Abstract
Background: Capsinoids from the Capsicum genus of plants are nonpungent capsaicin-related substances with effects on metabolism and body weight in animals.
Objectives: Our objectives were to explore the safety and efficacy of capsinoids taken orally (6 mg/d) for weight loss, fat loss, and change in metabolism and to examine whether candidate genes are predictors of capsinoid response.
Design: This was a 12-wk, placebo-controlled, double-blind, randomized study. Eligibility criteria included a body mass index (BMI; in kg/m2) of 25–35. Body weight was measured, and dual-energy X-ray absorptiometry, indirect calorimetry (men only), and genotyping were conducted.
Results: Forty women and 40 men with a mean (± SD) age of 42 ± 8 y and BMI of 30.4 ± 2.4 were randomly assigned to a capsinoid or placebo group. Capsinoids were well tolerated. Mean (± SD) weight change was 0.9 ± 3.1 and 0.5 ± 2.4 kg in the capsinoid and placebo groups, respectively (P = 0.86). There was no significant group difference in total change in adiposity, but abdominal adiposity decreased more (P = 0.049) in the capsinoid group (−1.11 ± 1.83%) than in the placebo group (−0.18 ± 1.94%), and this change correlated with the change in body weight (r = 0.46, P < 0.0001). Changes in resting energy expenditure did not differ significantly between groups, but fat oxidation was higher at the end of the study in the capsinoid group (least-squares mean difference: 21.0 mg/min; P = 0.06). Of 13 genetic variants tested, TRPV1 Val585Ile and UCP2 −866 G/A correlated significantly with change in abdominal adiposity.
Conclusions: Treatment with 6 mg/d capsinoids orally appeared to be safe and was associated with abdominal fat loss. Capsinoid ingestion was associated with an increase in fat oxidation that was nearly significant. We identified 2 common genetic variants that may be predictors of therapeutic response.
INTRODUCTION
Capsinoids are nonpungent capsaicin-related substances found in all tested variants of the Capsicum genus of plants (1–3). The 3 capsinoids capsiate, dihydrocapsiate, and nordihydrocapsiate are structurally identical to the pungent Capsicum constituents capsaicin, dihydrocapsaicin, and nordihydrocapsaicin, respectively, except that their 2 moieties are connected by an ester bond rather than an amide bond. Although capsaicin is perceived as “hot” in the oral cavity because it activates TRPV1 receptors (transient receptor potential cation channel, subfamily V, member 1) located in neurons on the tongue (4), the capsinoids lack this sensory quality because they are hydrolyzed as they cross the oral mucosa. Nevertheless, orally administered capsiate replicates in an equipotent fashion the thermogenic effect of capsaicin in animals, which involves activation of TRPV1 receptors on vagal afferents in the gut (5, 6) and results in increased sympathetic efferent activity (7) and up-regulation of uncoupling proteins (8). Moreover, capsinoids suppress body fat accumulation in mice (9). Some of the findings from animal studies have been reproduced in small, short-term human studies (10, 11).
The primary aim of the present investigation was to explore the safety and efficacy of capsinoids for loss of body weight and body fatness in 80 overweight or obese humans, who, in addition to lifestyle recommendations, were randomly assigned in a double-blind fashion to receive either capsinoids (6 mg/d orally) or placebo for 12 wk. To obtain mechanistic insight, we assessed accompanying changes in resting metabolism (in men only) and examined candidate genes for response.
SUBJECTS AND METHODS
Subjects
The study was carried out in northern New Jersey. Enrollment was open to a total of 80 participants of either sex who wanted to lose weight, who were between 30 and 60 y of age, and whose body mass index (BMI; in kg/m2) was between 25 and 35. Key exclusion criteria were diabetes mellitus, eating disorders (eg, anorexia or bulimia), recent significant weight gain or loss, recent use of prescription or nonprescription weight-loss or appetite-altering medications or supplements, history of gastric bypass surgery, or any other serious or chronic medical or surgical condition that might confer an increased risk of harm from participation or confound the study results.
The study was approved by the Essex Institutional Review Board (Lebanon, NJ) and conformed to the principles in the Helsinki Declaration and applicable legislation regarding research involving human subjects. All subjects gave written informed consent. Enrollment took place from 13 April through 17 July 2006.
Study visits
The study included 5 scheduled visits over 13 wk (day −7 to 84). Screening was conducted at visit 1 (day −7). Randomization and the baseline measurements were conducted at visit 2 (day 0). Efficacy was assessed 4, 8, and 12 wk after randomization at visit 3 (day 28), visit 4 (day 56), and visit 5 (day 84), respectively.
Screening and randomization
At visit 1, after providing informed consent, the subjects provided their medical history and underwent a physical examination that included a 12-lead electrocardiogram. At this and at each subsequent visit, vital signs and anthropomorphic characteristics were measured as follows. Heart rate, blood pressure, and temperature were measured while the subjects were in a seated position after a 15-min rest with the use of an automated vital signs monitor (Dinamap; Tampa, FL). Body weight was obtained to the nearest 0.1 kg with a calibrated digital scale (Seca North America, Hanover, MD) while the subjects were wearing underwear and a hospital gown and no shoes. Waist circumference was measured to the nearest 0.1 cm (in triplicate and averaged) with the use of the 2-person observer-recorder procedure and the anatomic landmarks used in the National Health and Nutrition Examination Survey (12). At each visit, fasting blood and urine samples were collected for analysis at a clinical laboratory (Quest Diagnostics, Madison, NJ). At visit 2, individuals who qualified for study participation were assigned the next available study identifier number. In a double-blind, randomized fashion, each number corresponded to a 12-wk supply of prepackaged vials containing either capsinoids or placebo.
Test substance
The capsinoid intervention consisted of an extract from the Capsicum anuum L. [Solanacae (pepper fruit)] variety CH-19 Sweet. Capsinoid oil was extracted as follows: dried fruit was treated with hexane, and fruit sediment was removed by filtration, followed by evaporation and distillation with medium-chain triglycerides and column chromatography to yield purified capsinoids, which consisted of capsiate, dihydrocapsiate, and nordihydrocapsiate in a 63:27:10 ratio by the HPLC method; this ratio is in close agreement with the ratio previously reported (13). The purified capsinoids were dissolved in rapeseed oil and encapsulated in vegetarian softgel capsules made of modified cornstarch, vegetable glycerin, and carageenan; each capsule contained 1 mg capsinoids and 199 mg of the oil mixed with rapeseed oil and medium-chain triglycerides. To mask the color of the capsinoids, both placebo and active softgels contained 0.015% marigold oil (0.03 mg/capsule), a commonly used yellow food coloring. A total of 6 capsules were ingested with ≈240 mL water each day: 3 within 30 min before the morning meal and 3 within 30 min before the evening meal. This dosage regimen was based on the previous observation that a single dose (0.1 g · kg body wt−1 · d−1) of CH-19 sweet peppers (containing 0.3–1.0 mg capsinoids/1 g peppers) significantly increased 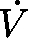 O2 in humans (10) and that chronic administration of capsules containing 3 or 10 mg/d capsinoids increased
O2 in humans (10) and that chronic administration of capsules containing 3 or 10 mg/d capsinoids increased 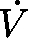 O2 in humans (13). A sufficient supply of the assigned study product to last until the next visit was dispensed at visit 2 and subsequent visits along with instructions for product use and product-use diaries. Compliance with the study products was determined by capsule count and product-use diary review. All capsules were manufactured in one batch. Preceding stability tests had determined that the product would be stable beyond the duration of the trial.
O2 in humans (13). A sufficient supply of the assigned study product to last until the next visit was dispensed at visit 2 and subsequent visits along with instructions for product use and product-use diaries. Compliance with the study products was determined by capsule count and product-use diary review. All capsules were manufactured in one batch. Preceding stability tests had determined that the product would be stable beyond the duration of the trial.
Dietary advice and physical activity
At visit 2, subjects met individually for 1 h with a dietitian, who obtained a dietary history and instructed subjects on how to modify their diet to induce a mild energy deficit of 300–600 kcal/d, which was to be maintained for the duration of the study. The energy content of this diet was calculated on the basis of work published by the Food and Agriculture Organization of the United Nations (14) as estimated basal metabolic rate multiplied by an activity factor of 1.3 (sedentary or light-activity lifestyle) or 1.5 (active or moderately active lifestyle), respectively. Because self-monitoring is associated with greater success at weight loss (15), the subjects were given food and activity diaries to complete daily. At each visit, the subjects were reminded by staff to continue the energy-reduced diet.
Body composition
Body-composition measurements were obtained by dual-energy X-ray absorptiometry (DXA) with the use of a Hologic QDR 4500 W scanner with Windows XP–based software (Hologic Inc, Bedford, MA) at visit 2 (baseline) and visit 5 (final visit). Body fat was quantified as overall and abdominal. The quantification of abdominal fat was done by using the region-of-interest function in the Hologic software to define a quadrilateral box in which the upper and lower bounds coincided with the L1/L2 and L4/L5 intervertebral spaces, respectively. The lateral bounds were located in the free air between the trunk and the arms (16). Abdominal region-of-interest analysis of DXA scans was validated in comparison with computed tomography as a measure of total abdominal fat (17) and in comparison with magnetic resonance imaging as a measure of intraabdominal fat (16, 18).
Indirect calorimetric measurement
Resting metabolic rate and substrate oxidation were measured at each visit in men. Women did not participate in this part of the investigation because the timing of the menstrual cycle would make interpretation difficult. A Vmax29 (Sensormedics Inc, Yorba Linda, CA) metabolic cart was used in ventilated-hood mode. At visit 1, subjects were tested to familiarize themselves with the equipment; this was done to help eliminate the effects of first exposure on the measurements on visit 2, which constituted the baseline. Subjects were tested in the supine position. The test was conducted until the cart software indicated steady state or that 25 min had elapsed, whichever occurred first. For visits 2 through 5, men were admitted on the day before the visit, and the metabolic measurements were performed after the subjects awoke after a mandatory bathroom visit. The morning dose of the test substance was withheld until after the indirect calorimetry study to exclude acute effects of capsinoids. Results from the metabolic cart test were captured in the form of mean resting energy expenditure (REE), respiratory quotient, 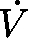 O2, and
O2, and 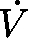 CO2 for the last 7 min of testing. Fat oxidation (in g/min) was calculated as 1.689 [
CO2 for the last 7 min of testing. Fat oxidation (in g/min) was calculated as 1.689 [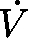 O2 (L/min) –
O2 (L/min) – 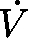 CO2 (L/min)] (19).
CO2 (L/min)] (19).
Genotyping
To identify genetic variants that could intensify or attenuate the response, we assessed the relation between response and candidate genotypes in subjects who had been assigned to the capsinoid group. The following 13 genetic polymorphisms were selected from the National Center for Biotechnology Information database on the basis of biological plausibility and an anticipated minor allele frequency of >10% in the studied population: TRPV1 [transient receptor potential cation channel, subfamily V, member 1; also known as vanilloid receptor 1 (VR1) or capsaicin receptor] Val585Ile, Met315Ile, Thr469Ile; PPARG (peroxisome proliferator-activated receptor-γ) Pro12Ala; UCP1 (uncoupling protein 1) −3826 A/G, Leu229Met; UCP2 (uncoupling protein 2) −866 G/A, UCP3 (uncoupling protein 3) −55 C/T, ADRB1 (β1-adrenergic receptor) Arg389Gly; ADRB2 (β2-adrenergic receptor) Arg16Gly, Gln27Glu; ADRB3 (β3-adrenergic receptor) Trp64Arg; and ADRA2B (α2b-adrenergic receptor) Glu9/Glu12 insertion/deletion. The 5′ nuclease-based assay (TaqMan; Applied Biosystems, Foster City, CA) was used to genotype single nucleotide polymorphisms, and pyrosequencing was used for the insertion-deletion variant.
Safety assessment
All subjects who received at least one dose of the study agent were included in the safety analysis (safety population). Study personnel evaluated the laboratory test results and classified adverse events by type, incidence, severity, timing, seriousness, and relatedness to study agent. After the study, adverse events were tabulated and summarized by an external reviewer.
Efficacy assessment
The primary prespecified endpoints were change from baseline in body weight and body composition at the end of the study (12 wk). To obtain mechanistic insight, secondary prespecified endpoints were change from baseline in REE and substrate oxidation.
Statistical analysis
Differences in proportions were analyzed by a chi-square test. The efficacy analysis was conducted as an analysis of covariance, which was adjusted for the baseline value of the dependent variable, and group differences were expressed as least-squares means. Pearson's correlation coefficients were used to analyze relations between continuous traits. SAS software version 9 (SAS Institute, Cary, NC) was used for the analysis. Because postbaseline DXA measures were available only for the population that completed the 12-wk assessment (study end), all nonsafety outcome analyses were performed on this population. For the genetic analysis, primary analysis was conducted with the use of SAS PROC GLM (SAS Institute), with the allele effects modified in an additive fashion. Thus, the number of tested genetic hypotheses was 13 (the number of tested polymorphisms). Polymorphisms yielding a P value <0.05 under the additive model were further explored in dominant and recessive models.
RESULTS
Eighty of 213 subjects screened were randomly assigned to the study groups. The subjects' disposition is shown in Table 1. The number of discontinued subjects did not differ significantly between the groups as determined by a chi-square test (P = 0.12). Of the subjects randomly assigned to capsinoids who did not complete the study, one was withdrawn because of erroneous enrollment despite a below-normal plasma concentration of thyroid-stimulating hormone. The remainder discontinued participation for reasons that neither involved an adverse event nor were considered to be related to the test substance.
TABLE 1.
Subject disposition
| Capsinoids | Placebo | Total | |
| n | |||
| Study group (randomized) | 41 | 39 | 80 |
| Dosed (safety population)1 | 37 | 38 | 75 |
| Completed assessments at 4 wk | 33 | 38 | 71 |
| Completed assessments at 8 wk | 31 | 37 | 68 |
| Completed the study (12 wk) | 31 | 36 | 67 |
Dosed subjects are those subjects who received at least one dose of study drug.
The baseline characteristics of the safety population are shown in Table 2. By self-identification, 44% of participants were Hispanic; 41% were white, not Hispanic; 13% were black, not Hispanic; and 2% were in other categories.
TABLE 2.
Baseline characteristics of all dosed subjects1
| Capsinoids (n = 37) | Placebo (n = 38) | |
| Age (y) | 43 ± 82 | 41 ± 8 |
| Females (%) | 51.4 | 52.6 |
| Body weight (kg) | 88.9 ± 13.3 | 84.7 ± 12.5 |
| BMI (kg/m2) | 30.6 ± 2.4 | 30.3 ± 2.4 |
| Waist girth (cm) | 104 ± 7 | 101 ± 8 |
| Total fat (%) | 37.1 ± 7.4 | 36.8 ± 7.4 |
| Abdominal fat (%) | 41.9 ± 6.3 | 39.6 ± 6.7 |
| REE (kcal/d) | 1811 ± 216 | 1753 ± 172 |
| Fat oxidation (mg/min) | 59 ± 40 | 53 ± 24 |
Dosed subjects are those subjects who received at least one dose of study drug. REE, resting energy expenditure. No significant group differences were observed (t test).
Mean ± SD (all such values).
There were 17 treatment-emergent adverse events in the capsinoid group and 26 events in the placebo group; none of the events was considered serious, and none led to withdrawal. The higher number of events in the placebo group was significantly different from the null hypothesis of a proportionate number of events in the 2 groups as determined by a chi-square test (P = 0.0001). Of the 43 treatment-emergent adverse events, 14 were considered possibly related to the study substance. After unblinding, organ systems were identified in which adverse events were more prominent among the capsinoid group. In the gastrointestinal system, there were 2 cases of dyspepsia, 1 case of bowel irregularities, and 1 case of diarrhea compared with none in the placebo group. In addition, there was one case of skin rash in the capsinoid group compared with none in the placebo group. Mean values of systolic and diastolic blood pressure, heart rate, and body temperature did not differ significantly between visits or between capsinoid and placebo groups.
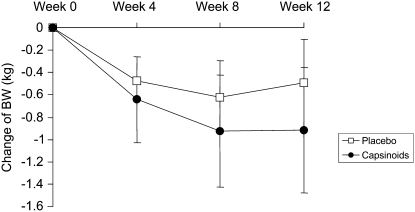
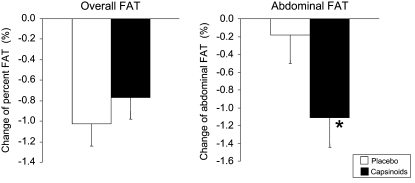
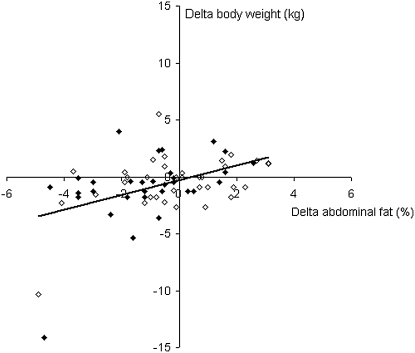
Changes in body weight are shown in Figure 1. Mean (± SD) weight change at 12 wk was –0.92 ± 3.12 kg in the capsinoid group and –0.49 ± 2.37 kg in the placebo group (P = 0.86 for group difference). Results of the body-composition measures by DXA, which were performed twice, at baseline and after 12 wk, are shown in Figure 2. No difference in overall percentage body fat was observed between the placebo and capsinoid groups (P = 0.67), but abdominal adiposity decreased to a greater extent in the capsinoid group than in the placebo group (–1.11± 1.83% compared with –0.18 ± 1.94%; least-squares mean difference: –0.95%; 95% CI: –1.89, –0.0007; P = 0.049). To explore whether the statistically significant reduction in abdominal fat might result from random measurement error, we examined the correlation between change in abdominal fat by DXA and change in overall fat by weighing. A correlation between the 2 measurements, obtained by entirely different methods, would corroborate the DXA finding. Indeed, the 2 measurements were correlated (r = 0.46, P < 0.0001) (Figure 3). The mean change in waist girth was –3.0 ± 4.6 and –2.1 ± 3.9 cm in the capsinoid and placebo groups, respectively (least-squares mean difference: –0.4 cm; 95% CI: –2.4, 1.6; P = 0.68). The change in lean tissue mass by DXA did not differ significantly between the capsinoid and placebo groups (–156 ± 257 compared with –41 ± 239 g; least-squares mean difference: –115 g; 95% CI: –818, 588; P = 0.74).
FIGURE 1.
Mean (± SE) changes in body weight (BW) from baseline. n = 37 (capsinoids week 0), 33 (capsinoids week 4), 31 (capsinoids weeks 8 and 12), 38 (placebo weeks 0 and 4), 37 (placebo week 8), and 36 (placebo week 12). P = 0.86 for group difference at 12 wk by ANCOVA, adjusted for baseline.
FIGURE 2.
Mean (± SE) changes in overall fat and abdominal fat from baseline in the placebo (n = 36) and capsinoid (n = 31) groups. P = 0.67 (overall fat) and *P = 0.049 (abdominal fat) for group difference by ANCOVA, adjusted for baseline.
FIGURE 3.
Correlation between change in body weight determined by weighing and abdominal fatness determined by dual-energy X-ray absorptiometry in the capsinoid group (solid diamonds; n = 31) and the placebo group (open diamonds; n = 36); r = 0.46, P < 0.0001. The correlation was robust to the removal of the 2 subjects who lost >10 kg body weight (r = 0.29, P = 0.02).
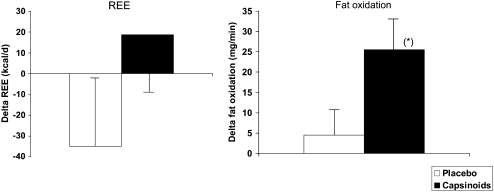
REE, which was measured in men only (Figure 4), trended toward an increase in the capsinoid group and a decrease in the placebo group, such that at the end of the trial it was 54 kcal/d higher in the capsinoid group than in the placebo group (least-squares mean difference: 95% CI: −29, 136; P = 0.19). Fat oxidation (Figure 4) was higher in the capsinoid group than in the placebo group (least-squares mean difference: 21.0 mg/min; 95% CI: –1.1, 43.1; P = 0.06). Changes in plasma lipids (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) did not differ significantly between the 2 groups.
FIGURE 4.
Least-squares mean (± SE) changes in resting energy expenditure (REE) and in fat oxidation from baseline in the placebo (n = 16) and capsinoid (n =15) groups. P = 0.19 (REE) and *P = 0.06 (fat oxidation) for group difference by ANCOVA, adjusted for baseline.
Of the 13 genetic variants, 2 correlated significantly with the change in abdominal adiposity under an additive model, namely TRPV1 Val585Ile and UCP2 –866 G/A (Table 3). Further exploration of the recessive and dominant models for these variants showed that the effects were also significant for TRPV1 Val585Ile when all Val/− carriers were collapsed and for UCP2 –866 G/A when all –866 G/− carriers were collapsed (data not shown).
TABLE 3.
Change in abdominal fat as a function of the TRPV1 Val585Ile and UCP2 −866 G/A variants1
| Variant | ΔAbdominal fat |
| % | |
| TRPV1 5852 | |
| Ile/Ile (n = 14) | 0.12 ± 1.2 |
| Val/Ile (n = 13) | −2.03 ± 1.82 |
| Val/Val (n = 2) | −2.60 ± 1.27 |
| UCP2 −8663 | |
| G/G (n = 4) | 0.30 ± 1.28 |
| G/A (n = 13) | −0.65 ± 2.00 |
| A/A (n = 12) | −1.88 ± 1.53 |
All values are means ± SDs (all such values).
Genotype effects under additive model, P = 0.0008.
Genotype effects under additive model, P = 0.02.
DISCUSSION
In this small double-blind, randomized, placebo-controlled trial, we found that treatment of overweight or obese humans with 6 mg/d capsinoids orally safely promoted loss of abdominal fat. In addition, we identified genetic polymorphisms associated with the response.
With regard to safety, none of the adverse events was considered serious and none led to withdrawal. Likewise, no laboratory or electrocardiogram findings caused withdrawal. After unblinding, it was shown that most of the treatment-emergent adverse events occurred in the placebo group. In contrast, gastrointestinal events were observed exclusively in the capsinoid group in which 4 mild and diverse events were observed. On the basis of the indications of the available safety data, it appears that the administration of 6 mg/d capsinoids to humans is safe.
The efficacy analysis yielded some interesting findings. The point estimates for body weight change in the capsinoid and placebo groups were –0.9 and –0.5 kg, respectively. These differences were not statistically significant and within the range of what might be explained by unobserved group differences in adherence to the lifestyle recommendations. However, the DXA measurements of abdominal fat showed a statistically significant change of –1.1 percentage points in the capsinoid group compared with –0.2 percentage points in the placebo group. Moreover, the change in body weight and the change in abdominal adiposity (as measured by independent methods) were correlated. In combination, these findings suggest that the point estimate for body weight change is accurate despite its lack of statistical significance. Thus, when planning a study of longer duration, one could use the point estimate in the capsinoid group to set expectations for the rate of weight loss. If the point estimate (–0.9 kg in 12 wk) is upheld, a medically significant weight loss would be achievable in 1–2 y or, as discussed below, possibly sooner in individuals with certain genotypes. However, even on its own, the statistically significant reduction in abdominal fat observed is encouraging because of its role in metabolic pathophysiology (20). Although we did not find an improvement in plasma lipids in the present study, we note that administration of capsinoids to hyperlipidemic rats resulted in a lowering of total cholesterol and triglycerides as well as a reduction of liver fat mass (21). The point estimate for lean tissue loss in the capsinoid group was 156 g, which was modest compared with a total body weight loss of 0.92 kg. This finding suggests the possibility that the lean-tissue-sparing effect of capsinoids observed in animals (5) may apply to humans.
In the indirect calorimetry studies, we observed a downward trend of REE in the placebo group, consistent with what one might expect secondary to dieting (22), whereas the upward trend in the capsinoid group was consistent with the effect of capsinoids in animals (5). Although not statistically significant, the point estimate of the difference at 12 wk, 54 kcal/d, should be seen in the context of calculations that show that the general increase in body weight observed in the United States over the last decade can be explained by an imbalance of as little as just 50 kcal/d (23). With borderline significance (P = 0.06), fat oxidation increased to a greater extent in the capsinoid group, which was analogous to studies of capsinoids in animals (5, 24) and of capsaicin, the pungent relative of capsinoids, in humans (25).
The genetic studies showed associations between 2 variants and the change in abdominal adiposity among the capsinoid-treated subjects. The variant with the most pronounced effect was TRPV1 Val585Ile, ie, a valine to leucine substitution in the TRPV1 receptor responsible for the activation of vagal afferents by capsinoids. Subjects with the Val/Val and Val/Ile variants lost about twice as much abdominal fat as the study average, whereas Ile/Ile subjects lost almost none. The TRPV1 Val585Ile polymorphism was first reported by Hayes et al (26) and has been associated with functional differences (27). Moreover, in cross-species comparisons, the inherent insensitivity to the pungency of capsaicin among birds and rabbits has been attributed to variation in areas of TRPV1 in some proximity to residue 585 (28, 29). The other variant associated with abdominal fat loss was −866 G/A in the promoter region of UCP2. Up-regulation of UCP2 appears to be a mediator of the effects of capsinoids on metabolism (5), and the −866 G/A variant has previously been associated with both obesity (30) and lipid oxidation (31). If the importance of these variants is confirmed, one could imagine the use of genotyping tests to identify those most likely to benefit from capsinoid administration.
Limitations of the study include its short duration (although it was longer than previous studies of the same substance) and the fact that the sample size was reduced by one-half for the metabolic studies because women did not participate in this part of the study.
In conclusion, treatment with 6 mg/d capsinoids orally appears to be safe and well tolerated and is associated with loss of abdominal fat. Capsinoid ingestion was associated with an increase in fat oxidation that was nearly significant. We identified 2 common single nucleotide polymorphisms that may be predictors of the therapeutic response. Future research directions may include studies of longer duration to examine the potential of capsinoids to control body weight with a continued emphasis on genetic determinants of response.
Acknowledgments
We thank the study participants and the clinical staff. Christopher Lee of Ajinomoto provided valuable input to the design of the protocol and provided medical supervision of the trial. Tatsuyuki Kakuma of the Kurume University School of Medicine, Japan, provided statistical advice. Keith Tanner of the University of Maryland School of Medicine provided technical assistance. Coleen Damcott of the University of Maryland School of Medicine helped select candidate single nucleotide polymorphisms.
The authors' responsibilities were as follows—SS, Y Fujishima, XP-S, and MT: design of the study; SS, Y Fujishima, and SO: collection of data; SS, H Shen, Y Furuhata, H Sato, and MT: analysis of data; and SS, Y Fujishima, XP-S, and MT: writing of the manuscript. H Shen, SO, and XP-S had no conflicts to report. Y Fujishima, Y Furuhata, H Sato, and MT are employees of Ajinomoto. SS receives research support from Ajinomoto.
REFERENCES
- 1.Yazawa S, Suetome N, Okamoto K, Namiki T. Content of capsaicinoids and capsaicinoid-like substances in fruit of pepper (Capsicum annuum L.) hybrids made with “CH-19 Sweet” as a parent. J Jpn Soc Hort Sci 1989;58:601–7 [Google Scholar]
- 2.Kobata K, Todo T, Yazawa S, Iwai K, Watanabe T. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, form the fruits of a nonpungent cultivar, CH-19 Sweet, of pepper (Capsicum annuum L.). J Agric Food Chem 1998;46:1695–7 [Google Scholar]
- 3.Yazawa S, Yoneda H, Hosokawa M, Fushiki T, Watanabe T. Novel capsaicinoid like substances in the fruits of the non-pungent cultivar “CH-19 Sweet” of pepper (Capsicum annuum). Capsicum and Eggplant Newsletter 2004;23:17–20 [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24 [DOI] [PubMed] [Google Scholar]
- 5.Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Biochem 2001;65:2735–40 [DOI] [PubMed] [Google Scholar]
- 6.Iida T, Moriyama T, Kobata K, et al. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003;44:958–67 [DOI] [PubMed] [Google Scholar]
- 7.Iwai K, Yazawa A, Watanabe T. Roles as metabolic regulators of the non-nutrients, a non-pungent capsaicin-like compound, capsiate. Proc Jpn Acad Ser B Phys Biol Sci 2003;79:207–12 [Google Scholar]
- 8.Masuda Y, Haramizu S, Oki K, et al. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol 2003;95:2408–15 [DOI] [PubMed] [Google Scholar]
- 9.Ohnluki K, Haramizu S, Watanabe T, Yazawa S, Fushiki T. CH-19 Sweet, nonpungent cultivar of red pepper, increased body temperature in mice with vanilloid receptor stimulation by capsiate. J Nutr Sci Vitaminol (Tokyo) 2001;47:295–8 [DOI] [PubMed] [Google Scholar]
- 10.Ohnuki K, Niwa S, Maeda S, Inoue N, Yazawa S, Fushiki T. CH-19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Biosci Biotechnol Biochem 2001;65:2033–6 [DOI] [PubMed] [Google Scholar]
- 11.Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH-19 sweet, a non-pungent cultivar of red pepper, in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem 2006;70:2824–35 [DOI] [PubMed] [Google Scholar]
- 12.Westat Inc. National Health and Nutrition Survey III: body measurements (anthropometry). Version current October 1988. Available from: www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf (cited 16 September 2008)
- 13.Inoue N, Matsunaga Y, Satoh H, Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci Biotechnol Biochem 2007;71:380–9 [DOI] [PubMed] [Google Scholar]
- 14.Human energy requirements: report of a joint FAO/WHO/UNU Expert Consultation. United Nations University World Health Organization Food and Agriculture Organization of the United Nations. Rome, 17–24 October 2001. Rome, Italy, 2004 [Google Scholar]
- 15.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res 2004;12:1426–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord 2002;26:978–83 [DOI] [PubMed] [Google Scholar]
- 17.Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Appl Physiol 2004;97:509–14 [DOI] [PubMed] [Google Scholar]
- 18.Kamel EG, McNeill G, Van Wijk MC. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res 2000;8:36–42 [DOI] [PubMed] [Google Scholar]
- 19.Consolazio CF, Johnson RE, Pecora LJ. Physiological measurements of metabolic functions in man. New York, NY: McGraw-Hill, 1963:316 [Google Scholar]
- 20.Tracy RP. Is visceral adiposity the “enemy within”? Arterioscler Thromb Vasc Biol 2001;21:881–3 [DOI] [PubMed] [Google Scholar]
- 21.Tani Y, Fujioka T, Sumioka M, Furuichi Y, Hamada H, Watanabe T. Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J Nutr Sci Vitaminol (Tokyo) 2004;50:351–5 [DOI] [PubMed] [Google Scholar]
- 22.Ravussin E, Burnand B, Schutz Y, Jéquier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr 1985;41:753–9 [DOI] [PubMed] [Google Scholar]
- 23.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5 [DOI] [PubMed] [Google Scholar]
- 24.Haramizu S, Mizunoya W, Masuda Y, et al. Capsiate, a nonpungent capsaicin analog, increases endurance swimming capacity of mice by stimulation of vanilloid receptors. Biosci Biotechnol Biochem 2006;70:774–81 [DOI] [PubMed] [Google Scholar]
- 25.Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr 2003;90:651–9 [DOI] [PubMed] [Google Scholar]
- 26.Hayes P, Meadows HJ, Gunthorpe MJ, et al. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain 2000;88:205–15 [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Neubert JK, San Miguel A, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 2004;109:488–96 [DOI] [PubMed] [Google Scholar]
- 28.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 2002;108:421–30 [DOI] [PubMed] [Google Scholar]
- 29.Gavva NR, Klionsky L, Qu Y, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem 2004;279:20283–95 [DOI] [PubMed] [Google Scholar]
- 30.Dalgaard LT, Andersen G, Larsen LH, et al. Mutational analysis of the UCP2 core promoter and relationships of variants with obesity. Obes Res 2003;11:1420–7 [DOI] [PubMed] [Google Scholar]
- 31.Le Fur S, Le Stunff C, Dos Santos C, Bougnères P. The common −866 G/A polymorphism in the promoter of uncoupling protein 2 is associated with increased carbohydrate and decreased lipid oxidation in juvenile obesity. Diabetes 2004;53:235–9 [DOI] [PubMed] [Google Scholar]