and arterial cells are implicated in atherogenesis (209). However, the mechanisms for atherogenic oxidative alterations in vivo, such as LDL oxidation, have not been fully revealed. The mechanisms by which O2•− might modify LDL could involve catalysis by transition metal ions such as Cu and iron (15) or ceruloplasmin (161) or by the reactive ONOO− formed through the reaction between NO and O2•−(46). In contrast, it has been suggested that the O2•− can react with the lipid peroxy radical and alkoxy radical formed during lipid peroxidation and that at least the latter reaction might lead to chain termination (166). Such a reaction might balance other pro-oxidant reactions.
SOD catalyzes the dismutation of O2•− to H2O2, which further reduces to H2O by catalase, GPX, and Prxs (Fig. 1). Thus, SOD has been proposed to be involved in atherogenesis by inhibition of oxidative alterations caused by O2•−(29), prevention of O2•−-mediated removal of NO, thereby facilitating endothelium-dependent vasorelaxation (237) inhibition of leukocyte adhesion to the vascular endothelium (134), and altered vascular cellular responses [vascular smooth muscle cell (VSMC) and endothelial cell (EC) apoptosis, VSMC proliferation, hypertrophy, and migration (145, 185)], as shown in Figures 7 and 8. These findings indicate the potential for a protective role of SODs in atherosclerosis. Of note, in some cases, mice with genetically altered SOD function showed rather proatherogenic effects, as shown in Table 2. In this section, we will summarize and discuss role of SODs in atherosclerosis.
Table 2.
Role of Superoxide Dismutases in Atherosclerosis
| |
|
|
|
Fold change of lesion |
|
||
|---|---|---|---|---|---|---|---|
| SODs | Gene altered | Genetic background | Diet and intervention | Increase | No change | Decrease | Reference |
| SOD1 (Cu/ZnSOD) | |||||||
| 1 | SOD1 Tg | B6 | High fat (add irradiation) | 2 | 219 | ||
| 2 | SOD1 Tg | B6 | High fat | 2.2 | 220 | ||
| 3 | SOD1 Tg | ApoE −/−/B6 | high fat | NS | 245 | ||
| SOD1 + catalase Tg | ApoE −/−/B6 | High fat | 4 | ||||
| SOD2 (MnSOD) | |||||||
| 4 | SOD2+/− | ApoE−/−/B6 | High fat | 2.5 | 12 | ||
| SOD3 (ecSOD) | |||||||
| 5 | SOD3−/− | ApoE−/−/B6 | Normal chow | NS (after 3M) | 1.7 (after 1M) | 204 | |
| High fat | NS | ||||||
SOD1 and atherosclerosis
Many previous studies have shown that increased expression of SOD1 confers protection against acute or chronic oxidative injury, including atherosclerosis (139, 219). However, pathological role of SOD1 has been reported (58, 220). For example, transgenic mice that overexpress SOD1 develop more extensive fatty-streak deposition than control mice when fed a high cholesterol diet (220). This may due to the possibility that high SOD activity could enhance oxidative injury by increasing rates of formation of distal oxidants. For example, SOD1 will convert O2•− to H2O2, which can readily cross cellular membrane and form hydroxyl radicals or related metal-associated reactive species through its interaction with redox-active transitional metals (248), which may have proatherogenic properties. Consistent with this, overexpression of catalase alone or overexpression of SOD1 and catalase in combination reduced the level of plasma and aortic F2-isoprostane and retarded the development of atherosclerosis in ApoE−/− mice, whereas overexpression of SOD1 alone did not significantly reduce the level of F2-isoprostane and atherosclerosis in these mice (245). Alternatively, SOD also could increase oxidant formation by interacting directly with ONOO− in a reaction that increases highly reactive nitronium ion (NO2+) (99). It is possible that effects of SOD are dose dependent, which is characterized by a bell-shaped curve (166, 177). Or localization of SOD may be critical to its protective effects (i.e., extracellular vs. intracellular). Indeed, oxidative damage to lipoproteins and the vascular endothelium are attributed primarily to oxygen-derived species that are either generated extracellularly or released into the extracellular space as cellular oxidative waste or in association with the oxidative burst. These processes are more likely to be under the control of SOD3, which is located primarily within the interstitial matrix or anchored to cell-surface HSPGs (67). Finally, the atherogenic role of O2•− may vary depending on animal models. For example, O2•− contributes to the development of atherosclerosis in X-ray-exposed mice (219), but not to the nonirradiated models such as fat-fed mice and ApoE−/− mice (220).
SOD2 and atherosclerosis
Superoxide anions in the mitochondrial matrix are quickly dismutated to H2O2 by SOD2, whereas those in the mitochondrial IMS are converted by SOD1 (175). Thus, it is conceivable that SOD1 and SOD2 may play a role in atherosclerosis through regulation of mitochondrial O2•− as well as available mitochondrial NO by preventing oxidative inactivation. Indeed, mitochondrial dysfunction, resulting from SOD2 deficiency, increased mtDNA damage and accelerated atherosclerosis in ApoE−/− mice (12). Of note, mitochondrial DNA damage not only correlated with the extent of atherosclerosis in human specimens and aortas from ApoE−/− mice but also preceded atherogenesis in young ApoE−/− mice. These findings suggest that increased ROS production and DNA damage in mitochondria are early events in the initiation of atherosclerosis (146). In addition, SOD2 regulates endothelial dysfunction/apoptosis and VSMC proliferation/apoptosis, leading to the development of atherosclerosis by controlling mitochondrial ROS and NO (146).
SOD3 and atherosclerosis
Several findings suggest that SOD3 may play a role in atherosclerosis. We and others have demonstrated that SOD3 is abundantly present in the vascular wall and synthesized in atherosclerotic lesions by smooth muscle cells and macrophages (68, 141, 143). Indeed, immunohistochemical studies showed that SOD3 staining colocalizes with lipid-laden macrophages in atherosclerotic vessels of ApoE−/− mice. In contrast, SOD3 activity is decreased in connective tissue-rich human atherosclerotic lesions (124, 143). Thus, in atherosclerosis, SOD3 expression is increased in lipid-laden macrophages, whereas it is decreased in other cellular components such as VSMCs (68). Interestingly, SOD3 is expressed in iNOS-positive, macrophage-rich lesions colocalized with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins (143). Thus, it is conceivable that high SOD3 expression in the arterial wall may prevent not only deleterious effects of O2•− but also ONOO− formation. This is supported by the findings that SOD3 attenuates tyrosine nitration and enhances nitrite formation in rabbit balloon injury model (135). In cell culture studies, SOD3 markedly reduced LDL oxidation by endothelial cells (127, 215). Wang et al. reported that low plasma SOD3 was independently associated with an increase of history of myocardial infarction (230). Moreover, activity of SOD3, but not that of SOD1 or SOD2, is reduced in coronary artery segments in patients with coronary artery disease compared with normal subjects (143). Adachi reported that the SOD3 affinity to endothelial surface heparan sulfate was decreased in coronary artery disease (3). Note that the R213G polymorphism in the SOD3 gene, which reduces binding to endothelial surface and increases serum SOD3 levels, has been linked to an increase in cardiovascular risk (110).
In atherosclerotic vessels, SOD3 is regulated in multifaceted ways. First, SOD3 expression is regulated or associated with NO bioavailability, as discussed above (69, 124, 179). Second, the expression level and distribution of SOD3 is regulated by proteolytic removal of heparin binding domain. Level of low heparin affinity forms of SOD3 was increased in atherosclerotic patients, whereas the amount of the high-heparin affinity, C form of SOD3 was decreased (3). It has been shown that proteolytic processing leading to the appearance of truncated SOD3 can occur both intracellularly and in the extracellular space (59). In this context, coronary artery concentration of heparan sulfate, the physiological ligand for SOD3, tends to decrease with the atherosclerotic lesions, whereas the content of chondroitin sulfate increases (247). Third, SOD3 activity is modulated by H2O2 produced by lipid-laden macrophages due to its peroxidase activity, because lipid laden macrophage produces a large amount of both O2•− and SOD3, resulting in the accumulation of H2O2. In apolipoprotein E−/− mice, SOD3 activity is inactivated, which can be restored by increasing the plasma concentration of urate (89). This peroxidase-like activity of SOD1 has been shown to inactivate the enzyme (92, 93). Fourth, SOD3 expression is regulated by inflammatory cytokines in VSMCs, such that TNFα decreases SOD3 expression, whereas interferon γ and interleukin-4 increase its expression (212). Fifth, homocysteine, an independent risk factor for atherosclerosis (169, 244), decreases SOD3 expression and the binding of SOD3 to vascular endothelial surface, resulting in a loss of the ability to protect endothelial surfaces from oxidative stress. Further, homocysteine induces ER stress, and SOD3 activity is regulated by disulfide bridge arrangements (188). Thus, it is conceivable that homocysteine decreases the secretion of SOD3 by disturbing the disulfide bond formation and/or inhibiting the glycosylation, resulting in the incorrect assembly of the protein. Sixth, the methylation status of the 5′ flanking region of the SOD3 gene may be responsible for enhanced expression of SOD3 enzyme in the early stages of atherosclerosis (128, 221). Indeed, reduction in the methylation status of SOD3 promoters is characteristic of atherosclerotic lesions (128, 221). Finally, SOD3 activity is critically regulated by Cu chaperone/transcription factor Atox1 and Cu transporter ATP7A (105, 190), since the activity of SOD3 requires a catalytic Cu to scavenge O2•−, as mentioned above (105). Interestingly, Atox1 and ATP7A are highly expressed in atherosclerotic vessels (105, 190), suggesting that they may regulate SOD3 activity in atherosclerotic vessels.
The functional significance of SOD3 activity in the development of atherosclerosis remains still unclear. Sentman et al. observed that genetic deletion of SOD3 paradoxically caused a slight increase in atherosclerotic lesions in apo(E)-deficient mice after 1-month atherogenic diet, while having no effect after 3 months on the atherogenic diet or after 8 months on standard chow (204). The authors concluded that SOD3 may enhance or have little effect on the development of atherosclerotic lesions. It should be noted that mice with a life-long deficiency in an enzyme may have multiple adaptations. Indeed, mice with the embryonic deletion of SOD3 can tolerate ambient oxygen, whereas those with acute deletion of SOD3 in ambient oxygen can lead to severe lung damage and high mortality (75).
SOD and Hypertension
Hypertension is associated with increased ROS formation, in particular O2•−, in multiple organs, including the brain, the vasculature, and the kidney, all of which could contribute to hypertension. Further, the adaptive immune system contributes to hypertension by interacting with these organs. A large body of evidence using exogenous SOD support the notion that O2•− and/or SOD plays a role in hypertension. For example, membrane permeable SOD or SOD mimetic improves hypertension, oxidation markers, endothelium-dependent relaxation, media/lumen ratio, kidney damage, glomerular filtration, and NOx excretion in models such as Ang II infusion (14, 113, 168), SHR (165, 234), endothelin-1 infusion (203), DOCA-salt (16, 252), the one-kidney, one-clip (54), and Dahl salt-sensitive (91). In contrast, SOD has no effect on the response to norepinephrine, which is not accompanied by O2•− production (14).
Mechanisms by which SOD improves hypertension include modulation of vasodilation, vasoconstriction, vascular remodeling, cardiac hypertrophy, renal sodium handling including tubuloglomerular feedback, and neuronal control of sympathetic activity. As discussed in the previous section, O2•− induces vascular dysfunction in various models of hypertension by its well described interaction with NO. In addition, SOD mimetic inhibits medial thickening in vivo, as well as VSMC hypertrophy and cell proliferation in vitro. The Cu chelator DETC, an inhibitor of Cu/ZnSODs (SOD1 and SOD3), increases blood pressure after intravenous administration or infusion into the kidney medulla (148), whereas in Ang II–infused hypertensive rats, acute SOD mimetic tempol injections increased renal blood flow, glomular filtration rate, and Na excretion (120). Endogenous SOD expression and activity are altered by hypertensive stimuli, such as Ang II (70). These findings suggest that SOD plays an important role in both development and the maintenance of chronic hypertension in various organs. Role of each isoform of SOD in hypertension has been investigated by using mice lacking the enzyme and adenovirus-mediated gene transfer as described below.
SOD1 and hypertension
Transgenic mice overexpressing SOD1 show inhibition of the increase in vascular O2•− and arterial pressure in response to Ang II without affecting Ang II-induced hypertrophic response (229). In contrast, SOD1 knockout mice exhibit no effect or even lower blood pressure as compared to control mice, in spite of increases in vascular O2•− and contractile responses to vasoconstrictors, as well as impaired endothelial-dependent relaxation (51). This paradoxical effect may be due to compensatory mechanisms.
SOD1 may play a role in hypertension via neuronal mechanism, rather than vascular or renal. Hypertension is also linked to increased brain levels of Ang II, which mediates its effects partially via O2•− and intracellular Ca2+changes. Overexpression of SOD1 attenuates increased voltage-sensitive intracellular Ca2+ in response to Ang II in neuroblastoma cells (255). ROS signaling to hypertension is also implicated in the NTS. Compared with Wistar-Kyoto rats, stroke-prone spontaneously hypertensive rats (SHR) exhibit elevated activity of Rac1, a regulator of Nox1 and Nox2, in the NTS, and adenoviral-mediated expression of SOD1 decreases BP, heart rate, and urinary norepinephrine excretion (171). Seminal work by Davisson and coworkers has shown that intracerebroventricular injection of SOD1-expressing adenoviruses inhibits increases in heart rate, BP, and drinking behavior induced by Ang II in mice (254). Interestingly, Chan et al. observed that NADPH oxidase stimulated mitochondrial ROS production through an ROS-induced ROS release mechanism (32). Further, they found that overexpression of SOD1 in rostral ventrolateral medulla (RVLM) restored mitochondrial electron transport chain (ETC) activity, reduced mitochondrial ROS production, and prevented hypertension in the spontaneously hypertensive rat. By contrast, intracerebroventricular administration of ETC inhibitors stimulated mitochondrial ROS production and produced hypertension. Thus, neuronal SOD1 in RVLM may play a role in regulating mitochondrial ROS by regulating NADPH oxidase-derived O2•− in the cytoplasm, which contributes to regulation of sympathetic vasomotor tone and hypertension.
SOD2 and hypertension
In SHRs and Ang II-infused Wistar-Kyoto rats, mitochondrial dysfunction in the rostral ventrolateral medulla and the subsequent production of mitochondrial-derived ROS play a critical role in hypertension (32). Coenzyme Q10 treatment restores electron transport capacity and reduces blood pressure and sympathetic neurogenic vasomotor tone. Interestingly, p22phox antisense, SOD2, and catalase prevent Ang II-induced ROS generation, suggesting the existence of a feed-forward effect of Nox on mitochondrial function. The importance of neuronal SOD2 in hypertension is also demonstrated by that intracerebroventricular injection of SOD2-expressing adenoviruses inhibits Ang II-induced increase in heart rate, blood pressure, and drinking behavior in mice in a similar fashion to SOD1, as mentioned above (253). Mice lacking SOD2 die of a cardiomyopathy within 10 days of birth and mice lacking one allele of SOD2 (SOD2+/− mice) develop hypertension with aging and in response to a high-salt diet (197). Mitochondria-targeted SOD mimetic mitoTEMPO or overexpression of SOD2 improves endothelial function and reduces hypertension and oxidative stress in mice with Ang II– or DOCA salt-induced hypertension, but not in normotensive mice (52). These findings indicate that mitochondrial O2•− play an important role in endothelial function and hypertension. In this case, mitochondrion is the initial source of free radicals in response to Ang II, and then NADPH oxidase is secondarily activated by mitochondrial ROS. MitoTEMPO had no effect on blood pressure in normotensive animals. The Framingham Heart Study demonstrated a maternal influence on blood pressure, suggesting that hypertension may be transferred to offspring via inheritance of maternal mitochondrial DNA (246). These findings from SOD2 transgenic mice may explain this maternal heritability of blood pressure by linking it to systemic oxidative stress and inappropriate activation of NADPH oxidase in hypertension.
Archer et al. recently reported that epigenetic attenuation of SOD2 contribute to pulmonary arterial hypertension (PAH) by promoting cell proliferation and impaired apoptosis of pulmonary artery smooth muscle cells (9). Thus, enhanced mitochondrial H2O2 generation by epigenetic SOD2 deficiency plays a role in initiating and/or sustaining PAH, implicating SOD2 as a potential therapeutic target.
SOD3 and hypertension
Because of its extracellular location and O2•− reacts with NO• at an almost diffusion-controlled rate, SOD3 plays an important role in regulating blood pressure by modulating bioactivity of NO• (67). Indeed, gene transfer of SOD3 reduces O2•− and restores impairment of endothelium dependent relaxation and renal sodium handling, resulting in the decrease in arterial pressure in a genetic model of hypertension (34, 35). SOD3 deficiency promotes O2•− increases and impairment of endothelium-dependent relaxation in aortas from mice with the two-kidney and one-clip (2K1C) model (high renin induced hypertension) and in small mesenteric arteries from mice with chronic Ang II infusion, which is associated with an enhanced blood pressure (76, 108). In mice bearing a mutation in the Cu transporter ATP7A, SOD3 activity is reduced because of impaired incorporation of Cu, leading to impaired endothelium-dependent vasorelaxation, further supporting a role for SOD3 in vascular protection (189). In a similar fashion as SOD2, SOD3 deficiency or overexpression had no effect on blood pressure in normotensive animals. This is in keeping with the concept that O2•− does not affect hemodynamics under normal physiological conditions but begins to play a role in pathophysiological states (131, 165).
In prooxidant pathological states such as atherosclerosis and hypertension, SOD3 is inactivated probably due its peroxidase activity (89, 109, 231). In this case, H2O2, the dismutation product of O2•−, can inactivate SOD3 through reacting with the copper center of SOD3, thereby forming the Cu–OH radical and leading to enzyme inactivation (89). This effect can be prevented by scavenging the Cu–OH radical with small anionic antioxidants, such as urate or nitrite. In the one-kidney and one-clip (1K1C) model of low renin and high-volume hypertension, SOD3 is inactivated by H2O2 probably by its peroxidase activity, resulting in promoting the increase in extracellular O2•− levels, which contribute to enhancing blood pressure and impaired endothelium-dependent vasodilation in high-volume hypertension (109). Indeed, PEG-catalase and the hydroxyl radical scavenger uric acid reduced these responses in this hypertension model. Similarly, in a lamb model of persistent pulmonary hypertension of the newborn (PPHN), Wedgwood et al. demonstrated that H2O2 generated by PPHN and hyperoxia inactivates SOD3, and intratracheal catalase enhances enzyme function (231).
Lob et al. show that subfornical organ (SFO)-targeted ablation of endogenous SOD3 causes a significant elevation in basal blood pressure (140). Note that cells lining the third ventricle highly express SOD3 under normal conditions, which may explain why earlier studies failed to show additional overexpression effect of SOD3 in the SFO to inhibit the pressor effects of Ang II. These regions lack a well-formed blood–brain barrier and are therefore affected by circulating signals like Ang II. In addition, deletion of SOD3 in the SFO increases the sensitivity to systemic low-dose Ang II. More importantly, this central manipulation caused an increase in the percent of T cells with an activated phenotype, and markedly increased the vascular inflammation associated with Ang II infusion. Analysis of heart rate and blood pressure variability indicated that deletion of SOD3 in the circumventricular organ (CVO) enhanced sympathetic outflow (140). These data are in keeping with prior studies by Ganta et al., suggesting that sympathetic outflow can promote T cell activation (71), and a feed-forward loop exists between organ systems. Given that all three SOD isozymes play an important role in neuronal regulation of hypertension, future analysis of the relative expression, distribution, and functional role of these three SOD isozymes in SFO will be important in understanding the mechanisms of central redox signaling and how it regulates hemodynamics and inflammation.
Overexpression of SOD3 ameliorated pulmonary hypertension in monocrotaline-treated rats (111) and attenuated pulmonary vascular remodeling in mice exposed to chronic hypoxia (170). In pulmonary artery smooth muscle cells and lung extracts of fetal PPHN lambs relative to fetal controls, SOD3 specific activity is decreased without altering specific activities of SOD1 and SOD2 (231). In the same model, intratracheal administration of SOD improved oxygenation and reduced O2•− levels (122). Taken together, these findings further highlight the potential importance of SOD3 in protecting against O2•−-induced pulmonary hypertension.
SOD and Vascular Remodeling
Vascular repair reaction after injury is characterized by neointimal growth as well as vascular remodeling. Such events contribute not only to restenosis after angioplasty but also to vascular diseases, such as atherosclerosis, hypertension, and diabetes mellitus. Balloon angioplasty induces an acute increase in systemic oxidative stress, local O2•− generation, and altered vascular SOD activity (185). Given that only a few reports about role of SOD1 or SOD2 (45, 121), role of SOD3 in vascular remodeling will be discussed here.
Several studies using SOD3 gene transfer show its protective effect on balloon-induced or cuff-induced neointima formation (23, 126, 182) and constrictive remodeling (135) as well as cardioprotective properties (4) such as recovery of the endothelial layer in arterial wall. These SOD3-induced protective effects are associated with reduced inflammatory cell accumulation, decreased VSMC proliferation, and altered NO levels through iNOS or eNOS expression (23, 126, 135, 182). They may be by either regulating extracellular O2•− level or by preserving NO level by protecting its oxidative inactivation. Interestingly, SOD3 reduced collagen content in response to vascular injury (182), which may contribute to prevention of constrictive remodeling. Consistent with this, liposomal SOD delivery reduces the redox-dependent expression of TGF beta1 and collagen in dermal myofibroblasts (228).
Role of SOD in Angiogenesis and Stem/Progenitor Function
Neovascularization is involved in physiological process such as development and wound repair as well as pathophysiologies such as ischemic heart/limb diseases, and atherosclerosis. Postnatal new blood vessel formation involves not only angiogenesis but also vasculogenesis, which is mediated through mobilization of bone marrow (BM)-derived angiogenic stem/progenitor cells as well as their homing to the ischemic tissues (101). Angiogenesis is dependent on cell proliferation, migration, and capillary tube formation in endothelial cells (ECs). BM-derived stem/progenitor cells or mesenchymal stem cells (MSCs) have been used for cell-based therapy to promote revascularization after peripheral or myocardial ischemia. ROS play an important role in redox signaling linked to angiogenesis in ECs as well as stem/progenitor cell mobilization, homing, and differentiation, thereby promoting neovascularization (222, 225). Although physiological levels of ROS are required for normal function, excess amounts of ROS produced in various pathophysiology contribute to dysfunction of endothelial progenitor cell (EPC) and stem/progenitor cells (225). Number and functional capacity of EPCs are reduced in oxidative stress-dependent cardiovascular diseases, including hypertension, atherosclerosis, diabetes, coronary artery disease, and heart failure as well as comorbid risk factors such as aging, hypercholesterolemia, and cigarette smoking (225). Role of SODs in postnatal angiogenesis and stem/progenitor function has been demonstrated, as shown below.
SODs and postnatal angiogenesis
Studies using SOD3-deficient mice show that SOD3 plays an essential role in reparative neovascularization in response to ischemic injury by protecting ischemic tissues from overproduction of O2•− (117) or generating H2O2 (180). Gene transfer of SOD3 promotes neovascularization with an increase in H2O2 (180) as well as tissue injury recovery by activating mitogenic Ras-ERK and PI3kinase-Akt pathways leading to increased VEGF and cyclin D1 expression (129). These responses are associated with reduced adhesion molecule and cytokine expression and inflammatory cell recruitment (130) in ischemic tissues in hindlimb ischemia model. Consistent with SOD3−/− mice, SOD1-deficient mice show impaired neovascularization, as assessed by reduction of blood flow recovery and capillary density in ischemic muscle (80). Overexpression of human SOD1 in mouse NIH 3T3 fibroblasts increased SOD activity, enhanced intracellular generation of H2O2, and significantly stimulated VEGF production (81). FGF-induced angiogenesis and tumor development are enhanced in SOD1 transgenic mice (150). Connor et al. (40) reported that overexpression of SOD2 promotes mitochondrial H2O2 production, thereby stimulating EC sprouting and neovascularization in the angiogenesis assay. Further, VEGF-induced ROS via activation of Rac1 upregulate SOD2 expression in ECs (1), which could represent a feed-forward mechanism by which ROS-induced H2O2 enhances angiogenesis. Thus, all three SOD isoforms serve as not only O2•−scavenger but also H2O2-generating angiogenic enzymes. Thus, SODs are required for postnatal angiogenesis and SODs treatment is essential therapeutic approach for treatment of angiogenesis-dependent cardiovascular diseases.
SODs and stem cells/progenitor cells function
In SOD3−/− mice, EPCs in both peripheral blood and BM are reduced, which is associated with decreased differentiation of BM cells into EC-like cells as well as levels of NO in the BM (117). Moreover, defective neovascularization in SOD3−/− mice was rescued by SOD mimetic infusion as well as transplantation of BM from WT mice (117). In human mesenchymal stem cells, secreted SOD3 play an important role in their neuroprotective effect by promoting cell survival (114, 115). These results strongly suggest that SOD3 in BM-derived cells plays an important in protecting overproduction of O2•− or generating signaling molecule H2O2 to maintain the normal function of BM in mediating stem/progenitor cells mobilization and function. SOD1-deficient mice also show a reduction in the number of EPCs in the BM and spleen. The impaired neovascularization in these mice is rescued by wild type, but not SOD1−/−, EPC supplementation (80). Functionally, SOD1−/− EPCs show increased O2•− levels, decreased NO production, and a reduced ability to migrate and integrate into capillary-like networks in vitro. Marrotte recently reported that EPCs-mediated augmentation of angiogenesis and wound repair are functionally impaired in diabetes due to decreased SOD2 expression in EPCs, which is restored by SOD2 gene therapy (154). Dihydropyridine calcium antagonist, which reduces cardiovascular events, improves migratory ability of circulating EPCs via upregulation of SOD2 (186). Taken together, SODs in BM-derived stem/progenitor cells and EPCs play an important role in protecting against oxidative stress, which appears to be an important for their normal function.
Conclusions and Future Directions
SODs play a critical role in endothelial and mitochondrial function by inhibiting oxidative inactivation of bioavailable NO, and thus preventing ONOO formation or OH radical formation via inhibition of oxidation of Fe-S cluster containing enzymes. Since SODs catalyze dismutation of O2•− to H2O2 and localize at distinct compartments (cytosol (for SOD1), mitochondria (for SOD2), and extracellular matrix (for SOD3)), they participate in compartmentalized redox signaling to regulate many vascular function. Dysregulation of these signaling pathways leads to endothelial dysfunction, altered vascular tone, vascular inflammation, vascular remodeling, enhanced vascular permeability, and increased platelet aggregation, which contribute to impaired angiogenesis as well as various vascular diseases such as atherosclerosis and hypertension. Lack of SOD1 contributes to vascular abnormality (increased vasoconstriction and endothelial dysfunction) and impaired angiogenesis (13, 51, 80, 119). The role of SOD2 in maintaining mitochondrial function is demonstrated by the neonatal lethality of mice lacking SOD2 (104, 133, 138). The importance of SOD3 is shown by 85% mortality of mice within a week of induction of SOD3 ablation (75). A prospective, population-based study from the Copenhagen City Heart Study showed that heterozygosity for SOD3 with R213G is associated with increased risk of ischemic heart disease (110). Given the importance of SODs in cardiovascular disease, the concept of antioxidant therapies, that is, reinforcement of endogenous antioxidant defenses to protect more effectively against oxidative stress, is of substantial interest.
Abundant data from epidemiological observational studies have shown an inverse association between antioxidant intake or body status and the risk of cardiovascular diseases (149, 242). By contrast, clinical trials, such as the GISSI and HOPE trials, have found no benefits of vitamin E supplementation on cardiovascular disease risk (149, 242). Given the compartmentalized redox signaling, this may be because agents such as vitamin E and vitamin C are not targeted to sites of ROS generation that are most important in pathological conditions. Further, effect of SOD may depend on cell type, context specific, and disease states. For example, we found that SOD3 is essential for angiogenesis either by preserving NO bioactivity or by dismutating O2•− to H2O2 (117, 180). In contrast, SOD3 overexpression inhibits, instead of increasing, tumor angiogenesis and tumor invasion (31, 236). In diabetic mice, Nox2 deficiency improved neovascularization (55), whereas it impairs angiogenesis in wild-type mice (217). This may be explained by the concept that optimal levels of ROS are required but excess or lower amounts of ROS are inhibitory for biological responses. Note that in hypertensive rats or mice, SOD reduces blood pressure, but in normal rats or mice, SOD had no pressure effect (35, 52, 131, 165). Thus, therapeutic efficacy of SOD may be varied depending on the diseases states. Finally, in pro-oxidant pathological conditions such as atherosclerosis and hypertension, SOD1 and SOD3 seem to be inactivated by H2O2 derived from ecSOD due to its peroxidase activity (89, 109, 231). Thus, further detailed studies of SODs and other antioxidant defense systems are needed.
The evidence discussed in this review supports the notion that an increase in O2•−, H2O2, and ONOO−, which critically regulated by SODs, contributes to modulation of cell signals related to vascular physiologies and pathophysiologies. Thus, antioxidant enzymes such as SODs are potential candidate drugs (199). However, effective protection against vascular oxidative stress by antioxidant enzymes requires prolonged half-life and specific recognition of cell types such as endothelium and targets to specific subcellular compartments. In this regard, vascular immunotargeting and gene transfer would be a promising approach (87, 164). Given the importance of Cu importers, transporters, and chaperones in regulating activity of SODs, understanding the processes and effects of metal ion insertion on SOD function is essential and subject of further investigation. Because of the compartmental localization of each SOD, approaches to target their site-specific expression will be very important and could be used to aid the development of novel SOD-dependent therapeutic strategies.
Abbreviations Used
- 1K1C
1-kidney-1-clip
- 2K1C
two-kidney and one-clip
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- Atox1
antioxidant-1
- ATP7A
Menkes ATPase, MNK
- BM
bone marrow
- CCO
cytochrome c oxidase
- CCS
Cu chaperone for SOD1
- ClC-3
chloride channels-3
- Cu
copper
- CVO
circumventricular organ
- EC
endothelial cell
- EDHF
endothelium-derived hyperpolarization factor
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- ER
endoplasmic reticulum
- ETC
electron transport chain
- Fe-S
iron-sulfur
- GPx
glutathione peroxidases
- H2O2
hydrogen peroxide
- HBD
heparin-binding domain
- HSPGs
heparan sulfate proteoglycans
- ICAM1
intercellular adhesion molecule 1
- IMS
intermembrane space
- iNOS
inducible NOS
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MMPs
matrix metalloproteinases
- Mn
manganese
- MSCs
mesenchymal stem cells
- MT
metallothioneins
- mtDNA
mitochondrial DNA
- NO
nitric oxide
- NO2+
nitronium ion
- Nox
NADPH oxidase
- Nox2
gp91phox
- O2•−
superoxide anion
- OH•
hydroxyl radical
- ONOO−
peroxynitrite
- PAH
pulmonary arterial hypertension
- PARP
poly (ADP-ribose) polymerase
- PPHN
persistent pulmonary hypertension of the newborn
- Prxs
peroxiredoxins
- PTPs
protein tyrosine phosphatases
- ROS
reactive oxygen species
- RVLM
rostral ventrolateral medulla
- SFO
subfornical organ
- sGC
soluble guanylate cyclase
- SHR
spontaneously hypertensive rats
- SOD1
cytoplasmic Cu/Zn SOD
- SOD2
mitochondrial MnSOD
- SOD3
extracellular Cu/ZnSOD, ecSOD
- SODs
superoxide dismutases
- TGN
trans-Golgi network
- VCAM1
vascular cellular adhesion molecule 1
- VSMC
vascular smooth muscle cell
- Zn
zinc
Acknowledgments
This research was supported by NIH R01 HL070187 (T.F.) and NIH R01 HL077524 (to M.U.-F.).
References
- 1.Abid MR. Tsai JC. Spokes KC. Deshpande SS. Irani K. Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 2.Abreu IA. Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim Biophys Acta. 2010;1804:263–274. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Adachi T. Yamazaki N. Tasaki H. Toyokawa T. Yamashita K. Hirano K. Changes in the heparin affinity of extracellular-superoxide dismutase in patients with coronary artery atherosclerosis. Biol Pharm Bull. 1998;21:1090–1093. doi: 10.1248/bpb.21.1090. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal RS. Muangman S. Layne MD. Melo L. Perrella MA. Lee RT. Zhang L. Lopez-Ilasaca M. Dzau VJ. Pre-emptive gene therapy using recombinant adeno-associated virus delivery of extracellular superoxide dismutase protects heart against ischemic reperfusion injury, improves ventricular function and prolongs survival. Gene Ther. 2004;11:962–969. doi: 10.1038/sj.gt.3302250. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez B. Demicheli V. Duran R. Trujillo M. Cervenansky C. Freeman BA. Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Andresen JJ. Faraci FM. Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol. 2004;287:H1141–H1148. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- 7.Antonyuk SV. Strange RW. Marklund SL. Hasnain SS. The structure of human extracellular copper-zinc superoxide dismutase at 1.7 A resolution: insights into heparin and collagen binding. J Mol Biol. 2009;388:310–326. doi: 10.1016/j.jmb.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Archer SL. Gomberg-Maitland M. Maitland ML. Rich S. Garcia JG. Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 9.Archer SL. Marsboom G. Kim GH. Zhang HJ. Toth PT. Svensson EC. Dyck JR. Gomberg-Maitland M. Thebaud B. Husain AN. Cipriani N. Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada K. Yoshikawa K. Takahashi M. Maeda Y. Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975;250:2801–2807. [PubMed] [Google Scholar]
- 11.Balaban RS. Nemoto S. Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Ballinger SW. Patterson C. Knight-Lozano CA. Burow DL. Conklin CA. Hu Z. Reuf J. Horaist C. Lebovitz R. Hunter GC. McIntyre K. Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 13.Baumbach GL. Didion SP. Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke. 2006;37:1850–1855. doi: 10.1161/01.STR.0000227236.84546.5a. [DOI] [PubMed] [Google Scholar]
- 14.Bech Laursen J. Rajagopalan S. Tarpey M. Freeman BA. Harrison DG. A role of superoxide in angiotensin II—but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 15.Bedwell S. Dean RT. Jessup W. The action of defined oxygen-centred free radicals on human low-density lipoprotein. Biochem J. 1989;262:707–712. doi: 10.1042/bj2620707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beswick RA. Zhang H. Marable D. Catravas JD. Hill WD. Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- 17.Beyer WF., Jr. Fridovich I. Effect of hydrogen peroxide on the iron-containing superoxide dismutase of Escherichia coli. Biochemistry. 1987;26:1251–1257. doi: 10.1021/bi00379a008. [DOI] [PubMed] [Google Scholar]
- 18.Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN. Kohl HW., 3rd Barlow CE. Paffenbarger RS., Jr. Gibbons LW. Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 20.Bogdan C. Rollinghoff M. Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 21.Borgstahl GE. Parge HE. Hickey MJ. Beyer WF., Jr. Hallewell RA. Tainer JA. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992;71:107–118. doi: 10.1016/0092-8674(92)90270-m. [DOI] [PubMed] [Google Scholar]
- 22.Boveris A. Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasen JH. Leppanen O. Inkala M. Heikura T. Levin M. Ahrens F. Rutanen J. Pietsch H. Bergqvist D. Levonen AL. Basu S. Zeller T. Kloppel G. Laukkanen MO. Yla-Herttuala S. Extracellular superoxide dismutase accelerates endothelial recovery and inhibits in-stent restenosis in stented atherosclerotic Watanabe heritable hyperlipidemic rabbit aorta. J Am Coll Cardiol. 2007;50:2249–2253. doi: 10.1016/j.jacc.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Brookes PS. Levonen AL. Shiva S. Sarti P. Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 25.Brown GC. Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 26.Brown KA. Chu Y. Lund DD. Heistad DD. Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2006;290:H2600–H2605. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 27.Carroll MC. Girouard JB. Ulloa JL. Subramaniam JR. Wong PC. Valentine JS. Culotta VC. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci U S A. 2004;101:5964–5969. doi: 10.1073/pnas.0308298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassano S. Agnese S. D'Amato V. Papale M. Garbi C. Castagnola P. Ruocco MR. Castellano I. De Vendittis E. Santillo M. Amente S. Porcellini A. Avvedimento EV. Reactive oxygen species, Ki-Ras, and mitochondrial superoxide dismutase cooperate in nerve growth factor-induced differentiation of PC12 cells. J Biol Chem. 2010;285:24141–24153. doi: 10.1074/jbc.M109.098525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cathcart MK. McNally AK. Morel DW. Chisolm GM., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989;142:1963–1969. [PubMed] [Google Scholar]
- 30.Chaiswing L. Oberley TD. Extracellular/microenvironmental redox state. Antioxid Redox Signal. 2010;13:449–465. doi: 10.1089/ars.2009.3020. [DOI] [PubMed] [Google Scholar]
- 31.Chaiswing L. Zhong W. Cullen JJ. Oberley LW. Oberley TD. Extracellular redox state regulates features associated with prostate cancer cell invasion. Cancer Res. 2008;68:5820–5826. doi: 10.1158/0008-5472.CAN-08-0162. [DOI] [PubMed] [Google Scholar]
- 32.Chan SH. Wu KL. Chang AY. Tai MH. Chan JY. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension. 2009;53:217–227. doi: 10.1161/HYPERTENSIONAHA.108.116905. [DOI] [PubMed] [Google Scholar]
- 33.Chang LY. Slot JW. Geuze HJ. Crapo JD. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988;107:2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu Y. Alwahdani A. Iida S. Lund DD. Faraci FM. Heistad DD. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation. 2005;112:1047–1053. doi: 10.1161/CIRCULATIONAHA.104.531251. [DOI] [PubMed] [Google Scholar]
- 35.Chu Y. Iida S. Lund DD. Weiss RM. DiBona GF. Watanabe Y. Faraci FM. Heistad DD. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res. 2003;92:461–468. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 36.Chu Y. Piper R. Richardson S. Watanabe Y. Patel P. Heistad DD. Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol. 2006;26:1985–1990. doi: 10.1161/01.ATV.0000234921.88489.5c. [DOI] [PubMed] [Google Scholar]
- 37.Cleeter MW. Cooper JM. Darley-Usmar VM. Moncada S. Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 38.Cobine PA. Ojeda LD. Rigby KM. Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem. 2004;279:14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 39.Cobine PA. Pierrel F. Bestwick ML. Winge DR. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem. 2006;281:36552–36559. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 40.Connor KM. Subbaram S. Regan KJ. Nelson KK. Mazurkiewicz JE. Bartholomew PJ. Aplin AE. Tai YT. Aguirre-Ghiso J. Flores SC. Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 41.Cooke CL. Davidge ST. Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol. 2002;282:C395–C402. doi: 10.1152/ajpcell.00295.2001. [DOI] [PubMed] [Google Scholar]
- 42.Crapo JD. Oury T. Rabouille C. Slot JW. Chang LY. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csiszar A. Labinskyy N. Orosz Z. Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses. 2006;67:904–908. doi: 10.1016/j.mehy.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Culotta VC. Yang M. O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalle Lucca JJ. Saari JT. Falcone JC. Schuschke DA. Neointima formation in the rat carotid artery is exacerbated by dietary copper deficiency. Exp Biol Med (Maywood) 2002;227:487–491. [PubMed] [Google Scholar]
- 46.Darly-Usmar VM. Hogg N. O'Leary VJ. Wilson MT. Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Rad Res Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 47.Didion SP. Faraci FM. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler Thromb Vasc Biol. 2005;25:90–95. doi: 10.1161/01.ATV.0000149868.74075.5d. [DOI] [PubMed] [Google Scholar]
- 48.Didion SP. Kinzenbaw DA. Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 49.Didion SP. Kinzenbaw DA. Fegan PE. Didion LA. Faraci FM. Overexpression of CuZn-SOD prevents lipopolysaccharide-induced endothelial dysfunction. Stroke. 2004;35:1963–1967. doi: 10.1161/01.STR.0000132764.06878.c5. [DOI] [PubMed] [Google Scholar]
- 50.Didion SP. Kinzenbaw DA. Schrader LI. Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–1079. doi: 10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- 51.Didion SP. Ryan MJ. Didion LA. Fegan PE. Sigmund CD. Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 52.Dikalova AE. Bikineyeva AT. Budzyn K. Nazarewicz RR. McCann L. Lewis W. Harrison DG. Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dionisi O. Galeotti T. Terranova T. Azzi A. Superoxide radicals and hydrogen peroxide formation in mitochondria from normal and neoplastic tissues. Biochim Biophys Acta. 1975;403:292–300. doi: 10.1016/0005-2744(75)90059-5. [DOI] [PubMed] [Google Scholar]
- 54.Dobrian AD. Schriver SD. Prewitt RL. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38:361–366. doi: 10.1161/01.hyp.38.3.361. [DOI] [PubMed] [Google Scholar]
- 55.Ebrahimian TG. Heymes C. You D. Blanc-Brude O. Mees B. Waeckel L. Duriez M. Vilar J. Brandes RP. Levy BI. Shah AM. Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edlund A. Edlund T. Hjalmarsson K. Marklund SL. Sandstrom J. Stromqvist M. Tibell L. A non-glycosylated extracellular superoxide dismutase variant. Biochem J. 1992;288:451–456. doi: 10.1042/bj2880451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekelund LG. Haskell WL. Johnson JL. Whaley FS. Criqui MH. Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 58.Elroy-Stein O. Bernstein Y. Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986;5:615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enghild JJ. Thogersen IB. Oury TD. Valnickova Z. Hojrup P. Crapo JD. The heparin-binding domain of extracellular superoxide dismutase is proteolytically processed intracellularly during biosynthesis. J Biol Chem. 1999;274:14818–14822. doi: 10.1074/jbc.274.21.14818. [DOI] [PubMed] [Google Scholar]
- 60.Epstein CJ. Avraham KB. Lovett M. Smith S. Elroy-Stein O. Rotman G. Bry C. Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erusalimsky JD. Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 62.Esposito LA. Melov S. Panov A. Cottrell BA. Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–4825. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fattman CL. Schaefer LM. Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 64.Fennell JP. Brosnan MJ. Frater AJ. Hamilton CA. Alexander MY. Nicklin SA. Heistad DD. Baker AH. Dominiczak AF. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther. 2002;9:110–117. doi: 10.1038/sj.gt.3301633. [DOI] [PubMed] [Google Scholar]
- 65.Folz RJ. Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- 66.Fridovich I. Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- 67.Fukai T. Folz RJ. Landmesser U. Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 68.Fukai T. Galis ZS. Meng XP. Parthasarathy S. Harrison DG. Vascular expression of extracellular superoxide dismutase in atherosclerosis. J Clin Invest. 1998;101:2101–2111. doi: 10.1172/JCI2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukai T. Siegfried MR. Ushio-Fukai M. Cheng Y. Kojda G. Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukai T. Siegfried MR. Ushio-Fukai M. Griendling KK. Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res. 1999;85:23–28. doi: 10.1161/01.res.85.1.23. [DOI] [PubMed] [Google Scholar]
- 71.Ganta CK. Lu N. Helwig BG. Blecha F. Ganta RR. Zheng L. Ross CR. Musch TI. Fels RJ. Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–H1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 72.Ghafourifar P. Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 73.Giulivi C. Poderoso JJ. Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 74.Go YM. Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gongora MC. Lob HE. Landmesser U. Guzik TJ. Martin WD. Ozumi K. Wall SM. Wilson DS. Murthy N. Gravanis M. Fukai T. Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gongora MC. Qin Z. Laude K. Kim HW. McCann L. Folz JR. Dikalov S. Fukai T. Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Pacheco FR. Deudero JJ. Castellanos MC. Castilla MA. Alvarez-Arroyo MV. Yague S. Caramelo C. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol. 2006;291:H1395–H1401. doi: 10.1152/ajpheart.01277.2005. [DOI] [PubMed] [Google Scholar]
- 78.Gorecki M. Beck Y. Hartman JR. Fischer M. Weiss L. Tochner Z. Slavin S. Nimrod A. Recombinant human superoxide dismutases: production and potential therapeutical uses. Free Radic Res Commun. 1991;(12–13 Pt 1):401–410. doi: 10.3109/10715769109145810. [DOI] [PubMed] [Google Scholar]
- 79.Griffith TM. Edwards DH. Newby AC. Lewis MJ. Henderson AH. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986;20:7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- 80.Groleau J. Dussault S. Haddad P. Turgeon J. Menard C. Chan JS. Rivard A. Essential role of copper-zinc superoxide dismutase for ischemia-induced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:2173–2181. doi: 10.1161/ATVBAHA.110.212530. [DOI] [PubMed] [Google Scholar]
- 81.Grzenkowicz-Wydra J. Cisowski J. Nakonieczna J. Zarebski A. Udilova N. Nohl H. Jozkowicz A. Podhajska A. Dulak J. Gene transfer of CuZn superoxide dismutase enhances the synthesis of vascular endothelial growth factor. Mol Cell Biochem. 2004;264:169–181. doi: 10.1023/b:mcbi.0000044386.45054.70. [DOI] [PubMed] [Google Scholar]
- 82.Guo W. Adachi T. Matsui R. Xu S. Jiang B. Zou MH. Kirber M. Lieberthal W. Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- 83.Guzik TJ. Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Hambrecht R. Wolf A. Gielen S. Linke A. Hofer J. Erbs S. Schoene N. Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 85.Harraz MM. Marden JJ. Zhou W. Zhang Y. Williams A. Sharov VS. Nelson K. Luo M. Paulson H. Schoneich C. Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawkins BJ. Madesh M. Kirkpatrick CJ. Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heistad DD. Gene therapy for vascular disease. Vascul Pharmacol. 2006;45:331–333. doi: 10.1016/j.vph.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Higashi Y. Sasaki S. Kurisu S. Yoshimizu A. Sasaki N. Matsuura H. Kajiyama G. Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 89.Hink HU. Santanam N. Dikalov S. McCann L. Nguyen AD. Parthasarathy S. Harrison DG. Fukai T. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22:1402–1408. doi: 10.1161/01.atv.0000027524.86752.02. [DOI] [PubMed] [Google Scholar]
- 90.Hjalmarsson K. Marklund SL. Engstrom A. Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987;84:6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoagland KM. Maier KG. Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension. 2003;41:697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- 92.Hodgson EK. Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975;14:5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- 93.Hodgson EK. Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975;14:5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- 94.Hornig B. Maier V. Drexler H. Physical training improves endothelial function in patients with chronic heart failure [see comments] Circulation. 1996;93:210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 95.Hsu JL. Hsieh Y. Tu C. O'Connor D. Nick HS. Silverman DN. Catalytic properties of human manganese superoxide dismutase. J Biol Chem. 1996;271:17687–17691. doi: 10.1074/jbc.271.30.17687. [DOI] [PubMed] [Google Scholar]
- 96.Iadecola C. Zhang F. Niwa K. Eckman C. Turner SK. Fischer E. Younkin S. Borchelt DR. Hsiao KK. Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 97.Iida S. Chu Y. Francis J. Weiss RM. Gunnett CA. Faraci FM. Heistad DD. Gene transfer of extracellular superoxide dismutase improves endothelial function in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H525–H532. doi: 10.1152/ajpheart.00108.2005. [DOI] [PubMed] [Google Scholar]
- 98.Inoue N. Ramasamy S. Fukai T. Nerem RM. Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res. 1996;79:32–37. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- 99.Ischiropoulos H. Zhu L. Chen J. Tsai M. Martin JC. Smith CD. Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 100.Ishibashi M. Hiasa K. Zhao Q. Inoue S. Ohtani K. Kitamoto S. Tsuchihashi M. Sugaya T. Charo IF. Kura S. Tsuzuki T. Ishibashi T. Takeshita A. Egashira K. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 101.Isner JM. Losordo DW. Therapeutic angiogenesis for heart failure. Nat Med. 1999;5:491–492. doi: 10.1038/8374. [DOI] [PubMed] [Google Scholar]
- 102.Itoh S. Kim HW. Nakagawa O. Ozumi K. Lessner SM. Aoki H. Akram K. McKinney RD. Ushio-Fukai M. Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008;283:9157–9167. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Itoh S. Ozumi K. Kim HW. Nakagawa O. McKinney RD. Folz RJ. Zelko IN. Ushio-Fukai M. Fukai T. Novel mechanism for regulation of extracellular SOD transcription and activity by copper: role of antioxidant-1. Free Radic Biol Med. 2009;46:95–104. doi: 10.1016/j.freeradbiomed.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jang YC. Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Jeney V. Itoh S. Wendt M. Gradek Q. Ushio-Fukai M. Harrison DG. Fukai T. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res. 2005;96:723–729. doi: 10.1161/01.RES.0000162001.57896.66. [DOI] [PubMed] [Google Scholar]
- 106.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 107.Juarez JC. Manuia M. Burnett ME. Betancourt O. Boivin B. Shaw DE. Tonks NK. Mazar AP. Donate F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci U S A. 2008;105:7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung O. Marklund SL. Geiger H. Pedrazzini T. Busse R. Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 109.Jung O. Marklund SL. Xia N. Busse R. Brandes RP. Inactivation of extracellular superoxide dismutase contributes to the development of high-volume hypertension. Arterioscler Thromb Vasc Biol. 2007;27:470–477. doi: 10.1161/01.ATV.0000254823.15843.1f. [DOI] [PubMed] [Google Scholar]
- 110.Juul K. Tybjaerg-Hansen A. Marklund S. Heegaard NH. Steffensen R. Sillesen H. Jensen G. Nordestgaard BG. Genetically reduced antioxidative protection and increased ischemic heart disease risk: the Copenhagen City Heart Study. Circulation. 2004;109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 111.Kamezaki F. Tasaki H. Yamashita K. Tsutsui M. Koide S. Nakata S. Tanimoto A. Okazaki M. Sasaguri Y. Adachi T. Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 112.Kamii H. Kato I. Kinouchi H. Chan PH. Epstein CJ. Akabane A. Okamoto H. Yoshimoto T. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn-superoxide dismutase. Stroke. 1999;30:867–871. doi: 10.1161/01.str.30.4.867. discussion 872, [DOI] [PubMed] [Google Scholar]
- 113.Kawada N. Imai E. Karber A. Welch WJ. Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 114.Kemp K. Gray E. Mallam E. Scolding N. Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev. 2010;6:548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 115.Kemp K. Hares K. Mallam E. Heesom KJ. Scolding N. Wilkins A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem. 2010;114:1569–1580. doi: 10.1111/j.1471-4159.2009.06553.x. [DOI] [PubMed] [Google Scholar]
- 116.Kim BE. Nevitt T. Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 117.Kim HW. Lin A. Guldberg RE. Ushio-Fukai M. Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 118.Kojda G. Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 119.Kondo T. Reaume AG. Huang TT. Carlson E. Murakami K. Chen SF. Hoffman EK. Scott RW. Epstein CJ. Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kopkan L. Castillo A. Navar LG. Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F80–F86. doi: 10.1152/ajprenal.00090.2005. [DOI] [PubMed] [Google Scholar]
- 121.Kuo MD. Bright IJ. Wang DS. Ghafouri P. Yuksel E. Hilfiker PR. Miniati DN. Dake MD. Local resistance to oxidative stress by overexpression of copper-zinc superoxide dismutase limits neointimal formation after angioplasty. J Endovasc Ther. 2004;11:585–594. doi: 10.1583/04-1310.1. [DOI] [PubMed] [Google Scholar]
- 122.Lakshminrusimha S. Russell JA. Wedgwood S. Gugino SF. Kazzaz JA. Davis JM. Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamb AL. Torres AS. O'Halloran TV. Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol. 2001;8:751–755. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 124.Landmesser U. Merten R. Spiekermann S. Buttner K. Drexler H. Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 125.Lassegue B. Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Laukkanen MO. Kivela A. Rissanen T. Rutanen J. Karkkainen MK. Leppanen O. Brasen JH. Yla-Herttuala S. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2002;106:1999–2003. doi: 10.1161/01.cir.0000031331.05368.9d. [DOI] [PubMed] [Google Scholar]
- 127.Laukkanen MO. Lehtolainen P. Turunen P. Aittomaki S. Oikari P. Marklund SL. Yla-Herttuala S. Rabbit extracellular superoxide dismutase: expression and effect on LDL oxidation. Gene. 2000;254:173–179. doi: 10.1016/s0378-1119(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 128.Laukkanen MO. Mannermaa S. Hiltunen MO. Aittomaki S. Airenne K. Janne J. Yla-Herttuala S. Local hypomethylation in atherosclerosis found in rabbit ec-sod gene. Arterioscler Thromb Vasc Biol. 1999;19:2171–2178. doi: 10.1161/01.atv.19.9.2171. [DOI] [PubMed] [Google Scholar]
- 129.Laurila JP. Castellone MD. Curcio A. Laatikainen LE. Haaparanta-Solin M. Gronroos TJ. Marjamaki P. Martikainen S. Santoro M. Laukkanen MO. Extracellular superoxide dismutase is a growth regulatory mediator of tissue injury recovery. Mol Ther. 2009;17:448–454. doi: 10.1038/mt.2008.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laurila JP. Laatikainen LE. Castellone MD. Laukkanen MO. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laursen JB. Rajagopalan S. Galis Z. Tarpey M. Freeman BA. Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension [see comments] Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 132.Laursen JB. Somers M. Kurz S. McCann L. Warnholtz A. Freeman BA. Tarpey M. Fukai T. Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 133.Lebovitz RM. Zhang H. Vogel H. Cartwright J., Jr. Dionne L. Lu N. Huang S. Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lehr HA. Becker M. Marklund SL. Hubner C. Arfors KE. Kohlschutter A. Messmer K. Superoxide-dependent stimulation of leukocyte adhesion by oxidatively modified LDL in vivo. Arterioscler Thromb. 1992;12:824–829. doi: 10.1161/01.atv.12.7.824. [DOI] [PubMed] [Google Scholar]
- 135.Leite PF. Danilovic A. Moriel P. Dantas K. Marklund S. Dantas AP. Laurindo FR. Sustained decrease in superoxide dismutase activity underlies constrictive remodeling after balloon injury in rabbits. Arterioscler Thromb Vasc Biol. 2003;23:2197–2202. doi: 10.1161/01.ATV.0000093980.46838.41. [DOI] [PubMed] [Google Scholar]
- 136.Levanon D. Lieman-Hurwitz J. Dafni N. Wigderson M. Sherman L. Bernstein Y. Laver-Rudich Z. Danciger E. Stein O. Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985;4:77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Y. Huang TT. Carlson EJ. Melov S. Ursell PC. Olson JL. Noble LJ. Yoshimura MP. Berger C. Chan PH. Wallace DC. Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 139.Liu JQ. Zelko IN. Folz RJ. Reoxygenation-induced constriction in murine coronary arteries: the role of endothelial NADPH oxidase (gp91phox) and intracellular superoxide. J Biol Chem. 2004;279:24493–24497. doi: 10.1074/jbc.M402920200. [DOI] [PubMed] [Google Scholar]
- 140.Lob HE. Marvar PJ. Guzik TJ. Sharma S. McCann LA. Weyand C. Gordon FJ. Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loenders B. Van Mechelen E. Nicolai S. Buyssens N. Van Osselaer N. Jorens PG. Willems J. Herman AG. Slegers H. Localization of extracellular superoxide dismutase in rat lung: neutrophils and macrophages as carriers of the enzyme. Free Radic Biol Med. 1998;24:1097–1106. doi: 10.1016/s0891-5849(97)00434-6. [DOI] [PubMed] [Google Scholar]
- 142.Luk E. Jensen LT. Culotta VC. The many highways for intracellular trafficking of metals. J Biol Inorg Chem. 2003;8:803–809. doi: 10.1007/s00775-003-0482-3. [DOI] [PubMed] [Google Scholar]
- 143.Luoma JS. Stralin P. Marklund SL. Hiltunen TP. Sarkioja T. Yla-Herttuala S. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler Thromb Vasc Biol. 1998;18:157–167. doi: 10.1161/01.atv.18.2.157. [DOI] [PubMed] [Google Scholar]
- 144.MacMillan-Crow LA. Crow JP. Kerby JD. Beckman JS. Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Madamanchi NR. Moon SK. Hakim ZS. Clark S. Mehrizi A. Patterson C. Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol. 2005;25:950–956. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 146.Madamanchi NR. Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 147.Maejima Y. Kuroda J. Matsushima S. Ago T. Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol. 50:408–416. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Makino A. Skelton MM. Zou AP. Roman RJ. Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 149.Marchioli R. Antioxidant vitamins and prevention of cardiovascular disease: laboratory, epidemiological and clinical trial data. Pharmacol Res. 1999;40:227–238. doi: 10.1006/phrs.1999.0480. [DOI] [PubMed] [Google Scholar]
- 150.Marikovsky M. Nevo N. Vadai E. Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. Int J Cancer. 2002;97:34–41. doi: 10.1002/ijc.1565. [DOI] [PubMed] [Google Scholar]
- 151.Marklund SL. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990;266:213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984;74:1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marrotte EJ. Chen DD. Hakim JS. Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010;120:4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McCord JM. Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 156.Miao L. St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miller FJ., Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 158.Miller FJ., Jr. Gutterman DD. Rios CD. Heistad DD. Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 159.Mizuno K. Whittaker MM. Bachinger HP. Whittaker JW. Calorimetric studies on the tight binding metal interactions of Escherichia coli manganese superoxide dismutase. J Biol Chem. 2004;279:27339–27344. doi: 10.1074/jbc.M400813200. [DOI] [PubMed] [Google Scholar]
- 160.Morikawa K. Shimokawa H. Matoba T. Kubota H. Akaike T. Talukder MA. Hatanaka M. Fujiki T. Maeda H. Takahashi S. Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mukhopadhyay CK. Ehrenwald E. Fox PL. Ceruloplasmin enhances smooth muscle cell- and endothelial cell-mediated low density lipoprotein oxidation by a superoxide-dependent mechanism. J Biol Chem. 1996;271:14773–14778. doi: 10.1074/jbc.271.25.14773. [DOI] [PubMed] [Google Scholar]
- 162.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release. 2001;71:1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 165.Nakazono K. Watanabe N. Matsuno K. Sasaki J. Sato T. Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nelson SK. Bose SK. McCord JM. The toxicity of high-dose superoxide dismutase suggests that superoxide can both initiate and terminate lipid peroxidation in the reperfused heart. Free Radic Biol Med. 1994;16:195–200. doi: 10.1016/0891-5849(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 167.Nguyen AD. Itoh S. Jeney V. Yanagisawa H. Fujimoto M. Ushio-Fukai M. Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95:1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 168.Nishiyama A. Fukui T. Fujisawa Y. Rahman M. Tian RX. Kimura S. Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin ii-infused hypertensive rats. Hypertension. 2001;37:77–83. doi: 10.1161/01.hyp.37.1.77. [DOI] [PubMed] [Google Scholar]
- 169.Nonaka H. Tsujino T. Watari Y. Emoto N. Yokoyama M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation. 2001;104:1165–1170. doi: 10.1161/hc3601.093976. [DOI] [PubMed] [Google Scholar]
- 170.Nozik-Grayck E. Suliman HB. Majka S. Albietz J. Van Rheen Z. Roush K. Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nozoe M. Hirooka Y. Koga Y. Sagara Y. Kishi T. Engelhardt JF. Sunagawa K. Inhibition of Rac1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension. 2007;50:62–68. doi: 10.1161/HYPERTENSIONAHA.107.087981. [DOI] [PubMed] [Google Scholar]
- 172.O'Halloran TV. Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 173.Ohashi M. Runge MS. Faraci FM. Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 174.Ohta H. Adachi T. Hirano K. Internalization of human extracellular-superoxide dismutase by bovine aortic endothelial cells. Free Radic Biol Med. 1994;16:501–507. doi: 10.1016/0891-5849(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 175.Okado-Matsumoto A. Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 176.Oliver FJ. Menissier-de Murcia J. Nacci C. Decker P. Andriantsitohaina R. Muller S. de la Rubia G. Stoclet JC. de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Omar BA. Gad NM. Jordan MC. Striplin SP. Russell WJ. Downey JM. McCord JM. Cardioprotection by Cu,Zn-superoxide dismutase is lost at high doses in the reoxygenated heart. Free Radic Biol Med. 1990;9:465–471. doi: 10.1016/0891-5849(90)90123-z. [DOI] [PubMed] [Google Scholar]
- 178.Ookawara T. Imazeki N. Matsubara O. Kizaki T. Oh-Ishi S. Nakao C. Sato Y. Ohno H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol. 1998;275:C840–C847. doi: 10.1152/ajpcell.1998.275.3.C840. [DOI] [PubMed] [Google Scholar]
- 179.Oppermann M. Balz V. Adams V. Dao VT. Bas M. Suvorava T. Kojda G. Pharmacologic induction of vascular extracellular superoxide dismutase expression in-vivo. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oshikawa J. Urao N. Kim HW. Kaplan N. Razvi M. McKinney R. Poole LB. Fukai T. Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oury TD. Day BJ. Crapo JD. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest. 1996;75:617–636. [PubMed] [Google Scholar]
- 182.Ozumi K. Tasaki H. Takatsu H. Nakata S. Morishita T. Koide S. Yamashita K. Tsutsui M. Okazaki M. Sasaguri Y. Adachi T. Nakashima Y. Extracellular superoxide dismutase overexpression reduces cuff-induced arterial neointimal formation. Atherosclerosis. 2005;181:55–62. doi: 10.1016/j.atherosclerosis.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 183.Paffenbarger RS., Jr. Hyde RT. Wing AL. Lee IM. Jung DL. Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 184.Pantoliano MW. Valentine JS. Burger AR. Lippard SJ. A pH-dependent superoxide dismutase activity for zinc-free bovine erythrocuprein. Reexamination of the role of zinc in the holoprotein. J Inorg Biochem. 1982;17:325–341. doi: 10.1016/s0162-0134(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 185.Papaharalambus CA. Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17:48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Passacquale G. Desideri G. Croce G. Murgo S. Mancarelli MM. Zazzeroni F. Alesse E. Ferri C. Nifedipine improves the migratory ability of circulating endothelial progenitor cells depending on manganese superoxide dismutase upregulation. J Hypertens. 2008;26:737–746. doi: 10.1097/HJH.0b013e3282f4d1bd. [DOI] [PubMed] [Google Scholar]
- 187.Petersen SV. Oury TD. Ostergaard L. Valnickova Z. Wegrzyn J. Thogersen IB. Jacobsen C. Bowler RP. Fattman CL. Crapo JD. Enghild JJ. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 188.Petersen SV. Oury TD. Valnickova Z. Thogersen IB. Hojrup P. Crapo JD. Enghild JJ. The dual nature of human extracellular superoxide dismutase: one sequence and two structures. Proc Natl Acad Sci U S A. 2003;100:13875–13880. doi: 10.1073/pnas.2436143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qin Z. Gongora MC. Ozumi K. Itoh S. Akram K. Ushio-Fukai M. Harrison DG. Fukai T. Role of Menkes ATPase in angiotensin II-induced hypertension: a key modulator for extracellular superoxide dismutase function. Hypertension. 2008;52:945–951. doi: 10.1161/HYPERTENSIONAHA.108.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qin Z. Itoh S. Jeney V. Ushio-Fukai M. Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J. 2006;20:334–336. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 191.Qin Z. Reszka KJ. Fukai T. Weintraub NL. Extracellular superoxide dismutase (ecSOD) in vascular biology: an update on exogenous gene transfer and endogenous regulators of ecSOD. Transl Res. 2008;151:68–78. doi: 10.1016/j.trsl.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rae TD. Schmidt PJ. Pufahl RA. Culotta VC. O'Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 193.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 194.Rigo A. Terenzi M. Viglino P. Calabrese L. Cocco D. Rotilio G. The binding of copper ions to copper-free bovine superoxide dismutase. Properties of the protein recombined with increasing amounts of copper ions. Biochem J. 1977;161:31–35. doi: 10.1042/bj1610031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Robinson NJ. Winge DR. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rodman DM. Mallet J. McMurtry IF. Difference in effect of inhibitors of energy metabolism on endothelium-dependent relaxation of rat pulmonary artery and aorta. Am J Respir Cell Mol Biol. 1991;4:237–242. doi: 10.1165/ajrcmb/4.3.237. [DOI] [PubMed] [Google Scholar]
- 197.Rodriguez-Iturbe B. Sepassi L. Quiroz Y. Ni Z. Wallace DC. Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol. 2007;102:255–260. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 198.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 199.Salvemini D. Riley DP. Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 200.Sanchez-Quesada JL. Homs-Serradesanferm R. Serrat-Serrat J. Serra-Grima JR. Gonzalez-Sastre F. Ordonez-Llanos J. Increase of LDL susceptibility to oxidation occurring after intense, long duration aerobic exercise. Atherosclerosis. 1995;118:297–305. doi: 10.1016/0021-9150(95)05617-3. [DOI] [PubMed] [Google Scholar]
- 201.Sandstrom J. Karlsson K. Edlund T. Marklund SL. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J. 1993;294:853–857. doi: 10.1042/bj2940853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schmidt PJ. Rae TD. Pufahl RA. Hamma T. Strain J. O'Halloran TV. Culotta VC. Multiple protein domains contribute to the action of the copper chaperone for superoxide dismutase. J Biol Chem. 1999;274:23719–23725. doi: 10.1074/jbc.274.34.23719. [DOI] [PubMed] [Google Scholar]
- 203.Sedeek MH. Llinas MT. Drummond H. Fortepiani L. Abram SR. Alexander BT. Reckelhoff JF. Granger JP. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension. 2003;42:806–810. doi: 10.1161/01.HYP.0000084372.91932.BA. [DOI] [PubMed] [Google Scholar]
- 204.Sentman ML. Brannstrom T. Westerlund S. Laukkanen MO. Yla-Herttuala S. Basu S. Marklund SL. Extracellular superoxide dismutase deficiency and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2001;21:1477–1482. doi: 10.1161/hq0901.094248. [DOI] [PubMed] [Google Scholar]
- 205.Sessa WC. Pritchard K. Seyedi N. Wang J. Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 206.Shern-Brewer R. Santanam N. Wetzstein C. White-Welkley J. Parthasarathy S. Exercise and cardiovascular disease: a new perspective. Arterioscler Thromb Vasc Biol. 1998;18:1181–1187. doi: 10.1161/01.atv.18.7.1181. [DOI] [PubMed] [Google Scholar]
- 207.Soriano FG. Virag L. Szabo C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J Mol Med. 2001;79:437–448. doi: 10.1007/s001090100236. [DOI] [PubMed] [Google Scholar]
- 208.Stasch JP. Schmidt PM. Nedvetsky PI. Nedvetskaya TY. HS AK. Meurer S. Deile M. Taye A. Knorr A. Lapp H. Muller H. Turgay Y. Rothkegel C. Tersteegen A. Kemp-Harper B. Muller-Esterl W. Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stocker R. Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 210.Stralin P. Jacobsson H. Marklund SL. Oxidative stress, NO* and smooth muscle cell extracellular superoxide dismutase expression. Biochim Biophys Acta. 2003;1619:1–8. doi: 10.1016/s0304-4165(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 211.Stralin P. Karlsson K. Johansson BO. Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 212.Stralin P. Marklund SL. Multiple cytokines regulate the expression of extracellular superoxide dismutase in human vascular smooth muscle cells. Atherosclerosis. 2000;151:433–441. doi: 10.1016/s0021-9150(99)00427-x. [DOI] [PubMed] [Google Scholar]
- 213.Sturtz LA. Diekert K. Jensen LT. Lill R. Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 214.Suliman HB. Ali M. Piantadosi CA. Superoxide dismutase-3 promotes full expression of the EPO response to hypoxia. Blood. 2004;104:43–50. doi: 10.1182/blood-2003-07-2240. [DOI] [PubMed] [Google Scholar]
- 215.Takatsu H. Tasaki H. Kim HN. Ueda S. Tsutsui M. Yamashita K. Toyokawa T. Morimoto Y. Nakashima Y. Adachi T. Overexpression of EC-SOD suppresses endothelial-cell-mediated LDL oxidation. Biochem Biophys Res Commun. 2001;285:84–91. doi: 10.1006/bbrc.2001.5114. [DOI] [PubMed] [Google Scholar]
- 216.Tan RJ. Lee JS. Manni ML. Fattman CL. Tobolewski JM. Zheng M. Kolls JK. Martin TR. Oury TD. Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury. Am J Respir Cell Mol Biol. 2006;34:226–232. doi: 10.1165/rcmb.2005-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tojo T. Ushio-Fukai M. Yamaoka-Tojo M. Ikeda S. Patrushev N. Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 218.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 219.Tribble DL. Barcellos-Hoff MH. Chu BM. Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol. 1999;19:1387–1392. doi: 10.1161/01.atv.19.6.1387. [DOI] [PubMed] [Google Scholar]
- 220.Tribble DL. Gong EL. Leeuwenburgh C. Heinecke JW. Carlson EL. Verstuyft JG. Epstein CJ. Fatty streak formation in fat-fed mice expressing human copper-zinc superoxide dismutase. Arterioscler Thromb Vasc Biol. 1997;17:1734–1740. doi: 10.1161/01.atv.17.9.1734. [DOI] [PubMed] [Google Scholar]
- 221.Turunen MP. Aavik E. Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790:886–891. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 222.Urao N. Inomata H. Razvi M. Kim HW. Wary K. McKinney R. Fukai T. Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 225.Ushio-Fukai M. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 227.Valentine JS. Pantoliano MW. McDonnell PJ. Burger AR. Lippard SJ. pH-dependent migration of copper(II) to the vacant zinc-binding site of zinc-free bovine erythrocyte superoxide dismutase. Proc Natl Acad Sci U S A. 1979;76:4245–4249. doi: 10.1073/pnas.76.9.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vozenin-Brotons MC. Sivan V. Gault N. Renard C. Geffrotin C. Delanian S. Lefaix JL. Martin M. Antifibrotic action of Cu/Zn SOD is mediated by TGF-beta1 repression and phenotypic reversion of myofibroblasts. Free Radic Biol Med. 2001;30:30–42. doi: 10.1016/s0891-5849(00)00431-7. [DOI] [PubMed] [Google Scholar]
- 229.Wang HD. Johns DG. Xu S. Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282:H1697–H1702. doi: 10.1152/ajpheart.00914.2001. [DOI] [PubMed] [Google Scholar]
- 230.Wang XL. Adachi T. Sim AS. Wilcken DE. Plasma extracellular superoxide dismutase levels in an Australian population with coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1915–1921. doi: 10.1161/01.atv.18.12.1915. [DOI] [PubMed] [Google Scholar]
- 231.Wedgwood S. Lakshminrusimha S. Fukai T. Russell JA. Schumacker PT. Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal. 2011;15:1497–1506. doi: 10.1089/ars.2010.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Weir CJ. Gibson IF. Martin W. Effects of metabolic inhibitors on endothelium-dependent and endothelium-independent vasodilatation of rat and rabbit aorta. Br J Pharmacol. 1991;102:162–166. doi: 10.1111/j.1476-5381.1991.tb12147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Weisiger RA. Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- 234.Welch WJ. Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int. 2001;59:1257–1263. doi: 10.1046/j.1523-1755.2001.0590041257.x. [DOI] [PubMed] [Google Scholar]
- 235.Wenzel P. Schuhmacher S. Kienhofer J. Muller J. Hortmann M. Oelze M. Schulz E. Treiber N. Kawamoto T. Scharffetter-Kochanek K. Munzel T. Burkle A. Bachschmid MM. Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wheeler MD. Smutney OM. Samulski RJ. Secretion of extracellular superoxide dismutase from muscle transduced with recombinant adenovirus inhibits the growth of B16 melanomas in mice. Mol Cancer Res. 2003;1:871–881. [PubMed] [Google Scholar]
- 237.White CR. Brock TA. Chang LY. Crapo J. Briscoe P. Ku D. Bradley WA. Gianturco SH. Gore J. Freeman BA, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Whittaker JW. The irony of manganese superoxide dismutase. Biochem Soc Trans. 2003;31:1318–1321. doi: 10.1042/bst0311318. [DOI] [PubMed] [Google Scholar]
- 239.Whittaker JW. Metal uptake by manganese superoxide dismutase. Biochim Biophys Acta. 2010;1804:298–307. doi: 10.1016/j.bbapap.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Williams MD. Van Remmen H. Conrad CC. Huang TT. Epstein CJ. Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 241.Wintjens R. Noel C. May AC. Gerbod D. Dufernez F. Capron M. Viscogliosi E. Rooman M. Specificity and phenetic relationships of iron- and manganese-containing superoxide dismutases on the basis of structure and sequence comparisons. J Biol Chem. 2004;279:9248–9254. doi: 10.1074/jbc.M312329200. [DOI] [PubMed] [Google Scholar]
- 242.Witztum JL. Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med. 2001;11:93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 243.Yamakura F. Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim Biophys Acta. 2010;1804:318–325. doi: 10.1016/j.bbapap.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 244.Yamamoto M. Hara H. Adachi T. Effects of homocysteine on the binding of extracellular-superoxide dismutase to the endothelial cell surface. FEBS Lett. 2000;486:159–162. doi: 10.1016/s0014-5793(00)02260-2. [DOI] [PubMed] [Google Scholar]
- 245.Yang H. Roberts LJ. Shi MJ. Zhou LC. Ballard BR. Richardson A. Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 246.Yang Q. Kim SK. Sun F. Cui J. Larson MG. Vasan RS. Levy D. Schwartz F. Maternal influence on blood pressure suggests involvement of mitochondrial DNA in the pathogenesis of hypertension: the Framingham Heart Study. J Hypertens. 2007;25:2067–2073. doi: 10.1097/HJH.0b013e328285a36e. [DOI] [PubMed] [Google Scholar]
- 247.Yla-Herttuala S. Sumuvuori H. Karkola K. Mottonen M. Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest. 1986;54:402–407. [PubMed] [Google Scholar]
- 248.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 249.Zanetti M. Sato J. Katusic ZS. O'Brien T. Gene transfer of superoxide dismutase isoforms reverses endothelial dysfunction in diabetic rabbit aorta. Am J Physiol Heart Circ Physiol. 2001;280:H2516–H2523. doi: 10.1152/ajpheart.2001.280.6.H2516. [DOI] [PubMed] [Google Scholar]
- 250.Zelko IN. Folz RJ. Extracellular superoxide dismutase functions as a major repressor of hypoxia-induced erythropoietin gene expression. Endocrinology. 2005;146:332–340. doi: 10.1210/en.2004-1007. [DOI] [PubMed] [Google Scholar]
- 251.Zelko IN. Mariani TJ. Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 252.Zheng JS. Yang XQ. Lookingland KJ. Fink GD. Hesslinger C. Kapatos G. Kovesdi I. Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation. 2003;108:1238–1245. doi: 10.1161/01.CIR.0000089082.40285.C3. [DOI] [PubMed] [Google Scholar]
- 253.Zimmerman MC. Lazartigues E. Lang JA. Sinnayah P. Ahmad IM. Spitz DR. Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 254.Zimmerman MC. Lazartigues E. Sharma RV. Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 255.Zimmerman MC. Sharma RV. Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- 256.Zorov DB. Juhaszova M. Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 257.Zou MH. Shi C. Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]