Abstract
One mechanism through which bioactive food components may exert anticancer effects is by reducing the expression of the proinflammatory gene cyclooxygenase-2 (COX-2), which has been regarded as a risk factor in tumor development. Rosmarinic acid (RA) is a phenolic derivative of caffeic acid present in rosemary (Rosmarinus officinalis). Previous research documented that RA may exert antiinflammatory effects. However, the mechanisms of action of RA on COX-2 expression have not been investigated. Here, we report that in colon cancer HT-29 cells, RA (5, 10, and 20 μmol/L) reduced the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced COX-2 promoter activity (P < 0.05) and protein levels (P < 0.05). In addition, the cotreatment with RA reduced (5 μmol/L, P < 0.05; 10 and 20 μmol/L, P < 0.01) TPA-induced transcription from a control activator protein-1 (AP-1) promoter-luciferase construct and repressed binding of the AP-1 factors c-Jun (10 μmol/L; P < 0.01) and c-Fos (10 μmol/L; P < 0.05) to COX-2 promoter oligonucleotides harboring a cAMP-response element (CRE). The anti-AP1 effects of RA were also examined in a nonmalignant breast epithelial cell line (MCF10A) in which RA antagonized the stimulatory effects of TPA on COX-2 protein expression (5 μmol/L, P < 0.05; 10 and 20 μmol/L, P < 0.01), the recruitment of c-Jun and c-Fos (10 μmol/L; P < 0.01) to the COX-2/CRE oligonucleotides, and activation of the extracellular signal-regulated protein kinase-1/2 (ERK1/2) (10 μmol/L; P < 0.01), a member of the mitogen-activated protein kinase pathway. Additionally, RA antagonized ERK1/2 activation in colon HT-29 and breast MCF-7 cancer cells (10 μmol/L; P < 0.01). Thus, we propose that RA may be an effective preventative agent against COX-2 activation by AP-1-inducing agents in both cancer and nonmalignant mammary epithelial cells.
Introduction
The cyclooxygenase (COX)3 enzyme system comprises the isoenzymes COX-1 and COX-2, which are responsible for the rate-limiting step in the conversion of arachidonic acid into prostaglandins (1). The COX-1 and COX-2 genes are constitutively expressed in many tissues. However, growth factors, tumor promoters, hormones, bacterial endotoxin, cytokines, and physiological stress have been shown to induce COX-2 gene expression (2), which has been recognized as a feature of epithelial tumors (3,4). The use of nonsteroidal antiinflammatory drugs (NSAID) that inhibit COX activity has been linked with a reduced incidence of colon (5) and breast cancers (6). In addition, selective COX-2 inhibitors have been shown to reduce tumor formation in animal models (7,8). However, the chronic use of NSAID has been associated with gastrointestinal ulceration and bleeding (9) and the long-term use of selective COX-2 inhibitors has been linked to increased risk of cardiovascular toxicity (10).
The expression of the human COX-2 gene is regulated at the transcriptional level through several cis-acting elements located within the proximal 5′-flanking region of the COX-2 gene (11). These elements include an E-box and activating transcription factor/cAMP response element (CRE) sequences, the nuclear factor/interluekin-6 CAAT enhancer binding sequence, and 2 nuclear factor κB binding sites (12). The CRE (5′-TGACGTCA-3′) is activated by hetero- and homodimers of the c-Fos, c-Jun, and ATF families of bZIP proteins [activator protein-1 (AP-1)], and the cAMP regulatory binding protein (13,14). The CRE sequence in the COX-2 promoter is homologous to the 12-O-tetradecanoylphorbol-13-acetate (TPA)-responsive element (TRE) (5′-TGA(C/G)TCA-3′) site located within the collagenase-1 promoter (15). A broad range of physiological and pathological stimuli such as cytokines, growth factors, stress, and oncogenic signals have been shown to regulate AP-1 activity (16). The tumor promoter and proinflammatory agent, TPA has been used as a prototype compound to induce binding of AP-1 proteins to the CRE and TRE elements (17).
In the recent past, there has been growing interest in the chemopreventative and chemotherapeutic ability of bioactive food components to prevent chronic diseases and cancer (18). A variety of dietary polyphenolic agents, such as resveratrol, genistein, curcumin, quercetin, and epigallocatechin gallate, have been shown to possess chemoprotective effects (19). Rosmarinic acid [(RA) α-o-caffeoyl-3, 4-dihydroxyphenyl lactic acid] is a polyphenolic compound found in Lamiaceae herbs such as Perilla frutescens, oregano, sage, mint, sweet basil, and perilla (20). RA has been observed to possess antioxidant and antiinflammatory properties (21–23). Several studies reported that RA exerted antiinflammatory effects by inhibiting complement activation (24) and COX (25) activity. However, the mechanisms underlying the RA-induced effects on COX-2 expression remain unknown. Therefore, in this study, we examined the effects of RA on COX-2 expression in colon and breast cancer cells and in nonmalignant mammary epithelial cells. We propose that at least in part, RA may exert antiinflammatory and anticarcinogenic effects by antagonizing AP-1–dependent activation of COX-2 gene expression.
Materials and Methods
Reagents and cell culture.
Colon HT-29 and breast MCF-7 cancer cells and nonmalignant breast epithelial MCF10A cells were obtained from the American Type Culture Collection. TPA, RA, and all other chemicals and cell culture media were from Sigma Chemical. All cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), penicillin (100 kU/L), and streptomycin (100 mg/L) at 37°C, 95% relative humidity, and 5% CO2. All experiments were performed with cells cultured in DMEM supplemented with 0.5% FBS, because the growth factors contained in FBS have been shown to induce COX-2 (1).
Western blot analysis.
Western blot analysis was performed as previously described (26). Equal amounts of proteins were subjected to SDS-PAGE analysis and subsequent immunoblotting was carried out with antibodies raised against COX-2 (Cayman Chemical), c-Jun, c-Fos, and phosphorylated extracellular signal-regulated protein kinase-1/2 (ERK1/2) (Cell Signaling Technology). Levels of immunocomplexes for glyceraldehyde-3-phosphate (GADPH) (Cell Signaling Technology) were used as an internal standard for equal loading. The immunocomplexes were detected using the enhanced chemiluminescence (GE Healthcare/Amersham).
Transient transfections and luciferase assay.
The pGL3–3.9-Kb COX-2 luciferase reporter construct was a gift of Dr. Tom McIntyre (University of Utah, Salt Lake City, UT) (27). The human collagenase-1 promoter (−73 to +63) luciferase construct harboring a single AP-1 site (p1xAP-1) was a gift from Dr. G. T. Bowden, University of Arizona, Tucson, AZ (28). Cells [1 × 105 in 2 mL DMEM (10% FBS) per well] were plated in 24-well Costar tissue culture plates. Transient transfections were performed using the Lipofectamine-Plus procedure according to the manufacturer's instruction (Life Technologies). Briefly, 24 h after plating, cells were cotransfected with 1.6 μg of COX-2 plasmid and 0.4 μg of the internal control plasmid pRL-TK (renilla luciferase gene) to account for variations in transfection efficiency. Cells were incubated with the DNA-liposome complex for 3 h at 37°C in 5% CO2. Following transfection, cells were maintained in DMEM (10% FBS) and allowed to recover for 48 h. Cells were then treated in DMEM (0.5% FBS) containing either control (dimethyl sulfoxide vehicle) or various concentrations of RA for the times indicated. Following treatment with selected agents, luciferase reporter activity was monitored in cell lysates of transfected cells with a Luminometer 20/20 (Turner Biosystems) and expressed as relative expression units corrected for the internal control renilla (Luc/Ren).
DNA protein-binding assay.
The binding assay was performed as previously described (26). Biotinylated oligonucleotides were synthesized by Sigma Genosys using the nucleotide sequences from a segment of the human COX-2 promoter harboring the COX-2 CRE: 5′-AAACAGTCATTTCGTCACATGGGCTTG-3′ (sense) and 5′-CAAGCCCATGTGACGAAATGACTGTTT-3′ (antisense) (26). Nuclear extracts were harvested from cells that were cotreated for 3 h with 10 μmol/L of RA plus TPA (0.1 μmol/L). The binding assay was performed by incubating 200 μg of the nuclear extracts, 2 μg biotin-labeled double-stranded DNA oligonucleotides, and 40 μL of 4% beaded-agarose conjugated with streptavidin in 600 μL of PBS buffer containing multiple protease inhibitors: 1 mmol/L Na3VO4, 10 mmol/L NaF, 25 mmol/L β-glycerophosphate, 0.1 mmol/L phenylmethanesulphonylfluoride, 0.06 g/L aprotinin, 1 g/L leupeptin, 0.5 mmol/L dithiothreitol) for 2 h with shaking at room temperature. Beads were pelleted by centrifugation at 550 × g for 1 min and then washed 3 times with cold PBS buffer containing multiple protease inhibitors. Nuclear proteins were dissociated by incubating the mixture at 95°C for 5 min. The binding proteins were separated on a 4–12% SDS-PAGE and subsequently subjected to Western blot analysis with an antibody that detected either total c-Jun or c-Fos protein regardless of phosphorylation state.
Statistical analysis.
We used Statview, the SAS Institute statistical analysis software, for analysis of results from Western blotting, transfection, and binding experiments. Data from factorial experiments were analyzed by 2-way ANOVA. When main effects and interactions were significant, post hoc multiple comparisons were conducted using Fisher's protected least significant different test. Differences were considered significant at P ≤ 0.05. Data are presented as means + SE.
Results
RA and COX-2 protein expression data.
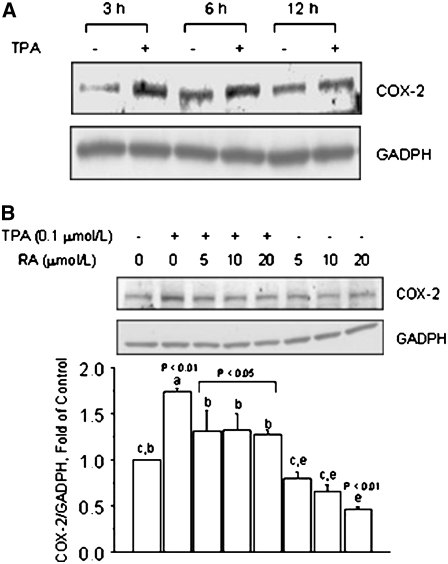
Previous studies (29) have documented that the expression of COX-2 is induced by agents that activate the AP-1 pathway. We characterized in HT-29 colon cancer cells the temporal regulation of COX-2 expression and observed greater induction by TPA (P < 0.01) of COX-2 protein levels at 3 and 6 h than at 12 h (Fig. 1A). The cotreatment with RA counteracted the stimulatory effects of TPA on COX-2 protein expression, which was reduced (5, 10, and 20 μmol/L; P < 0.05) to near control levels (Fig. 1B). The treatment with RA alone at the concentrations of 5 and 10 μmol/L reduced COX-2 protein to levels that approximated those measured in untreated HT-29 cells, whereas at the concentration of 20 μmol/L, COX-2 protein levels were lower (P < 0.01) than control cells.
FIGURE 1 .
RA reduces TPA-induced COX-2 expression in colon cancer HT-29 cells. (A) Time course of COX-2 protein induction by TPA. HT-29 cells were cultured in basal medium plus vehicle (−) or in the presence (+) of TPA (0.1 μmol/L) for 3, 6, and 12 h. (B) Effects of RA on basal and TPA-induced COX-2 protein expression. HT-29 cells were pretreated for 1 h with RA and then cultured for 6 h in the presence of TPA (0.1 μmol/L), RA (5, 10, or 20 μmol/L), or their combination. In A and B, bands represent COX-2 and GADPH (loading control) immunocomplexes. Values are means + SE of triplicates from 2, n = 2, independent experiments. Means without a common letter differ, P < 0.05.
RA represses AP-1–dependent transcriptional activation.
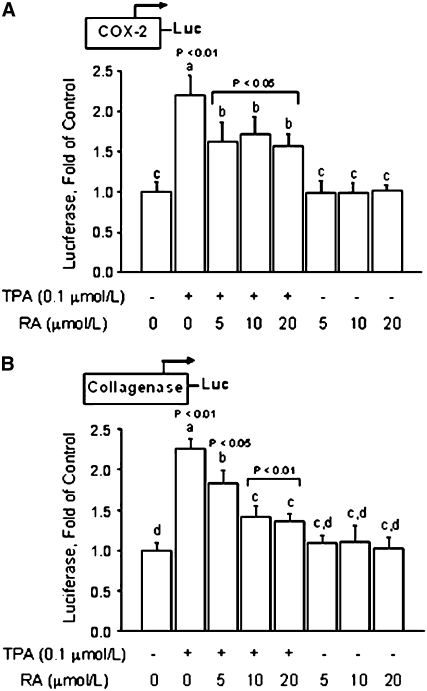
Based on the COX-2 protein expression results (Fig. 1), we examined the effects of RA on COX-2 transcription. We found that in colon cancer HT-29 cells transfected with a 3.9-Kb COX-2 promoter-luciferase construct, the luciferase reporter activity was induced (∼120%; P < 0.01) by TPA (Fig. 2A). The treatment with RA at the concentrations of 5, 10, and 20 μmol/L did not influence basal COX-2 transcription activity, but it reduced (P < 0.05) the TPA-dependent activation of the COX-2 promoter by ∼30%.
FIGURE 2 .
RA represses transcription activity in colon HT-29 cancer cells. Cells were transiently transfected with a 3.9-Kb fragment of the human COX-2 promoter (A) or a plasmid (p1xAP-1) containing a segment of the collagenase-1 gene promoter that harbors a consensus TRE (B). Transfected cells were pretreated for 1 h with various amounts (5, 10, and 20 μmol/L) of RA, followed by cotreatment with TPA (0.1 μmol/L) for 6 h. Values are means + SE of triplicates from 2, n = 2, independent experiments. Means without a common letter differ, P < 0.05.
Because TPA is a known inducer of the AP-1 transcription factor, we examined the effects of RA on regulation of transcription from a control luciferase reporter construct (p1xAP-1) containing a fragment of the collagenase-1 gene promoter, which harbors an AP-1 responsive element (28). The transfection results (Fig. 2B) indicated that the treatment of transfected HT-29 cells with TPA induced (P < 0.01) the transcription activity of the collagenase-1 promoter construct. However, the cotreatment with RA reduced the TPA-induced luciferase reporter activity by ∼20% at 5 μmol/L (P < 0.05) and by ∼40% at 10 and 20 μmol/L (P < 0.01). The treatment with RA alone did not influence basal transcription activity in cells transfected with p1xAP-1, regardless of the concentration used. Overall, these data suggested that RA may prevent COX-2 expression by antagonizing the AP-1–dependent activation of the COX-2 promoter.
RA antagonizes AP-1 binding to COX-2 promoter oligonucleotides.
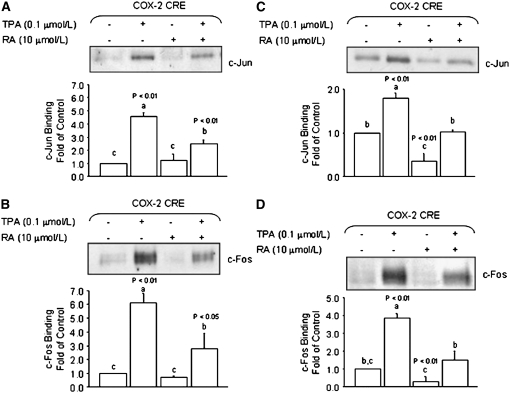
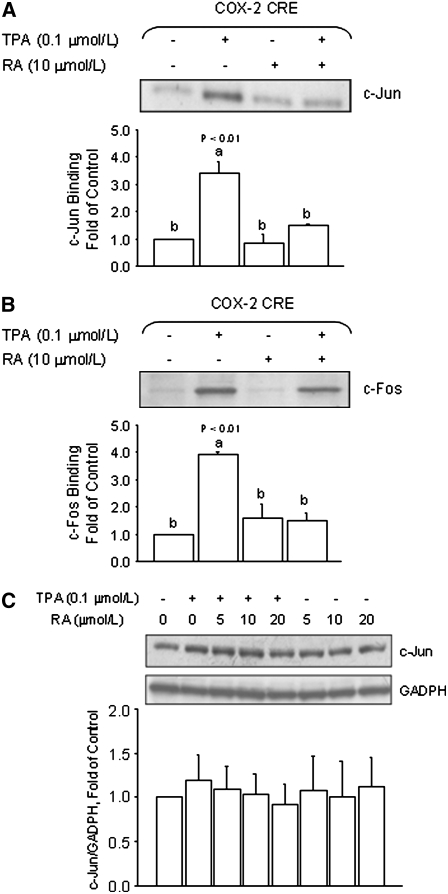
To investigate whether or not RA reduced the TPA-induced activation of COX-2 transcription through an AP-1–dependent mechanism, we examined the effects of RA on recruitment of AP-1 factors to a region of the COX-2 gene harboring the CRE element. In DNA pull-down experiments with nuclear extracts obtained from HT-29 colon cancer cells treated with TPA, we observed increased binding of c-Jun (P < 0.01) and c-Fos (P < 0.01) to COX-2 promoter oligonucleotides (Fig. 3A,B). The treatment with RA (10 μmol/L) alone had negligible effects on the basal association of c-Jun and c-Fos with the COX-2 oligonucleotides. However, the cotreatment with RA reduced the TPA-induced binding of c-Jun (P < 0.01) and c-Fos (P < 0.05). These results were confirmed using nuclear extracts from breast cancer MCF-7 cells (Fig. 3C,D), in which the cotreatment with RA (10 μmol/L) reduced the TPA-induced association of c-Jun and c-Fos with the COX-2 promoter oligonucleotides to control levels, whereas the treatment with RA alone reduced (P < 0.01) the basal recruitment of c-Jun and c-Fos.
FIGURE 3 .
RA reduces the recruitment of AP-1 factors to the COX-2/CRE. The binding of c-Jun and c-Fos to a promoter segment of the human COX-2 promoter harboring a CRE (5′-TTCGTCA-3′) was examined in colon HCT-29 (A,B) and breast MCF-7 (C,D) cancer cells using a DNA-protein binding assay. Nuclear extracts were obtained from cells cotreated for 3 h with TPA (0.1 μmol/L) in the presence (+) or absence (−) of RA (10 μmol/L). Values are means + SE of triplicates from 2, n = 2, independent experiments. Means without a common letter differ, P < 0.05.
RA reduces TPA-induced COX-2 protein in nontransformed mammary epithelial cells.
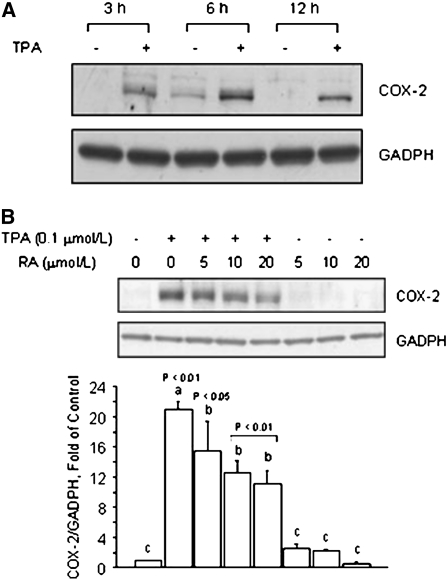
The results obtained with colon HT-29 cancer cells suggested that RA may effectively antagonize the activation of COX-2 expression by AP-1. Based on the notion that higher constitutive levels of COX-2 are associated with biologically aggressive and invasive phenotypes (29), we examined whether or not RA could exert protective effects against COX-2 activation in nontransformed cells. For this purpose, we used the MCF10A cell line, which has been used as a normal, nontumorigenic, mammary epithelial model (30). In MCF10A cells, the treatment with TPA effectively induced at 3, 6, and 12 h COX-2 protein expression, with maximal expression at 6 h (Fig. 4A). The cotreatment of MCF10A cells with RA (5, 10, and 20 μmol/L) reduced (5 μmol/L, P < 0.05; 10 and 20 μmol/L, P < 0.01) TPA-induced COX-2 protein levels (Fig. 4B), whereas COX-2 protein expression did not change in MCF-10A cells treated with RA alone.
FIGURE 4 .
RA reduces TPA-induced COX-2 expression in nonmalignant breast epithelial MCF10A cells. (A) Time course of COX-2 protein induction by TPA. MCF10A cells were cultured in basal medium plus vehicle (−) or in the presence (+) of TPA (0.1 μmol/L) for 3, 6, and 12 h. (B) Effects of RA on basal and TPA-induced COX-2 protein. MCF10A cells were pretreated for 1 h with RA and then cultured for 6 h in the presence of TPA (0.1 μmol/L), RA (5, 10, 20 μmol/L), or their combination. (A,B) Bands represent COX-2 and GADPH (loading control) immunocomplexes. Values are means + SE of triplicates from 2, n = 2, independent experiments. Means without a common letter differ, P < 0.05.
These cumulative results illustrated that RA was effective in reducing the TPA-induced increase in COX-2 expression in nonmalignant MCF-10A cells. We also confirmed that the treatment of MCF10A cells with RA (10 μmol/L) reduced the TPA-induced binding of c-Jun (P < 0.01) and c-Fos (P < 0.01) to the COX-2 promoter oligonucleotides (Fig. 5A,B). In control experiments, we examined the effects of RA in MCF10A cells on the expression levels of c-Jun. Compared with MCF10A cells cultured in control medium, c-Jun protein levels did not differ following the treatment with RA. These data suggested that the reduced c-Jun binding in MCF10A cells cotreated with RA was not due to indirect effects of RA on the expression or cellular content of the c-Jun protein (Fig. 5C).
FIGURE 5 .
RA antagonizes TPA-induced binding of AP-1 to the COX-2 promoter in nonmalignant MCF10A breast epithelial cells. The binding of c-Jun (A) and c-Fos (B) to a promoter segment of the human COX-2 promoter harboring a CRE (5′-TTCGTCA-3′) was examined in MCF10A cells. Nuclear protein extracts were obtained from cells cotreated for 3 h with TPA (0.1 μmol/L) in the presence (+) or absence (−) of RA (10 μmol/L). (C) Western blot analysis of total cell lysates obtained from MCF10A cells cultured in the presence of TPA or in combination with various concentrations of RA (5, 10, and 20 μmol/L) for 6 h. Bands represent immunocomplexes for c-Jun and GADPH (loading control). In A–C, values are means + SE of triplicates from 2, n = 2, independent experiments. Means without a common letter differ, P < 0.05.
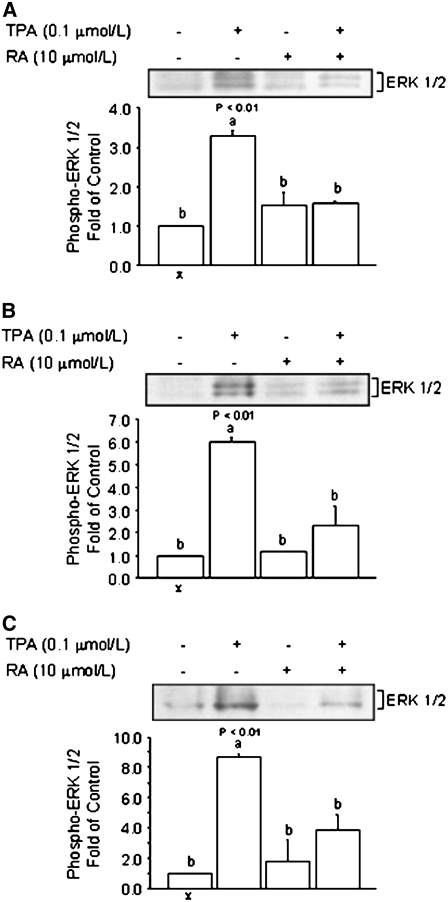
RA inhibits the activation of phosphorylated ERK1/2 in cancer and nonmalignant cells.
The reduced recruitment of AP-1 to the COX-2 oligonucleotides (Fig. 3A–D and Fig. 5A,B) suggested that the effects of RA could be mediated at least in part through repression of cellular pathways that activate AP-1. This contention was addressed in follow-up experiments in which we examined the effects of RA on the cellular levels of phosphorylated ERK1/2 in colon cancer HT-29, breast cancer MCF-7, and breast nonmalignant MCF10A cells. The ERK1/2 is an upstream mediator of AP-1 activation (31) and it is upregulated in various types of cancer (32). We examined the effects of RA on the cytoplasmic levels of phosphorylated ERK1/2 at 15 min, because phosphorylated ERK1/2 has been shown to be activated by TPA at this time point in previous studies (33). Expression data (Fig. 6A) indicated that in HT-29 cells, the treatment with TPA induced (P < 0.01), whereas the cotreatment with RA (10 μmol/L) reduced to basal levels, phosphorylated ERK1/2. The phosphorylated ERK1/2 was visualized as a doublet representing 2 isoforms of 43 (ERK1) and 41 (ERK2) kDa. The treatment with RA alone (10 μmol/L) did not affect basal levels of phosphorylated ERK1/2. The repressive effects of RA on phosphorylated ERK1/2 expression were confirmed in breast cancer MCF-7 (10 μmol/L; P < 0.01) (Fig. 6B) and nontransformed MCF-10A (10 μmol/L; P < 0.01) mammary epithelial cells (Fig. 6C). Because the transactivating properties of the transcription factor AP-1 are influenced, among other pathways, by phosphorylation through ERK1/2, these data provided a potential mechanism through which RA may antagonize the activation of AP-1 and, ultimately, the expression of the COX-2 gene.
FIGURE 6 .
Comparison of the repressive effects of RA on ERK1/2 activation by TPA in HT-29, MCF-7, and MCF 10A cells. (A) HT-29 colon and (B) MCF-7 breast cancer cells and (C) MCF 10A nonmalignant breast epithelial cells were cultured for 15 min in the presence of TPA (0.1 μmol/L), RA (10 μmol/L), or their combination. Bands represent phosphorylated ERK1/2 and GADPH (loading control). Values are means + SE of triplicates from 2, n = 2, independent experiments. Means without a common letter differ, P < 0.05.
Discussion
The activation of COX-2 expression has been regarded as a causative factor in the onset of several inflammatory conditions and etiology of colorectal (4) and breast (34) tumors. COX-2 has been a target for therapies based on NSAID and selective COX-2 inhibitors. Because NSAID have been shown to enhance gastrointestinal ulceration (9) and selective COX-2 inhibitors increased the risk for cardiovascular diseases (10), interest has been generated toward the development of alternative and prophylactic anti-COX-2 strategies that lack these negative effects (35).
Preparations from the plant Rosmarinus officinalis have been recently investigated for their ability to exert antiproliferative and antioxidant properties (36,37) and protect against skin tumorigenesis (38) and DNA damage (21). Earlier studies reported that rosemary extracts inhibited 7,12-dimethyl-benz[a]anthracene-induced DNA adducts and mammary tumors in female Sprague-Dawley rats (39–41) and benzo[a]pyrene-induced genotoxicity in bronchial cells (42). The topical application of rosemary extracts inhibited benzo[a]pyrene- and dimethyl-benz[a]anthracene–induced initiation of tumors in mouse skin as well as TPA-induced tumor promotion (43). Some of the protective effects of rosemary extracts were attributed to enhancement of xenobiotic detoxification (44).
Rosemary extracts contain several polyphenolic components, including carnosic acid, carnosol, and RA. The latter is an esterification product of caffeic acid with 3,4-dihydroxyphenyllactic acid, which is also found in sage, peppermint, and lemon balm (20). In previous investigations, the pretreatment with RA was shown to reduce COX-2 mRNA expression in a TPA-challenged skin mouse model (45). Therefore, in this study, we examined the mechanisms through which RA may antagonize COX-2 expression. We found that the cotreatment of colon cancer HT-29 cells with RA reduced TPA-induced COX-2 promoter activity and protein levels. RA antagonized the AP-1–dependent activation of COX-2 transcription, as evidenced by its ability to repress transcription from a collagenase AP-1-luciferase reporter construct transfected into colon HT-29 cancer cells. Moreover, the cotreatment of HT-29 cells with RA repressed the TPA-induced recruitment of c-Jun and c-Fos proteins to COX-2 promoter oligonucleotides. The anti-AP-1 effects of RA were not specific to HT-29 cells, because RA counteracted the binding of c-Jun and c-Fos in nuclear extracts obtained from breast cancer MCF-7 cells. Finally, we documented in nontransformed MCF10A mammary epithelial cells that RA reduced the TPA-induced accumulation of COX-2 protein and recruitment of c-Jun and c-Fos to the COX-2 promoter oligonucleotides.
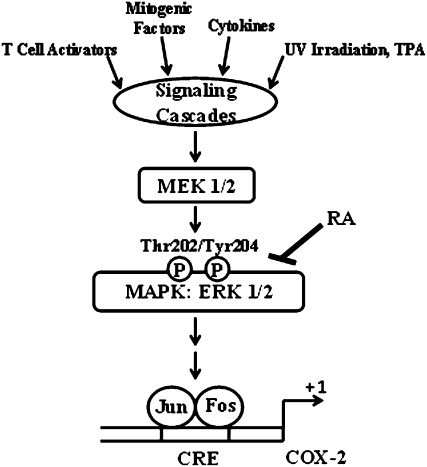
We further examined the effects of RA on signal transduction pathways that are known to activate AP-1 (46). The cotreatment of HT-29, MCF-7, and MCF10A cells with RA reduced the cellular levels of ERK1/2, a component of the mitogen-activated protein kinase pathway. The model (Fig. 7) suggests that the preventative effects of RA against TPA-induced COX-2 activation may be attributable at least in part to repression of signaling pathways that participate in activation of ERK, thus preventing the downstream activation of AP-1. Our data parallel those of recent investigations (47) reporting that the treatment of H9c2 cardiac muscle cells with higher concentrations of RA than those used in this study (55 vs. 10 μmol/L) for up to 2 h antagonized the adriamycin-dependent activation of c-Jun N-terminal kinase and ERK and partially inhibited the binding of AP-1 members to a control AP-1 oligonucleotide. However, our results contrast with those of other studies (48) documenting that the cotreatment with RA (30 μmol/L for 16 h) did not prevent, but rather slightly induced, TPA-dependent AP-1 activation in transfected Jurkat T cells. One possible interpretation for these contrasting results is that the ability of RA to either stimulate or repress AP-1 activity may be due to cell-specific differences or related to higher doses and longer times of incubation. This interpretation is consistent with that of previous investigations with human colon cancer HT-29 cells documenting that the chemopreventative functions of various compounds on signal transduction pathways such as AP-1 may be highly dose dependent (49). It is also feasible that the effect of RA on AP-1 activity may be due to indirect effects of the metabolites ferulic and caffeic acid. In fact, ferulic acid dimer (50) and chlorogenic acid (51), an esterification product of caffeic acid with quinic acid, but not caffeic acid (52), have been shown to inhibit the TPA-dependent activation of AP-1.
FIGURE 7 .
A schematic model depicting the potential preventative effects of RA against AP-1–dependent activation of COX-2 expression. Under the current model, several stimuli, including TPA, may activate signaling cascades that lead to the subsequent activation of mitogen-activated protein kinase pathways, including the ERK1/2. The ERK1/2 pathway contributes to the recruitment of members of the AP-1 factor, which comprises members of the Jun and Fos family of transcription factors, to the COX-2 promoter. RA may exert preventative effects against the induction of COX-2 transcription by antagonizing the activation of ERK1/2 and the recruitment of AP-1 factors to the CRE harbored in the proximal COX-2 promoter. Arrows indicate activation and the bar indicates repression by RA. Thr-202 and Tyr-204 indicate phosphorylation sites on ERK1/2. The +1 indicates the initiation of transcription of the COX-2 gene.
One important question pertains to the physiological significance of these results. Little information is available concerning the plasma values of RA achievable in humans. Previous research that measured RA levels in healthy men after a single oral administration of 200 mg RA reported plasma values of ∼1.2 μmol/L (53). However, the sample size of these studies was limited to 6 individuals and large variations in plasma concentrations were observed. The same group reported plasma values of ∼5 μmol/L after oral administration of RA (50 mg/kg body weight) to Sprague-Dawley male rats (54). In our studies, we used concentrations of RA ranging from 5 to 20 μmol/L. At the lowest concentration tested (5 μmol/L), which approximates the plasma levels previously documented (53), we observed that RA reduced COX-2 expression, reduced AP-1 activation, and antagonized ERK1/2 activation. While future studies should investigate the effects of RA through supplementation or cumulative intake from various herbal sources of RA on plasma levels achievable in humans, our study provides novel evidence that RA represses AP-1–dependent activation of COX-2 expression. Nevertheless, the proposed effects of RA on AP-1 activation and COX-2 expression await further confirmation in other cancer cell lines and in vivo models. Given the role of COX-2 in inflammation and carcinogenesis, and the role of AP-1 in proliferation and transformation, this study provides mechanistic evidence that RA merits further investigation as a natural bioactive component to modulate AP-1 activity and COX-2 gene expression.
Supported by a grant from the Undergraduate Honors College, the University of Arizona, Tucson, a fellowship from Graduate Training Program T32 ES-07091-24, and grant 0819 from the Arizona Biomedical Research Commission, Phoenix, AZ.
Author disclosures: K. A. Scheckel, S. C. Degner, and D. F. Romagnolo, no conflicts of interest.
Abbreviations used: AP-1, activator protein-1; COX-2, cyclooxygenase-2; CRE, cAMP-response element; ERK1/2, extracellular signal-regulated protein kinase-1/2; FBS, fetal bovine serum; GADPH, glyceraldehyde-3-phosphate; NSAID, nonsteroidal antiinflammatory drug; RA, rosmarinic acid; TPA, 12-O-tetradecanoylphorbol-13-acetate.
References
- 1.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. [DOI] [PubMed] [Google Scholar]
- 2.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–40. [DOI] [PubMed] [Google Scholar]
- 3.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–45. [DOI] [PubMed] [Google Scholar]
- 4.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. [DOI] [PubMed] [Google Scholar]
- 6.Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1213–7. [PubMed] [Google Scholar]
- 7.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–12. [PubMed] [Google Scholar]
- 8.Yoshinaka R, Shibata MA, Morimoto J, Tanigawa N, Otsuki Y. COX-2 inhibitor celecoxib suppresses tumor growth and lung metastasis of a murine mammary cancer. Anticancer Res. 2006;26:4245–54. [PubMed] [Google Scholar]
- 9.Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol. 1993;35:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–9. [DOI] [PubMed] [Google Scholar]
- 11.Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem. 1994;221:889–97. [DOI] [PubMed] [Google Scholar]
- 12.Tazawa R, Xu XM, Wu KK, Wang LH. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochem Biophys Res Commun. 1994;203:190–9. [DOI] [PubMed] [Google Scholar]
- 13.Sassone-Corsi P, Ransone LJ, Verma IM. Cross-talk in signal transduction: TPA-inducible factor jun/AP-1 activates cAMP-responsive enhancer elements. Oncogene. 1990;5:427–31. [PubMed] [Google Scholar]
- 14.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–68. [DOI] [PubMed] [Google Scholar]
- 15.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–52. [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. [DOI] [PubMed] [Google Scholar]
- 17.Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–39. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RG, Pezzuto JM. Discovery of cancer preventive agents from natural products: from plants to prevention. Curr Oncol Rep. 2002;4:478–86. [DOI] [PubMed] [Google Scholar]
- 19.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals: promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–8. [DOI] [PubMed] [Google Scholar]
- 20.al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol. 1999;37:124–30. [PubMed] [Google Scholar]
- 21.Slamenova D, Kuboskova K, Horvathova E, Robichova S. Rosemary-stimulated reduction of DNA strand breaks and FPG-sensitive sites in mammalian cells treated with H2O2 or visible light-excited Methylene Blue. Cancer Lett. 2002;177:145–53. [DOI] [PubMed] [Google Scholar]
- 22.Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry. 2003;62:121–5. [DOI] [PubMed] [Google Scholar]
- 23.Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006;239:271–80. [DOI] [PubMed] [Google Scholar]
- 24.Sahu A, Rawal N, Pangburn MK. Inhibition of complement by covalent attachment of rosmarinic acid to activated C3b. Biochem Pharmacol. 1999;57:1439–46. [DOI] [PubMed] [Google Scholar]
- 25.Osakabe N, Takano H, Sanbongi C, Yasuda A, Yanagisawa R, Inoue K, Yoshikawa T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors. 2004;21:127–31. [DOI] [PubMed] [Google Scholar]
- 26.Degner SC, Kemp MQ, Bowden GT, Romagnolo DF. Conjugated linoleic acid attenuates cyclooxygenase-2 transcriptional activity via an anti-AP-1 mechanism in MCF-7 breast cancer cells. J Nutr. 2006;136:421–7. [DOI] [PubMed] [Google Scholar]
- 27.Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem. 1999;274:8328–34. [DOI] [PubMed] [Google Scholar]
- 28.Tang Q, Chen W, Gonzales MS, Finch J, Inoue H, Bowden GT. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene. 2001;20:5164–72. [DOI] [PubMed] [Google Scholar]
- 29.Liu XH, Rose DP. Differential expression and regulation of cyclooxygenase-1 and -2 in two human breast cancer cell lines. Cancer Res. 1996;56:5125–7. [PubMed] [Google Scholar]
- 30.Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA. Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif. 2008;41:59–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–400. [DOI] [PubMed] [Google Scholar]
- 32.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–47. [DOI] [PubMed] [Google Scholar]
- 33.Lee KW, Kang NJ, Heo YS, Rogozin EA, Pugliese A, Hwang MK, Bowden GT, Bode AM, Lee HJ, et al. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni S, Patil DB, Diaz LK, Wiley EL, Morrow M, Khan SA. COX-2 and PPARgamma expression are potential markers of recurrence risk in mammary duct carcinoma in-situ. BMC Cancer. 2008;8:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degner SC, Kemp MQ, Hockings JK, Romagnolo DF. Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer MCF-7 cells: repressive effects of conjugated linoleic acid. Nutr Cancer. 2007;59:248–57. [DOI] [PubMed] [Google Scholar]
- 36.Cheung S, Tai J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol Rep. 2007;17:1525–31. [PubMed] [Google Scholar]
- 37.Frankel EN, Huang SW, Aeschbach R, Prior E. Antioxidant activity of rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oils and oil-in-water emulsion. J Agric Food Chem. 1996;44:131–5. [Google Scholar]
- 38.Sancheti G, Goyal PK. Effect of Rosmarinus officinalis in modulating 7,12-dimethylbenz(a)anthracene induced skin tumorigenesis in mice. Phytother Res. 2006;20:981–6. [DOI] [PubMed] [Google Scholar]
- 39.Singletary KW, Nelshoppen JM. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and of in vivo formation of mammary DMBA-DNA adducts by rosemary extract. Cancer Lett. 1991;60:169–75. [DOI] [PubMed] [Google Scholar]
- 40.Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–8. [DOI] [PubMed] [Google Scholar]
- 41.Amagase H, Sakamoto K, Segal ER, Milner JA. Dietary rosemary suppresses 7,12-dimethylbenz(a)anthracene binding to rat mammary cell DNA. J Nutr. 1996;126:1475–80. [DOI] [PubMed] [Google Scholar]
- 42.Offord EA, Macé K, Ruffieux C, Malnoë A, Pfeifer AM. Rosemary components inhibit benzo[a]pyrene-induced genotoxicity in human bronchial cells. Carcinogenesis. 1995;16:2057–62. [DOI] [PubMed] [Google Scholar]
- 43.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–8. [PubMed] [Google Scholar]
- 44.Singletary KW, Rokusek JT. Tissue-specific enhancement of xenobiotic detoxification enzymes in mice by dietary rosemary extract. Plant Foods Hum Nutr. 1997;50:47–53. [DOI] [PubMed] [Google Scholar]
- 45.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–57. [DOI] [PubMed] [Google Scholar]
- 46.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–6. [DOI] [PubMed] [Google Scholar]
- 47.Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ, Chae SW. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005;70:1066–78. [DOI] [PubMed] [Google Scholar]
- 48.Kang MA, Yun SY, Won J. Rosmarinic acid inhibits Ca2+-dependent pathways of T-cell antigen receptor-mediated signaling by inhibiting the PLC-gamma 1 and Itk activity. Blood. 2003;101:3534–42. [DOI] [PubMed] [Google Scholar]
- 49.Jeong WS, Kim IW, Hu R, Kong AN. Modulation of AP-1 by natural chemopreventive compounds in human colon HT-29 cancer cell line. Pharm Res. 2004;21:649–60. [DOI] [PubMed] [Google Scholar]
- 50.Murakami Y, Ito S, Atsumi T, Fujisawa S. Theoretical prediction of the relationship between phenol function and COX-2/AP-1 inhibition for ferulic acid-related compounds. In Vivo. 2005;19:1039–43. [PubMed] [Google Scholar]
- 51.Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem. 2005;280:27888–95. [DOI] [PubMed] [Google Scholar]
- 52.Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, Kim SH, Kim JG, Kim CH. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–81. [DOI] [PubMed] [Google Scholar]
- 53.Baba S, Osakabe N, Natsume M, Yasuda A, Muto Y, Hiyoshi K, Takano H, Yoshikawa T, Terao J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. Eur J Nutr. 2005;44:1–9. [DOI] [PubMed] [Google Scholar]
- 54.Baba S, Osakabe N, Natsume M, Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004;75:165–78. [DOI] [PubMed] [Google Scholar]