Abstract
Mitochondria are well known for their central roles in ATP production, calcium homeostasis, and heme and steroid biosynthesis. However, mitochondrial reactive oxygen species (ROS), including superoxide and hydrogen peroxide, once thought to be toxic byproducts of mitochondrial physiologic activities, have recently been recognized as important cell-signaling molecules in the vascular endothelium, where their production, conversion, and destruction are highly regulated. Mitochondrial reactive oxygen species appear to regulate important vascular homeostatic functions under basal conditions in a variety of vascular beds, where, in particular, they contribute to endothelium-dependent vasodilation. On exposure to cardiovascular risk factors, endothelial mitochondria produce excessive ROS in concert with other cellular ROS sources. Mitochondrial ROS, in this setting, act as important signaling molecules activating prothrombotic and proinflammatory pathways in the vascular endothelium, a process that initially manifests itself as endothelial dysfunction and, if persistent, may lead to the development of atherosclerotic plaques. This review concentrates on emerging appreciation of the importance of mitochondrial ROS as cell-signaling molecules in the vascular endothelium under both physiologic and pathophysiologic conditions. Future potential avenues of research in this field also are discussed. Antioxid. Redox Signal. 15, 1517–1530.
Introduction
The endothelium is well recognized as a central regulator of vascular homeostasis, and the development of endothelial dysfunction is known to precede the development of atherosclerosis and portend cardiovascular risk (168). The role of oxidative stress in the development of endothelial dysfunction and subsequent atherosclerotic disease has been the subject of extensive study over the past three decades. Initial work in this field considered reactive oxygen species (ROS) as the primarily toxic metabolic by-products with adverse effects on vascular function from direct damage to key cellular proteins and overall reduction in bioavailable endothelium-derived nitric oxide (NO). However, in recent years, a novel emerging view identifies endothelial cell ROS as prominent signaling molecules in cellular regulatory cascades, important under in both normal and pathophysiologic states (51, 77, 84, 120, 146, 150, 157, 172, 173).
As a major cellular source of ROS, mitochondria have recently garnered increased attention for their contribution to the detrimental effects of cardiovascular risk factors (7, 95, 126). Common cardiovascular risk factors, including diabetes, hypertension, and hyperlipidemia, induce pathologic alterations to the vascular phenotype through signaling pathways that require increases in mitochondrial ROS production above basal levels (113, 167, 184). These findings suggest excessive mitochondrial ROS act to encourage pathologic cell-signaling cascades under cellular conditions of overall excessive oxidative stress (125, 150, 180). However, mitochondrial-derived ROS also exhibit important physiological modulating effects on vasomotor tone in response to mechanical forces in both normal and diseased microvascular beds (90, 91). These data strongly suggest that homeostatic levels of mitochondrial ROS production act as important cell-signaling molecules, even in the absence of excessive oxidative stress.
We review the growing literature implicating mitochondrial ROS as key regulators of the vascular response to homeostatic and pathological stimuli. We discuss the regulation of the production, distribution, and destruction of mitochondrial ROS in the vascular endothelium, their role in regulating signaling cascades important in vasomotor activity, and roles in pathogenic signaling cascades in the setting of traditional cardiovascular risk factors that ultimately lead to the development of endothelial dysfunction and atherosclerosis.
Finally, we identify potential targets for favorably modulating endothelial mitochondrial ROS production for therapeutic intent.
Mitochondrial ROS Production
General characteristics
Mitochondria reign as a major source of cellular ROS, with reports that between 0.2% and 2% of cellular mitochondrial oxygen consumption generates superoxide (24, 31, 129). Based on data from isolated mitochondrial samples, organelle concentrations of oxygen and superoxide range between 3 and 30 μM and 10 and 200 pM, respectively (4, 171). The rate of hydrogen peroxide production in isolated mitochondria varies between approximately 0.1 and 0.2 nmol/min/mg protein (136). The local concentrations of superoxide and hydrogen peroxide likely vary among tissues, based on local conditions (44). Exact tissue-specific superoxide concentrations and production rates in vivo remain only estimates, as our ability to make in vivo measurements of mitochondrial ROS remains limited.
Multiple potential mitochondrial sites of reactive oxygen species generation exist. These include Krebs cycle enzymes involved in redox reactions such as α-ketoglutarate dehydrogenase and aconitase (116), glycerol-3-phosphate dehydrogenase from the glycerol phosphate shuttle (108), and monoamine oxidase (19, 162). However, the vast majority of superoxide from mitochondria is produced by, or modulated by the complexes of the electron-transport chain (ETC) (110).
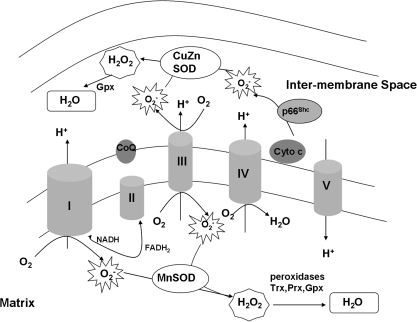
The ETC consists of four protein complexes embedded in the mitochondrial inner membrane. The spatial orientation is shown in Fig. 1. The ETC accepts electrons from reducing equivalents (NADH to complex I and FADH2 produced from the glucose and fatty acid oxidation, as well as from the glycerol phosphate shuttle related to glycolysis to complex II). Donated electrons are passed down their electrochemical gradient through the complexes of the ETC, including reactive intermediaries (ubiquinone and cytochrome c), ultimately reducing O2 to water. At multiple steps in this process, protons are pumped from the mitochondrial matrix into the intermembrane space, generating a proton motive force (Δp) that is a combination of the mitochondrial membrane potential created by the voltage gradient across the inner mitochondrial membrane (Δψm) and the pH gradient created by proton transport during electron transfer. Δp is central to ATP production (139).
FIG. 1.
Electron-transport chain and reactive oxygen species production. The process of oxidative phosphorylation receives reducing equivalents from the Krebs cycle (NADH to complex I and FADH2 to complex II) and passes these electrons down the transport chain, ultimately to reduce oxygen to water. The fidelity of the process is incomplete, and the relative fidelity of the process depends on local environmental conditions. As such, oxygen can be reduced to O2- in at least three sites within the mitochondria: complexes, I, III, and through p66Shc. O2- that does not escape the mitochondria at this point is rapidly reduced to H2O2 by manganese superoxide dismutase (MnSOD) and copper-zinc superoxide dismutase (CuZnSOD) in the matrix and intermembrane space, respectively. H2O2 either may leave the mitochondria and react with mitochondria proteins, or may be reduced to H2O by local peroxidase enzymes. CoQ, coenzyme Q/ubiquinone; cyto C,cytochrome c.
At the individual protein-complex level, the rate of production of superoxide by the mitochondrial ETC depends on several factors, including the availability of ROS sites of production, as well as oxygen tension (68, 78).
ETC sources of ROS
The major ETC sources of ROS from mitochondria are complexes I and III (24, 182). Complex II may also generate ROS (182), but this may not be independent of complex I. Local environmental conditions, including type and amount of substrate, pH, local oxygen tension, and the overall level of oxidative stress, strongly influence the site specificity and concentration of ROS produced (24).
Complex I is the initial entry point for NADH reducing equivalents and is comprised of more than 40 proteins (66, 137). Under physiologic conditions favorable to ATP production, the complex I flavin mononucleotide exposed in the mitochondrial matrix receives electrons from nicotine adenine dinucleotide (NADH) and passes these electrons through several iron-sulfur centers to the complex I ubiquinone-binding site, where electrons can reduce ubiquinone to semiquinone and subsequently to ubiquinol. In states of high NADH/NAD+ ratios and minimal ATP need (i.e., a fuel-replete state), electrons can be passed from the fully reduced FMN site on complex I to O2 to produce superoxide. This mechanism is proposed as an important in vivo source of oxidative damage to ETC proteins (147, 164).
Alternatively, in the setting of high Δp and succinate levels and a low NADH/NAD+ ratio, as may be seen when fatty acids are the primary fuel of mitochondria (87), electrons entering the ETC through complex II via FADH2 can backflow to complex I through reactive quinone intermediates and produce superoxide (65, 76). This reverse electron transport appears sensitive to reductions in the pH gradient between the matrix and the intermembrane space (89). The exact site of superoxide production by this mechanism is not known, but may be the ubiquinone binding site or a distal iron-sulfur cluster in complex I (87). Regardless of the mechanism, complex I–derived ROS appear to be formed primarily in the mitochondrial matrix.
Complex III also contributes to overall mitochondrial ROS production (23, 155, 182) through the two-step Q cycle. In this process, electrons are passed from ubiquinol to cytochrome c with reactive semiquinone intermediates that face both the matrix and the intermembrane space. Complex III can produce superoxide in either the matrix or the intermembrane space during Q-cycle inhibition, depending on whether uncoupling occurs at the matrix or the intermembrane space component of the Q cycle (59, 109). The in vivo physiologic importance of complex III relative to complex I ROS production remains controversial (110, 149).
A third and novel mechanism for mitochondrial ROS generation has been reported to involve p66Shc, which can transfer electrons directly from cytochrome c to oxygen to form superoxide (54). This mechanism appears to be important in settings of cellular oxidative stress and particularly for apoptotic signaling (153).
Recent data suggest that ROS may be produced in bursts from mitochondria, related to brief periods of inner-membrane depolarization (165). The relation between these bursts of superoxide production and ETC complex ROS production remains to be elucidated.
Mitochondrial ROS as Cell-Signaling Molecules
Mitochondrial ROS as vascular cell-signaling molecules has significant biologic plausibility
For years, the pervasive impression has been that mitochondrial ROS are toxic by-products of energy production. However, a unique metabolic feature of endothelial cells provides the flexibility to modulate the mitochondrial respiratory rate and closely to regulate ROS production. Specifically, under most metabolic conditions, energy requirements of endothelial cells are met through anaerobic glycolysis (36) rather than through oxidative phosphorylation (37, 100, 142). Thus, endothelial mitochondrial ROS production within the ETC can be modulated by second-messenger systems without jeopardizing cellular energy requirements.
Mitochondrial ROS can escape from both the intermembrane space and the matrix into the cytosol. Superoxide in the intermembrane space may leave the mitochondria through voltage-dependent anion channels located in the outer membrane (58). However, given its electrophilic nature and short half-life, superoxide is a poor candidate molecule for mitochondrial-based cell signaling. However, superoxide is rapidly reduced hydrogen peroxide (H2O2) in both the intermembrane space via copper-zinc superoxide dismutase (CuZn SOD, SOD1) and the matrix, via manganese superoxide dismutase (MnSOD,SOD2) (24, 115). MnSOD is posttranslationally targeted specifically to the mitochondrial matrix and appears to be centrally important in maintaining vascular homeostasis (170). ApoE-knockout mice also made deficient in MnSOD have greater impairment in endothelial function compared with ApoE-null mice (114).
An advantage of H2O2 as a cell-signaling molecule is its greater stability, leading to a longer in vivo half-life. The concentration of H2O2 in mitochondria is 100 times greater than that of superoxide (24). Most important, hydrogen peroxide's lack of charge allows it to pass freely through cell membranes. Emerging evidence suggests that H2O2 transport from mitochondria to the cytosol is enhanced by the presence of aquaporins, particularly aquaporin 8, on mitochondrial membranes (14, 15, 25). The presence of these aquaporins in the inner mitochondrial membrane appears important to maintaining cell viability through mitochondrial volume regulation (86).
Mitochondrial ROS production and removal are highly regulated
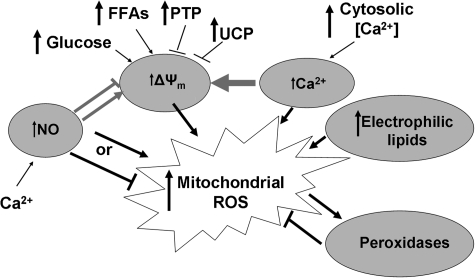
The presence of multiple pathways involved in regulating mitochondrial ROS production and destruction argues strongly for a significant role of mitochondrial ROS in cell signaling. These regulators include novel mitochondria-specific features (mitochondrial membrane potential, ΔΨm, dismutase and peroxidase enzymes), molecules well recognized for their importance in cell-signaling cascades (NO, calcium), and non-mitochondrial sources of cellular ROS. As described later and demonstrated in Fig. 2, these regulatory stimuli interact in coordinated fashion to modulate overall levels of mitochondrial ROS.
FIG. 2.
Regulation of mitochondrial ROS production. Major regulators of mitochondrial ROS production include nitric oxide (NO), calcium, the mitochondrial membrane potential (ΔΨm), and electrophilic lipids. Interestingly, these effects are often coordinated. ΔΨm is highly influenced by the cellular fuel supply, and uncoupling proteins (UCPs) and the permeability transition pore (PTP) also play significant roles in modulation of mitochondrial ROS through ΔΨm.
Mitochondrial membrane potential
As described previously, the mitochondrial membrane potential (ΔΨm) is the portion of the proton motive force accounted for by the transfer of electrons through the ETC. Therefore, ΔΨm is sensitive to ETC substrate conditions (46, 140) pH, and the fidelity of electron transport, with higher, more polarized ΔΨm generally associated with greater mitochondrial superoxide production (140). When ΔΨm is maximal, protons are less avidly pumped from the matrix to the intermembrane space, reducing ETC flux and increasing the half-life of redox unstable intermediates (150). Regulatory proteins in the mitochondrial inner membrane also play a key role in modulating ΔΨm. Specifically, mitochondrial uncoupling proteins (UCPs) can open in the inner membrane, leading to membrane depolarization and partial uncoupling of ATP synthesis from oxidative phosphorylation by a reduction in the proton gradient. Suppression of UCP expression increases ROS production (43), whereas overexpression of UCPs may attenuate mitochondrial ROS production, dependent on cellular ATP status (46, 83). Further, UCP2 transcription is significantly upregulated in states of excessive oxidative stress and elevated ΔΨm, suggesting a role in feedback regulation of ΔΨm (122), whereas UCP overexpression reverses excessive substrate-induced oxidative stress (113). The mitochondrial permeability transport pore (mPTP), which plays an important role in apoptotic signaling, may also play a role in ROS signal regulation (35, 165), but further data are needed, the better to elucidate its importance in ROS signaling in viable cells and tissues (69, 185).
Dismutase and peroxidase enzymes
Peroxidases are a group of compounds that enzymatically convert H2O2 to water. In the mitochondrial matrix, this group of compounds includes thioredoxin-2 (148), peroxiredoxin-3 (32), and glutaredoxin-2 (93). These compounds are upregulated in the setting of cellular oxidative stress likely to defend against the toxic consequences of elevated H2O2 levels (73, 131, 154). Coordinated regulation of peroxidase and MnSOD appears to be in part controlled by the transcriptional regulator PGC-1α (158), which in turn is upregulated by NO, another key controller of mitochondrial homeostasis in endothelial cells (18, 45). Peroxidase enzymes also are modulated by ambient calcium (104, 132).
Nitric oxide
The complex role that NO plays in regulating mitochondrial homeostasis in the endothelium has been the subject of a recent excellent review (45). NO encourages mitochondrial biogenesis through PGC-1α activation (175), and the identification of eNOS within the mitochondrial outer membrane suggests that local mitochondrial NO production may serve a mitochondrial-specific regulatory purpose (52).
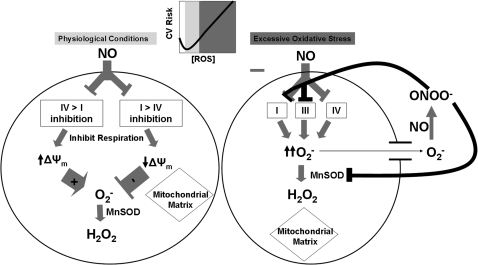
NO is a known inhibitor of the respiratory chain, and the relatively high basal concentrations of NO in endothelial cells suggests that endothelial mitochondrial respiration is restricted under basal conditions (45). The relative effects of NO on respiration, ΔΨm, and mitochondrial ROS production are shown in Fig. 3. NO competes directly with O2 at complex IV, reversibly inhibiting this complex and inducing ROS production (27, 128). NO also can inhibit complex I through S-nitrosylation (22, 28, 34, 38, 110). This is more likely to occur in settings of glutathione depletion and appears to reduce mitochondrial ROS. Interactions between NO and complex IV occur rapidly and reversibly, playing an important role in short-term regulation of respiration and ROS production, whereas complex I inhibition by nitroso compounds is longer lasting, suggesting a greater role in long-term regulation of respiration and ROS generation.
FIG. 3.
Regulation of mitochondrial ROS in endothelium by NO. The overall effect of NO on mitochondrial ROS production is highly dependent on the relative inhibition of complex IV versus complex I under normal physiologic conditions. Complex IV inhibition occurs rapidly, and complex I inhibition appears to occur over a more prolonged time span. With excessive oxidative stress, as in the setting of cardiovascular risk factors (CV Risk) and atherosclerosis, superoxide reacts rapidly with NO to form peroxynitrite (ONOO-), which inhibits multiple complexes of the respiratory chain as well as MnSOD. MnSOD is overwhelmed, leading to a significant increase in mitochondria-centered superoxide production. Importantly, an optimal level of ROS generation seems to exist, such that both excessive production of ROS (with reduced bioavailability of NO) and insufficient ROS (with attendant impaired physiologic signaling) can lead to a proinflammatory state and promote atherosclerosis.
The situation becomes even further complicated in the setting of elevated systemic oxidative stress, as is seen with cardiovascular risk factors. NO reacts rapidly with excess superoxide to form peroxynitrite, which can irreversibly inhibit multiple complexes of the respiratory chain, as well as dismutase enzymes, leading initially to increased oxidative stress and increased ΔΨm (53).
Calcium
The role of calcium (Ca2+) in regulating mitochondrial ROS production in endothelial cells is integrally linked with other regulatory factors, including NO and ΔΨm. Increased calcium bioavailability from either intra- or extracellular sources increases NO production from eNOS (41). Ca2+ also increases flux through the Krebs cycle, leading to increased concentrations of reducing equivalents available for oxidative phosphorylation (98). Increases in cytosolic Ca2+ drives Ca2+ entry into mitochondria through calcium uniporter channels and potentially mitochondria-based ryanodine receptors on the inner mitochondrial membrane (13, 39). Ca2+ influx into the matrix is also directly related to the magnitude of ΔΨm (39). Increased calcium uptake into mitochondria also leads to an increase in mitochondrial ROS production (26). Taken together, these data suggest that a regulatory nexus of Ca2+, NO, ΔΨm, and peroxidase enzymes coordinately modulate mitochondrial homeostasis in endothelial cells and the subsequent balance of mitochondrial ROS (Fig. 2).
Nonmitochondrial ROS sources
Emerging data suggest that nonmitochondrial-generated ROS can stimulate mitochondrial ROS production. Increased ROS production from specific, nonmitochondrial ROS-producing enzymes in the vasculature, including NADPH oxidase (42, 49, 85, 166, 174), xanthine oxidase (11), and uncoupled eNOS (30), are capable of coordinating ROS expression by mitochondria. Although this communication between nonmitochondrial ROS sources and mitochondria has been established, the optimal conditions and the mechanisms for this ROS-induced ROS release remain to be elucidated. Lipid peroxidation products with electrophilic centers may be one potential factor in this type of communication (80).
Mitochondrial ROS participate in vascular responses to mechanical stimuli
Mitochondrial ROS are tightly regulated in endothelial cells, providing the potential for a signaling role in vascular regulation, during both physiologic and pathologic stimuli. As a clinically relevant example, ROS generated from mitochondria are responsible for the vasodilator response to shear stress in patients with coronary artery disease (180).
In the microvasculature, important regulators of vascular homeostasis include not only NO and prostanoids, but also endothelium-derived hyperpolarizing factor (EDHF). Although the identity of EDHF may differ between vascular beds and in different pathologic states, H2O2 has been identified as an EDHF in multiple vascular beds including human coronary arterioles from patients with CAD (91, 102, 124), as well as human and mouse mesenteric arteries (96, 97).
The mechanism of flow-induced dilation in the human heart is unique. Isolated human coronary arterioles from discarded samples of the right atrial appendage ligated at the time of cardiopulmonary bypass, cannulated, pressurized, and subjected to laminar shear show an increase in superoxide and hydrogen peroxide production that is reversed by exposure to rotenone, a mitochondrial complex I inhibitor. Inhibition of complex I or complex III markedly reduces laminar shear–induced vasodilation. The mechanism of shear-induced vasodilation increases mitochondrial ROS and appears to involve an increase ΔΨm that is opposed by NO-dependent ETC inhibition (60, 88). These alterations are consistent with established effects of both ΔΨm and NO on mitochondrial ROS. In animal models, laminar shear upregulates both MnSOD and peroxiredoxin in the endothelium, suggesting a compensatory mechanism for tempering overall mitochondrial superoxide and H2O2 concentrations (1, 107).
While the mechanism for transduction of the shear signal from the cell surface to the mitochondria remains to be fully elucidated, the endothelial cytoskeleton appears to play a prominent role. Mechanical signals from the endothelial cell surface are transduced to cellular organelles through actin filaments, linking focal adhesion kinases to integrins located on the cell surface and at the abluminal basement membrane (40, 63). Cytoskeletal involvement in transducing shear-mediated dilation is observed in rat muscle arterioles, where NO and prostaglandins mediate the dilation (145), and in diseased human coronary arterioles, where hydrogen peroxide plays a key role (102). Human coronary arterioles exposed to inhibitors of actin and microtubule formation demonstrate blunted shear-induced vasodilation with a concomitant loss of superoxide and H2O2 production (90). The mechanism by which this signal is transmitted to the mitochondria is under investigation but may involve increases in endothelial cell calcium that are modulated by local NO production (99).
Mitochondrial ROS production in endothelial cells also appears to be important in regulating vascular responses to other mechanical stimuli. For example, both cyclic strain and stretch, important mechanical forces on the pulmonary vasculature, activate signaling cascades that stimulate production of NF-κB and endothelial cell adhesion molecules. This activation requires the production of mitochondrial ROS and an intact cytoskeleton (2, 3, 176). Redundancy exists with regard to cellular ROS sources important in shear-stress vasomotion. For example, NADPH oxidase ROS production appears to be an important mediator of pathologic cell signaling because of flow reversal and turbulent flow (55), as well as oscillatory shear (70, 71, 141).
Mitochondrial ROS and pathologic vascular stressors
Excessive vascular oxidative stress not only reduces bioavailable NO through direct inactivation via production of peroxynitrite, but can also lead to eNOS uncoupling and further ROS production (81). NF-κB and protein kinase C (PKC) activation occur secondary to excessive mitochondrial ROS production in the endothelium, precipitating a range of proinflammatory and prothrombotic alterations in the endothelial phenotype (2, 9, 121, 127,156). Taken together, these data suggest that pathologic stressors common to cardiovascular risk factors can induce excessive mitochondrial ROS levels in the endothelium, leading to alterations in cell signaling and direct losses of bioavailable NO, hallmarks of endothelial dysfunction (Fig. 4).
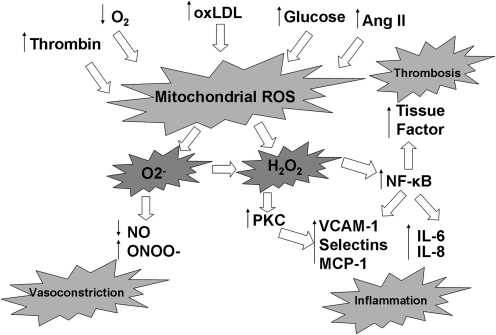
FIG. 4.
Endothelial cell mitochondrial ROS response to pathologic stressors. A wide variety of pathologic stressors associated with cardiovascular risk factors are known to increase mitochondrial ROS production, including thrombin, hypoxemia, oxidized LDL, elevated angiotensin II levels, and elevated glucose levels. These have the effect of increasing both superoxide and hydrogen peroxide levels. This results in inactivation of endothelium-derived NO synthase and consumes any NO that is produced, unleashing proinflammatory signaling pathways. The consequence is increased expression of endothelial cell-adhesion molecules (VCAM-1, selectins, MCP-1), inflammatory cytokines (IL-6 and IL-8), and prothrombotic tissue factor.
These data support the concept that mitochondrial ROS levels serve as a barometer of overall vascular health in pathologic settings (Fig. 5) (163). As such, targeting mechanisms to reduce excessive mitochondrial ROS production may improve endothelial function and reduce cardiovascular events in those at high risk. The following sections review our current knowledge regarding the relation between cardiovascular risk factors and mitochondrial ROS, as well as the body of evidence supporting a pathogenic role for excessive mitochondrial ROS in the development of atherosclerotic disease through pathologic cell signaling.
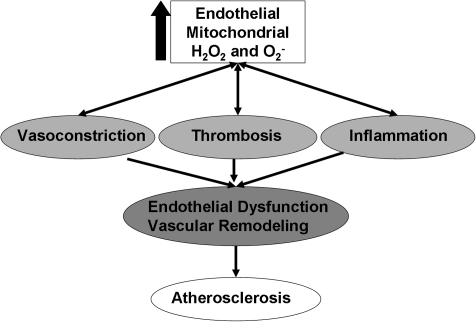
FIG. 5.
Cascade of risk from excessive endothelial mitochondrial ROS production. Excessive mitochondrial ROS production induces endothelial dysfunction through multiple cell-signaling pathways. The endothelium develops this pathologic phenotype characterized by vasoconstriction, thrombosis, and inflammation. This condition is a precursor to the development of clinically relevant atherosclerosis and predicts future cardiovascular events.
The ability of endothelial cells to regulate mitochondrial ROS within a narrow physiologic range is important to normal vascular homeostasis. When antioxidant balancing systems are overwhelmed, as with pathologic vascular stressors, mitochondrial ROS production accelerates, leading to direct cellular oxidative damage and activation of pathologic cell-signaling pathways, as well as inactivation of protective mechanisms (Fig. 4). Seemingly disparate pathologic stressors, including thrombin activation of the PAR1/2 receptor (9, 33), leptin (177), hyperglycemia (113), angiotensin II (127), and hypoxemia (74, 121) all appear to induce excessive mitochondrial ROS production, leading to subsequent expression of a dysfunctional endothelial phenotype. Mechanistically, the excessive production of mitochondrial ROS reduces NO bioavailability (81) and leads to activation of NF-κB and protein kinase C (20, 156). This in turn leads to increased expression of cell-adhesion molecules, including ICAM-1, P-selectin, and E-selectin (72). Depletion of mitochondrial glutathione stores as an antioxidant defense produces a similar proinflammatory milieu with overexpression of E-selectin and VCAM-1, resulting in increased monocyte recruitment by endothelial cells (33). Mitochondrial ETC ROS production activates NF-κB resulting in increased production of the inflammatory cytokines IL-6 and IL-8 (121, 143) and prothrombotic tissue factor (9). In summary, multiple well-recognized pathophysiologic stimuli that act on the endothelium to produce a dysfunctional phenotype act at least in part through signaling pathways involving increases in mitochondrial ROS.
Diabetes and mitochondrial ROS
A growing recognition exists of a link between mitochondrial morphology and function (133). This is best seen in the conditions of diabetes and insulin resistance, which are characterized by alterations in mitochondrial morphology, including smaller mitochondria with less-complex structure and reduced capacity for oxidative phosphorylation, elevated mitochondrial membrane potential, and reduced mitochondrial mass (106, 123). Microarray analyses of skeletal muscle from diabetic patients demonstrate reduced expression of oxidative phosphorylation–pathway elements. This can be reversed by peroxisome proliferator–activated receptor α coactivator 1α (PGC-1α) (105). PGC-1α plays an integral role in the regulation of mitochondrial biogenesis, and inhibition of PGC-1α activity leads to reductions in mitochondrial number (175). Prior in vitro cell culture and muscle biopsy data demonstrate decreased mitochondrial mass under hyperglycemic conditions, and this derangement also is present in the insulin-resistant offspring of type 2 diabetic patients (106).
Insulin resistance and diabetes are characterized by elevated circulating levels of glucose and free fatty acids (17, 130). In endothelial cells, hyperglycemia induces mitochondrial fission with the subsequent development of excessive mitochondrial ROS production, reduced ATP production, and blunted cell growth (179). Endothelial cell-culture data demonstrate that exposure to high glucose (30 mM for 7 days) increases endothelial ROS production. This phenomenon is primarily related to increased mitochondrial ROS, because blunting of this effect occurs only with pharmacologic inhibition of complex II, overexpression of UCP1, or exposure to MnSOD. Indicative of the cell-signaling capacity of mitochondrial ROS, mitochondrial ROS inhibitors also reduce NF-κB and protein kinase C activation, and blunt the production of toxic advanced glycation end products and sorbitol (113). Expression of cell-adhesion molecules on the endothelial cell surface is dependent on mitochondrial ROS in the setting of high glucose (10, 143).
Exposure of endothelial cells to high concentrations of free fatty acids (lysophosphatidylcholine and linoleic acid) increases ΔΨm, mitochondrial ROS, and NF-κB in human aortic endothelial cells (83). Each of these responses is inhibited by overexpression of UCP2, showing that reductions in mitochondrial ROS, linked to reductions in ΔΨm, are responsible for the observed improvement in endothelial function. Similar findings were noted in intact vessels in which overexpression of UCP2 in the rat aorta reversed the endothelial dysfunction induced by lysophosphatidylcholine) (83, 169).
Excessive mitochondrial ROS production in diabetes has been implicated as a “master switch” for activation of discrete pathologic signaling pathways leading to subsequent endothelial dysfunction through (a) protein kinase C activation, (b) increased age-related glycation end-product formation, and (c) increased polyol and hexosamine pathway flux (21). In vivo human studies have verified that hyperglycemia induces such a state of endothelial dysfunction through excessive oxidative stress. Hyperglycemia has been shown to inhibit endothelial function in the forearm microvasculature through suppression of NO bioavailability (169). This reduced dilation during acute hyperglycemia also is observed in patients with diabetes and can be improved with antioxidant therapy (12, 151, 152). The relation between human endothelial dysfunction and alterations in endothelial and inflammatory cell mitochondrial homeostasis in diabetes is relatively uncharted, although preliminary work from our laboratory suggests that altered mitochondrial homeostasis plays an important pathophysiologic role in diabetic endothelial dysfunction (unpublished results).
Dyslipidemia and mitochondrial ROS
Oxidized LDL (oxLDL) contributes fundamentally to the development of atherosclerosis (144). Mitochondrial ROS are important in the generation of oxLDL in endothelial cells (94). These oxidized lipids in turn modulate complex I activity, resulting in an increase in the expression of antioxidant genes, which attenuates the excessive oxidative stress (28, 183).
Recent data demonstrating electrophilic lipid-associated induction of mitochondrial ROS production suggest that oxidized lipids can act as messengers between nonmitochondrial sources of ROS and mitochondria (184). This concept has been buoyed by recent data demonstrating significant mitochondrial respiratory chain inhibition after exposure to extensively oxidized LDL, resulting in an increase in mitochondrial ROS production (134). The relevance of this coordinate expression of ROS to abnormalities of the vascular phenotype in vivo remains to be determined.
Hypertension and mitochondrial ROS
Hypertension (HTN) has a clear etiologic foundation based on enhanced oxidative stress in key target organs, including brain, kidney, and the vasculature (61). The development of HTN is associated with elevation in vascular tissue levels of superoxide, whereas infusions of superoxide scavengers can reduce blood pressure in animal models of HTN (82, 103, 111, 138). A key role for NADPH oxidase in the genesis of HTN-associated oxidative stress is well established (61, 67). However, both NADPH oxidase and mitochondria coordinately generate excessive ROS in multiple different animal models of HTN, including angiotensin II–related models (127, 167). Overexpression of mitochondrial matrix-based thioredoxin-2 in a transgenic mouse model reduces overall superoxide production and blunts the hypertensive effects of angiotensin II infusion (167). Dietary supplementation with a mitochondria-targeted antioxidant, MitoQ, attenuates the age-related hypertensive response in a spontaneous hypertensive rat model (56). These data provide feasibility for antihypertensive therapeutic interventions that target reductions in mitochondrial ROS.
Smoking and mitochondrial ROS
The adverse effects of cigarette smoke on mitochondrial homeostasis in lung tissue and myocardium have long been recognized and likely involve multiple components of tobacco smoke (57, 160). Cigarette smoking intake reduces inner mitochondrial membrane fluidity, inhibits respiration, and reduces ATP production through a switch from mitochondrial state 3 to state 4 respiration (50, 75). Recent data also demonstrate that acrolein, a reactive aldehyde in cigarette smoke, induces hyperpolarization of ΔΨm with a resultant increase in ROS production (135). In addition, lipid-soluble components of cigarette smoke induce excessive mitochondrial ROS production in lung epithelial cells, contributing to toxicity in that organ (159).
The adverse effects of smoking on vascular function are well established, and both direct and second-hand exposure to cigarette smoke rapidly induces endothelial dysfunction (29, 62). Functionally, acute exposure of endothelial cells to tobacco smoke leads to a rapid reduction in ΔΨm and subsequent cell death (161, 178). Further, exposure of endothelial cells to the contents of cigarette smoke leads to a short-term upregulation of proinflammatory and compensatory heat-shock and antioxidant genes (64). The inflammatory response can be reversed by the addition of the thiol antioxidant N-acetylcysteine, suggesting oxidative stress as a mechanism for the cigarette-smoke–related effects on the endothelium.
Similar to the alterations seen in isolated mitochondria and lung cells, cigarette smoke alters both mitochondrial morphology and function in endothelial cells, leading to mitochondrial DNA damage and cell death. Endothelial cells in the umbilical arteries of newborns of smoking mothers demonstrate an increased in mitochondrial content relative to those of nonsmoking mothers (6). In a mouse model of atherosclerosis, exposure to second-hand smoke accelerates mitochondrial DNA damage, oxidative impairment of mitochondrial enzymes, and accelerated atherogenesis (159).
Atherosclerosis and mitochondrial ROS
Multiple lines of evidence support the concept that excessive mitochondrial ROS production and its subsequent downstream signaling effects are contributory in the pathogenesis of atherosclerosis (95). Mitochondrial DNA (mtDNA) integrity, an index of mitochondrial oxidative stress, is inversely correlated with the development of atherogenesis. mtDNA is circular and contains 13 genes critical to the respiratory chain (5). The mitochondrial DNA lack of protective histones, poor DNA-repair mechanisms, and close proximity to ROS being produced in matrix and intermembrane space make it exquisitely sensitive as an indicator of oxidative damage, especially compared with genomic DNA (92). Oxidative damage to mtDNA encoding for proteins important to complex I or ATP synthase leads to further increases in mitochondrial ROS production, a process known as ROS-induced ROS release, and demonstrates a direct link between mtDNA damage and excess mitochondrial ROS (112). Increased mtDNA damage is observed histologically to a much greater degree in atherosclerotic human arterial tissue than in nonatherosclerotic regions from the same patients (8). In an ApoE-knockout mouse model, mtDNA damage precedes the development of atherosclerotic lesions, establishing temporal plausibility for cause and effect. When a genetic deficiency of MnSOD is superimposed on this model through breeding to create mice that were also heterozygous knockouts for MnSOD, accelerated atherosclerosis is observed (8). The role of excessive mitochondrial ROS in the pathogenesis of atherosclerosis also is reinforced by ApoE-knockout mice studies, in which transgenic overexpression of thioredoxin-2 improves endothelial function and reduces the formation of atherosclerotic plaques in part by reducing oxidative stress (181), as well as human epidemiologic data suggesting that genetic polymorphisms leading to reduced MnSOD function are associated with increased atherosclerotic risk (48).
Another approach that has been used to implicate mitochondrial ROS in atherosclerosis is through modulation of mitochondrial membrane potential. As mentioned earlier, hyperpolarization leads to enhanced ROS generation. When an atherogenic mouse model undergoes bone-marrow transplant of marrow from a UCP2-knockout mouse, accelerated atherosclerosis results (16). Thus monocyte mitochondrial homeostasis can contribute to atherosclerotic plaque formation. UCP2-knockout mice fed an atherogenic diet show significantly greater aortic atheroma formation relative to control mice, suggesting that the well-known link between dietary fuel sources and atherosclerosis is critically dependent on mitochondrial homeostasis and membrane potential. This mechanistic study was recently corroborated by a genetic epidemiologic study in humans, demonstrating that a loss of function polymorphism in UCP2 is associated with elevations in cardiovascular risk in a selected population (79).
Conclusions and Future Directions
Based on the available body of evidence, mitochondrial ROS levels are tightly regulated. Regulators include mitochondria-specific enzymes, mitochondrial energetics, well-characterized second-messenger signaling molecules, and novel mechanisms for coordination of total cellular ROS production. Basal levels of mitochondrial ROS are important for maintaining vascular homeostasis in the human coronary microvasculature under normal physiological conditions. Traditional cardiovascular risk factors, which are associated with excessive vascular oxidative stress and endothelial dysfunction, exert at least a portion of their adverse effects on vascular homeostasis in cell culture and animal models through signaling pathways involving increases in mitochondrial ROS concentrations to levels significantly higher than those seen in the basal state. Finally, cell-culture and animal studies provide strong evidence that excessive mitochondrial ROS turn on mechanisms important in the pathogenesis of atherosclerosis.
A multitude of questions remain unanswered regarding the roles of mitochondrial ROS in cell signaling under both physiologic and pathophysiologic conditions. These include determining the differential expression and signaling of mitochondrial ROS under a variety of fuel-substrate states (i.e., high succinate vs. high malate fuel states), the mechanisms of coordinate expression of ROS from mitochondrial and nonmitochondrial sources, and the role of mitochondria in regulating vascular endothelial function under normal physiologic conditions in conduit vessels relevant to the development of clinical atherosclerosis. Given the clear propensity for increased mitochondrial ROS production in fuel-replete states or under the influence of external regulators leading to a similar state of mitochondrial respiration, determination of mechanisms to alter mitochondrial fuel states will likely have an important impact on our understanding of the regulation of mitochondria ROS production under normal physiologic and pathophysiologic conditions. From a simplistic standpoint, exercise or limiting food intake may operate through the same mechanism for atheroprotection by reducing mitochondrial membrane potential and ROS production.
To apply the knowledge gained from the laboratory bench, translational studies are needed to determine whether mitochondrial ROS signaling pathways are relevant to the development of endothelial dysfunction and atherosclerosis in humans. Such human studies will necessitate the design and testing of novel, mitochondria-specific interventions. Potential therapeutic targets are listed in Table 1. Recent data from animal studies show that MitoQ, a mitochondria-targeted antioxidant, is an effective antihypertensive agent, demonstrating the potential promise of this approach (56). Data demonstrating an increased life span and protection from age-related endothelial dysfunction in p66Shc-knockout animals suggest p66Shc as a potential target as well (47, 101). Recent data also support a role for mitochondria as generators of signaling molecules in the circulation, affecting systemic endothelial function and blood pressure (117–119). Investigations into the potential role of mitochondrial proteins as circulating hormone-like regulators of systemic vascular homeostasis (e.g., coupling factor 6) may be of clinical importance.
Table 1.
Potential Therapeutic Targets for Reducing Excessive Mitochondrial ROS
| Reduce excess mitochondrial O2− production | ↓ΔΨm |
| ↓p66Shc | |
| ↓Mitochondrial fission | |
| Quenching of excess mitochondrial O2− production | ↑MnSOD |
| Excess mitochondrial H2O2 destruction | ↑Grx |
| ↑Prx | |
| ↑Trx | |
| Quench excess mitochondrial O2− | Mitochondrial Targeted Antioxidants |
| Inhibit ROS from nonmitochondrial sources | ↓NADPH Oxidase |
| ↓oxLDL |
Although numerous conceptual and methodologic challenges remain, investigations into the mechanisms and roles of mitochondrial ROS in regulating vascular homeostasis are likely to yield important, clinically relevant findings.
Abbreviations Used
- Δψm
mitochondrial membrane potential
- Δp
proton motive force
- ApoE
apolipoprotein E
- ETC
electron-transport chain
- FMN
flavin mononucleotide
- Grx
glutathione reductase
- mPTP
mitochondrial permeability transition pore
- mtDNA
mitochondrial DNA
- oxLDL
oxidized LDL
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- Trx
thioredoxin
- UCP
uncoupling protein
Acknowledgments
Dr. Widlansky is supported by K23HL089326, 10GRNT3880044, (supported by a T. Franklin Williams Scholars Award with funding supplied by Atlantic Philanthropies, Inc., the American Heart Association, the John A. Hartford Foundation, and the Association of Specialty Physicians), and a grant from the Greater Milwaukee Foundation. Dr. Gutterman is supported by HL094971 and HL080704.
References
- 1.Ai L. Rouhanizadeh M. Wu JC. Takabe W. Yu H. Alavi M. Li R. Chu Y. Miller J. Heistad DD. Hsiai TK. Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration. Am J Physiol Cell Physiol. 2008;294:C1576–C1585. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali MH. Mungai PT. Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol. 2006;291:L38–L45. doi: 10.1152/ajplung.00287.2004. [DOI] [PubMed] [Google Scholar]
- 3.Ali MH. Pearlstein DP. Mathieu CE. Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L486–L496. doi: 10.1152/ajplung.00389.2003. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez S. Valdez LB. Zaobornyj T. Boveris A. Oxygen dependence of mitochondrial nitric oxide synthase activity. Biochem Biophys Res Commun. 2003;305:771–775. doi: 10.1016/s0006-291x(03)00818-0. [DOI] [PubMed] [Google Scholar]
- 5.Anderson S. Bankier AT. Barrell BG. de Bruijn MH. Coulson AR. Drouin J. Eperon IC. Nierlich DP. Roe BA. Sanger F. Schreier PH. Smith AJ. Staden R. Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 6.Asmussen I. Chondrial proliferation in endothelium: observations on umbilical arteries from newborn children of smoking mothers. Atherosclerosis. 1984;50:203–208. doi: 10.1016/0021-9150(84)90023-6. [DOI] [PubMed] [Google Scholar]
- 7.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Ballinger SW. Patterson C. Knight-Lozano CA. Burow DL. Conklin CA. Hu Z. Reuf J. Horaist C. Lebovitz R. Hunter GC. McIntyre K. Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 9.Banfi C. Brioschi M. Barbieri SS. Eligini S. Barcella S. Tremoli E. Colli S. Mussoni L. Mitochondrial reactive oxygen species: a common pathway for. J Thromb Haemost. 2009;7:206–216. doi: 10.1111/j.1538-7836.2008.03204.x. [DOI] [PubMed] [Google Scholar]
- 10.Basta G. Lazzerini G. Del Turco S. Ratto GM. Schmidt AM. De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 11.Baudry N. Laemmel E. Vicaut E. In vivo reactive oxygen species production induced by ischemia in muscle arterioles of mice: involvement of xanthine oxidase and mitochondria. Am J Physiol Heart Circ Physiol. 2008;294:H821–H828. doi: 10.1152/ajpheart.00378.2007. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JA. Goldfine AB. Gordon MB. Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103:1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- 13.Beutner G. Sharma VK. Lin L. Ryu SY. Dirksen RT. Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 15.Bienert GP. Schjoerring JK. Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Blanc J. Yves-Guerra MC. Esposito B. Rousset S. Gourdy P. Ricquier D. Tedgui A. Miroux B. Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 17.Bogardus C. Lillioja S. Howard BV. Reaven G. Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984;74:1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borniquel S. Valle I. Cadenas S. Lamas S. Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- 19.Bortolato M. Chen K. Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60:1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 21.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 22.Burwell LS. Nadtochiy SM. Tompkins AJ. Young S. Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cadenas E. Boveris A. Ragan CI. Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 24.Cadenas E. Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 25.Calamita G. Ferri D. Gena P. Liquori GE. Cavalier A. Thomas D. Svelto M. The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem. 2005;280:17149–17153. doi: 10.1074/jbc.C400595200. [DOI] [PubMed] [Google Scholar]
- 26.Camello-Almaraz C. Gomez-Pinilla PJ. Pozo MJ. Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 27.Cassina A. Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 28.Ceaser EK. Ramachandran A. Levonen AL. Arley-Usmar VM. Oxidized low-density lipoprotein and 15-deoxy-delta 12,14-PGJ2 increase mitochondrial complex I activity in endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H2298–H2308. doi: 10.1152/ajpheart.00508.2003. [DOI] [PubMed] [Google Scholar]
- 29.Celermajer DS. Sorensen KE. Georgakopoulos D. Bull C. Thomas O. Robinson J. Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 30.Ceylan-Isik AF. Guo KK. Carlson EC. Privratsky JR. Liao SJ. Cai L. Chen AF. Ren J. Metallothionein abrogates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension. 2009;53:1023–1031. doi: 10.1161/HYPERTENSIONAHA.108.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chance B. Sies H. Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 32.Chang TS. Cho CS. Park S. Yu S. Kang SW. Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 33.Chen KH. Reece LM. Leary JF. Mitochondrial glutathione modulates TNF-alpha-induced endothelial cell dysfunction. Free Radic Biol Med. 1999;27:100–109. doi: 10.1016/s0891-5849(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 34.Clementi E. Brown GC. Feelisch M. Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 36.Culic O. Gruwel ML. Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997;273:C205–C213. doi: 10.1152/ajpcell.1997.273.1.C205. [DOI] [PubMed] [Google Scholar]
- 37.Dagher Z. Ruderman N. Tornheim K. Ido Y. Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ Res. 2001;88:1276–1282. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- 38.Dahm CC. Moore K. Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 39.Davidson SM. Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 40.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dedkova EN. Ji X. Lipsius SL. Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2004;286:C406–C415. doi: 10.1152/ajpcell.00155.2003. [DOI] [PubMed] [Google Scholar]
- 42.Doughan AK. Harrison DG. Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 43.Duval C. Negre-Salvayre A. Dogilo A. Salvayre R. Penicaud L. Casteilla L. Increased reactive oxygen species production with antisense oligonucleotides directed against uncoupling protein 2 in murine endothelial cells. Biochem Cell Biol. 2002;80:757–764. doi: 10.1139/o02-158. [DOI] [PubMed] [Google Scholar]
- 44.Erecinska M. Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 45.Erusalimsky JD. Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 46.Fink BD. Reszka KJ. Herlein JA. Mathahs MM. Sivitz WI. Respiratory uncoupling by UCP1 and UCP2 and superoxide generation in endothelial cell mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E71–E79. doi: 10.1152/ajpendo.00332.2004. [DOI] [PubMed] [Google Scholar]
- 47.Francia P. delli Gatti C. Bachschmid M. Martin-Padura I. Savoia C. Migliaccio E. Pelicci PG. Schiavoni M. Luscher TF. Volpe M. Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 48.Fujimoto H. Taguchi J. Imai Y. Ayabe S. Hashimoto H. Kobayashi H. Ogasawara K. Aizawa T. Yamakado M. Nagai R. Ohno M. Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoprotein-induced apoptosis of macrophages and coronary artery disease. Eur Heart J. 2008;29:1267–1274. doi: 10.1093/eurheartj/ehm500. [DOI] [PubMed] [Google Scholar]
- 49.Fukai T. Mitochondrial thioredoxin: novel regulator for NADPH oxidase and angiotensin II-induced hypertension. Hypertension. 2009;54:224–225. doi: 10.1161/HYPERTENSIONAHA.109.134403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gairola C. Aleem HM. Cigarette smoke: in vitro effects of condensate fractions on mitochondrial respiration. Life Sci. 1974;14:2199–2207. doi: 10.1016/0024-3205(74)90102-7. [DOI] [PubMed] [Google Scholar]
- 51.Gao L. Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 52.Gao S. Chen J. Brodsky SV. Huang H. Adler S. Lee JH. Dhadwal N. Cohen-Gould L. Gross SS. Goligorsky MS. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem. 2004;279:15968–15974. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- 53.Geng Y. Hansson GK. Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992;71:1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- 54.Giorgio M. Migliaccio E. Orsini F. Paolucci D. Moroni M. Contursi C. Pelliccia G. Luzi L. Minucci S. Marcaccio M. Pinton P. Rizzuto R. Bernardi P. Paolucci F. Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Godbole AS. Lu X. Guo X. Kassab GS. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol. 2009;296:H152–H158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham D. Huynh NN. Hamilton CA. Beattie E. Smith RA. Cocheme HM. Murphy M P. Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 57.Gvozdjakova A. Kucharska J. Gvozdjak J. Effect of smoking on the oxidative processes of cardiomyocytes. Cardiology. 1992;81:81–84. doi: 10.1159/000175780. [DOI] [PubMed] [Google Scholar]
- 58.Han D. Antunes F. Canali R. Rettori D. Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 59.Han D. Williams E. Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Z. Chen YR. Jones CI., III Meenakshisundaram G. Zweier JL. Alevriadou BR. Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells. Am J Physiol Cell Physiol. 2007;292:C1103–C1112. doi: 10.1152/ajpcell.00389.2006. [DOI] [PubMed] [Google Scholar]
- 61.Harrison D/G. Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 62.Heiss C. Amabile N. Lee AC. Real WM. Schick SF. Lao D. Wong ML. Jahn S. Angeli FS. Minasi P. Springer ML. Hammond SK. Glantz SA. Grossman W. Balmes JR. Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Helmke BP. Davies PF. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann Biomed Eng. 2002;30:284–296. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- 64.Henderson B. Csordas A. Backovic A. Kind M. Bernhard D. Wick G. Cigarette smoke is an endothelial stressor and leads to cell cycle arrest. Atherosclerosis. 2008;201:298–305. doi: 10.1016/j.atherosclerosis.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 65.Hinkle PC. Butow RA. Racker E. Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation, XV: reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem. 1967;242:5169–5173. [PubMed] [Google Scholar]
- 66.Hirst J. Carroll J. Fearnley IM. Shannon RJ. Walker JE. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochim Biophys Acta. 2003;1604:135–150. doi: 10.1016/s0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 67.Hoch NE. Guzik TJ. Chen W. Deans T. Maalouf SA. Gratze P. Weyand C. Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffman DL. Salter JD. Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 69.Huser J. Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343:311–317. [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang J. Ing MH. Salazar A. Lassegue B. Griendling K. Navab M. Sevanian A. Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang J. Saha A. Boo YC. Sorescu GP. McNally JS. Holland SM. Dikalov S. Giddens DP. Griendling KK. Harrison DG. Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 72.Ichikawa H. Kokura S. Aw TY. Role of endothelial mitochondria in oxidant production and modulation of neutrophil adherence. J Vasc Res. 2004;41:432–444. doi: 10.1159/000081466. [DOI] [PubMed] [Google Scholar]
- 73.Imai H. Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 74.Ishida I. Kubo H. Suzuki S. Suzuki T. Akashi S. Inoue K. Maeda S. Kikuchi H. Sasaki H. Kondo T. Hypoxia diminishes Toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–2075. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- 75.Kennedy JR. Elliott AM. Cigarette smoke: the effect of residue on mitochondrial structure. Science. 1970;168:1097–1098. doi: 10.1126/science.168.3935.1097. [DOI] [PubMed] [Google Scholar]
- 76.Krishnamoorthy G. Hinkle PC. Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J Biol Chem. 1988;263:17566–17575. [PubMed] [Google Scholar]
- 77.Kunsch C. Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 78.Kussmaul L. Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Labayen I. Ortega FB. Sjostrom M. Nilsson TK. Olsson LA. Ruiz JR. Association of common variants of UCP2 gene with low-grade inflammation in Swedish children and adolescents; the European Youth Heart Study. Pediatr Res. 2009;66:350–354. doi: 10.1203/PDR.0b013e3181b1bd35. [DOI] [PubMed] [Google Scholar]
- 80.Landar A. Zmijewski JW. Dickinson DA. Le Goffe C. Johnson MS. Milne GL. Zanoni G. Vidari G. Morrow JD. Arley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- 81.Landmesser U. Dikalov S. Price SR. McCann L. Fukai T. Holland SM. Mitch WE. Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laursen JB. Rajagopalan S. Galis Z. Tarpey M. Freeman BA. Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 83.Lee KU. Lee IK. Han J. Song DK. Kim YM. Song HS. Kim HS. Lee WJ. Koh EH. Song KH. Han SM. Kim MS. Park IS. Park JY. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005;96:1200–1207. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- 84.Lee MY. Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee SB. Bae IH. Bae YS. Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem. 2006;281:36228–36235. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- 86.Lee WK. Bork U. Gholamrezaei F. Thevenod F. Cd(2+)-induced cytochrome c release in apoptotic proximal tubule cells: role of mitochondrial permeability transition pore and Ca(2+) uniporter. Am J Physiol Renal Physiol. 2005;288:F27–F39. doi: 10.1152/ajprenal.00224.2004. [DOI] [PubMed] [Google Scholar]
- 87.Lenaz G. Fato R. Genova ML. Bergamini C. Bianchi C. Biondi A. Mitochondrial complex I: structural and functional aspects. Biochim Biophys Acta. 2006;1757:1406–1420. doi: 10.1016/j.bbabio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 88.Li R. Beebe T. Cui J. Rouhanizadeh M. Ai L. Wang P. Gundersen M. Takabe W. Hsiai TK. Pulsatile shear stress increased mitochondrial membrane potential: implication of Mn-SOD. Biochem Biophys Res Commun. 2009;388:406–412. doi: 10.1016/j.bbrc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- 90.Liu Y. Li H. Bubolz AH. Zhang DX. Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput. 2008;46:469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y. Zhao H. Li H. Kalyanaraman B. Nicolosi AC. Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 92.Luft R. Landau BR. Mitochondrial medicine. J Intern Med. 1995;238:405–421. doi: 10.1111/j.1365-2796.1995.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 93.Lundberg M. Johansson C. Chandra J. Enoksson M. Jacobsson G. Ljung J. Johansson M. Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 94.Mabile L. Meilhac O. Escargueil-Blanc I. Troly M. Pieraggi MT. Salvayre R. Negre-Salvayre A. Mitochondrial function is involved in LDL oxidation mediated by human cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1575–1582. doi: 10.1161/01.atv.17.8.1575. [DOI] [PubMed] [Google Scholar]
- 95.Madamanchi NR. Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 96.Matoba T. Shimokawa H. Kubota H. Morikawa K. Fujiki T. Kunihiro I. Mukai Y. Hirakawa Y. Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 97.Matoba T. Shimokawa H. Nakashima M. Hirakawa Y. Mukai Y. Hirano K. Kanaide H. Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCormack JG. Halestrap AP. Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 99.Mendoza SA. Fang J. Gutterman DD. Wilcox DA. Bubolz AH. Li R. Suzuki M. Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mertens S. Noll T. Spahr R. Krutzfeldt A. Piper HM. Energetic response of coronary endothelial cells to hypoxia. Am J Physiol. 1990;258:H689–H694. doi: 10.1152/ajpheart.1990.258.3.H689. [DOI] [PubMed] [Google Scholar]
- 101.Migliaccio E. Giorgio M. Mele S. Pelicci G. Reboldi P. Pandolfi PP. Lanfrancone L. Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 102.Miura H. Bosnjak JJ. Ning G. Saito T. Miura M. Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 103.Miyagawa K. Ohashi M. Yamashita S. Kojima M. Sato K. Ueda R. Dohi Y. Increased oxidative stress impairs endothelial modulation of contractions in arteries from spontaneously hypertensive rats. J Hypertens. 2007;25:415–421. doi: 10.1097/HJH.0b013e3280115b96. [DOI] [PubMed] [Google Scholar]
- 104.Monteiro G. Kowaltowski AJ. Barros MH. Netto LE. Glutathione and thioredoxin peroxidases mediate susceptibility of yeast mitochondria to Ca(2+)-induced damage. Arch Biochem Biophys. 2004;425:14–24. doi: 10.1016/j.abb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Mootha VK. Lindgren CM. Eriksson KF. Subramanian A. Sihag S. Lehar J. Puigserver P. Carlsson E. Ridderstrale M. Laurila E. Houstis N. Daly MJ. Patterson N. Mesirov JP. Golub TR. Tamayo P. Spiegelman B. Lander ES. Hirschhorn JN. Altshuler D. Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 106.Morino K. Petersen KF. Dufour S. Befroy D. Frattini J. Shatzkes N. Neschen S. White MF. Bilz S. Sono S. Pypaert M. Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mowbray AL. Kang DH. Rhee SG. Kang SW. Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622–1627. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- 108.Mracek T. Pecinova A. Vrbacky M. Drahota Z. Houstek J. High efficiency of ROS production by glycerophosphate dehydrogenase in mammalian mitochondria. Arch Biochem Biophys. 2009;481:30–36. doi: 10.1016/j.abb.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 109.Muller FL. Liu Y. Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 110.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakazono K. Watanabe N. Matsuno K. Sasaki J. Sato T. Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nijtmans LG. Henderson NS. Attardi G. Holt IJ. Impaired ATP synthase assembly associated with a mutation in the human ATP synthase subunit 6 gene. J Biol Chem. 2001;276:6755–6762. doi: 10.1074/jbc.M008114200. [DOI] [PubMed] [Google Scholar]
- 113.Nishikawa T. Edelstein D. Du XL. Yamagishi S. Matsumura T. Kaneda Y. Yorek MA. Beebe D. Oates PJ. Hammes HP. Giardino I. Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 114.Ohashi M. Runge MS. Faraci FM. Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 115.Okado-Matsumoto A. Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 116.Orrenius S. Gogvadze V. Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 117.Osanai T. Kamada T. Fujiwara N. Katoh T. Takahashi K. Kimura M. Satoh K. Magota K. Kodama S. Tanaka T. Okumura K. A novel inhibitory effect on prostacyclin synthesis of coupling factor 6 extracted from the heart of spontaneously hypertensive rats. J Biol Chem. 1998;273:31778–31783. doi: 10.1074/jbc.273.48.31778. [DOI] [PubMed] [Google Scholar]
- 118.Osanai T. Okada S. Sirato K. Nakano T. Saitoh M. Magota K. Okumura K. Mitochondrial coupling factor 6 is present on the surface of human vascular endothelial cells and is released by shear stress. Circulation. 2001;104:3132–3136. doi: 10.1161/hc5001.100832. [DOI] [PubMed] [Google Scholar]
- 119.Osanai T. Tanaka M. Kamada T. Nakano T. Takahashi K. Okada S. Sirato K. Magota K. Kodama S. Okumura K. Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor. J Clin Invest. 2001;108:1023–1030. doi: 10.1172/JCI11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paravicini TM. Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 121.Pearlstein DP. Ali MH. Mungai PT. Hynes KL. Gewertz BL. Schumacker PT. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler Thromb Vasc Biol. 2002;22:566–573. doi: 10.1161/01.atv.0000012262.76205.6a. [DOI] [PubMed] [Google Scholar]
- 122.Pecqueur C. Alves-Guerra MC. Gelly C. Levi-Meyrueis C. Couplan E. Collins S. Ricquier D. Bouillaud F. Miroux B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- 123.Petersen KF. Dufour S. Befroy D. Garcia R. Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Phillips SA. Hatoum OA. Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292:H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 125.Poyton RO. Ball KA. Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 126.Puddu P. Puddu GM. Galletti L. Cravero E. Muscari A. Mitochondrial dysfunction as an initiating event in atherogenesis: a plausible hypothesis. Cardiology. 2005;103:137–141. doi: 10.1159/000083440. [DOI] [PubMed] [Google Scholar]
- 127.Pueyo ME. Gonzalez W. Nicoletti A. Savoie F. Arnal JF. Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 128.Quintero M. Colombo SL. Godfrey A. Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raha S. Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 130.Reaven GM. Hollenbeck C. Jeng CY. Wu MS. Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 131.Rhee SG. Kang S/W. Chang TS. Jeong W. Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 132.Rigobello MP. Vianello F. Folda A. Roman C. Scutari G. Bindoli A. Differential effect of calcium ions on the cytosolic and mitochondrial thioredoxin reductase. Biochem Biophys Res Commun. 2006;343:873–878. doi: 10.1016/j.bbrc.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 133.Riva A. Tandler B. Loffredo F. Vazquez E. Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2005;289:H868–H872. doi: 10.1152/ajpheart.00866.2004. [DOI] [PubMed] [Google Scholar]
- 134.Roy Chowdhury SK. Sangle GV. Xie X. Stelmack GL. Halayko AJ. Shen GX. Effects of extensively oxidized low-density lipoprotein on mitochondrial function and reactive oxygen species in porcine aortic endothelial cells. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00433.2009. [DOI] [PubMed] [Google Scholar]
- 135.Roy J. Pallepati P. Bettaieb A. Tanel A. Averill-Bates DA. Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chem Biol Interact. 2009;181:154–167. doi: 10.1016/j.cbi.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 136.Sarkela TM. Berthiaume J. Elfering S. Gybina AA. Giulivi C. The modulation of oxygen radical production by nitric oxide in mitochondria. J Biol Chem. 2001;276:6945–6949. doi: 10.1074/jbc.M007625200. [DOI] [PubMed] [Google Scholar]
- 137.Sazanov LA. Respiratory complex I: mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry. 2007;46:2275–2288. doi: 10.1021/bi602508x. [DOI] [PubMed] [Google Scholar]
- 138.Schnackenberg CG. Welch WJ. Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 139.Skulachev VP. Chemiosmotic concept of the membrane bioenergetics: what is already clear and what is still waiting for elucidation? J Bioenerg Biomembr. 1994;26:589–598. doi: 10.1007/BF00831533. [DOI] [PubMed] [Google Scholar]
- 140.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 141.Sorescu GP. Song H. Tressel SL. Hwang J. Dikalov S. Smith DA. Boyd NL. Platt M. O, Lassegue B, Griendling KK, and Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 142.Spahr R. Krutzfeldt A. Mertens S. Siegmund B. Piper HM. Fatty acids are not an important fuel for coronary microvascular endothelial cells. Mol Cell Biochem. 1989;88:59–64. doi: 10.1007/BF00223424. [DOI] [PubMed] [Google Scholar]
- 143.Srinivasan S. Yeh M. Danziger EC. Hatley ME. Riggan AE. Leitinger N. Berliner JA. Hedrick CC. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ Res. 2003;92:371–377. doi: 10.1161/01.RES.0000061714.74668.5C. [DOI] [PubMed] [Google Scholar]
- 144.Stocker R. Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 145.Sun D. Huang A. Sharma S. Koller A. Kaley G. Endothelial microtubule disruption blocks flow-dependent dilation of arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2087–H2093. doi: 10.1152/ajpheart.2001.280.5.H2087. [DOI] [PubMed] [Google Scholar]
- 146.Suvorava T. Kojda G. Reactive oxygen species as cardiovascular mediators: lessons from endothelial-specific protein overexpression mouse models. Biochim Biophys Acta. 2009;1787:802–810. doi: 10.1016/j.bbabio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 147.Takeshige K. Minakami S. Inhibition of the mitochondrial respiratory chain by alanine in rat cerebral cortex. Biochem J. 1979;180:129–135. [Google Scholar]
- 148.Tanaka T. Hosoi F. Yamaguchi-Iwai Y. Nakamura H. Masutani H. Ueda S. Nishiyama A. Takeda S. Wada H. Spyrou G. Yodoi J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Therade-Matharan S. Laemmel E. Carpentier S. Obata Y. Levade T. Duranteau J. Vicaut E. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1756–R1762. doi: 10.1152/ajpregu.00480.2004. [DOI] [PubMed] [Google Scholar]
- 150.Thomas SR. Witting PK. Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 151.Timimi FK. Ting HH. Haley EA. Roddy MA. Ganz P. Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1998;31:552–557. doi: 10.1016/s0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- 152.Ting HH. Timimi FK. Boles KS. Creager SJ. Ganz P. Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Trinei M. Giorgio M. Cicalese A. Barozzi S. Ventura A. Migliaccio E. Milia E. Padura IM. Raker VA. Maccarana M. Petronilli V. Minucci S. Bernardi P. Lanfrancone L. Pelicci PG. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 154.Trujillo M. Mauri P. Benazzi L. Comini M. De Palma A. Flohe L. Radi R. Stehr M. Singh M. Ursini F. Jaeger T. The mycobacterial thioredoxin peroxidase can act as a one-cysteine peroxiredoxin. J Biol Chem. 2006;281:20555–20566. doi: 10.1074/jbc.M601008200. [DOI] [PubMed] [Google Scholar]
- 155.Turrens JF. Alexandre A. Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 156.Ungvari Z. Orosz Z. Labinskyy N. Rivera A. Xiangmin Z. Smith K. Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 157.Ungvari Z. Wolin MS. Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid Redox Signal. 2006;8:1121–1129. doi: 10.1089/ars.2006.8.1121. [DOI] [PubMed] [Google Scholar]
- 158.Valle I. Alvarez-Barrientos A. Arza E. Lamas S. Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 159.van der Toorn M. Rezayat D. Kauffman HF. Bakker SJ. Gans RO. Koeter GH. Choi AM. van Oosterhout AJ. Slebos DJ. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L109–L114. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Jaarsveld H. Kuyl JM. Alberts DW. Exposure of rats to low concentration of cigarette smoke increases myocardial sensitivity to ischaemia/reperfusion. Basic Res Cardiol. 1992;87:393–399. doi: 10.1007/BF00796524. [DOI] [PubMed] [Google Scholar]
- 161.Vayssier-Taussat M. Camilli T. Aron Y. Meplan C. Hainaut P. Polla BS. Weksler B. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am J Physiol Heart Circ Physiol. 2001;280:H1293–H1300. doi: 10.1152/ajpheart.2001.280.3.H1293. [DOI] [PubMed] [Google Scholar]
- 162.Vindis C. Seguelas MH. Lanier S. Parini A. Cambon C. Dopamine induces ERK activation in renal epithelial cells through H2O2 produced by monoamine oxidase. Kidney Int. 2001;59:76–86. doi: 10.1046/j.1523-1755.2001.00468.x. [DOI] [PubMed] [Google Scholar]
- 163.Vita JA. Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 164.Votyakova TV. Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 165.Wang W. Fang H. Groom L. Cheng A. Zhang W. Liu J. Wang X. Li K. Han P. Zheng M. Yin J. Wang W. Mattson MP. Kao JP. Lakatta EG. Sheu SS. Ouyang K. Chen J. Dirksen RT. Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wenzel P. Mollnau H. Oelze M. Schulz E. Wickramanayake JM. Muller J. Schuhmacher S. Hortmann M. Baldus S. Gori T. Brandes RP. Munzel T. Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 167.Widder JD. Fraccarollo D. Galuppo P. Hansen JM. Jones DP. Ertl G. Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of thioredoxin 2. Hypertension. 2009;54:338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Widlansky ME. Gokce N. Keaney JF., Jr Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 169.Williams SB. Goldfine AB. Timimi FK. Ting HH. Roddy MA. Simonson DC. Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 170.Wispe JR. Clark JC. Burhans MS. Kropp KE. Korfhagen TR. Whitsett JA. Synthesis and processing of the precursor for human mangano-superoxide dismutase. Biochim Biophys Acta. 1989;994:30–36. doi: 10.1016/0167-4838(89)90058-7. [DOI] [PubMed] [Google Scholar]
- 171.Wittenberg BA. Wittenberg JB. Transport of oxygen in muscle. Annu Rev Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 172.Wolin MS. Activated oxygen metabolites as regulators of vascular tone. Klin Wochenschr. 1991;69:1046–1049. doi: 10.1007/BF01645156. [DOI] [PubMed] [Google Scholar]
- 173.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol. 2009;296:H539–H549. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wosniak J., Jr Santos CX. Kowaltowski AJ. Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 175.Wu Z. Puigserver P. Andersson U. Zhang C. Adelmant G. Mootha V. Troy A. Cinti S. Lowell B. Scarpulla RC. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 176.Wung BS. Cheng JJ. Hsieh HJ. Shyy YJ. Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 177.Yamagishi SI. Edelstein D. Du XL. Kaneda Y. Guzman M. Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 178.Yang YM. Liu GT. Damaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanism. Biomed Environ Sci. 2004;17:121–134. [PubMed] [Google Scholar]
- 179.Yu T. Robotham JL. Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang DX. Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 181.Zhang H. Luo Y. Zhang W. He Y. Dai S. Zhang R. Huang Y. Bernatchez P. Giordano FJ. Shadel G. Sessa WC. Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang L. Yu L. Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem. 1998;273:33972–33976. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- 183.Zhao R. Shen GX. Functional modulation of antioxidant enzymes in vascular endothelial cells by glycated LDL. Atherosclerosis. 2005;179:277–284. doi: 10.1016/j.atherosclerosis.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 184.Zmijewski JW. Landar A. Watanabe N. Dickinson DA. Noguchi N. Arley-Usmar VM. Cell signalling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem Soc Trans. 2005;33:1385–1389. doi: 10.1042/BST20051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zorov DB. Filburn CR. Klotz LO. Zweier JL. Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]