Abstract
Previous studies showed that dietary l-arginine supplementation decreased white fat mass in genetically obese rats. This study tested the effectiveness of l-arginine in diet-induced obesity. Male Sprague-Dawley rats were fed for 15 wk a high-fat (HF) (40% energy) or low-fat (LF) (10% energy) diet beginning at 4 wk of age, resulting in 18% higher body weight gains and 74% higher weights of major white fat pads (retroperitoneal, epididymal, subcutaneous, and mesenteric adipose tissues) in HF than in LF fed rats. Starting at 19 wk of age, rats in each dietary group were supplemented for 12 wk with 1.51% l-arginine-HCl or 2.55% l-alanine (isonitrogenous control) (n = 8 per treatment) in drinking water and arginine groups were individually pair-fed to alanine controls. Despite similar energy intake, absolute weights of white fat pads increased by 98% in control rats over a 12-wk period but only by 35% in arginine-supplemented rats. The arginine treatment reduced the relative weights of white fat pads by 30% and enhanced those of soleus muscle by 13%, extensor digitorum longus muscle by 11%, and brown fat by 34% compared with control rats. Serum concentrations of insulin, adiponectin, growth hormone, corticosterone, triiodothyronine, and thyroxine did not differ between control and arginine-supplemented rats. However, arginine treatment resulted in lower serum concentrations of leptin, glucose, triglycerides, urea, glutamine, and branched-chain amino acids, higher serum concentrations of nitric-oxide metabolites, and improvement in glucose tolerance. Thus, dietary arginine supplementation shifts nutrient partitioning to promote muscle over fat gain and may provide a useful treatment for improving the metabolic profile and reducing body white fat in diet-induced obese rats.
Introduction
Obesity is caused by a chronic imbalance in energy metabolism, namely a greater energy intake than energy expenditure (1). This metabolic disorder has continued to increase worldwide at an alarming rate in the past decade and affects both adults and children (2). Particularly, obesity is closely associated with many diseases and is a major risk factor for insulin resistance, type II diabetes, atherosclerosis, stroke, hypertension, and some types of cancer (3). The prevalence of obesity and the tremendous costs of its treatment necessitate the search for new alternative nutritional means.
l-Arginine, a conditionally essential amino acid (AA)7 for adult mammals, is a precursor for the synthesis of biologically important molecules, including nitric oxide (NO), polyamines, creatine, agmatine, proline, and glutamate (4). Available evidence shows that physiological levels of arginine and NO promote fat oxidation and decrease fat synthesis in a tissue-specific manner (5). Mice with the knockout of endothelial NO synthase had a higher body fat weight than the wild-type mice with normal expression of the protein despite similar food intake between the 2 groups (6). Additionally, inhibition of systemic NO synthesis increased circulating levels of triglycerides and body fat mass in rats (7). In our recent studies aimed at improving vascular function in Zucker diabetic fatty (ZDF) rats, we found that dietary supplementation with l-arginine selectively reduced white fat mass while increasing expression of the genes for AMP-activated protein kinase and PPARγ coactivator-1α (master regulators of mitochondrial oxidation) (8). The ZDF rat has a defect in the leptin receptor and is a genetically obese animal model of type II diabetes (9). At present, the relevance of our finding of the fat-reducing effect of arginine in ZDF rats to more common diet-induced obesity is not known. Therefore, this study determined if dietary arginine supplementation could decrease fat gain and improve glucose tolerance in diet-induced obese (DIO) rats.
Materials and Methods
Chemicals.
Hexokinase and glucose-6-phosphate dehydrogenase were obtained from Roche Diagnostics. HPLC-grade methanol and water were purchased from Fisher Scientific. Unless indicated, all other chemicals were obtained from Sigma-Aldrich.
Animals and diets.
This study was approved by the Institutional Animal Care and Use Committee of Texas A&M University. Male Sprague-Dawley rats (23-d-old, 80–100 g) were purchased from Harlan Laboratories. Upon arrival at the Texas A&M University Kleberg animal facilities, all rats were housed individually in carbonate cages in a temperature- and humidity-controlled room on a 12-h- light:12-h-dark cycle. After a 5-d period of adaptation during which rats were fed a regular nonpurified diet (product catalog no. 8604, Harlan Teklad), they were randomly assigned to either a low-fat (LF) or high-fat (HF) diet (n = 24/diet) obtained from Research Diets (Table 1). The LF (4.3% fat) and HF (23.6% fat) diets provided 10 and 40% of total energy as lipids (mainly lard), respectively, and contained no nitrite or nitrate. The ratios of protein, vitamins, minerals, and fiber to energy were constant in the LF and HF diets. The body weight of rats assigned to the LF and HF groups at 4 wk of age were 98.2 ± 2.0 and 98.5 ± 1.8 g, respectively. Body weight and food intake were recorded on a weekly basis between 4 and 19 wk of age (the phase of obesity induction). After the 15-wk HF or LF feeding, 8 rats from each diet group were killed to obtain tissues (10) and the remaining rats in the HF or LF group were divided randomly into 2 subgroups, which continued to be fed their same respective diets and received drinking water containing either 1.51% l-arginine-HCl or 2.55% l-alanine (isonitrogenous control) (n = 8/sub-group). Thus, there were 4 treatment groups: LF diet + l-alanine supplementation (LF-Ala); LF diet + l-arginine supplementation (LF-Arg); HF diet + l-alanine supplementation (HF-Ala); HF-diet + l-arginine supplementation (HF-Arg). The drinking water was provided to rats daily. The dosages of arginine and alanine were chosen on the basis of our previous studies with nondiabetic and diabetic rats (8,11). l-Alanine was chosen as isonitrogenous control primarily because of its extensive catabolism in the body, its safety, and its inability as a precursor for endogenous synthesis of arginine (4). In a separate study, we found that supplementing l-alanine (2.55% in drinking water) to 19-wk-old lean or DIO rats for 12 wk did not affect body weight or the weights of white fat, brown fat, and skeletal muscle compared with rats that did not receive supplementation of any AA in drinking water (G. Wu and C.J. Meininger, unpublished data).
TABLE 1.
Composition of LF and HF diets
| LF diet1 |
HF diet2 |
|||
|---|---|---|---|---|
| Ingredient | Composition, % | kJ/kg diet | Composition, % | kJ/kg diet |
| Casein | 18.96 | 3173 | 23.31 | 3901 |
| l-Cystine | 0.284 | 48 | 0.350 | 59 |
| Corn starch | 29.85 | 4996 | 8.48 | 1419 |
| Maltodextrin-10 | 3.32 | 556 | 11.65 | 1950 |
| Sucrose | 33.17 | 5552 | 20.14 | 3371 |
| Cellulose | 4.74 | 0 | 5.83 | 0 |
| Soybean oil | 2.37 | 892 | 2.91 | 1096 |
| Lard | 1.90 | 715 | 20.68 | 7788 |
| Mineral mix S100263 | 0.95 | 63 | 1.17 | 78 |
| Dicalcium phosphate | 1.23 | 0 | 1.51 | 0 |
| Calcium carbonate | 0.521 | 0 | 0.641 | 0 |
| Potassium citrate | 1.56 | 0 | 1.92 | 0 |
| Vitamin mix V100014 | 0.95 | 159 | 1.17 | 196 |
| Choline bitartrate | 0.19 | 0 | 0.233 | 0 |
| Yellow dye | 0.005 | 0 | — | — |
| Red dye | — | — | 0.006 | 0 |
| Total | 100 | 16,155 | 100 | 19,858 |
Containing 67.3% carbohydrate, 4.3% fat, and 19.2% protein on an as-fed basis.
Containing 41.0% carbohydrate, 23.6% fat, and 20.89% protein on an as-fed basis.
Containing the following (g/kg mineral mix): magnesium oxide, 41.9; magnesium sulfate.7H2O, 257.6; sodium chloride, 259; chromium KSO4.12H2O, 1.925; cupric carbonate, 1.05; potassium iodate, 0.035; ferric citrate, 21; manganous carbonate, 12.25; sodium selenite, 0.035; zinc carbonate, 5.6; sodium fluoride, 0.20; ammonium molybdate.4H2O, 0.30; sucrose, 399.105. Sucrose in the mineral mix provided 63 kJ energy/kg diet.
Containing the following (g/kg vitamin mix): retinyl palmitate, 0.80; cholecalciferol, 1.0; all-rac-α-tocopheryl acetate, 10; menadione sodium bisulfite, 0.08; biotin (1.0%), 2.0; cyancocobalamin (0.1%), 1.0; folic acid, 0.20; nicotinic acid, 3.0; calcium pantothenate, 1.6; pyridoxine-HCl, 0.70; riboflavin, 0.60; thiamin-HCl, 0.60; and sucrose, 978.42. Sucrose in the vitamin mix provided 159 kJ energy/kg diet.
Because our pilot studies showed that arginine-supplemented rats tended to eat more than control rats, arginine-supplemented rats within the LF or HF diet were individually pair-fed with alanine-supplemented rats on a kilogram-body weight basis to ensure similar intakes of all nutrients (except for arginine and alanine) between the 2 groups. Body weight, food intake, and water intake of each rat were recorded on a daily basis throughout this amino-acid supplementation phase of the study. No spillage of food (pellet form) was noted for any group of rats. After 12 wk of arginine supplementation, rats were food deprived for 5 h to obtain blood samples (100 μL) from the tail vein using a microhematocrit (12) for analyses of serum glucose and AA. Rats were then immediately anesthetized with CO2 and killed by cervical dislocation. Cardiac blood samples were collected and centrifuged immediately to obtain sera for analyses of lipids and hormones. In addition, retroperitoneal (RP), epididymal (EP), subcutaneous (SC; inguinal), and mesenteric (MT) fat tissues, as well as brown adipose tissue (located in the interscapular region), extensor digitorum longus (EDL), and soleus muscles, brain, kidney, and other tissues were dissected and weighed. The intestinal lumen content was removed before weighing. Small portions of each tissue were either used freshly for metabolic assays or snap-frozen rapidly in liquid nitrogen for storage at −80°C.
Oral glucose tolerance test.
An oral glucose tolerance test (OGTT) was performed at 10 wk after the initiation of arginine supplementation as described by Vital et al. (13) with modifications. After a 5-h period of food deprivation, glucose (2 g/kg body weight) in water was administrated orally into stomach by gavage. Blood samples (20 μL) were obtained from the tail vein into plain tubes using microhematocrit capillary tubes at 0, 30, 60, 90, 120, 150, and 180 min postgavage, as previously described (12). Blood samples were centrifuged immediately at 10,000 × g; 1 min to obtain sera, which were stored at −80°C until analysis for glucose. Because of insufficient serum samples, insulin assays were not performed in the OGTT.
Biochemical analyses of serum.
Glucose was measured enzymatically using a fluorometric method involving hexokinase and glucose-6-phosphate dehydrogenase (11). AA were quantified using fluorometric HPLC methods after a derivatization reaction with o-phthaldialdehyde (14). Urea was analyzed using a colorimetric method involving urease, phenol, and hypochlorite (15). Nitrite and nitrate (oxidation products of NO) were measured using a fluorescence HPLC method (16). Assay kits were employed for the analysis of triglycerides (catalog no. 2780–250; Thermo DMA), total cholesterol (catalog no. 2350–250; Thermo DMA), FFA (catalog no. 994–75409; Wako Chemicals), total triiodothyronine (catalog no. 06-B256447; MP Biomedicals), total thyroxine (catalog no. 06-B254011; MP Biomedicals), insulin (catalog no. RI-13K for rat; Linco), leptin (catalog no. RL-83K for rat; Linco), adiponectin (catalog no. MADP-60HK for mouse; Linco), growth hormone (catalog no. RGH-45HK for rat; Linco), and corticosterone (catalog no. 07–120002 for rat; MP Biomedicals). The precision of the assays (agreement between replicate measurements) was as follows: 0.8% for glucose, 1.3–1.7% for AA (14), 1.8% for urea, 0.9% for nitrite (16), 0.4% for nitrate (16), 1.7% for triglycerides, 2.2% for total cholesterol, 1.1% for FFA, 2.9% for total triiodothyronine, 2.6% for total thyroxine, 2.5% for insulin, 3.0% for leptin, 3.3% for adiponectin, 3.6% for growth hormone, and 3.2% for corticosterone. The accuracy of the assays (the nearness of an experimental value to the true value) was as follows: 1.2% for glucose, 1.6–2.2% for AA (14), 2.0% for urea, 1.5% for nitrite (16), 1.7% for nitrate (16), 2.1% for triglycerides, 1.8% for total cholesterol, 1.5% for FFA, 3.3% for total triiodothyronine, 3.6% for total thyroxine, 2.8% for insulin, 4.4% for leptin, 5.6% for adiponectin, 4.9% for growth hormone, and 5.1% for corticosterone.
Determination of cellularity of RP adipose tissue.
Adipocytes were isolated from RP fat pad using collagenase digestion, as described by Rodbell (17). Adipocyte size and density were measured according to the microscopic method of DiGirolamo et al. (18).
Biochemical analyses of liver, skeletal muscle, and adipose tissue.
Lipids in liver, gastrocnemius muscle, and RP adipose tissue were determined using the Folch method, in which a chloroform and methanol mixture (2:1, v:v) was used to extract lipid from tissues (19). Glycogen in liver and gastrocnemius muscle was measured by an enzymatic method involving amyloglucosidase, as described previously (20). Glutathione, an indicator of oxidative stress (21), was determined by an HPLC method after derivatization with dansyl chloride (22). Tetrahydrobiopterin, an essential factor for NO synthesis (23), was analyzed using an HPLC method (24).
Calculations and statistical analysis.
Results were expressed as means ± SEM. Data on body weight were analyzed using the growth curve model, namely a mixed-effect model that fits fixed effects and random effects (25,26). The fixed effects include diet, AA treatment, their interaction (diet × AA), a 3rd order polynomial of age in weeks (slope by age effect, curvature by age × age, and 3rd order effect by age × age × age), different slopes by diet (age × diet), and different slopes by treatment (age × AA). The random effects allowed different rats to have different slopes (random age). We used the SAS PROC MIXED procedure to fit the mixed effects model (SAS Institute). The OGTT was assessed by calculating the area under the curve (AUC) (13). Data on tissue weights, biochemical metabolites, and OGTT were analyzed statistically by 2-way ANOVA using a General Linear Model by SPSS software (version 12.0). The Tukey multiple comparison test was used to identify which specific means differed when the F test for treatment effect was significant. Data on body weights and tissue weights of rats killed at 19 wk of age were analyzed by unpaired t test. Probability values ≤ 0.05 were considered significant.
Results
Intakes of food, water, and energy.
The HF-fed rats consumed the same amount of dietary energy as the LF-fed rats per kg body weight during the 12-wk period of arginine supplementation (Table 2). Neither HF diet nor arginine supplementation affected water consumption by rats (Table 2). As a result, intakes of energy or nutrients (including alanine and arginine) from enteral diets did not differ among the 4 groups of rats during the entire period of arginine supplementation (Table 2).
TABLE 2.
Intakes of food, water, and energy by rats during a 12-wk period of receiving a LF or HF diet and water containing either 1.51% l-arginine·HCl or 2.55% l-alanine1
| Groups |
P-value |
||||||
|---|---|---|---|---|---|---|---|
| Intake | LF-Ala | LF-Arg | HF-Ala | HF-Arg | Diet | AA | Diet × AA |
| Food, g/(kg body wt·d) | 36.4 ± 0.5a | 36.2 ± 0.6a | 29.7 ± 0.3b | 29.5 ± 0.3b | <0.01 | 0.78 | 0.95 |
| Water, mL/(kg body wt·d) | 70.8 ± 1.1 | 77.7 ± 1.8 | 74.8 ± 1.8 | 73.8 ± 1.9 | 0.97 | 0.46 | 0.30 |
| Protein, g/(kg body wt·d) | 6.19 ± 0.1 | 6.15 ± 0.1 | 6.20 ± 0.1 | 6.16 ± 0.06 | 0.71 | 0.64 | 0.78 |
| Minerals,2mg/(kg body wt·d) | 208 ± 3.1 | 207 ± 3.4 | 209 ± 2.2 | 207 ± 2.3 | 0.88 | 0.82 | 0.92 |
| Vitamins,3mg/(kg body wt·d) | 7.61 ± 0.11 | 7.57 ± 0.13 | 7.64 ± 0.08 | 7.59 ± 0.09 | 0.74 | 0.71 | 0.80 |
| Energy,4kJ/(kg body wt·d) | 611 ± 8.8 | 602 ± 9.6 | 615 ± 6.2 | 601 ± 6.5 | 0.96 | 0.82 | 0.32 |
| Alanine from food, mg/(kg body wt·d) | 160 ± 2.4 | 159 ± 2.6 | 160 ± 1.7 | 159 ± 1.8 | 0.79 | 0.69 | 0.79 |
| Alanine from drinking water, mg/(kg body wt·d) | 1805 ± 28 | – | 1907 ± 46 | – | 0.11 | – | – |
| Arginine from food, mg/(kg body wt·d) | 222 ± 3.3 | 221 ± 3.7 | 223 ± 2.3 | 221 ± 2.5 | 0.81 | 0.71 | 0.81 |
| Arginine from drinking water, mg/(kg body wt·d) | – | 969 ± 23 | – | 922 ± 24 | 0.64 | – | – |
Values are means ± SEM, n = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
Provided from mineral mix S10026.
Provided from vitamin mix V10001.
Total energy intake from diet plus drinking water.
Body weights at the beginning and end of arginine supplementation.
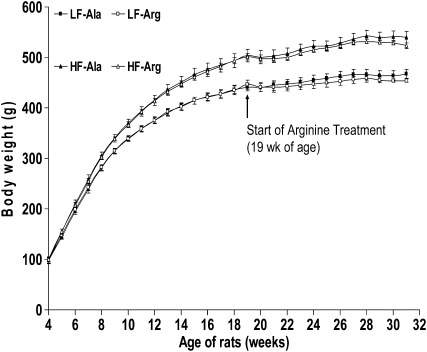
At 19 wk of age (the beginning of arginine supplementation), the body weights of rats fed the LF and HF diets for 15 wk were 443.5 ± 3.2 and 502.5 ± 4.0 g (n = 16), respectively (P < 0.01). Rats fed the HF diet gained more body weight (P < 0.01) than rats fed the LF diet between 4 and 19 wk of age (Fig. 1). The 15-wk HF feeding resulted in an 18% higher weight gain (P < 0.01) in the HF-fed than in LF-fed rats (Table 3). At 31 wk of age (the end of the study), body weights were 467.5 ± 8.8, 454.0 ± 3.1, 540.2 ± 12, and 523.2 ± 5.7 g (n = 8), respectively, for LF-Ala, LF-Arg, HF-Ala, and HF-Arg groups, and the body weight of the HF-fed rats was 15.3% greater (P < 0.01) than that of the LF-fed rats (Fig. 1). Arginine supplementation for 12 wk decreased (P < 0.01) the body weight gains of LF- and HF-fed rats by 60 and 40%, respectively, compared with control groups (Table 4). The long-term arginine treatment did not result in any adverse effect on LF or HF rats.
FIGURE 1 .
Body weights of rats receiving a LF or HF diet before and after the initiation of supplementation with 1.51% l-arginine·HCl or 2.55% l-alanine via drinking water. Values are means ± SEM, n = 8. P (diet) < 0.01; P (AA) = 0.07; P (diet × AA) < 0.01; P (age) < 0.01; P (AA × age) = 0.01; P (diet × age) < 0.01; P (age × age) < 0.01; P (age × age × age) < 0.01; P (age × diet × AA) < 0.01.
TABLE 3.
Body weights, absolute tissue weights, and adiposity index of 19-wk-old rats at the end of a 15-wk period of LF or HF feeding1
| Body or tissue weight | LF diet | HF diet |
|---|---|---|
| Body weight, g | 442.6 ± 8.5 | 506.3 ± 10† |
| Tissue weight, g | ||
| RP adipose tissue | 3.29 ± 0.27 | 5.50 ± 0.42† |
| EP adipose tissue | 4.01 ± 0.31 | 6.63 ± 0.44† |
| SC adipose tissue | 2.96 ± 0.20 | 5.88 ± 0.39† |
| MT adipose tissue | 1.74 ± 0.13 | 2.80 ± 0.22† |
| Major white fat pads2 | 12.0 ± 0.78 | 20.9 ± 1.10† |
| Brown adipose tissue | 0.63 ± 0.05 | 0.66 ± 0.04 |
| Soleus muscle | 0.160 ± 0.004 | 0.183 ± 0.005* |
| EDL muscle | 0.168 ± 0.005 | 0.185 ± 0.007* |
| Adiposity index,3% | 2.70 ± 0.19 | 4.15 ± 0.24† |
Values are means ± SEM, n = 8. *Different from the LF group, P < 0.05; †P < 0.01.
The sum of RP, EP, SC, and MT adipose tissues.
(RP + EP + SC + MT adipose tissues)/body weight × 100.
TABLE 4.
Gains of body weight and major white fat pads, as well as the relative weights of adipose tissues and skeletal muscles in 31-wk-old rats at the end of a 12-wk period of consuming LF or HF diet and water containing either 1.51% l-arginine·HCl or 2.55% l-alanine1
|
P-value |
|||||||
|---|---|---|---|---|---|---|---|
| Body weight gains or tissue weights | LF-Ala | LF-Arg | HF-Ala | HF-Arg | Diet | AA | Diet × AA |
| Gains of body weights and major white fat pads,2g | |||||||
| Body weights | 26.3 ± 2.2b | 8.4 ± 0.86c | 36.7 ± 3.4a | 22.0 ± 1.9b | <0.01 | <0.01 | 0.47 |
| Major white fat pads3 | 12.8 ± 1.0b | 4.5 ± 0.05d | 19.4 ± 1.3a | 7.1 ± 0.52c | <0.01 | <0.01 | 0.63 |
| Relative weight of tissue (tissue weight/body weight x 100), % | |||||||
| RP adipose tissue | 1.58 ± 0.12b | 0.98 ± 0.06c | 2.08 ± 0.21a | 1.43 ± 0.08b | <0.01 | <0.01 | 0.60 |
| EP adipose tissue | 1.59 ± 0.11c | 1.29 ± 0.07d | 2.05 ± 0.13a | 1.67 ± 0.10b | <0.01 | <0.01 | 0.20 |
| SC adipose tissue | 1.46 ± 0.11c | 1.00 ± 0.06d | 2.46 ± 0.21a | 1.70 ± 0.14b | <0.01 | <0.01 | 0.31 |
| MT adipose tissue | 0.68 ± 0.07b | 0.38 ± 0.03c | 0.85 ± 0.05a | 0.55 ± 0.04b | <0.01 | <0.01 | 0.69 |
| Adiposity index4 | 5.29 ± 0.34b | 3.63 ± 0.20c | 7.46 ± 0.49a | 5.34 ± 0.31b | <0.01 | <0.01 | 0.65 |
| Brown adipose tissue | 0.122 ± 0.005b | 0.161 ± 0.006a | 0.115 ± 0.007b | 0.156 ± 0.008a | 0.42 | <0.01 | 0.93 |
| Soleus muscle | 0.034 ± 0.001b | 0.390 ± 0.001a | 0.034 ± 0.001b | 0.038 ± 0.001a | 0.77 | <0.01 | 0.77 |
| EDL muscle | 0.036 ± 0.001b | 0.040 ± 0.001a | 0.035 ± 0.001b | 0.039 ± 0.001a | 0.08 | <0.01 | 0.87 |
| Cellularity of RP adipose tissue | |||||||
| Adipocyte size,5μm | 90.4 ± 1.9b | 77.1 ± 3.1c | 99.9 ± 2.8a | 92.7 ± 2.5b | <0.01 | <0.01 | 0.26 |
| Total adipocytes, n × 10−6 | 15.6 ± 1.6 | 14.4 ± 0.7 | 17.2 ± 0.9 | 15.1 ± 1.1 | 0.33 | 0.14 | 0.70 |
| Adipocyte density, n × 10−6/g tissue | 2.13 ± 0.16b | 3.27 ± 0.27a | 1.62 ± 0.11c | 2.05 ± 0.14b | <0.01 | <0.01 | 0.22 |
Values are means ± SEM, n = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
During the period of AA supplementation between 19 and 31 wk of age.
Gains of major white fat pads (RP + EP + SC + MT adipose tissues) were calculated on the basis of the mean values for LF- and HF-fed rats at 19 wk of age (the beginning of AA supplementation).
(RP + EP + SC + MT adipose tissues)/body weight × 100.
Diameter of the cell.
Tissue weight at the beginning and end of arginine supplementation.
At the beginning of arginine supplementation (19 wk of age), rats fed the HF diet for 15 wk had heavier (P < 0.05) absolute weights of major white fat pads (RP + EP + SC + MT adipose tissue; 74%), soleus muscle (14%), and EDL muscle (10%), as well as a higher (P < 0.01) adiposity index (54%) than the LF groups (Table 3). This 15-wk HF feeding enhanced (P < 0.05) the absolute weights of heart (16%), lungs (17%), liver (13%), kidneys (10%), and small intestine (15%) but did not affect those of other tissues (spleen, brown adipose tissue, pancreas, testes, or brain) compared with the LF diet (data not shown).
At the end of the study (31 wk of age), the HF group had heavier weights of major white fat pads (65%), soleus muscle (14%), and EDL muscle (11%), as well as liver (14%), heart (18%), lungs (21%), kidneys (8%), and small intestine (15%) than the LF group (Supplemental Table 1). During the 12-wk period of AA supplementation, absolute major white fat pad weights increased (P < 0.01) by 107, 38, 92, and 33% in LF-Ala, LF-Arg, HF-Ala, and HF-Arg groups, respectively. However, except for white adipose tissue (Table 4), testes, and brain (Supplemental Table 2), the relative weights of other tissues (expressed as a percentage of the body weight) did not differ between the LF- and HF-fed rats (Supplemental Table 2).
Alanine-supplemented rats fed the LF or HF diet did not gain muscle weight between 19 and 31 wk of age (Supplemental Table 1). At the end of the AA supplementation, the absolute weights of major white fat pads were lower (−32%; P < 0.01; Table 4), but those of soleus muscle (8.7%), EDL muscle (6.4%), and brown fat (29%) were higher (P < 0.05) in arginine- than in alanine-supplemented rats (Supplemental Table 1). The 12-wk arginine treatment decreased (P < 0.01) the gains of major white fat pads by 64% compared with the control groups (Table 4). The relative weights of major white fat pads (adiposity index) were 30% lower (P < 0.01), whereas those of soleus muscle, EDL muscle, and brown fat were 13, 11, and 34% higher (P < 0.05), respectively, in arginine-supplemented than in control rats. Consequently, the adiposity index did not differ between HF-Arg and LF-Ala groups at the end of the study (Table 4).
The size and number of adipocytes in RP adipose tissue.
At the end of the study, the HF diet enhanced (P < 0.01) the size of adipocytes by 15% compared with the LF diet, whereas dietary arginine supplementation reduced (P < 0.01) the size of adipocytes by 11% compared with alanine-supplemented rats (Table 4). The total numbers of adipocytes in the entire RP adipose tissue did not differ among the 4 groups of rats (Table 4). However, the HF feeding reduced (P < 0.01) the density of adipocytes per g of RP adipose tissue compared with the LF diet and arginine supplementation enhanced (P < 0.01) the density of adipocytes compared with alanine-supplemented rats (Table 4).
Serum concentrations of AA.
Serum concentrations of most AA, including glutamine, citrulline, arginine, proline, cysteine, and branched-chain AA (BCAA), were greater (P < 0.05) in HF- than in LF-fed rats (Table 5). Serum concentrations of arginine (+105%), ornithine (+64%), and proline (+42%) were higher (P < 0.01), but those of glutamate (−28%), glutamine (−15%), and BCAA (−22%) were lower (P < 0.05) in arginine- than in alanine-supplemented rats (Table 5). Dietary arginine supplementation did not affect serum concentrations of other measured AA (Table 5). Serum concentrations of alanine were 65% higher (P < 0.01) in alanine- than in arginine-supplemented rats (Table 5).
TABLE 5.
Serum concentrations of AA in 31-wk-old rats at the end of a 12-wk period of consuming a LF or HF diet and water containing either 1.51% l-arginine·HCl or 2.55% l-alanine1
| Groups |
P-value |
||||||
|---|---|---|---|---|---|---|---|
| Serum AA | LF-Ala | LF-Arg | HF-Ala | HF-Arg | Diet | AA | Diet × AA |
| μmol/L | |||||||
| Aspartate | 27 ± 4.6 | 20 ± 1.7 | 24 ± 2.7 | 21 ± 2.5 | 0.73 | 0.17 | 0.66 |
| Glutamate | 92 ± 15a | 61 ± 4.9b | 85 ± 8.9a | 67 ± 6.1b | 0.96 | 0.03 | 0.57 |
| Asparagine | 48 ± 3.1b | 46 ± 2.3b | 59 ± 9.0a | 64 ± 8.3a | 0.03 | 0.81 | 0.60 |
| Serine | 240 ± 20b | 249 ± 14b | 286 ± 15a | 310 ± 29a | 0.02 | 0.46 | 0.75 |
| Glutamine | 657 ± 39b | 531 ± 28c | 769 ± 31a | 685 ± 36b | <0.01 | <0.01 | 0.57 |
| Histidine | 53 ± 4.0b | 49 ± 3.3b | 84 ± 8.4a | 77 ± 9.0a | <0.01 | 0.42 | 0.84 |
| Glycine | 209 ± 25b | 240 ± 16b | 323 ± 17a | 354 ± 29a | <0.01 | 0.23 | 0.99 |
| Threonine | 188 ± 12b | 203 ± 11b | 247 ± 13a | 214 ± 18ab | 0.02 | 0.53 | 0.11 |
| Citrulline | 60 ± 4.9b | 56 ± 4.0b | 77 ± 7.7a | 74 ± 11a | 0.03 | 0.69 | 0.99 |
| Arginine | 163 ± 20d | 371 ± 28b | 223 ± 27c | 488 ± 63a | 0.01 | <0.01 | 0.20 |
| β-Alanine | 11 ± 1.0b | 8.5 ± 0.6b | 13 ± 1.0a | 13 ± 1.5a | <0.01 | 0.50 | 0.18 |
| Taurine | 385 ± 41 | 369 ± 25 | 427 ± 20 | 417 ± 38 | 0.23 | 0.72 | 0.93 |
| Alanine | 603 ± 61a | 370 ± 60b | 755 ± 92a | 454 ± 62b | 0.13 | <0.01 | 0.65 |
| Tyrosine | 58 ± 4.2b | 62 ± 4.7b | 87 ± 8.5a | 69 ± 8.2ab | 0.01 | 0.33 | 0.14 |
| Tryptophan | 67 ± 3.9b | 66 ± 3.8b | 94 ± 12a | 74 ± 10b | 0.05 | 0.22 | 0.27 |
| Methionine | 34 ± 2.4b | 31 ± 2.5b | 64 ± 7.7a | 54 ± 6.3a | <0.01 | 0.25 | 0.47 |
| Valine | 158 ± 9.0b | 119 ± 9.4c | 236 ± 31a | 168 ± 24b | <0.01 | 0.02 | 0.51 |
| Phenylalanine | 53 ± 3.5b | 51 ± 2.4b | 77 ± 8.6a | 70 ± 8.7a | <0.01 | 0.17 | 0.29 |
| Isoleucine | 82 ± 5.0b | 62 ± 4.7c | 97 ± 9.4a | 80 ± 8.1b | 0.04 | 0.02 | 0.84 |
| Leucine | 131 ± 6.8b | 104 ± 7.0c | 161 ± 9.3a | 138 ± 8.4b | <0.01 | <0.01 | 0.85 |
| Ornithine | 31 ± 2.2b | 58 ± 6.2a | 36 ± 2.2b | 52 ± 6.5a | 0.92 | <0.01 | 0.24 |
| Lysine | 284 ± 20b | 307 ± 36b | 439 ± 36a | 394 ± 37a | <0.01 | 0.71 | 0.28 |
| Proline | 306 ± 18d | 451 ± 25b | 392 ± 20c | 538 ± 33a | <0.01 | <0.01 | 0.43 |
| Cysteine2 | 147 ± 10b | 160 ± 13b | 219 ± 17a | 202 ± 15a | <0.01 | 0.76 | 0.91 |
Values are means ± SEM, n = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
Including cysteine plus 1/2 cystine.
Serum concentrations of glucose, lipids, urea, NO metabolites, and hormones.
Serum concentrations of cholesterol and leptin were 45 and 49% higher (P < 0.01) in HF- than in LF-fed rats, but those of glucose, FFA, triglycerides, or urea did not differ between the 2 groups of rats (Table 6). Dietary arginine supplementation reduced (P < 0.05) serum concentrations of glucose, triglycerides, urea, and leptin by 5, 32, 20, and 37%, respectively, enhanced (P < 0.01) serum concentrations of nitrite plus nitrate by 50%, and did not affect serum concentrations of FFA and cholesterol, compared with alanine-supplemented rats (Table 6). Serum concentrations of insulin (Table 6), as well as adiponectin, growth hormone, triiodothyronine, thyroxine, and corticosterone (Supplemental Table 3), were not affected by either HF feeding or dietary arginine supplementation.
TABLE 6.
Serum concentrations of glucose, lipids, insulin, and leptin in 31-wk-old rats at the end of a 12-wk period of receiving a LF or HF diet and water containing either 1.51% l-arginine·HCl or 2.55% l-alanine 1
| Groups |
P-value |
||||||
|---|---|---|---|---|---|---|---|
| Variables | LF-Ala | LF-Arg | HF-Ala | HF-Arg | Diet | AA | Diet × AA |
| Glucose, mmol/L | 5.99 ± 0.09a | 5.76 ± 0.15b | 6.08 ± 0.15a | 5.74 ± 0.13b | 0.81 | 0.04 | 0.69 |
| Triglycerides, mmol/L | 0.93 ± 0.11a | 0.68 ± 0.07b | 1.05 ± 0.08a | 0.66 ± 0.07b | 0.57 | <0.01 | 0.45 |
| Cholesterol, mmol/L | 4.80 ± 0.27b | 4.45 ± 0.19b | 6.27 ± 0.54a | 7.11 ± 0.41a | <0.01 | 0.54 | 0.10 |
| FFA, mmol/L | 0.81 ± 0.12 | 0.62 ± 0.07 | 0.80 ± 0.13 | 0.72 ± 0.09 | 0.68 | 0.23 | 0.61 |
| Insulin, pmol/L | 95.9 ± 7.1 | 116 ± 9.6 | 113 ± 9.0 | 105 ± 13 | 0.62 | 0.56 | 0.20 |
| Leptin, μg/L | 7.73 ± 1.22b | 3.35 ± 0.36c | 9.22 ± 1.41a | 7.28 ± 0.94b | 0.02 | <0.01 | 0.27 |
| Urea, mmol/L | 2.64 ± 0.11a | 2.05 ± 0.25b | 2.35 ± 0.14a | 1.94 ± 0.10b | 0.23 | <0.01 | 0.69 |
| NOx,2μmol/L | 20.7 ± 0.81b | 29.2 ± 1.1a | 17.1 ± 0.90c | 27.6 ± 1.2a | 0.01 | <0.01 | 0.41 |
Values are means ± SEM, n = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
NO metabolites (nitrite plus nitrate).
Improvement of OGTT in arginine-supplemented rats.
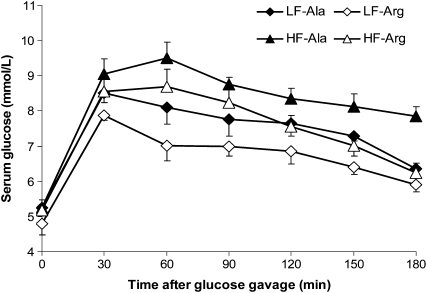
The basal serum concentrations of glucose in rats were ∼5 mmol/L and did not differ among the 4 treatment groups (Fig. 2). In response to oral gavage with glucose, serum concentrations of glucose increased (P < 0.01) to peak values (8–9 mmol/L) at 30 min in all the rats. Between 60 and 180 min postadministration of glucose, serum concentrations of glucose were higher (P < 0.01) in the HF-Ala group than in any other group of rats. At 60 min, serum concentrations of glucose in the LF-Arg group were lower (P < 0.01) than those in the LF-Ala and HF-Arg groups. At 180 min, serum concentrations of glucose in the LF-Ala group decreased (P < 0.05) to 6.5 mmol/L, whereas those in the HF-Ala group remained elevated (8.5 mmol/L). Based on AUC values (Fig. 2), glucose tolerance was lower (P < 0.01) in HF- than LF-fed rats. Dietary arginine supplementation improved (P < 0.05) glucose tolerance in both LF and HF rats.
FIGURE 2 .
OGTT in rats that received a LF or HF diet and water containing either 1.51% l-arginine·HCl or 2.55% l-alanine for 10 wk. Values are means ± SEM, n = 8. AUC values were 1137 ± 58,b 990 ± 69,c 1463 ± 27,a and 1244 ± 59b (h·mmol)·L−1, respectively, for LF-Ala, LF-Arg, HF-Ala, and HF-Arg groups. P (diet) < 0.01, P (AA) = 0.02, and P (diet × AA) = 0.20. Auc means without a common superscript letter (a–c) differ (P < 0.05).
Lipid, glycogen, glutathione, and tetrahydrobiopterin in tissues.
Concentrations of lipids in liver and gastrocnemius muscle were higher (P < 0.05), but concentrations of tetrahydrobiopterin and reduced glutathione in liver were lower (P < 0.05), in HF- than in LF-fed rats (Table 7). Concentrations of oxidized glutathione in liver were not detectable (<0.05 μmol/g tissue) in all groups of rats. Dietary arginine supplementation increased (P < 0.05) concentrations of tetrahydrobiopterin in liver compared with control rats (Table 7). Neither the HF diet nor arginine supplementation affected concentrations of lipids in adipose tissue or concentrations of glycogen in liver and gastrocnemius muscle (Table 7).
TABLE 7.
Concentrations of lipids, tetrahydrobiopterin, glycogen, and glutathione in tissues of 31-wk-old rats at the end of a 12-wk period of receiving a LF or HF diet and water containing either 1.51% l-arginine·HCl or 2.55% l-alanine1
| Groups |
P-value |
||||||
|---|---|---|---|---|---|---|---|
| Variables | LF-Ala | LF-Arg | HF-Ala | HF-Arg | Diet | AA | Diet × AA |
| Liver lipid, g/100 g | 3.85 ± 0.11b | 3.64 ± 0.06b | 4.59 ± 0.24a | 4.66 ± 0.17a | <0.01 | 0.67 | 0.40 |
| Muscle lipid, g/100 g | 1.60 ± 0.11b | 1.64 ± 0.12b | 1.94 ± 0.14a | 1.92 ± 0.15a | 0.05 | 0.42 | 0.52 |
| RP2 adipose tissue lipid, g/100 g | 80.6 ± 1.6 | 82.0 ± 1.8 | 83.1 ± 1.6 | 82.9 ± 0.6 | 0.27 | 0.72 | 0.60 |
| Liver BH4, nmol/g tissue | 4.02 ± 0.38b | 5.06 ± 0.43a | 3.13 ± 0.15c | 4.35 ± 0.13b | 0.02 | <0.01 | 0.79 |
| Liver glycogen, mg/g | 21.8 ± 1.7 | 18.6 ± 0.9 | 19.7 ± 0.5 | 19.1 ± 1.8 | 0.55 | 0.16 | 0.31 |
| Muscle glycogen, mg/g | 1.26 ± 0.19 | 1.13 ± 0.20 | 1.01 ± 0.13 | 1.05 ± 0.11 | 0.36 | 0.80 | 0.65 |
| Liver GSH, μmol/g | 6.09 ± 0.27a | 5.59 ± 0.33a | 4.41 ± 0.43b | 4.24 ± 0.33b | <0.01 | 0.41 | 0.67 |
Values are means ± SEM, n = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
Abbreviations used: BH4, tetrahydrobiopterin; GSH, reduced glutathione.
Discussion
The DIO rat provides a useful model to define an effect of dietary arginine supplementation on reducing fat mass in environmentally induced obesity. The measurement of fat pad weights is the most direct means to assess total and regional adiposity and there is a close correlation between the sum of fat pad weights to body fat measured by carcass analysis (27,28). Results of the present study demonstrate that dietary arginine supplementation was highly effective in reducing the gain of major white fat pad in DIO rats (Table 4), as reported for genetically obese ZDF rats (8). The decrease in the fat pad weight was accounted for by reduced adipocyte size, with no change in cell number (Table 4). Accordingly, serum concentrations of leptin were lower in arginine- than in alanine-supplemented rats (Table 6). We cannot exclude the unlikely possibility that fat accumulated in nonmajor fat depots or ectopically. However, visually, we did not note increased fat mass in other tissues of arginine-supplemented rats.
The percentage loss of MT adipose tissue was the greatest in arginine-supplemented rats, followed by RP, SC, and EP fat pads in decreasing order (Table 4; Supplemental Table 1). Fatty acids released from white adipose tissue likely undergo increased oxidation in liver, skeletal muscle, and brown adipose tissue. This would result in lower circulating levels of serum concentrations of triglycerides in arginine- than in alanine-supplemented rats (Table 6), as reported for pigs (29), as well as chemically induced diabetic rats (11) and ZDF rats (8). Importantly, the arginine treatment markedly increased the mass of brown adipose tissue in rats by 34% (Table 4), which is likely due to NO-induced mitochondrial biogenesis (6). In support of this view, serum concentrations of nitrite plus nitrate [an indicator of systemic NO synthesis (30)] were higher in arginine- than in alanine-supplemented rats regardless of HF or LF feeding (Table 6). Brown adipose tissue is rich in mitochondria, where fatty acid and glucose oxidation results in the production of heat rather than ATP because of the presence of uncoupling protein-1 (31).
HF feeding induced oxidative stress in rats (indicated by a lower concentration of reduced glutathione in liver; Table 7), compromised NO synthesis (indicated by reductions in serum NO metabolites and hepatic availability of tetrahydrobiopterin; Tables 6 and 7), and impaired insulin action (indicated by a reduced disposal of oral glucose; Fig. 2). Importantly, arginine supplementation enhanced glucose disposal (Fig. 2), reduced serum glucose concentrations, and augmented antioxidative capacity in DIO rats (Table 6), as reported for chemically induced diabetic rats (11) and ZDF rats (8). These beneficial effects of dietary arginine supplementation can result from both improved insulin sensitivity in skeletal muscle and an increase in its mass (Table 4; Supplemental Table 1).
Little is known about the effect of HF feeding on plasma concentrations of AA. Notably, serum concentrations of most AA (including glutamine and BCAA) were increased in HF-fed rats compared with LF-fed rats (Table 5) despite similar intakes of dietary protein (Table 2). This result may be explained by inhibition of tissue-specific AA oxidation due to mitochondrial dysfunction and insulin resistance in obese rats. High concentrations of glutamine in the serum of DIO rats may result partly from its elevated production by adipose tissue (32). This may have important implications for obesity-related metabolic defects, because glutamine is a substrate for the synthesis of glucosamine, which contributes to insulin resistance in skeletal muscle (33) and possibly endothelial cells (34). Of particular note, elevated levels of BCAA have recently been reported to be associated with changes in energy expenditure in mice (35). Thus, it would be important to investigate the roles for AA in regulating fat metabolism as well as insulin and mammalian target of rapamycin signaling pathways in DIO rats.
Another novel and important finding from this study is that dietary arginine supplementation reduced serum concentrations of BCAA (Table 5). There are 2 possibilities for this observation. First, the use of BCAA for lean tissue growth may be enhanced due to an anabolic effect of arginine on protein synthesis and deposition in skeletal muscle (36). Second, arginine may increase mitochondrial function and oxidative capacity in skeletal muscle for promoting BCAA degradation. Of further interest, arginine supplementation reduced serum concentrations of glutamine and urea in both LF- and HF-fed rats (Table 6) as reported for ZDF rats (8) and pigs (37). These results may be explained by reduced synthesis of glutamine in adipose and muscle tissues, as well as reduced availability of AA for oxidation and ammonia production.
The mass of skeletal muscle, which represents 40–45% of the body weight (5), was greater (both absolute and relative increases) in both LF- and HF-fed rats in response to arginine supplementation (Table 4; Supplemental Table 1). Importantly, such an anabolic effect of arginine was achieved independently of changes in serum concentrations of insulin (Table 6). Similarly, dietary arginine supplementation reduced white fat accretion but increased muscle gain in growing-finishing pigs without affecting body weight (38). These findings suggest that, through yet unknown signaling pathways, arginine may regulate intracellular protein turnover, favoring net protein deposition in skeletal muscle (39). Alternatively, through NO generation (40), dietary arginine supplementation may increase insulin sensitivity and amplify its signaling mechanisms on net protein synthesis (5). Thus, arginine supplementation regulates the repartitioning of dietary energy to favor muscle over fat gain in the body. Additional studies are warranted to determine the whole-body composition of control and arginine-supplemented rats.
In summary, results of the present study demonstrated for the first time, to our knowledge, that dietary arginine supplementation reduced white fat gain, increased skeletal-muscle mass, decreased serum concentrations of glucose and triglycerides, and improved insulin sensitivity in DIO rats. Arginine supplementation may represent a safe and efficient nutritional treatment for obesity. Future studies are warranted to determine whole-body energy expenditure and define the cellular and molecular mechanisms responsible for the beneficial effects of arginine in ameliorating the metabolic syndrome in obese subjects.
Supplementary Material
Acknowledgments
We thank Katherine Kelly for technical assistance and Frances Mutscher for help in manuscript preparation.
Supported by the AHA-TX grants (nos. 0655109Y and 0755024Y), an AHA postdoctoral fellowship, the National Research Initiative Competitive grant (no. 2008-35206-18762) from the USDA Cooperative State Research, Education, and Extension Service, The Clinical Nutrition Research Unit of Maryland (P30 DK072488), and the Geriatric Research, Education, and Clinical Core of Baltimore Veteran Administration Medical Center.
Author disclosures: W. Jobgen, C. J. Meininger, S. C. Jobgen, P. Li, M.-J. Lee, S. B. Smith, T. E. Spencer, S. K. Fried, and G. Wu, no conflicts of interest.
Supplemental Tables 1–3 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AA, amino acid; AUC, area under the curve; BCAA, branched-chain amino acid; DIO, diet-induced obese; EDL, extensor digitorum longus; EP, epididymal; HF, high-fat; HF-Ala, high-fat diet + l-alanine supplementation; HF-Arg, high-fat diet + l-arginine supplementation; LF-Ala, low-fat diet + l-alanine supplementation; LF-Arg, low-fat diet + l-arginine supplementation; LF, low-fat; MT, mesenteric; NO, nitric oxide; OGTT, oral glucose tolerance test; RP, retroperitoneal; SC, subcutaneous; ZDF, Zucker diabetic fatty.
References
- 1.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–17. [DOI] [PubMed] [Google Scholar]
- 3.Zou C, Shao J. Role of adipocytokines in obesity-associated insulin resistance. J Nutr Biochem. 2008;19:277–86. [DOI] [PubMed] [Google Scholar]
- 4.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–88. [DOI] [PubMed] [Google Scholar]
- 6.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–9. [DOI] [PubMed] [Google Scholar]
- 7.Khedara A, Goto T, Morishima M, Kayashita J, Kato N. Elevated body fat in rats by the dietary nitric oxide synthase inhibitor, L-Nω-nitroarginine. Biosci Biotechnol Biochem. 1999;63:698–702. [DOI] [PubMed] [Google Scholar]
- 8.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–21. [DOI] [PubMed] [Google Scholar]
- 9.Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rats. Proc Soc Exp Biol Med. 1983;173:68–75. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–5. [DOI] [PubMed] [Google Scholar]
- 11.Kohli R, Meininger CJ, Haynes TE, Yan W, Self JT, Wu G. Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J Nutr. 2004;134:600–8. [DOI] [PubMed] [Google Scholar]
- 12.Wu G. Nitric oxide synthesis and the effect of aminoguanidine and NG-monomethyl-L-arginine on the onset of diabetes in the spontaneously diabetic BB rat. Diabetes. 1995;44:360–4. [DOI] [PubMed] [Google Scholar]
- 13.Vital P, Larrieta E, Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol. 2006;190:425–32. [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008;440:177–89. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Borbolla AG, Knabe DA. The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr. 1994;124:2437–44. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Meininger CJ, Wu G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 2000;746:199–207. [DOI] [PubMed] [Google Scholar]
- 17.Rodbell M. The metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–80. [PubMed] [Google Scholar]
- 18.Di Girolamo M, Mendlinger S, Fertig JW. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971;221:850–8. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974;60:405–12. [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Chen LX, Li P, Li XL, Zhou HJ, Wang FL, Li DF, Yin YL, Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138:1025–32. [DOI] [PubMed] [Google Scholar]
- 23.Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41:415–33. [DOI] [PubMed] [Google Scholar]
- 24.Meininger CJ, Wu G. Regulation of endothelial cell proliferation by nitric oxide. Methods Enzymol. 2002;352:280–95. [DOI] [PubMed] [Google Scholar]
- 25.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag. 2000.
- 26.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Long-term high-fat feeding leads to severe insulin resistance but not diabetes in Wistar rats. Am J Physiol Endocrinol Metab. 2002;282:E1231–8. [DOI] [PubMed] [Google Scholar]
- 28.Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am J Physiol. 1995;268:E546–50. [DOI] [PubMed] [Google Scholar]
- 29.He QH, Kong XF, Wu G, Ren PP, Tang HR, Hao FH, Huang RL, Li TJ, Tan BE, et al. Metabolomic analysis of the response of growing pigs to dietary L-arginine supplementation. Amino Acids. 2008; doi: 10.1007/s00726-008-0192-9. [DOI] [PubMed]
- 30.Jobgen WS, Jobgen SC, Li H, Meininger CJ, Wu G. Analysis of nitrite and nitrate in biological samples using high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:71–82. [DOI] [PubMed] [Google Scholar]
- 31.Cannon B, Nedergaard J. Brown adipose tissue: functions and physiological significance. Physiol Rev. 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 32.Kowalski TJ, Wu G, Watford M. Rat adipose tissue amino acid metabolism in vivo as assessed by microdialysis and arterio-venous techniques. Am J Physiol. 1997;273:E613–22. [DOI] [PubMed] [Google Scholar]
- 33.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu G, Haynes TE, Li H, Yan W, Meininger CJ. Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J. 2001;353:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2008; doi:. [DOI] [PMC free article] [PubMed]
- 37.Kim SW, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–30. [DOI] [PubMed] [Google Scholar]
- 38.Tan BE, Yin YL, Liu ZQ, Li XG, Xu HJ, Kong XF, Huang RL, Tang WJ, Shinzato I, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2008; doi:. [DOI] [PubMed]
- 39.Frank JW, Escobar J, Nguyen HV, Jobgen SC, Jobgen WS, Davis TA, Wu G. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr. 2007;137:315–9. [DOI] [PubMed] [Google Scholar]
- 40.Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002;22:61–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.