Abstract
Background: In vitro and in vivo studies suggest that carotenoids may inhibit bone resorption and stimulate proliferation and differentiation of osteoblasts. Few studies have examined the association between carotenoid intake (other than β-carotene) and bone mineral density (BMD).
Objective: We evaluated associations between total and individual carotenoid intake (α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein+zeaxanthin) with BMD at the hip, spine, and radial shaft and the 4-y change in BMD.
Design: Both cross-sectional and longitudinal analyses were conducted in 334 men and 540 women (mean ± SD age: 75 ± 5 y) in the Framingham Osteoporosis Study. Energy-adjusted carotenoid intakes were estimated from the Willett food-frequency questionnaire. Mean BMD and mean 4-y BMD changes were estimated, for men and women separately, by quartile of carotenoid intake with adjustment for age, BMI, height, physical activity index, smoking (never compared with ever smokers), multivitamin use, season of BMD measurement (for cross-sectional analyses on BMD only), estrogen use (in women), and intakes of total energy, calcium, vitamin D, caffeine, and alcohol.
Results: Few cross-sectional associations were observed with carotenoid intake. Associations between lycopene intake and 4-y change in lumbar spine BMD were significant for women (P for trend = 0.03), as were intakes of total carotenoids, β-carotene, lycopene and lutein+zeaxanthin with 4-y change in trochanter BMD in men (P for trend = 0.0005, 0.02, 0.009, and 0.008, respectively).
Conclusions: Carotenoids showed protective associations against 4-y loss in trochanter BMD in men and in lumbar spine in women. No significant associations were observed at other bone sites. Although not consistent across all BMD sites examined, these results support a protective role of carotenoids for BMD in older men and women.
INTRODUCTION
It has been estimated that almost 10 million Americans have osteoporosis (1), and low bone mass is a major public health threat for almost 44 million people in the US population aged ≥50 y. Studies have consistently shown that higher fruit and vegetable intakes have positive effects on bone mineral status (2–4), and that fruit- and vegetable-specific antioxidants, such as carotenoids, may decrease oxidative stress (5–7) arising from reactive oxygen intermediates that may be involved in the bone-resorptive process (8–11). Therefore, carotenoids might help prevent osteoporosis (12).
Data from several in vitro (13–16) and in vivo (7, 17, 18) studies suggest that further investigation into the relation between carotenoids and bone health is warranted. To our knowledge, only one observational study has examined the association between dietary intake of individual carotenoids (other than β-carotene) and bone mineral density (BMD), and there were conflicting associations with individual carotenoids. One other study found no association between dietary β-carotene and BMD in women (19). The published literature shows that individual carotenoids are associated with chronic diseases. For example, a high concentration of serum lycopene correlates with a reduced risk of prostrate cancer (20) and digestive tract cancers (21). Therefore, the primary aim of this study was to further evaluate associations between intake of total carotenoids as well as individual carotenoids (α-carotene, total β-carotene, β-cryptoxanthin, lycopene, and lutein+zeaxanthin) with cross-sectional and longitudinal changes in BMD at the hip, spine, and radial shaft in men and women participants in the Framingham Osteoporosis Study. A secondary aim of this study was to examine these associations for effect modification by smoking status, because a previous study identified this as an important interaction (22).
SUBJECTS AND METHODS
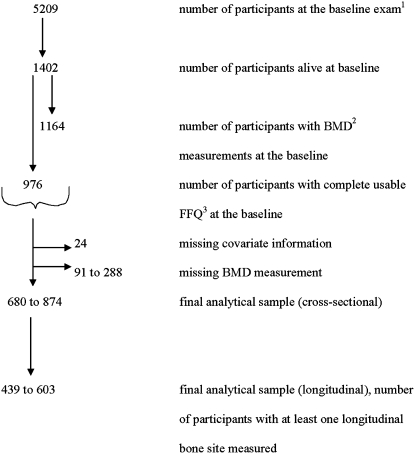
The sample included participants in the Framingham Osteoporosis Study, an ancillary study of the Framingham Heart Study. This population-based cohort study began in 1948 to examine risk factors for heart disease. The original subjects (5209 men and women aged 28–62 y) were selected as a population-based random sample of households in Framingham, MA (23). Of 5209 men and women who formed the original cohort, 1164 cohort members participated in the Framingham Osteoporosis Study when BMD measurements were made at the 20th biennial exam in 1988–1989 (Figure 1). We excluded subjects with missing food-frequency questionnaires (FFQs) (n = 333), with incomplete FFQs (based on the criteria of >12 food items left blank in the FFQ), or with energy intakes <2.51 or >16.74 MJ (<600 or >4000 kcal/d) (n = 92) at the 20th exam. Subjects with missing outcome variables (n = 111 for femoral neck BMD, 123 for trochanter BMD, 91 for radial shaft BMD, and 288 for lumbar spine BMD) or with missing covariate information on body mass index (BMI), physical activity, smoking status, multivitamin use, or current estrogen use (in women) (n = 24) were also excluded. The final analytic sample for cross-sectional analyses included 334 men and 540 women with complete FFQ and BMD measurements taken at the 20th exam. Longitudinal analyses were confined to 213 men and 390 women who had a complete FFQ at the 20th exam and at least one longitudinal bone site measured. Subjects whose follow up scans were on the opposite side from the original scan (due to fracture or joint replacement) were excluded from the longitudinal analyses (n = 30). All participants gave informed consent for their participation in the study. This study was approved by the Institutional Review Boards for Human Research at Boston University, Hebrew Rehabilitation Center, and Tufts University.
FIGURE 1.
Flow chart showing the total number of participants enrolled and the final number of participants included in the analyses. 1Framingham Heart Study. 2BMD, bone mineral density. 3FFQ, food-frequency questionnaire.
Assessment of BMD
BMD was measured in the original cohort in 1988–1989 and in 1992–1993 at the femur, spine, and radius as previously described (24). BMD of the proximal right femur (femoral neck and trochanter) and lumbar spine (average L2 to L4) was measured in g/cm2 with a Lunar DP3 dual-photon absorptiometer (Lunar Corporation, Madison, WI) at baseline. At the 4-y follow-up exam, BMD was measured by using dual-energy X-ray absorptiometry (DPX-L; Lunar Corporation). There were strong correlations between measures taken with dual-photon and dual-energy X-ray absorptiometry, but because of a small but consistent shift in BMD values between the 2 methods, femoral BMDs were adjusted for a change from DP3 to DPX-L technology with the use of published corrections (25). BMD at the radial shaft was measured in g/cm2 with a Lunar SP2 single-photon absorptiometer (Lunar Corporation) at both exams.
Assessment of dietary intake
Usual dietary intake was assessed in 1988–1989 with a semiquantitative, 126-item Willett FFQ (26). FFQs were mailed to the subjects, who were asked to complete them based on their intake over the previous year and to bring them to the exam site, where they were reviewed with the participants by the clinic staff. This FFQ was previously validated against biochemical measures for individual carotenoid intakes in this cohort (27). Pearson correlation coefficients for women and men, respectively, were as follows: α-carotene, 0.30 and 0.28; β-carotene, 0.34 and 0.31; β-cryptoxanthin, 0.45 and 0.36; lycopene, 0.36 and 0.31; and lutein+zeaxanthin, 0.24 and 0.14 (after adjustment for age, energy intake, BMI, plasma cholesterol concentrations, and smoking). The FFQ performed better among women than men. However, in men, the correlations improved after adjustment for confounders. The FFQ produces estimated intakes for each carotenoid in our study. However, the US Department of Agriculture's national nutrient database lists the combined content of lutein plus zeaxanthin (28). Therefore, these carotenoids were used as one observational unit in this study. The total carotenoid variable was calculated as a sum of the intake of 5 individual carotenoids examined in this study. Total β-carotene intake was calculated as the sum of dietary and supplemental intakes of β-carotene. Intakes of carotenoids (μg/d), total calcium (mg/d), vitamin D (IU/d), caffeine (mg/d), alcohol (g/d), potassium (mg/d), and total energy (MJ) were assessed by using the food list section of the FFQ. On the basis of alcohol intake, subjects were categorized as nondrinkers, moderate drinkers (<13.2 g alcohol/d for women and <26.4 g alcohol/d for men), or heavy drinkers (≥13.2 g alcohol/d for women and ≥ 26.4 g alcohol/d for men) based on the cutoffs recommended by the Dietary Guidelines for Americans (29). Intake of multivitamin supplements, as recorded on the supplement section of the FFQ, was coded as a yes-no variable.
Potentially confounding factors
Previous studies of this cohort have reported several risk factors for osteoporosis, including age (y), female sex (30), BMI (31), smoking (32), caffeine (33), alcohol (34), and current estrogen use in women (30). Other studies have reported low physical activity (35) and low intakes of calcium and vitamin D (36) as important predictors of bone loss and bone fracture. BMI (in kg/m2), a known risk factor for osteoporosis, was calculated in the Framingham Study from measurements of height at exam 1 (1948–1949) measured while subjects were shoeless (in inches); measurements of weight were taken at the 20th exam in pounds (and converted to kilograms) with a standardized balance-beam scale. Because BMI is a measure of relative weight, designed to be independent of height, we included both BMI and height in our equations to adjust for ponderosity and body stature, which may be related to dietary intake and BMD (4). A previous study reported that use of BMI and height in the model is an appropriate method to adjust for confounding by these variables (37). A recent article by Morin et al (38) also concluded that either weight or BMI could be used in the prediction of BMD or fractures.
The smoking status of the participants was assessed via questionnaire in 1988–1989 as current cigarette smoker (smoked regularly in the past year), former smoker, or never smoker. Because only a small proportion of participants were current smokers, former smokers and current smokers were combined into the group “ever smokers.” Physical activity was measured with the use of the Framingham physical activity index, which asked about the number of hours spent in heavy, moderate, light, or sedentary activity and the number of hours spent sleeping during a typical day (4). The physical activity index at the 1986–1987 exam (exam 19) was used for the subjects who had a missing physical activity index at the 1988–1989 exam (exam 20). For estrogen use, women were divided into 2 groups: those currently using (continuously for >1 y) compared with those who had never used or who had formerly used estrogen. Previous research has shown that there are seasonal changes in BMD in New England (39). Therefore, for the cross-sectional analyses, we created a categorical variable for the time of BMD measurement: July, August, and September were coded as summer; October, November, and December as fall; January, February, and March as winter; and April, May, and June as spring. A previous study by our group supported the hypothesis that alkaline-producing dietary components such as potassium, present in fruit and vegetables, play a beneficial role in bone health (40). Therefore, final models were adjusted for potassium intake to examine whether the association of carotenoid intake with BMD was independent of this measure of dietary quality.
Statistical analysis
We tested all associations for effect modification by sex. We also tested the associations for effect modification by smoking status, because previous studies identified this as an important interaction (22, 41). The Utah study of nutrition and bone health reported that smoking status (never/ever smoker) was an effect modifier of the association between antioxidant intake and hip fracture (41). This could be because tobacco smoke contains large amounts of oxidants and free radicals that induce oxidative stress—a condition associated with reduced BMD (11). Because we tested 12 interactions (6 in cross-sectional and 6 in longitudinal analyses) per bone site in men and women separately, only interactions that were significant at P < 0.01 were examined to avoid false-positive results. Carotenoid intakes were adjusted for total energy intake by using the residual method because this method is preferred when dietary variables are categorized for analysis (42). As per this method, carotenoid intakes were regressed on total energy intake to create residuals. Carotenoid intake residuals were then added to a constant, where the constant equals the predicted nutrient intake for the mean energy intake of the study population. Square root transformations were applied to the carotenoid intakes to achieve normality, before creating residuals. We calculated Pearson correlations between transformed primary exposures, nutrient covariates, and fruit and vegetable intakes. Mean BMD measures were estimated for men and women separately by quartile of carotenoid intake by using the general linear models procedure in SAS (SAS Institute, Cary, NC). We then regressed each of the 4 measures of BMD onto the continuous and categorical quartile of energy-adjusted total carotenoid intake as well as intake of α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein+zeaxanthin, adjusting for potential confounders and covariates. The analyses were adjusted for multiple comparisons by using Dunnett's adjustment. For analyses of the 4-y change in BMD from exam 20 to exam 22, we regressed the change in BMD (BMD at exam 22 − BMD at exam 20) onto the continuous and the categorical tertile form of energy-adjusted total carotenoid intake as well as intakes of α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein+zeaxanthin, with adjustment for potential confounders and covariates.
All models were adjusted for age, BMI, height at exam 1, total energy intake, physical activity index, alcohol intake, smoking (never compared with ever smokers), multivitamin use, season of BMD measurement (for cross sectional analyses on BMD only), estrogen use (in women), and intakes of total calcium, total vitamin D, and caffeine. Final models were adjusted for potassium intake. All analyses were performed by using SAS statistical software. The SAS user's guide (version 9.1, 2001; SAS Institute). A nominal 2-sided P value <0.05 was considered statistically significant for all of the analyses.
RESULTS
Sample characteristics
Women represented two-thirds (62%) of the study sample. The mean (±SD) age of the men and women was ≈75 ± 5 y, whereas the mean (±SD) BMI was 24.9 ± 4.7 (for women) and 26.2 ± 3.9 (for men) (Table 1). Approximately one-third of the women and men reported education beyond high school. Half of the women and two-thirds of the men reported alcohol use, whereas more than one-half of the women and three-fourths of the men reported ever having smoked cigarettes. Most of the women (95%) reported no estrogen use. Approximately one-fourth of the men and women reported multivitamin supplement use. The mean (±SD) intake of calcium was 831 ± 452 mg/d in women and 763 ± 389 mg/d in men. To ensure complete adjustment for body weight, we repeated our analyses with the replacement of height and BMI with height at exam 1 and weight at exam 20. These results did not differ from those adjusted for BMI and height; therefore, we present only the original models.
TABLE 1.
Descriptive characteristics of the Framingham Osteoporosis Study participants at the 20th exam in 1988–1989
| Descriptive variables1 | Men | Women |
| Age (y) | 75.2 ± 5.02 | 75.5 ± 5.0 |
| BMI (kg/m2) | 26.2 ± 3.93 | 24.9 ± 4.7 |
| Physical activity score | 33.7 ± 6.0 | 33.1 ± 4.9 |
| Education group (%) | ||
| No high school diploma | 32.9 | 29.1 |
| High school diploma | 33.4 | 38.2 |
| Higher education | 33.7 | 32.7 |
| Ever smoked (%) | 74.23 | 55.7 |
| Alcohol use (%) | ||
| None | 35.34 | 48.5 |
| Moderate | 44.44 | 34.4 |
| High | 20.34 | 17.1 |
| Multivitamin supplement use (%) | 21.55 | 27.6 |
| Current estrogen use (%)6 | ||
| Current user | — | 4.8 |
| Former user | — | 1.4 |
| Nonuser | — | 93.8 |
| Season of BMD measurement (%)6 | ||
| Winter | 27.1 | 28.4 |
| Spring | 28.0 | 28.1 |
| Summer | 24.6 | 21.3 |
| Fall | 20.3 | 22.2 |
| Intake6 | ||
| Total energy (MJ/d) | 7.8 ± 2.63 | 7.0 ± 2.3 |
| Total calcium (mg/d)7 | 763 ± 3893 | 831 ± 452 |
| Total vitamin D (IU/d)7 | 322 ± 2758 | 329 ± 254 |
| Total carotenoids (μg/d)910 | 15,186 ± 89023 | 16,502 ± 10,094 |
| α-Carotene (μg/d)9 | 685 ± 6213 | 913 ± 807 |
| Total β-carotene (μg/d)79 | 3892 ± 26513 | 4777 ± 3245 |
| β-Cryptoxanthin (μg/d)9 | 66.8 ± 668 | 76.4 ± 71 |
| Lycopene (μg/d)9 | 7740 ± 6128 | 7508 ± 6287 |
| Lutein+zeaxanthin (μg/d)9 | 2802 ± 23423 | 3228 ± 2773 |
| Fruit and vegetables (servings/d) | 5.0 ± 2.53 | 5.7 ± 2.8 |
| Bone mineral density at exam 20 (g/cm2)11 | ||
| Femoral neck | 0.878 ± 0.1463 | 0.720 ± 0.114 |
| Trochanter | 0.847 ± 0.1503 | 0.624 ± 0.127 |
| Radial shaft | 0.719 ± 0.0853 | 0.512 ± 0.091 |
| Lumbar spine | 1.33 ± 0.2273 | 1.07 ± 0.189 |
| Bone mineral density at exam 22 (g/cm2)12 | ||
| Femoral neck | 0.874 ± 0.1413 | 0.709 ± 0.113 |
| Trochanter | 0.849 ± 0.1473 | 0.612 ± 0.125 |
| Radial shaft | 0.698 ± 0.0793 | 0.492 ± 0.089 |
| Lumbar spine | 1.32 ± 0.2243 | 1.03 ± 0.219 |
Unless indicated otherwise, sample sizes varied from 368 to 374 for men and from 597 to 602 for women.
Mean ± SD (all such values).
Comparison with women (t tests were conducted): 3P < 0.0001, 4P < 0.001, 5P < 0.1, 8P < 0.01.
Sample sizes were 374 for men and 602 for women.
Total nutrient intake = dietary intake + intake from supplements.
Nutrient intake variables are presented in original scale; square root transformations were applied to the carotenoids before creating residuals. t Tests were conducted using energy-adjusted residuals.
Total carotenoid intake = sum of the intake of 5 individual carotenoids examined in this study.
Sample sizes ranged from 259 to 340 for men and 429 to 545 for women.
Sample sizes varied from 188 to 216 for men and from 348 to 395 for women.
Cross-sectional analyses of BMD
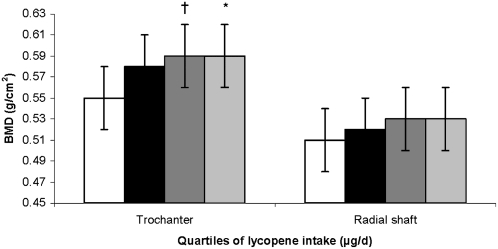
We observed strong Pearson correlations between α-carotene and β-carotene intakes and between β-carotene and lutein+zeaxanthin intakes (Table 2). All of the carotenoids, except for β-cryptoxanthin, were strongly correlated with total carotenoid intake. As expected, potassium (a marker of fruit and vegetable intake) was strongly correlated with average fruit and vegetable intake in this study. Because we observed a significant interaction with sex for lycopene intake (P for interaction = 0.003 at the trochanter), all analyses were stratified by sex. After adjustment for important BMD-related covariates, no associations were seen between intake of total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, and BMD at any site in men or women. Among women, higher lycopene intake was associated with higher trochanter BMD (P for trend = 0.02), and there was a significant trend for radial shaft BMD (P for trend = 0.07) (Figure 2). Women in the third and fourth quartiles of lycopene intake had a higher trochanter BMD than did those in the first quartile of intake (P = 0.06 and 0.04, respectively). In men, higher lycopene intake tended to be negatively associated with trochanter BMD (P for trend = 0.07) (data not shown). There were no associations with lycopene and the other BMD sites.
TABLE 2.
Pearson correlation coefficients for the primary exposures, nutrient covariates, and average fruit and vegetable intakes in the Framingham Osteoporosis Study participants (n = 976) at the 20th exam in 1988–19891
| Total carotenoids (μg/d) | α-Carotene (μg/d) | Total β-carotene (μg/d) | β-Cryptoxanthin (μg/d) | Lycopene (μg/d) | Lutein+zeaxanthin (μg/d) | Fruit and vegetables (servings/d) | Total energy (kcal/d) | Total calcium (mg/d) | Total vitamin D (mg/d) | Potassium (mg/d) | |
| Total carotenoids | 1.001 | 0.57 | 0.77 | 0.28 | 0.81 | 0.72 | 0.76 | 0.33 | 0.28 | 0.17 | 0.58 |
| α-Carotene | 1.00 | 0.82 | 0.20 | 0.17 | 0.46 | 0.52 | 0.18 | 0.18 | 0.16 | 0.37 | |
| Total β-carotene | 1.00 | 0.23 | 0.31 | 0.71 | 0.69 | 0.24 | 0.26 | 0.23 | 0.51 | ||
| β-Cryptoxanthin | 1.00 | 0.20 | 0.22 | 0.47 | 0.17 | 0.14 | 0.08 | 0.38 | |||
| Lycopene | 1.00 | 0.32 | 0.50 | 0.28 | 0.18 | 0.07 | 0.40 | ||||
| Lutein+zeaxanthin | 1.00 | 0.68 | 0.25 | 0.26 | 0.13 | 0.49 | |||||
| Fruit and vegetables | 1.00 | 0.40 | 0.32 | 0.19 | 0.71 | ||||||
| Total energy | 1.00 | 0.54 | 0.33 | 0.75 | |||||||
| Total calcium | 1.00 | 0.54 | 0.64 | ||||||||
| Total vitamin D | 1.00 | 0.42 | |||||||||
| Potassium | 1.00 |
Pearson correlation coefficients were calculated after square root transformation of carotenoid intakes (μg/d) and after logarithmic transformation of total vitamin D intake (mg/d).
FIGURE 2.
Adjusted mean (±SE) bone mineral density (BMD) at the trochanter (n = 527) and radial shaft (n = 540) by quartile (quartiles 1–4 from left to right) of lycopene intake in women. Analysis was based on a general linear model, with Dunnett's adjustment for multiple comparisons. P for trend = 0.02 for the trochanter and 0.08 for the radial shaft. Comparison with the lowest tertile: *P < 0.05, †P < 0.1. The primary predictor was energy-adjusted residuals added to a constant, where the constant equals the nutrient intake for the mean energy intake of the study population. Square root transformation was applied to the nutrient intake before the residuals were created. Models were adjusted for age (y) at exam 20, BMI (in kg/m2), height at exam 1 (inches), total energy intake (MJ/d), physical activity index at exam 20, alcohol intake [none, moderate (<13.2 g alcohol/d for women), or high (≥13.2 g alcohol/d for women)], smoking (never smoked or ever smoked), intake of total calcium (mg/d), intake of total vitamin D (IU/d), intake of caffeine (mg/d), season of BMD measurement, multivitamin use (yes or no), and current estrogen use at exam 20 (current compared with former/never user).
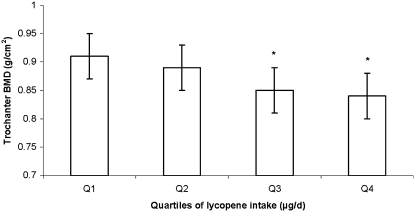
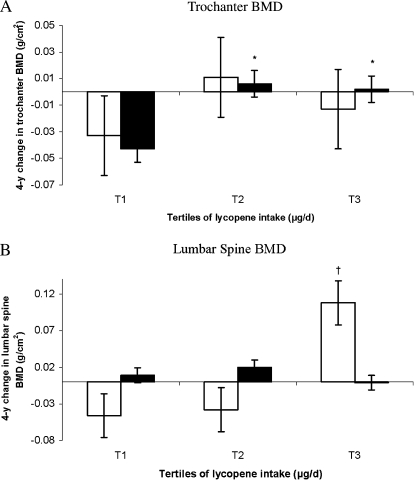
In women, after adjustment for potassium intake, positive associations of lycopene intake with trochanter and radial shaft BMD became nonsignificant (P > 0.1). In contrast, among men, the negative association of lycopene intake with trochanter BMD became more significant after adjustment for potassium intake (P for trend = 0.005). Men in the third and fourth quartiles of lycopene intake had a lower trochanter BMD than did subjects in the first quartile of intake (P for trend = 0.005) (Figure 3).
FIGURE 3.
Adjusted mean (±SE) bone mineral density (BMD) at the trochanter (n = 316), by quartile (Q) of lycopene intake in men. The analysis was based on a general linear model, with Dunnett's adjustment for multiple comparisons. P for trend = 0.005 for the trochanter. Significantly different from the lowest tertile: *P < 0.05. The primary predictor was energy-adjusted residuals added to a constant, where the constant equals the nutrient intake for the mean energy intake of the study population. Square root transformation was applied to the nutrient intake before the residuals were created. Models were adjusted for age (y) at exam 20, BMI (in kg/m2), height at exam 1 (inches), total energy intake (MJ/d), physical activity index at exam 20, alcohol intake [none, moderate (<26.4 g alcohol/d for men), or high (≥26.4 g alcohol/d for men)], smoking (never smoked or ever smoked), intake of total calcium (mg/d), intake of total vitamin D (IU/d), intake of caffeine (mg/d), season of BMD measurement, multivitamin use (yes or no), current estrogen use at exam 20 (current compared with former/never user).
4-y Change in BMD
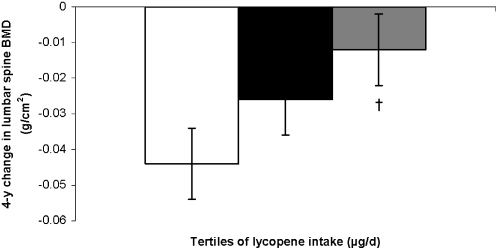
Again, results were stratified by sex, because significant interactions with sex were observed for total carotenoids (P for interaction = 0.0003 at the trochanter), β-carotene (P for interaction = 0.006 at the trochanter), lycopene (P for interaction = 0.006 at the trochanter), and lutein+zeaxanthin (P for interaction = 0.002 at the lumbar spine). Among women, a higher lycopene intake was associated with less bone loss from the lumbar spine (P for trend = 0.03) (Figure 4). Women in the highest tertile of lycopene intake had less loss in lumbar spine BMD from exam 20 to exam 22 than did those in the first tertile (P = 0.06).
FIGURE 4.
Adjusted mean (±SE) change in bone mineral density (BMD) at the lumbar spine, by tertile (tertiles 1–3 from left to right) of lycopene intake in women. The analysis was based on a general linear model, with Dunnett's adjustment for multiple comparisons. n = 281. P for trend = 0.03. Comparison with the lowest tertile: †P < 0.1. Models were adjusted for age (y) at exam 20, BMI (in kg/m2), height at exam 1 (inches), total energy intake (MJ/d), physical activity index at exam 20, alcohol intake [none, moderate (<13.2 g alcohol/d for women), or high (≥13.2 g alcohol/d for women)], smoking (never smoked or ever smoked), intake of total calcium (mg/d), intake of total vitamin D (IU/d), intake of caffeine (mg/d), multivitamin use (yes or no), and current estrogen use (current compared with former/never user).
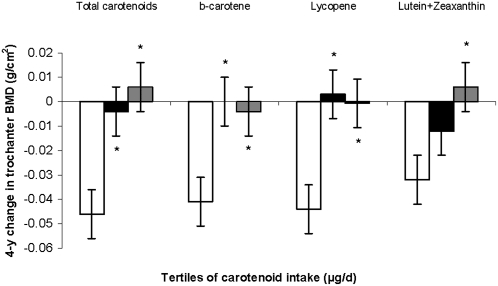
In men, a higher intake of total carotenoids (P for trend = 0.0005), β-carotene (P for trend = 0.02), lycopene (P for trend = 0.009), and lutein+zeaxanthin (P for trend = 0.008) were associated with less loss in trochanter BMD (Figure 5). A similar, but nonsignificant trend was seen for α-carotene and β-cryptoxanthin (P for trend > 0.10). There was a statistically significant interaction between lycopene intake and smoking status (never smokers compared with ever smokers) in men (P for interaction = 0.0005 for radial shaft BMD). In the analyses stratified by smoking status, the 2 groups showed protective tendencies at different bone sites, with the protective effect more apparent at the lumbar spine in never smokers (P for trend = 0.04) and at the trochanter in ever smokers (P for trend = 0.01) (Figure 6). None of the other associations was modified by smoking status (P for interaction > 0.01).
FIGURE 5.
Adjusted mean (±SE) change in bone mineral density (BMD) at the trochanter, by tertile (tertiles 1–3 from left to right) of carotenoid intakes in men. The analysis was based on a general linear model, with Dunnett's adjustment for multiple comparisons. n = 193. P for trend = 0.0005 (total carotenoid), 0.02 (β-carotene), 0.009 (lycopene), and 0.008 (lutein+zeaxanthin). Significantly different from the lowest tertile: *P < 0.05. Models were adjusted for age (y) at exam 20, BMI (in kg/m2), height at exam 1 (inches), total energy intake (MJ/d), physical activity index at exam 20, alcohol intake [none, moderate (<26.4 g alcohol/d for men), or high (≥ 26.4 g alcohol/d for men)], smoking status (never smoked or ever smoked), intake of total calcium (mg/d), intake of total vitamin D (IU/d), intake of caffeine (mg/d), and multivitamin use (yes or no).
FIGURE 6.
Adjusted mean (±SE) change in bone mineral density (BMD) at the trochanter (A) and lumbar spine (B), by tertile (T) of lycopene intake in men, stratified by smoking status. The analysis was based on a general linear model, with Dunnett's adjustment for multiple comparisons. n = 43 (lumbar spine) to 52 (trochanter) in never smokers and n = 115 (lumbar spine) to 141 (trochanter) in ever smokers. P for trend = 0.04 at the lumbar spine in never smokers and 0.01 at the trochanter in ever smokers. Comparison with the lowest tertile: *P < 0.05, †P < 0.1. Models were adjusted for age (y) at exam 20, BMI (in kg/m2), height at exam 1 (inches), total energy intake (MJ/d), physical activity index at exam 20, alcohol intake [none, moderate (<26.4 g alcohol/d for men), or high (≥26.4 g alcohol/d for men)], intake of total calcium (mg/d), intake of total vitamin D (IU/d), intake of caffeine (mg/d), and multivitamin use (yes or no).
In women, after adjustment for potassium intake, the protective association of lycopene intake with change in lumbar spine BMD persisted. In men, after adjustment for potassium intake, the protective associations of total carotenoid (P for trend = 0.002), lycopene (P for trend = 0.03), and lutein+zeaxanthin intake (P for trend = 0.03) with change in trochanter BMD remained. P values for these associations increased but remained significant at P < 0.05. However, the protective association of β-carotene intake (P for trend = 0.06) with trochanter BMD became weaker. After adjustment for potassium intake in the models of lycopene intake, stratified by smoking status, it was observed that lycopene intake was more protective for change in trochanter BMD among men who smoked; those in the higher tertiles of lycopene intake lost less bone from the trochanter than did those in the first tertile of intake. The mean 4-y change in BMD at tertile 1 was 0.038 ± 0.01, at tertile 2 was −0.008 ± 0.01 (P = 0.01), and at tertile 3 was −0.001 ± 0.01 (P = 0.04, P for trend = 0.05). No significant associations remained at any other bone sites or among never smokers.
DISCUSSION
Although few cross-sectional associations were identified between carotenoid intake and BMD in this population of older adults, protective effects against 4-y bone loss were seen for all carotenoids except β-cryptoxanthin and α-carotene at the trochanter in men and for lycopene intake in women at the lumbar spine. These associations remained significant after adjustment for potassium intake, which suggests that it is not due simply to confounding by dietary quality.
Studies have consistently shown that higher fruit and vegetable intakes have positive effects on bone mineral status (2–4, 40, 43, 44). Dietary antioxidants may play a role (45); therefore, more studies are required to clarify the role of fruit- and vegetable-specific carotenoids in the prevention of osteoporosis. One case-control study reported that all carotenoids, with the exception of lutein, were consistently lower in osteoporotic than in control women (46). To our knowledge, only one observational study has examined the association of individual dietary carotenoids (other than β-carotene) with BMD. That study, in an Australian population (n = 68 men and 137 women aged 26–86 y), reported that total-body and lumbar spine bone mass were positively related to lycopene intake in men and to lycopene and lutein+zeaxanthin intake in premenopausal women. In addition, a positive association of dietary β-carotene intake with lumbar spine bone mass was observed in postmenopausal women (47). A limitation of that study was a lack of control for total energy intake. The Women's Health Initiative Study (n = 11,068 women aged = 50–79 y) found no significant associations with serum carotenoids at any of the BMD sites, but reported a negative association of total β-carotene intake and BMD at the femoral neck (P = 0.03) (19). Another recent study reported weak but significant associations between serum β-cryptoxanthin, β-carotene, and radial BMD in postmenopausal Japanese women (48). Together, these epidemiologic studies suggest that carotenoids might play a protective role in bone health.
In the current study, we observed a protective association of total carotenoid intakes on 4-y bone loss in men. Additionally, we also observed several protective associations between individual carotenoid intakes and 4-y bone loss in both men and women.
β-Cryptoxanthin and bone health
In vivo and in vitro studies have suggested that β-cryptoxanthin has a unique anabolic effect on bone calcification (16, 17, 49). In vitro studies have shown that β-cryptoxanthin increases calcium content, alkaline phosphatase activity, and DNA content in the femoral tissues of rats (17). Uchiyama et al (17) showed that β-cryptoxanthin has a direct stimulatory effect on bone formation and an inhibitory effect on bone resorption. This was confirmed in a controlled human trial (n = 21 men and women aged 23–47 y) by the same group of investigators (18), who reported that the intake of β-cryptoxanthin–fortified juice caused a significant increase in β-carboxylated osteocalcin concentrations and a corresponding decrease in serum bone tartrate-resistant acid phosphatase (TRAP) activity (bone-specific alkaline phosphatases) and N-telopeptide of type I collagen—a marker of bone resorption. Despite strong evidence of the positive role of β-cryptoxanthin from previous studies, we found no cross-sectional or longitudinal associations between β-cryptoxanthin and BMD in men or women.
Lycopene and bone health
Laboratory studies have shown that lycopene inhibits the formation of osteoclasts and associated bone resorption. Furthermore, it stimulates proliferation and differentiation of osteoblasts (14, 15). Another cell culture study showed that lycopene had significant stimulatory effects on human osteoblast-like osteosarcoma cells (14). The effects of lycopene on alkaline phosphatase activity were dependent on the stage of cell differentiation. A small cross-sectional study (n = 33 postmenopausal women aged 50–60 y) by Rao et al (7) reported that high serum lycopene was associated with lower concentrations of N-telopeptides of type I collagen (P < 0.0005) and low protein oxidation (P < 0.05). In the current study, we observed a protective association of lycopene on 4-y loss in BMD at the lumbar spine in women and at the trochanter in nonsmoking men. However, we found a negative cross-sectional association between lycopene and trochanter BMD at baseline in men. Lycopene has often been found to differ in its health relations from other carotenoids, particularly in men (50). This may be because bioavailable sources of lycopene include tomato products, including pizza sauce and ketchup, which may sometimes be associated with otherwise less-than-healthy diets (51). Furthermore, we did not adjust for changes in diet over time; therefore, it is possible that, during the follow-up period, men may have adopted a healthier lifestyle and therefore might be getting lycopene from healthier food sources.
β-Carotene and bone health
Several prospective studies examining the association of serum β-carotene with fracture have reported null associations (52, 53). Similarly, in another study, dietary β-carotene intake was not significantly associated with change in BMD or fracture risk (54). In the current study, we observed a protective association of β-carotene on 4-y loss in trochanter BMD in men. This association was only weakly associated with 4-y loss in trochanter BMD in men, after adjustment for potassium intake.
Xanthophylls and bone health
Published reports on the xanthophylls lutein and zeaxanthin have shown that a high intake or status of these carotenoids is associated with a reduced risk of chronic diseases, such as cataract (55) and breast cancer (56). We observed a protective association of lutein+zeaxanthin intake with 4-y loss in trochanter BMD in men, which was independent of potassium intake.
We found that several carotenoids had a protective effect on 4-y bone loss in the men in our study. However, in women, only lycopene protected against 4-y bone loss. We have often seen different effects of dietary constituents on BMD between men and women. This sex difference may be due to different osteoporosis risk profiles, including hormonal interactions, or differences in distributions of intake. Because, with the exception of lycopene, men generally consume fewer fruits and vegetables than do women, it is likely that more men than women had a lower exposure to dietary carotenoids, which thus made the associations easier to observe.
Magnitude of effect
Our group previously reported an adjusted risk of fracture for each SD decrease in BMD of 2.5 (95% CI: 2.0, 3.5) at the hip (57). The results presented here for the relation between trochanter site and lycopene intake showed differences of ≈0.04 (7.3%) and 0.07 (7.7%) g/cm2 from baseline BMD for women and men, respectively, between the lowest and highest quartiles of intake. These differences are greater than those we have seen across categories of other dietary constituents (58, 59). Furthermore, there was a difference of 0.04 in 4-y loss in BMD for women from the lowest to the highest lycopene intakes. The SD of baseline BMD was 0.13 for women. Therefore, if losses continue at this rate, these differences in loss may be expected to translate to an estimated increase in relative risk of 2.5 for fracture in the low lycopene intake group, relative to the high intake group, over ≈17 y.
Carotenoids may protect the skeleton by reducing oxidative stress, which thereby inhibits bone resorption (5–7). Oxidative stress may increase bone resorption through activation of nuclear factor-κB protein, a crucial mediator of tumor necrosis factor-α, and osteoclastogenetic activity (60, 61). Other mechanisms may include nonantioxidant biological activities of carotenoids and their metabolites, such as retinoid-dependent signaling, stimulation of gap junction communications, impact on the regulation of cell growth, induction of detoxifying enzymes (62), up-regulation of the expression of genes such as connexin 43 (63), or by interacting synergistically (particularly lycopene) with vitamin D on cell proliferation, differentiation, and cell cycle progression (64). However, the impact of these mechanisms on bone metabolism still needs to be evaluated. There is another issue relevant to the effect of carotenoid intake on bone that deserves attention. Too little or too much retinol may increase age-related bone loss in women (65); therefore, provitamin A carotenoids such as α-carotene, β-carotene, and β-cryptoxanthin could possibly contribute to the bone-sparing activity of retinol (46). Furthermore, published reports on demonstrated synergy among carotenoids [eg, between β-carotene and lutein (66)] suggest that the strong protective role of total carotenoid intake among men in this study may have been due to a synergy among individual carotenoids.
The current study is unique in that it uses a large population-based cohort that included both elderly men and women. However, this study had some limitations. The dietary intake measures were derived from an FFQ, which is a semiquantitative instrument. However, these intakes were previously validated against plasma carotenoid concentrations in a subsample and were shown to have good predictive agreement for all carotenoids, except for lycopene in men. Another limitation of this study was that we examined only dietary intakes and not serum carotenoid concentrations. Complete dietary data were available only at baseline; therefore, we were unable to adjust for secular changes in diet during the follow-up period. Additionally, the enzyme carotenoid 15, 15′-monooxygenase (CMO1), catalyzes the first step in the conversion of dietary provitamin A carotenoids to vitamin A. In some cases, haploinsufficiency of the CMO1 enzyme can prevent this conversion (67) and can affect the proposed association of carotenoids with BMD. Finally, in any observational study, residual confounding may occur, despite control for several major potential confounders.
In summary, although we observed few cross-sectional associations between carotenoid intakes and BMD, we observed several inverse associations between carotenoids (except for β-cryptoxanthin and α-carotene) and 4-y loss in BMD in men and of lycopene and bone loss at the lumbar spine in women. These results suggest a possible protective effect of carotenoids, particularly of lycopene, against bone loss in older adults. It is therefore possible that carotenoids explain part of the previously observed protective effects of fruit and vegetable intake on BMD. More studies are needed to examine these associations in other populations.
Acknowledgments
The authors' responsibilities were as follows—SS, KLT, MTH, and JB: responsible for the conception and design of the study and the interpretation of the data; SS: conducted the statistical analyses and drafted the manuscript; KLT, MTH, and JB: supervised this work; and DPK and LAC: helped with the data acquisition, obtained funding, and provided administrative and technical support. All authors were responsible for critical revision of the manuscript for important intellectual content. No conflicts of interest were reported.
REFERENCES
- 1.Lanham-New SA. Fruit and vegetables: the unexpected natural answer to the question of osteoporosis prevention? Am J Clin Nutr 2006;83:1254–5 [DOI] [PubMed] [Google Scholar]
- 2.Tucker KL, Hannan MT, Kiel DP. The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. Eur J Nutr 2001;40:231–7 [DOI] [PubMed] [Google Scholar]
- 3.Prynne CJ, Mishra GD, O'Connell MA, et al. Fruit and vegetable intakes and bone mineral status: a cross sectional study in 5 age and sex cohorts. Am J Clin Nutr 2006;83:1420–8 [DOI] [PubMed] [Google Scholar]
- 4.Tucker KL, Chen H, Hannan MT, et al. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 2002;76:245–52 [DOI] [PubMed] [Google Scholar]
- 5.Astley SB, Hughes DA, Wright AJ, Elliott RM, Southon S. DNA damage and susceptibility to oxidative damage in lymphocytes: effects of carotenoids in vitro and in vivo. Br J Nutr 2004;91:53–61 [DOI] [PubMed] [Google Scholar]
- 6.Kiokias S, Gordon MH. Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur J Clin Nutr 2003;57:1135–40 [DOI] [PubMed] [Google Scholar]
- 7.Rao LG, Mackinnon ES, Josse RG, Murray TM, Strauss A, Rao AV. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos Int 2007;18:109–15 [DOI] [PubMed] [Google Scholar]
- 8.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 1990;85:632–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Key LL, Jr, Ries WL, Taylor RG, Hays BD, Pitzer BL. Oxygen derived free radicals in osteoclasts: the specificity and location of the nitroblue tetrazolium reaction. Bone 1990;11:115–9 [DOI] [PubMed] [Google Scholar]
- 10.Ries WL, Key LL, Jr, Rodriguiz RM. Nitroblue tetrazolium reduction and bone resorption by osteoclasts in vitro inhibited by a manganese-based superoxide dismutase mimic. J Bone Miner Res 1992;7:931–9 [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 2001;288:275–9 [DOI] [PubMed] [Google Scholar]
- 12.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition 1996;12:274–7 [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi M, Uchiyama S. beta-Cryptoxanthin stimulates bone formation and inhibits bone resorption in tissue culture in vitro. Mol Cell Biochem 2004;258:137–44 [DOI] [PubMed] [Google Scholar]
- 14.Kim L, Rao AV, Rao LG. Lycopene II–effect on osteoblasts: the carotenoid lycopene stimulates cell proliferation and alkaline phosphatase activity of SaOS-2 cells. J Med Food 2003;6:79–86 [DOI] [PubMed] [Google Scholar]
- 15.Rao LG, Krishnadev N, Banasikowska K, Rao AV. Lycopene I–effect on osteoclasts: lycopene inhibits basal and parathyroid hormone-stimulated osteoclast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures. J Med Food 2003;6:69–78 [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Uchiyama S. Effect of carotenoid on calcium content and alkaline phosphatase activity in rat femoral tissues in vitro: The unique anabolic effect of b-cryptoxanthin. Biol Pharm Bull 2003;26:1188–91 [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama S, Sumida T, Yamaguchi M. Oral administration of beta-cryptoxanthin induces anabolic effects on bone components in the femoral tissues of rats in vivo. Biol Pharm Bull 2004;27:232–5 [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Igarashi A, Uchiyama S, Morita S, Sugawara K, Sumida T. Prolonged intake of juice (Citrus unshiu) reinforced with β-cryptoxanthin has an effect on circulating bone biochemical markers in normal individuals. J Health Sci 2004;50:619–24 [Google Scholar]
- 19.Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women's Health Initiative. Am J Clin Nutr 2005;82:581–8 [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 1995;87:1767–76 [DOI] [PubMed] [Google Scholar]
- 21.Franceschi S, Bidoli E, La Vecchia C, Talamini R, D'Avanzo B, Negri E. Tomatoes and risk of digestive-tract cancers. Int J Cancer 1994;59:181–4 [DOI] [PubMed] [Google Scholar]
- 22.Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res 1999;14:129–35 [DOI] [PubMed] [Google Scholar]
- 23.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health 1951;41:279–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: results from the Framingham Osteoporosis Study. J Bone Miner Res 1992;7:547–53 [DOI] [PubMed] [Google Scholar]
- 25.Kiel DP, Mercier CA, Dawson-Hughes B, Cali C, Hannan MT, Anderson JJ. The effects of analytic software and scan analysis technique on the comparison of dual X-ray absorptiometry with dual photon absorptiometry of the hip in the elderly. J Bone Miner Res 1995;10:1130–6 [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26 (discussion 1127–36) [DOI] [PubMed] [Google Scholar]
- 27.Tucker KL, Chen H, Vogel S, Wilson PW, Schaefer EJ, Lammi-Keefe CJ. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J Nutr 1999;129:438–45 [DOI] [PubMed] [Google Scholar]
- 28.US Department of Agriculture, Agricultural Research Service USDA National Nutrient Database for Standard Reference, release 20. Nutrient Data Laboratory homepage. 2007. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl (cited16 January 2008)
- 29.US Department of Health and Human Services, US Department of Agriculture. Dietary guidelines for Americans 2005. 6th ed Washington, DC: US Government Printing Office, 2005 [Google Scholar]
- 30.Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:710–20 [DOI] [PubMed] [Google Scholar]
- 31.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt) 2006;15:1028–34 [DOI] [PubMed] [Google Scholar]
- 32.Kiel DP, Zhang Y, Hannan MT, Anderson JJ, Baron JA, Felson DT. The effect of smoking at different life stages on bone mineral density in elderly men and women. Osteoporos Int 1996;6:240–8 [DOI] [PubMed] [Google Scholar]
- 33.Kiel DP, Felson DT, Hannan MT, Anderson JJ, Wilson PW. Caffeine and the risk of hip fracture: the Framingham Study. Am J Epidemiol 1990;132:675–84 [DOI] [PubMed] [Google Scholar]
- 34.Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol 1995;142:485–92 [DOI] [PubMed] [Google Scholar]
- 35.Bakhireva LN, Barrett-Connor E, Kritz-Silverstein D, Morton DJ. Modifiable predictors of bone loss in older men: a prospective study. Am J Prev Med 2004;26:436–42 [DOI] [PubMed] [Google Scholar]
- 36.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670–6 [DOI] [PubMed] [Google Scholar]
- 37.Michels KB, Greenland S, Rosner BA. Does body mass index adequately capture the relation of body composition and body size to health outcomes? Am J Epidemiol 1998;147:167–72 [DOI] [PubMed] [Google Scholar]
- 38.Morin S, Tsang JF, Leslie WD. Weight and body mass index predict bone mineral density and fractures in women aged 40 to 59 years. Osteoporos Int (Epub ahead of print 17 July 2008) [DOI] [PubMed] [Google Scholar]
- 39.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 1991;115:505–12 [DOI] [PubMed] [Google Scholar]
- 40.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 1999;69:727–36 [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol 2006;163:9–17 [DOI] [PubMed] [Google Scholar]
- 42.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S (discussion 1229S–31S) [DOI] [PubMed] [Google Scholar]
- 43.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr 2004;79:155–65 [DOI] [PubMed] [Google Scholar]
- 44.New SA, Bolton-Smith C, Grubb DA, Reid DM. Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr 1997;65:1831–9 [DOI] [PubMed] [Google Scholar]
- 45.Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health (Larchmt) 2006;15:295–300 [DOI] [PubMed] [Google Scholar]
- 46.Maggio D, Polidori MC, Barabani M, et al. Low levels of carotenoids and retinol in involutional osteoporosis. Bone 2006;38:244–8 [DOI] [PubMed] [Google Scholar]
- 47.Wattanapenpaiboon N, Lukito W, Wahlqvist ML, Strauss BJ. Dietary carotenoid intake as a predictor of bone mineral density. Asia Pac J Clin Nutr 2003;12:467–73 [PubMed] [Google Scholar]
- 48.Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Yano M. Bone mineral density in post-menopausal female subjects is associated with serum antioxidant carotenoids. Osteoporos Int 2008;19:211–9 [DOI] [PubMed] [Google Scholar]
- 49.Uchiyama S, Sumida T, Yamaguchi M. Anabolic effect of β-cryptoxanthin on bone components in the femoral tissue of aged rats in vivo and in vitro. J Health Sci 2004;50:491–6 [DOI] [PubMed] [Google Scholar]
- 50.Hininger IA, Meyer-Wenger A, Moser U, et al. No significant effects of lutein, lycopene or beta-carotene supplementation on biological markers of oxidative stress and LDL oxidizability in healthy adult subjects. J Am Coll Nutr 2001;20:232–8 [DOI] [PubMed] [Google Scholar]
- 51.Hozawa A, Jacobs DR, Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem 2007;53:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker ME, McCloskey E, Saha S, et al. Serum retinoids and beta-carotene as predictors of hip and other fractures in elderly women. J Bone Miner Res 2005;20:913–20 [DOI] [PubMed] [Google Scholar]
- 53.Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med 2003;348:287–94 [DOI] [PubMed] [Google Scholar]
- 54.Rejnmark L, Vestergaard P, Charles P, et al. No effect of vitamin A intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int 2004;15:872–80 [DOI] [PubMed] [Google Scholar]
- 55.Chasan-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr 1999;70:509–16 [DOI] [PubMed] [Google Scholar]
- 56.Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst 1999;91:547–56 [DOI] [PubMed] [Google Scholar]
- 57.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucker KL, Hannan MT, Qiao N, et al. Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis Study. J Bone Miner Res 2005;20:152–8 [DOI] [PubMed] [Google Scholar]
- 59.Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. Am J Clin Nutr 2006;84:936–42 [DOI] [PubMed] [Google Scholar]
- 60.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 1991;10:2247–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J 1996;10:709–20 [DOI] [PubMed] [Google Scholar]
- 62.Stahl W, Ale-Agha N, Polidori MC. Non-antioxidant properties of carotenoids. Biol Chem 2002;383:553–8 [DOI] [PubMed] [Google Scholar]
- 63.Mehta PP, Hotz-Wagenblatt A, Rose B, Shalloway D, Loewenstein WR. Incorporation of the gene for a cell-cell channel protein into transformed cells leads to normalization of growth. J Membr Biol 1991;124:207–25 [DOI] [PubMed] [Google Scholar]
- 64.Amir H, Karas M, Giat J, et al. Lycopene and 1,25-dihydroxyvitamin D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutr Cancer 1999;33:105–12 [DOI] [PubMed] [Google Scholar]
- 65.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Retinol intake and bone mineral density in the elderly: the Rancho Bernardo Study. J Bone Miner Res 2002;17:1349–58 [DOI] [PubMed] [Google Scholar]
- 66.Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 1995;62:604–10 [DOI] [PubMed] [Google Scholar]
- 67.Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 2007;137:2346–50 [DOI] [PubMed] [Google Scholar]