Abstract
Current recommendations for vitamin A intake and liver stores (0.07 μmol/g) are based on maintaining normal vision. Higher levels may be required for maintaining normal immune function. The objective of this study was to assess the relationship between total body vitamin A stores in adult men and measures of adaptive immune function. We conducted an 8-wk residential study among 36 healthy Bangladeshi men with low vitamin A stores. Subjects received a standard diet and were randomized in a double-blind fashion to receive vitamin A (240 mg) or placebo during wk 2 and 3. Subjects received Yellow Fever Virus (YFV) and tetanus toxoid (TT) vaccines during wk 5. Vitamin A stores were estimated by isotopic dilution during wk 8. Vaccine-specific lymphocyte proliferation, cytokine production, and serum antibody responses were evaluated before and after vaccination. Vitamin A supplementation increased YFV- and TT-specific lymphocyte proliferation and YFV-specific interleukin (IL)-5, IL-10, and tumor necrosis factor-α production but inhibited development of a TT-specific IL-10 response. Both groups developed protective antibody responses to both vaccines. Some responses correlated positively with vitamin A stores. These findings indicate that the currently recommended vitamin A intake is sufficient to sustain a protective response to YFV and TT vaccination. However, YFV-specific lymphocyte proliferation, some cytokine responses, and neutralizing antibody were positively associated with liver vitamin A stores > 0.084 μmol/g. Such increases may enhance vaccine protection but raise the question of whether immune-mediated chronic diseases may by exacerbated by high-level dietary vitamin A.
Introduction
Several aspects of adaptive immunity are compromised by vitamin A deficiency (1). In particular, vitamin A deficiency impairs T cell-mediated responses (2,3) by impairing aspects of both T helper type 1 (Th1)6 and Th2 function (4,5) and by impairing B-cell function (6). Impaired T-cell–mediated antibody responses have been demonstrated in several animal models (2,3,7,8). Human studies have examined the effect of vitamin A supplementation on the antibody response to childhood vaccines. Most studies found no enhancement (9), although 1 study reported an increased serum antibody response to Tetanus Toxoid (TT) (10).
To our knowledge, no studies have used immune function as an index to evaluate dietary vitamin A requirements. The estimated average requirement (EAR) for vitamin A is based on maintenance of liver stores > 0.07 μmol retinol/g liver, which prevents development of eye signs of deficiency (an abnormal electroretinogram) for 4 mo while consuming a vitamin A-free diet (11). However, vitamin A supplementation reduces child mortality from infectious diseases even when children with eye signs (xerophthalmia) are excluded from such studies (12–14). This observation suggests that the maintenance of immune function associated with protection from infection may require higher liver stores than are needed to prevent eye signs of deficiency.
We hypothesized that vitamin A supplementation to increase liver vitamin A stores > 0.07 μmol/g would enhance the primary response to the live-virus Yellow Fever Virus (YFV) vaccine and the recall response to the inactivated TT vaccine. We also hypothesized that these responses would correlate positively with vitamin A stores. To test these hypotheses, we conducted a randomized, placebo-controlled intervention trial in Bangladeshi men with marginal vitamin A stores receiving a controlled diet low in vitamin A. Vitamin A stores were measured with the deuterated retinol dilution technique (15).
Materials and Methods
Study site and subjects.
This study was carried out at the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), located in Dhaka. The Institutional Review Board of the University of California, Davis and the Ethical Review Committee of ICDDR,B both approved the study procedures. All participants provided informed written consent. A total of 157 adult men (20–30 y of age) were screened for admission and 36 were recruited. Two weeks before collecting blood for screening, all subjects were treated with albendazole to clear intestinal helminth infections. The inclusion criteria were: serum retinol <1.22 μmol/L, a normal C-reactive protein concentration (<5mg/L), normal clinical chemistry and hematology values, and freedom from any infectious or chronic disease by medical history and physician exam. A previous study in Bangladesh by one of us (M. J. H.) (15) demonstrated that men meeting these serum retinol and C-reactive protein criteria had low liver vitamin A stores (<0.07 μmol/g). Subjects enrolled in the study lived near ICDDR,B during the study period and came to the ICDDR,B grounds to stay at the Advanced Biomedical Research Unit for 12 h/d, 7 d/wk, where they received and consumed all meals during the 2-mo residential study period. Study subjects were under the regular care of a physician who monitored them 3 times weekly for general health status and eye signs of vitamin A deficiency.
Study diet.
The diet was modeled on a traditional Bangladeshi diet and provided ∼40 μg/d retinol equivalents (6.4% of the EAR). The diet consisted of rice, wheat flat bread, lentils, small amounts of curried chicken or mutton, and pale fruits and vegetables, such as cauliflower, cabbage, white potatoes, white squash, and banana. Subjects consumed the diet ad libitum. These low, constant levels of dietary vitamin A stabilized the serum retinol concentration and eliminated any diet-induced variation among subjects during the isotope dilution period and during the assay of immunological parameters. The diet provided the recommended dietary allowance (RDA) for total energy and other major vitamins and minerals were based on food composition tables from the USDA and a Bangladeshi nutrient database (16). All subjects received a single dose (60 mg) of vitamin A at the end of the study.
Study design.
The study was a placebo-controlled, double-blind intervention trial using a randomized block design (with a block size of 6) among 36 men conducted in 2 phases (18 subjects per phase) between May and October 2005. Following a 1-wk stabilization on the study diet (d 1–7), subjects received either 4 vitamin A capsules (containing 60 mg retinol equivalents of retinyl palmitate per capsule) or 4 identical placebo capsules (containing corn oil), 1 capsule per day at 5-d intervals (d 7, 12, 17, and 22). One week after supplementation (d 29), all subjects were vaccinated with the YFV vaccine (5.04 Log10 plaque-forming units in a 0.5-mL dose; YF-VAX, Aventis Pasteur) subcutaneously in one arm and the TT vaccine (≥40 IU in a 0.5-mL dose; TETAVAX, Aventis Pasteur SA) i.m. in the other arm. One week after vaccination (d 36), a single dose (10 mg) of stable isotope-labeled vitamin A ([2H4]-retinyl acetate; Cambridge Isotope Laboratories) was given orally. Venous blood was obtained before (d 29), 1 wk (d 36), and 1 mo (d 57) after vaccination. Blood was collected after overnight food deprivation of ∼10 h between 0700 and 0800. The vitamin A and placebo groups will be referred to as the high Vitamin A (VA) and low VA groups, respectively.
Serum retinol and vitamin A reserves.
Serum was stored at −80°C for measurement of retinol by HPLC as described previously (17). Total body vitamin A pool size was estimated with the deuterated retinol dilution technique (18) using the equation of Furr et al. (19) on the day the isotopic dose was given (this calculation did not include the mass of the tracer dose), 1 wk after vaccination, and again 4 wk after vaccination at the final blood draw (when the 10-mg mass of the tracer dose was included in estimating vitamin A pool size). The liver vitamin A concentration was estimated by assuming that liver weight was 2.4% of body weight (20) and that 90% of vitamin A is found in the liver. In well-nourished persons, the liver contains >90% of total-body vitamin A (20,21), but in vitamin A-deficient individuals, liver vitamin A concentrations are lower, ∼50–90% of total stores, although vitamin A in other tissues remains relatively constant (21). In this study, we have used 90% as the percentage of total body vitamin A present in liver, because all subjects had serum retinol > 0.70 μmol/L and are thus not considered deficient using a standard definition (22). Whole-body and liver vitamin A stores at 1 mo postvaccination were estimated using the prediction equation with a modification to include the contribution of mass of tracer dose to the total body vitamin A pool size, because 4 hydrogen atoms in the isotopic retinol were replaced with 4 deuterium atoms probably retain the same biological activity as nonisotopic retinol.
Peripheral blood mononuclear cells proliferation and cytokine assay.
Peripheral blood mononuclear cells (PBMC) were separated from fresh heparinaized blood using a standard density gradient method (Ficoll-Paque-PLUS, Amersham Biosciences) cultured in standard Russ-10 media (3) supplemented with 10% heat-inactivated (56°C for 30 min) autologous plasma. Triplicate cultures (2 × 105 PBMC in a 96-well U-bottom plate) were stimulated with 3 levels of YFV vaccine (plaque-forming units) and 3 levels of limits of flocculation of TT antigen (02/126; NIBSC) for 6 d at 37°C and 5% CO2. Cell proliferation was measured by incorporation of [3H]-thymidine. Results were expressed as total counts or as a stimulation index (SI), the ratio of antigen-stimulated:unstimulated wells. An additional well of the highest antigen dose was collected at 6 d for analysis of supernatant levels of interleukin (IL)-2, IL-4, IL-10, interferon-γ (IFNγ), tumor necrosis factor-α (TNFα), and IL-5 by Luminex assay (Bio-Plex system, Bio-Rad Laboratories).
Serum and lymphocyte supernatant anti-TT IgG assay.
Unstimulated PBMC were cultured for 3 d to identify antibody-secreting lymphocytes essentially as described (23). Culture supernatants and plasma were both assayed for TT-specific IgG using EIA kits (The Binding Site). Results are expressed as mIU/L and levels above the 0.15 mIU/L threshold are considered protective (24).
Serum YFV specific plaque reduction neutralization test 50 assay.
The plaque reduction neutralization test (PRNT) to detect neutralizing antibody for the YFV 17D vaccine strain was carried out by Dr. Sutee Yoksan at the Center for Vaccine Development, Mahidol University, Thailand as described (25).
Statistical analysis.
Statistical analyses were conducted using SigmaStat 3.1 (Systat Software) and SPSS 13.0 for windows (SPSS). Distributions of study variables were analyzed to identify outliers and test assumptions of normality and equal variance. Data were transformed to produce normal distributions as needed.
Two subjects in the placebo group had implausibly high vitamin A pool size estimates (0.42 and 0.74 mmol). These levels are inconsistent with their reported dietary intakes and with pool size data from other subjects recruited in the same fashion in the same setting (M. J. Haskell, unpublished observations). Such overestimates of pool size are likely due to poor absorption of the tracer dose. One subject in the vitamin A group also demonstrated an unreasonably high estimate, 0.54 mmol, which was >3 SD higher than the mean value of this group. It is also likely that this subject had poor absorption of the tracer dose. Data from these 3 subjects were not used in the correlation analyses involving pool size estimates. Data from these subjects was used in the comparisons between low and high VA groups, because these comparisons do not depend on the pool size estimates.
Low-level prevaccination YFV antibody titers were detected in 2 placebo subjects, indicating previous exposure to a related virus (YFV is not found in Bangladesh). Because this previous exposure produced a memory response that was cross-reactive with YFV, their response to YFV immunization would be considered a secondary rather than a primary response (26). Hence, these subjects were not included in the analysis of the YFV response but were included in the analysis of the TT response. In addition, 1 subject in the vitamin A group did not respond with a measurable serum antibody titer or with an increased proliferative response to YFV 1 mo after YFV vaccination. This suggests that the subject was not vaccinated, although study records indicate the vaccination did occur. This subject was not included in analysis of the YFV data, because either a rare vaccine failure or failure to vaccinate would invalidate this subject in analyzing the effect of vitamin A on YFV immunity. This subject responded well to the TT vaccination and his data were included in that portion of the analysis.
Two-way repeated-measures ANOVA using the Holm-Sidak post hoc comparison proceure was used to compare vaccine-specific immune responses. The post hoc test was applied when significant differences were found between study groups, between study days, or their interaction. Between-group comparisons at a single time point were conducted using Student's t test. The overall significance level of these tests was set at P < 0.05.
Spearman rho correlation analysis was used to determine whether individual immune response variables were associated with vitamin A stores. Two-phase segmental linear regression was used to identify possible break points at which a linear association between stores and immune response variables might reach a plateau. Groups of similar immune response variables were converted to Z-scores (mean of 0, SD of 1) and analyzed together for the break-point analysis. For each vaccine, clusters of immune response variables measured at 1 wk or at 1 mo postvaccination were used. These clusters included: 1) the 3 levels of antigen-stimulated PBMC proliferation and serum antibody titer, because lymphocyte proliferation is a necessary prerequisite for producing a T-cell–mediated serum antibody response; 2) vaccine-specific Th1 cytokines (IL2, IFNγ, and TNFα), which stimulate cytotoxicity T cell responses and macrophage activation; and 3) vaccine-specific Th2 cytokines (IL-4, IL-5, and IL-10), which stimulate B cells for antibody production. GraphPad Prism 5 for Windows (GraphPad Software) was used for this analysis.
Results
Baseline characteristics.
Study subjects had normal anthropometric and hematologic measurements at baseline. The 2 groups did not differ in these characteristics (Table 1).
TABLE 1.
Baseline characteristics of the study subjects in the 2 groups1
| Study groups |
||
|---|---|---|
| Characteristics | Low VA | High VA |
| Age, y | 24.3 ± 2.70 | 23.5 ± 3.05 |
| Height, cm | 164 ± 7.71 | 161 ± 8.26 |
| Weight, kg | 53.1 ± 5.50 | 51.9 ± 7.49 |
| BMI, kg/m2 | 19.7 ± 1.60 | 19.7 ± 1.79 |
| WBC, ×109/L | 9.45 ± 1.92 | 9.00 ± 1.92 |
| Hemoglobin, g/L | 152 ± 10.9 | 152 ± 9.70 |
| MCV, limits of flocculation | 85.8 ± 5.01 | 84.4 ± 3.46 |
| MCH, pg/cell | 29.1 ± 3.06 | 29.6 ± 1.32 |
| MCHC, g/L | 335 ± 20.6 | 341 ± 16.0 |
Values are means ± SD, n = 18. MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell.
Vitamin A status.
Following supplementation, the mean serum retinol concentration in the high VA group increased significantly, whereas that of the low VA group did not change (Table 2). As expected, the estimated total body vitamin A pool size in the high VA group was significantly higher than the pool size in the low VA group (Table 2). All subjects in the high VA group had liver stores >0.07 μmol/g, the target level used for the RDA determination, whereas in the low VA group, all but 2 subjects (0.077 and 0.084 μmol/g) had liver stores <0.07 μmol/g. No subjects developed signs of clinical vitamin A deficiency.
TABLE 2.
Serum retinol concentration, estimated total body vitamin A pool size, and liver vitamin A concentration in low and high VA groups vaccinated with YFV and TT1
| Study groups |
||
|---|---|---|
| Low VA (n = 16) | High VA (n = 17) | |
| Serum retinol, μmol/L | ||
| Presupplementation | 1.52 ± 0.39 | 1.49 ± 0.37 |
| Postsupplementation2 | 1.42 ± 0.32 | 1.85 ± 0.34*# |
| Whole-body vitamin A,3mmol | 0.06 ± 0.04 | 0.23 ± 0.06* |
| Liver vitamin A,3μmol/g | 0.04 ± 0.03 | 0.16 ± 0.04* |
| Whole-body vitamin A,4mmol | 0.07 ± 0.04 | 0.24 ± 0.06* |
| Liver vitamin A,4μmol/g | 0.05 ± 0.03 | 0.17 ± 0.04* |
Values are means ± SD. *Different from low VA, P < 0.001; #different from presupplementation, P < 0.01.
Prevaccination (1-wk after the end of placebo/vitamin A supplementations).
1 wk postvaccination.
1 mo postvaccination.
Primary YFV vaccine response in low and high VA groups.
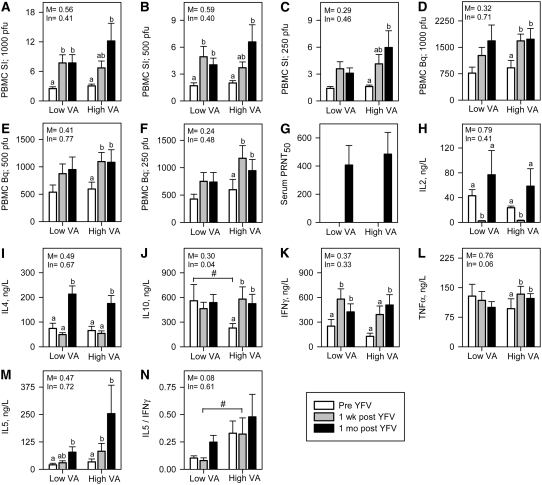
Vaccination induces development of antigen-specific T-cells that circulate in peripheral blood. Thus, assessment of antigen-specific lymphocyte proliferation in PBMC cultures is a useful index of response to immunization. In the present study, YFV-specific lymphocyte proliferation was assessed using 3 antigen doses at 1 wk and 1 mo after immunization. Proliferation was negligible before immunization and increased significantly in both groups at both 1 wk and 1 mo after immunization using at the 2 highest antigen doses (Fig. 1A,B). At the lowest antigen dose, the increase in SI was significant only in the high VA group and only 1 mo after immunization (Fig. 1C). The SI tended to differ between the low and high VA groups at 1 mo (P = 0.08). When proliferation was expressed as total Bq of 3H-thymidine incorporation, postvaccine incorporation increased significantly over the prevaccine levels for the high VA group but not for the low VA group at all antigen doses at both time points (Fig. 1D,F). Thus, the postvaccine increase in the YFV-specific proliferative response was greater in the high VA group than in the low VA group, although when the groups were compared directly, they did not significantly differ from one another (Fig. 1A–F).
FIGURE 1 .
Immune responses to live YFV vaccination in low VA (n = 16) and high VA (n = 17) groups. (A–C) PBMC SI in 3 different levels of YFV antigen. (D–F) 3H-thymidine incorporation in PBMC in 3 different levels of YFV antigen (G). Serum YFV-specific PRNT50; and (H–N) YFV-specific T-cell cytokines in PBMC. Values are means ± SE. Significance levels of the main effects (M) and interactions with time (In) are indicated. Labeled means within a group without a common letter differ, P < 0.05. #Groups differ at that time, P < 0.05.
Another important component of the vaccine-specific T-cell response is the production of growth (IL-2) and effector (IL-4, IL-5, IL-10, IFNγ, and TNFα) cytokines. Postvaccine increases in IL-2 concentrations ocurred 1 mo after immunization in both the low and high VA groups, although IL-2 levels were lower than baseline 1 wk after immunization (Fig. 1H).
YFV is a live virus vaccine and induces a predominant Th1 response. Two effector cytokines produced by Th1 cells were measured in this study, IFNγ and TNFα. Production of IFNγ increased significantly in both the high and low VA groups following YFV immunization and the increases were of similar magnitude (Fig. 1K). However, TNFα production increased significantly after immunization in the high VA group but not in the low VA group (Fig. 1L). The difference in the response trends was marginally significant (P = 0.06).
Il-4 and IL-5 are signature cytokines of Th2 cells. Both the high and low VA groups had significant postvaccine increases in IL-4 that were of similar magnitude (Fig. 1I). The postvaccine increase in IL-5 was significant in the low VA group 1 mo but not 1 wk after immunization, whereas this response was significant at both time points in the high VA group (Fig. 1M). When the IL-5:IFNγ ratio was evaluated as an index of the relative magnitude of the Th2 and Th1 responses, the high VA group had a significantly greater IL-5:IFNγ ratio 1 wk after immunization (Fig. 1N).
IL-10 is produced both by Th2 cells and regulatory T-cells. After YFV immunization, production of IL-10 increased significantly in the high VA group but not in the low VA group (Fig. 1J). Conversely, the baseline production of IL-10 was significantly greater in the low VA group than in the high VA group (Fig. 1J).
The principal indicator of YFV vaccine efficacy is the serum-neutralizing antibody response. This B-cell response is driven in part by cytokines produced by T-cells. In both the low and high VA groups, a substantial serum antibody response ocurred at 1 mo but not 1 wk after immunization (Fig. 1G). The magnitude of the responses did not differ between groups.
Recall TT vaccine responses in low and high VA groups.
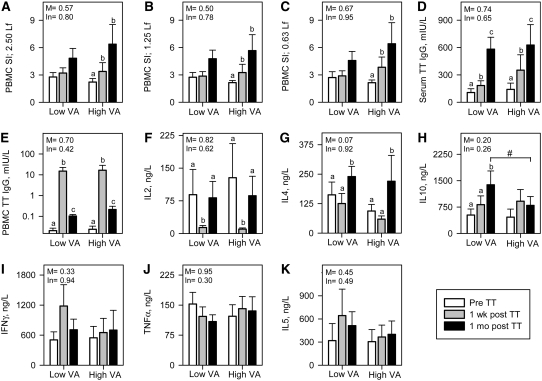
The TT vaccine is given routinely in Bangladesh and was used in our study to assess the effect of vitamin A on a secondary, or recall, response to immunization. Increased TT-specific lymphocyte proliferation was clearly evident at both 1 wk and 1 mo after immunization only in the high VA group (Fig. 2A–C). The magnitude of the responses did not differ between groups.
FIGURE 2 .
Immune responses to recall TT vaccination in low VA (n = 18) and high VA (n = 18) groups. (A–C) PBMC SI in 3 different levels of TT antigen. (D) Serum anti-TT IgG responses (E) PBMC-released spontenous anti-TT IgG. (F–K) TT-specific T-cell cytokines in PBMC. Values are means ± SE. Significance levels of the main effects (M) and interactions with time (In) are indicated. Labeled means within a group without a common letter differ, P < 0.05. #Groups differ at that time, P < 0.05.
Immunization did not induce significant TT-specific increases in the production of IL-2, IFNγ, TNFα, or IL-5 (Fig. 2F,I,J, and K, respectively) but did induce a significant increase in IL-4 in both the low and high VA groups (Fig. 2G). Interestingly, a TT-specific increase in IL-10 was seen in the low VA but not the high VA group and the concentration of IL-10 was significantly greater in the low than in the high VA group 1 mo after immunization (Fig. 2H).
Before vaccination, all subjects had serum anti-TT IgG titers above the minimum protective level (0.15 mIU/L). Serum titers increased significantly both 1 wk and 1 mo after immunization (Fig. 2D), as expected, and the magnitude of the responses did not differ between the 2 groups. In addition to measuring serum antibody, PBMC were cultured to allow production of TT-specific antibody by circulating plasma cells. The frequency of such cells was higher at 1 wk after immunization than at 1 mo after immunization judging by the antibody titers in culture supernatants (Fig. 2E). The magnitude of the response did not differ between the low and high VA groups.
Association of vaccine responses with vitamin A stores: correlation analysis.
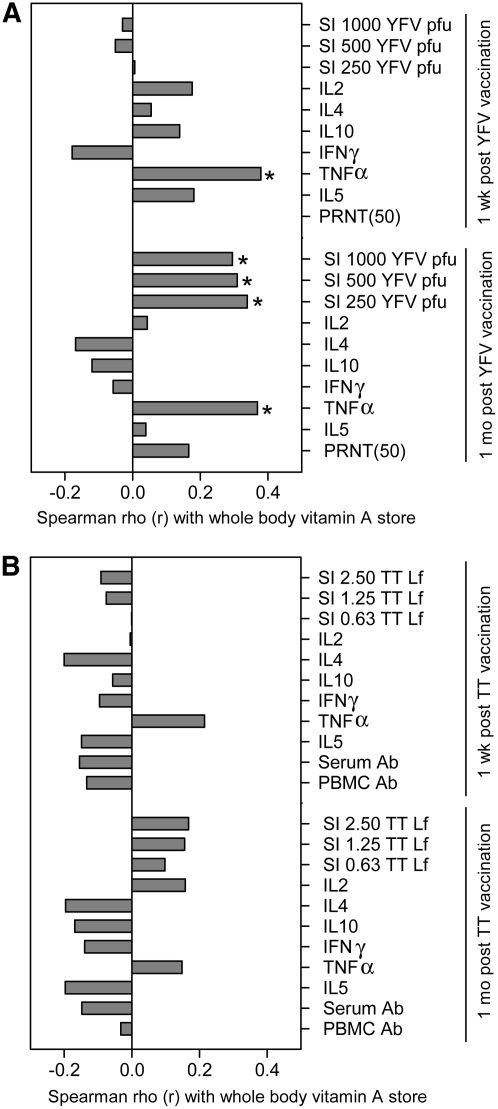
Correlation analysis was used to evaluate the association between vitamin A stores and immune function. We postulated that some measures of vaccine response would increase in a linear fashion with vitamin A stores. In agreement with this hypothesis, YFV-specific lymphocyte proliferation correlated significantly with liver vitamin A stores 1 mo but not 1 wk after immunization (Fig. 3A). In addition, YFV-stimulated production of TNFα correlated significantly with vitamin A stores at both time points (Fig. 3A). The IL-5:IFNγ ratio 1 wk after vaccination also had a marginally significant, positive correlation with vitamin A stores (r = 0.270; P = 0.07). Vitamin A stores and TT-specific responses were not associated, but the same variables that had positive associations with vitamin A stores for the YFV responses (i.e. antigen-specific proliferation and TNFα production) comprised 4 of the 5 TT response variables that also had positive rho values, although these correlations were not significant (P = 0.18–0.20) (Fig. 3B).
FIGURE 3 .
Spearman correlation between vaccine-specific immune responses and estimated whole body vitamin A pool size during 1 wk and 1 mo post vaccination: (A) YFV-specific responses, n = 30, and (B) TT-specific responses, n = 33. *P < 0.05.
Association of vaccine responses with vitamin A stores: segmented linear regression.
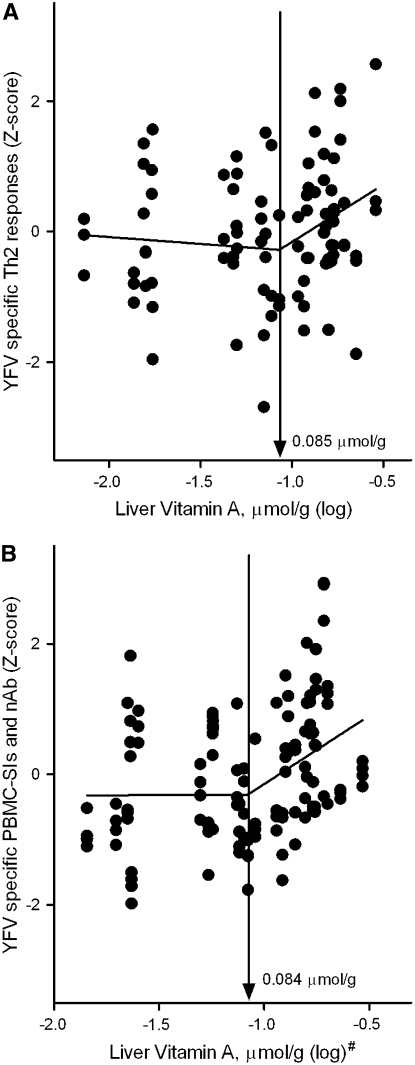
In addition to postulating that some immune response variables would have a linear association across all levels of vitamin A stores, we also postulated that a plateau effect might occur. Such a plateau could be detected by segmented linear regression. In mathematical terms, a plateau effect is a significant positive slope at low stores and a significantly lower slope (near 0, indicating a flat line) at higher stores. The point at which these 2 lines cross is the inflection point. Two inflection points were found for YFV-specific immune responses (Fig. 4). However, in contrast to our hypothesis, the inflection points represented a change from a slope near 0 at low vitamin A stores to a positive slope at higher stores. One inflection point was identified at a liver vitamin A concentration of 0.085 μmol/g (upper 95% CI: 0.246 μmol/g) in an analysis of the YFV-specific Th2 cytokines IL-4, IL-5, and IL-10 {regression line above break-point [slope (β) ± SE], 1.76 ± 0.79; P < 0.05; Fig. 4A}. The 2nd inflection point was at 0.084 μmol/g (upper 95% CI: 0.198 μmol/g) in an analysis of YFV-specific lymphocyte proliferation and serum neutralizing antibody titers {regression line above break-point [slope (β) ± SE], 2.09 ± 0.63; P < 0.05; Fig. 4B}. Inflection points were not found when TT-specific responses were analyzed.
FIGURE 4 .
Relationship between liver vitamin A concentrations and logical groupings of YFV vaccine responses (n = 30): (A) Th2 cytokines (IL-4, IL-5, and IL-10) at 1 wk postvaccination; and (B) 3 levels of antigen-induced PBMC SI and neutralizing antibody PRNT50 titer at 1 mo postvaccination. The Z-score-normalized responses for each group were plotted and the break-point estimates obtained from the 2-phase segmental linear regression model. #Liver vitamin A stores were estimated from total body vitamin A pool size 1 mo after vaccination (includeding oral isotopic dose) as described in Materials and Methods.
Discussion
Antigen-specific lymphocyte proliferation is a useful index of response to vaccination. By this measure, men in the high VA group had a marginally better response to both YFV and TT immunization than did men in the low VA group, as indicated by the significant increases in antigen-specific proliferation in the high VA but not low VA groups, and by the significant correlation of YFV-specific proliferation with vitamin A stores. Increased proliferation could be caused by several factors. IL-2 is an important growth factor for proliferating T-cells, but differences in IL-2 production were not seen between the low and high VA groups. However, the vitamin A metabolite retinoic acid can enhance IL-2–stimulated proliferation of T-cells by increasing cyclin D3 expression (27) and this mechanism could be responsible for the higher proliferation here. In addition, the low VA group had higher IL-10 levels in YFV-treated cultures before immunization than did the high VA group. This difference was not due to YFV-specific memory T-cells, because subjects were not yet immunized. The IL-10 could have come from regulating T-cells or monocytes stimulated by YFV via a pattern-recognition receptor such as toll-like receptor 3. IL-10 inhibits T-cell proliferation (28) and could thus have diminished YFV-stimulated T-cell proliferation, as reported for other viral infections (29,30). Vitamin A deficiency may enhance this phenomenon by further increasing IL-10 production. In addition, YFV-specific TNFα production was higher in the high VA group and can enhance proliferation by acting directly on T-cells (31,32) or indirectly by enhancing differentiation of antigen-presenting cells (33). Thus, the changes in YFV-specific IL-10 and TNFα production in these subjects could be responsible for the increased YFV-specific T-cell proliferation in the high VA group.
In these experiments, vaccine-specific IL-2 levels decreased significantly in both groups 1 wk after vaccination. This decrease presumably resulted from the rapid utilization of IL-2 by activated T-cells (34), thus triggering proliferation and differentiation into effector cells (35). In this experiment, we measured cytokine 6 d after stimulation. However, studies with other flavivirus-infected peripheral blood leukocyte cultures indicate the highest IL-2 release levels occur after 2 d and then diminish by 6 d (whereas the highest IL-10 response is detected at 6 d) (36). One month after immunization, an antigen-specific IL-2 response was seen in 6-d cultures, perhaps resulting from a greater number of IL-2–secreting memory cells at the later time point.
The YFV-specific IFNγ response did not differ between the 2 groups, but based on animal studies, we had predicted a decreased Th1 response in the high VA group (7). However, a modest shift toward a Th2 response occurred in the high VA group, which had a significantly higher YFV-specific IL-5 (particularly when evaluated as a ratio with IFNγ) and IL-10 responses than did the low VA group. This relative increase in IL-5 could also promote a greater serum antibody response.
We used TT immunization in this study to evaluate the effect of vitamin A on response to secondary immunization. As with YFV, the TT-specific increase in lymphocyte proliferation in response to immunization was significant in the high VA group but not in the low VA group. This finding is consistent with earlier results in an animal model where vitamin A enhanced TT-specific lymphocyte proliferation (37), although in 1 human study, there was no difference at 6 mo after vaccination (38).
Four of the 6 cytokines examined did not have TT-specific increases, including IL-2, IL-4, IL-5, IFNγ, and TNFα, although a significant TT-specific increase was seen for IL-4 1 mo after immunization for both groups. Interestingly, only the low VA group had a significant increase in TT-specific IL-10. This observation is reminiscent of a previous study in which a greater ovalbumin-specific IL-10 response was seen in vitamin A-deficient mice compared with mice receiving a control diet (4). These studies are similar in that both used a purified protein antigen given with a relatively noninflammatory adjuvant (alum in the case of the present study and incomplete Freund's in the previous study), suggesting that vitamin A deficiency may lead to an increased frequency of IL-10–producing T-cells in such cases.
The serum antibody response to TT immunization was not affected by vitamin A status in this study. Both groups mounted significant recall responses. Five other studies reported similar findings (39–43), although 1 study among children with confirmed vitamin A deficiency did report enhancement of the memory response to TT immunization (10). Subjects in the latter study had more profound vitamin A deficiency than did the subjects in the present study, as indicated by lower serum retinol concentrations and the presence of xerophthalmia in 1 of the study groups.
Segmented linear regression analysis was used to identify positive associations between vitamin A stores and 2 clusters of YFV-specific immune response variables 1): YFV-specific Th2 cytokine production (IL-4, IL-5, and IL-10); and 2) YFV-specific lymphocyte proliferation and serum antibody. These associations may be mechanistically related, because Th2 cytokines can promote both proliferation and antibody production. We had anticipated such responses would be positively correlated with vitamin A at the lowest storage levels and then might plateau at higher levels. However, the opposite trend occurred, in that a positive association was not seen at low storage levels but was seen at >0.084 μmol retinol/g liver. This observation suggests that the current 0.07 μmol/g index level for setting the EAR and RDA is adequate to maintain protective vaccine responses, particularly because all subjects developed protective antibody titers in response to both vaccines. However, increasing vitamin A stores above the 0.084 μmol/g level was associated with further increases in some measures of vaccine responsiveness. This observation raises the question of what immune function changes might be seen by further increasing stores to the even higher levels present in adults in the US and other industrialized countries (19,21,22,44–46). Might these high levels of vitamin A enhance chronic diseases mediated by antibody, Th2 cytokines, or TNFα? This possibility suggests that future evaluation of the tolerable upper intake for vitamin A intake might consider risk of adverse immune reactivity in addition to teratogenicity, hepatotoxicity, and fracture risk, which were used in setting the current upper intake (11).
This study had several strengths, including a double-blind, placebo-controlled design, a healthy and homogeneous group of subjects with low baseline vitamin A stores, a residential design to minimize environmental influences, and provision of a nutritionally balanced diet to eliminate dietary differences among subjects. In addition, a unique aspect of the study was the use of isotope dilution to provide a quantitative estimate of vitamin A stores. This allowed association of individual stores with immune function endpoints rather than relying solely on group comparisons. Such individualized data allowed the use of more powerful statistical techniques that revealed significant associations between pool size and immune function. Shortcomings of the study included the relatively small sample size and the lack of women among study subjects.
In summary, subjects with and without vitamin A deficiency (as defined by liver vitamin A stores < 0.07 μmol/g) responded adequately to immunization with primary YFV and secondary TT immunization in that all subjects developed protective antibody titers and these titers did not differ between the groups. However, vitamin A deficiency diminished vaccine-specific lymphocyte proliferation and the production of some cytokines, including IL-5, IL-10, and TNFα. The effect of vitamin A status on IL-10 differed by vaccine and may depend on the underlying level and type of immune stimulation produced by the vaccine adjuvant. Because these changes in immune response correlated directly with liver vitamin A stores, it would be prudent to consider if such “enhancement ” of immune function by high-level vitamin A intake might increase the risk of inflammatory diseases in subjects with genetic or other environmental risk factors that affect immune function.
Acknowledgments
We thank the following for their help and expertise: Xiaowen Jiang for estimating cytokines by multiplexed luminex assay, Alina Wettstein for measuring serum retinol, Emmanuel Aklamati for carrying out isotopic retinol measurements, Janet M. Peerson for advice on statistical analysis, and Proteim Sarker for conducting flow analysis and helping during in vitro method development. We also appreciate Dr. M. Jobayer Chisti and Dr. Kazi Jamil for monitoring health of the study subjects at ICDDR,B. Dhaka, Bangladesh.
Supported by the USDA Current Research Information System Project no. 5306-51530-013-00D and Specific Cooperative Agreement 58-5306-4-034F with International Centre for Diarrheal Disease Research, Bangladesh, Dhaka, Bangladesh. On-campus doctoral training of S.M.A. at UC Davis was supported by NIH research grant D43 TW01267, funded by the Fogarty International Center and the National Institute of Child Health and Human Development.
Author disclosures: S. Ahmad, M. Haskell, R. Raqib, and C. Stephensen, no conflicts of interest.
Abbreviations used: EAR, estimated average requirement; ICDDR,B, International Centre for Diarrheal Disease Research, Bangladesh; IFNγ, interferon-γ; IL, interleukin; PBMC, peripheral blood mononuclear cell; PRNT, plaque reduction neutralization test; RDA, recommended dietary allowance; SI, stimulation index; Th1, T helper type 1; TNFα, tumor necrosis factor-α; TT, tetanus toxoid; VA, vitamin A group; YFV, yellow fever virus.
References
- 1.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Hayes CE. Contrasting impairments in IgM and IgG responses of vitamin A-deficient mice. Proc Natl Acad Sci USA. 1987;84:5878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasatiempo AM, Kinoshita M, Taylor CE, Ross AC. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J. 1990;4:2518–27. [DOI] [PubMed] [Google Scholar]
- 4.Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr. 2004;134:2660–6. [DOI] [PubMed] [Google Scholar]
- 5.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–22. [PubMed] [Google Scholar]
- 6.Chen Q, Ross AC. Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci USA. 2005;102:14142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephensen CB, Blount SR, Schoeb TR, Park JY. Vitamin A deficiency impairs some aspects of the host response to influenza A virus infection in BALB/c mice. J Nutr. 1993;123:823–33. [DOI] [PubMed] [Google Scholar]
- 8.Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80:581–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semba RD, Muhilal, Scott AL, Natadisastra G, Wirasasmita S, Mele L, Ridwan E, West KP Jr, Sommer A. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr. 1992;122:101–7. [DOI] [PubMed] [Google Scholar]
- 11.Panel on Micronutrients, Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001. [PubMed]
- 12.Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta-analysis. JAMA. 1993;269:898–903. [PubMed] [Google Scholar]
- 13.Glasziou PP, Mackerras DE. Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ. 1993;306:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates CJ. Vitamin A. Lancet. 1995;345:31–5. [DOI] [PubMed] [Google Scholar]
- 15.Haskell MJ, Mazumder RN, Peerson JM, Jones AD, Wahed MA, Mahalanabis D, Brown KH. Use of the deuterated-retinol-dilution technique to assess total-body vitamin A stores of adult volunteers consuming different amounts of vitamin A. Am J Clin Nutr. 1999;70:874–80. [DOI] [PubMed] [Google Scholar]
- 16.Darnton-Hill I, Hassan N, Karim R, Duthie MR. Tables of nutrient composition of Bangladesh foods (particular emphasis on vitamin A content). Dhaka: Helen Keller International, Bangladesh and World Food Programme; 1988.
- 17.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI Jr, Gammon RB Jr. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr. 1994;60:388–92. [DOI] [PubMed] [Google Scholar]
- 18.Haskell MJ, Jamil KM, Hassan F, Peerson JM, Hossain MI, Fuchs GJ, Brown KH. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr. 2004;80:705–14. [DOI] [PubMed] [Google Scholar]
- 19.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR III, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr. 1989;49:713–6. [DOI] [PubMed] [Google Scholar]
- 20.Olson JA. Recommended dietary intakes (RDI) of vitamin A in humans. Am J Clin Nutr. 1987;45:704–16. [DOI] [PubMed] [Google Scholar]
- 21.Raica N Jr, Scott J, Lowry L, Sauberlich HE. Vitamin A concentration in human tissues collected from five areas in the United States. Am J Clin Nutr. 1972;25:291–6. [DOI] [PubMed] [Google Scholar]
- 22.Underwood BA. Hypovitaminosis A: international programmatic issues. J Nutr. 1994;124:S1467–72. [DOI] [PubMed] [Google Scholar]
- 23.Chang HS, Sack DA. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin Diagn Lab Immunol. 2001;8:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. The immunological basis for immunization series. Module 3: tetanus. Geneva: WHO; 1993.
- 25.Russell PK, Nisalak A. Dengue virus identification by the plaque reduction neutralization test. J Immunol. 1967;99:291–6. [PubMed] [Google Scholar]
- 26.Martin NC, Pardo J, Simmons M, Tjaden JA, Widjaja S, Marovich MA, Sun W, Porter KR, Burgess TH. An immunocytometric assay based on dengue infection via DC-SIGN permits rapid measurement of anti-dengue neutralizing antibodies. J Virol Methods. 2006;134:74–85. [DOI] [PubMed] [Google Scholar]
- 27.Engedal N, Gjevik T, Blomhoff R, Blomhoff HK. All-trans retinoic acid stimulates IL2-mediated proliferation of human T lymphocytes: early induction of cyclin D3. J Immunol. 2006;177:2851–61. [DOI] [PubMed] [Google Scholar]
- 28.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–71. [PubMed] [Google Scholar]
- 29.Brady MT, MacDonald AJ, Rowan AG, Mills KH. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL10 production from monocytes. Eur J Immunol. 2003;33:3448–57. [DOI] [PubMed] [Google Scholar]
- 30.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL10 production and T cell regulation. Proc Natl Acad Sci USA. 2004;101:7669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shalaby MR, Espevik T, Rice GC, Ammann AJ, Figari IS, Ranges GE, Palladino MA Jr. The involvement of human tumor necrosis factors-alpha and -beta in the mixed lymphocyte reaction. J Immunol. 1988;141:499–503. [PubMed] [Google Scholar]
- 32.Yokota S, Geppert TD, Lipsky PE. Enhancement of antigen- and mitogen-induced human T lymphocyte proliferation by tumor necrosis factor-alpha. J Immunol. 1988;140:531–6. [PubMed] [Google Scholar]
- 33.Geissmann F, Revy P, Brousse N, Lepelletier Y, Folli C, Durandy A, Chambon P, Dy M. Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med. 2003;198:623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii M, Sugamura K, Sano K, Nakai M, Sugita K, Hinuma Y. High-affinity receptor-mediated internalization and degradation of interleukin 2 in human T cells. J Exp Med. 1986;163:550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL2. Nat Rev Immunol. 2004;4:665–74. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi UC, Elbishbishi EA, Agarwal R, Raghupathy R, Nagar R, Tandon R, Pacsa AS, Younis OI, Azizieh F. Sequential production of cytokines by dengue virus-infected human peripheral blood leukocyte cultures. J Med Virol. 1999;59:335–40. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA. 2005;102:13556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer TR, Udomkesmalee E, Dhanamitta S, Sirisinha S, Charoenkiatkul S, Tuntipopipat S, Banjong O, Rojroongwasinkul N, Smith JC Jr. Lymphocyte responsiveness of children supplemented with vitamin A and zinc. Am J Clin Nutr. 1993;58:566–70. [DOI] [PubMed] [Google Scholar]
- 39.Newton S, Cousens S, Owusu-Agyei S, Filteau S, Stanley C, Linsell L, Kirkwood B. Vitamin a supplementation does not affect infants' immune responses to polio and tetanus vaccines. J Nutr. 2005;135:2669–73. [DOI] [PubMed] [Google Scholar]
- 40.Brown KH, Rajan MM, Chakraborty J, Aziz KM. Failure of a large dose of vitamin A to enhance the antibody response to tetanus toxoid in children. Am J Clin Nutr. 1980;33:212–7. [DOI] [PubMed] [Google Scholar]
- 41.Kutukculer N, Akil T, Egemen A, Kurugol Z, Aksit S, Ozmen D, Turgan N, Bayindir O, Caglayan S. Adequate immune response to tetanus toxoid and failure of vitamin A and E supplementation to enhance antibody response in healthy children. Vaccine. 2000;18:2979–84. [DOI] [PubMed] [Google Scholar]
- 42.Rahman MM, Mahalanabis D, Hossain S, Wahed MA, Alvarez JO, Siber GR, Thompson C, Santosham M, Fuchs GJ. Simultaneous vitamin A administration at routine immunization contact enhances antibody response to diphtheria vaccine in infants younger than six months. J Nutr. 1999;129:2192–5. [DOI] [PubMed] [Google Scholar]
- 43.Bhaskaram P, Jyothi SA, Rao KV, Rao BSN. Effects of subclinical vitamin a deficiency and administration of vitamin-A as a single large dose on immune function in children. Nutr Res. 1989;9:1017–25. [Google Scholar]
- 44.Hoppner K, Phillips WE, Erdody P, Murray TK, Perrin DE. Vitamin A reserves of Canadians. Can Med Assoc J. 1969;101:84–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell GV, Young M, Seward CR. Vitamin A and carotene levels of a selected population in metropolitan Washington, D. C. Am J Clin Nutr. 1973;26:992–7. [DOI] [PubMed] [Google Scholar]
- 46.Schindler R, Friedrich DH, Kramer M, Wacker HH, Feldheim W. Size and composition of liver vitamin A reserves of human beings who died of various causes. Int J Vitam Nutr Res. 1988;58:146–54. [PubMed] [Google Scholar]