Abstract
Background
There has been some difficulty getting standard laboratory rats to voluntarily consume large amounts of ethanol without the use of initiation procedures. It has previously been shown that standard laboratory rats will voluntarily consume high levels of ethanol if given intermittent-access to 20% ethanol in a 2-bottle-choice setting [Wise, Psychopharmacologia 29 (1973), 203]. In this study, we have further characterized this drinking model.
Methods
Ethanol-naïve Long–Evans rats were given intermittent-access to 20% ethanol (three 24-hour sessions per week). No sucrose fading was needed and water was always available ad libitum. Ethanol consumption, preference, and long-term drinking behaviors were investigated. Furthermore, to pharmacologically validate the intermittent-access 20% ethanol drinking paradigm, the efficacy of acamprosate and naltrexone in decreasing ethanol consumption were compared with those of groups given continuous-access to 10 or 20% ethanol, respectively. Additionally, ethanol consumption was investigated in Wistar and out-bred alcohol preferring (P) rats following intermittent-access to 20% ethanol.
Results
The intermittent-access 20% ethanol 2-bottle-choice drinking paradigm led standard laboratory rats to escalate their ethanol intake over the first 5 to 6 drinking sessions, reaching stable baseline consumption of high amounts of ethanol (Long–Evans: 5.1 ± 0.6; Wistar: 5.8 ± 0.8 g/kg/24 h, respectively). Furthermore, the cycles of excessive drinking and abstinence led to an increase in ethanol preference and increased efficacy of both acamprosate and naltrexone in Long–Evans rats. P-rats initiate drinking at a higher level than both Long–Evans and Wistar rats using the intermittent-access 20% ethanol paradigm and showed a trend toward a further escalation in ethanol intake over time (mean ethanol intake: 6.3 ± 0.8 g/kg/24 h).
Conclusion
Standard laboratory rats will voluntarily consume ethanol using the intermittent-access 20% ethanol drinking paradigm without the use of any initiation procedures. This model promises to be a valuable tool in the alcohol research field.
Keywords: Animal Models, Alcohol, Addiction, Rats, Acamprosate, Naltrexone
It has been a challenge to get standard laboratory rats to voluntarily consume high amounts of ethanol without the use of initiation procedures. In an attempt to increase oral ethanol consumption, various procedures had been developed to initiate ethanol drinking including sucrose fading (Samson, 1986), food and water deprivation (Meisch and Thompson, 1972), pairing drinking with electrical brain stimulation (Amit et al., 1970; Wayner and Greenberg, 1972), and limiting access to ethanol (Sinclair et al., 1992). Despite these efforts, rats rarely maintain high ethanol intake following the removal of the initiation procedure. Other attempts to increase ethanol consumption in rats has been made through the use of different concentrations of ethanol (Amit et al., 1970; Cicero and Myers, 1968; Kiefer et al., 1994; Myers et al., 1998; Wolffgramm and Heyne, 1995) and using various rats strains selectively bred for high preference for ethanol (Eriksson, 1968; Li et al., 1987; Mardones and Segovia-Riquelme, 1983).
During the 1970s, several studies showed that intermittent-access to ethanol induced high voluntary ethanol consumption (Pinel and Huang, 1976; Wayner and Greenberg, 1972; Wise, 1973). More recently, Tomie et al. (2002, 2006) have showed that intermittent-access to an ethanol sipper in an operant setting outside the home cage, induced elevated ethanol intake relative to continuous-access. In this study, we have characterized an intermittent-access 20% ethanol 2-bottle-choice drinking paradigm to determine its potential as a preclinical animal model of drinking.
MATERIAL AND METHODS
Animals and Housing
Adult, ethanol-naïve, male Long–Evans and male Wistar rats (Harlan, Indianapolis, IN), weighing 150 to 175 g upon arrival, were individually housed in ventilated Plexiglas cages in a climate-controlled room on a 12-hour reversed light/dark cycle (lights off at 10 AM) and given at least 1 week to acclimatize to the individual housing conditions and handling. Male ethanol-naïve, out-bred alcohol preferring (P) rats (Indiana University, IN), 45-day old upon arrival, were group housed until 3 months of age and thereafter individually housed in ventilated Plexiglas cages in a climate-controlled room on a 12-hour reverse light/dark cycle (lights off at 7 AM). Experiments were not commenced until Long–Evans, Wistar, and P-rats reached adulthood. Food and water were available ad libitum and all procedures were pre-approved by the Ernest Gallo Clinic and Research Center and the Medical University of South Carolina Institutional Animal Care and Use Committees, respectively, and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Ethanol Intake Procedures
All fluids were presented in 100-ml graduated glass cylinders with stainless-steel drinking spouts inserted through 2 grommets in front of the cage 15 minutes after the lights went off in the reversed light/-dark cycle room. Bottles were weighed 30 minutes and 24 hours after the fluids were presented, and measurements were taken to the nearest gram. The weight of each rat was measured daily Monday through Friday to calculate the grams of ethanol intake per kilogram of body weight. The preference for ethanol over water (the ratio of ethanol to total fluid intake) was calculated only at the 24-hour time point because of the minimal water intake during the first 30 minutes of access to the bottles. Ethanol solutions were prepared in tap water from 95% (v/v) ethanol (Gold Shield Chemical Ac., Hayward, CA).
Intermittent-Access 20% Ethanol 2-Bottle-Choice Drinking Paradigm
The intermittent-access 20% ethanol drinking paradigm was adapted from Wise (1973) and the animals were given access to ethanol without sweeteners during three 24-hour-sessions per week. On the Monday following the end of the housing acclimatization period, each ethanol-naïve rat from each strain was given access to 1 bottle of 20% v/v ethanol and 1 bottle of water. After 24 hours, the ethanol bottle was replaced with a second water bottle that was available for the next 24 hours. This pattern was repeated on Wednesdays and Fridays. The rats had unlimited access to 2 bottles of water over the weekend after the 24-hour measurements were taken on Saturday morning. The placement of the ethanol bottle was alternated each ethanol drinking session to control for side preferences.
Continuous-Access 10 or 20% Ethanol 2-Bottle-Choice Drinking Paradigms
One group of ethanol-naïve Long–Evans rats (n = 7) was given continuous-access to 1 bottle containing a 20% ethanol solution (v/v) and 1 bottle of water. A second group of ethanol-naïve Long–Evans rats (n = 7) was given continuous-access to a 10% ethanol solution (v/v) and 1 bottle of water. No initiation procedures were used before the first ethanol drinking session. The placement of the ethanol bottle was alternated daily to control for side preferences.
Experiment 1—Ethanol Consumption in Long–Evans Rats Using the Intermittent-Access 20% Ethanol Drinking Paradigm
The aim of the experiment was to determine if standard laboratory rats (Long–Evans) will voluntarily consume ethanol when given intermittent-access to 20% ethanol using a 2-bottle-choice drinking paradigm without the use of sucrose fading. Twelve ethanol-naïve Long–Evans rats (360 ± 7 g) were given intermittent-access to 20% ethanol, as described above, for 20 drinking sessions (45 days). The ethanol and water intake was recorded on each ethanol day (no data collected during the 12th drinking session). In addition, the intake of water was recorded on the water days to enable comparison of the total fluid intake between the ethanol days (ethanol + water) and the water days.
Experiment 2—Effect of a Prolonged Period of Abstinence (40 days) From Drinking Ethanol on Subsequent Ethanol Consumption
We determined if the high ethanol intake induced using the intermittent-access 20% ethanol 2-bottle-choice drinking paradigm would be maintained following a prolonged period of abstinence. A 40-daylong abstinence period was introduced after the 20th ethanol drinking session (day 45 after the first ethanol session) using the rats described in Experiment 1. Following this period, the rats were again given intermittent-access to 20% ethanol as described above. Comparisons were made between the mean ethanol consumption (g/kg/24 hours) and preference, respectively, during the 10 ethanol drinking sessions immediately before and after the abstinence period.
Experiment 3—The Effect of Acamprosate and Naltrexone on Ethanol Consumption Using the Intermittent-Access 20% Ethanol Drinking Paradigm
We next compared the effects of the 2 currently FDA-approved medications for alcohol use disorders (AUD), naltrexone (Sigma, St Louis, MO) and acamprosate (calcium acetylhomotaurinate; EstechPharma, Seoul, South Korea), to reduce ethanol consumption in each of 3 different drinking paradigms, intermittent-access 20% ethanol, continuous-access 10%, or continuous-access 20% ethanol 2-bottle-choice drinking paradigms. Two separate groups of Long–Evans rats (separate from the groups in Experiments 1 and 2; n =12 per group) were given intermittent-access to 20% ethanol as described above. When the rats had been exposed to 20% ethanol for approximately 2 months (body weights 511 ± 12 and 529 ± 16, respectively), the 2 groups were administered either acamprosate (0, 100, and 200 mg/kg i.p.) or naltrexone (0, 0.3, 1, and 3 mg/kg s.c.), respectively. Two separate groups of Long–Evans rats (n = 7 per group) were given continuous-access to either 10 or 20% ethanol, respectively as described above. When the rats in the continuous-access groups had been exposed to ethanol for approximately 2 months (body weights: 442 ± 23 and 440 ± 17, respectively), acamprosate (0, 100, or 200 mg/kg i.p.) and naltrexone (0, 0.3, 1, and 3 mg/kg s.c.) were administered. Each rat in the 2 separate groups received all doses of acamprosate with a 1-week wash-out period between injections using a Latin square design. Following a 2-week wash-out period after the last acamprosate injection, all doses of naltrexone were administered to each rat using a Latin square design with a 1-week wash-out period between each injection. Acamprosate and naltrexone were both dissolved in saline, in a volume of 1 ml/kg, and given 30 minutes before ethanol and water bottles were presented. All drug solutions were prepared immediately before each injection.
Experiment 4—Ethanol Consumption in Wistar and P-Rats Using the Intermittent-Access 20% Ethanol Drinking Paradigm
We then determined if Wistar and P-rats would escalate their consumption of ethanol using the intermittent-access 20% ethanol 2-bottle-choice drinking paradigm. One group of ethanol-naïve Wistar rats (274 ± 4, n = 11) and 1 group of ethanol-naïve P-rats (520 ± 7, n = 6) were given intermittent-access to 20% ethanol for 20 ethanol sessions as described above.
Experiment 5—Blood Ethanol Concentrations Obtained Following Voluntary Oral Ethanol Consumption Using the Intermittent-Access 20% Ethanol Drinking Paradigm
The objective of this experiment was to measure the blood ethanol concentration (BECs) following voluntary oral ethanol intake using the intermittent-access 20% ethanol drinking paradigm in Long–Evans, Wistar, and P-rats. When the rats had maintained a stable baseline in the intermittent-access 20% ethanol drinking paradigm, blood samples were collected from the lateral tail vein following 30 minutes access to 20% ethanol (approximately 45 minutes after the beginning of the dark cycle). The samples were centrifuged at 4°C for 20 minutes at 8,000 rpm and BECs were analyzed in plasma using gas chromatography (Doyon et al., 2003). Two samples from the Long–Evans and Wistar groups were excluded from the analysis due to insufficient amount of plasma. The BECs were correlated with the ethanol consumed (g/kg/30 min) prior to the blood sampling.
Statistics
Statistical analysis was performed using GraphPadPrism software (GraphPad, San Diego, CA). Behavioral data were analyzed using one-way ANOVA, or repeated measures one-way ANOVA where appropriate, followed by Newman–Keuls post hoc analysis when a significant overall main effect was found (p < 0.05). The data for ethanol consumption and preference for ethanol before and after the 40-day period of abstinence using the intermittent-access 20% ethanol paradigm, was compared by measuring the mean of the 10 drinking sessions before and after the 40-day period (days 25 to 45 and 85 to 105 after the first ethanol exposure, respectively) and analyzed using paired Student’s t-test. The acamprosate and naltrexone data within each separate treatment group were analyzed by repeated measures one-way ANOVA followed by Newman–Keuls post hoc analysis when a significant overall main effect was found (p < 0.05). The BEC data within each strain of rats were analyzed by linear regression and the mean ethanol intake prior to the BEC measurements (g/kg/30 min) was compared between strains using one-way ANOVA followed by Newman–Keuls post hoc analysis.
RESULTS
Experiment 1—Ethanol Consumption in Long–Evans Rats Using the Intermittent-Access 20% Ethanol Drinking Paradigm
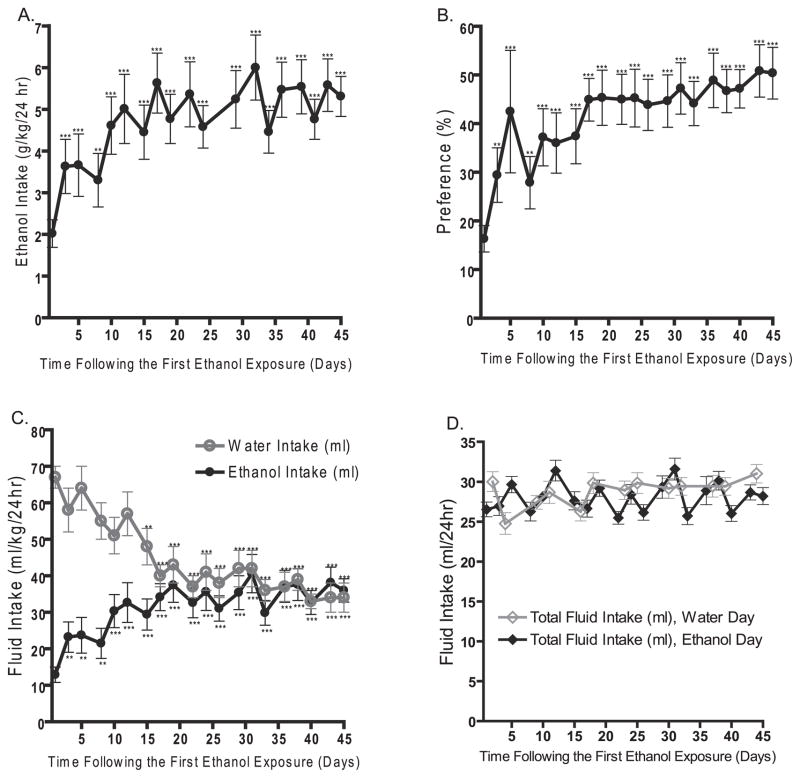
The intermittent-access 20% ethanol 2-bottle-choice drinking paradigm resulted in a steady escalation in ethanol consumption in Long–Evans rats (Fig. 1A). There was an overall main effect on the ethanol intake (g/kg/24 h) over time [F(19,11) = 11, p < 0.0001, n = 12] and post hoc analysis revealed that the mean ethanol intake was higher at all drinking sessions compared with the first drinking session. Following the fifth drinking session, there was no difference in the ethanol intake between any of the subsequent drinking sessions. Thus, the Long–Evans rats reached a stable baseline consumption of 5.1 ± 0.6 g/kg/24 h following the fifth exposure of ethanol. The mean baseline consumption for each rat ranged from 2.4 to 9.9 g/kg/24 h with 58% of the rats reaching a baseline above the group mean.
Fig. 1.
The intermittent-access 20% ethanol drinking paradigm in Long–Evans rats induces a robust escalation in (A) ethanol intake and (B) preference for ethanol over water, reaching a baseline ethanol consumption of 5.1 ± 0.6 g/kg/24 h. There was a significant decrease in the water intake and a significant increase in ethanol intake over time on the ethanol days (C). There was no significant difference between the total fluid intake on the ethanol compared with the water days (D). The values are expressed as mean ethanol intake (g/kg/24 h), fluid intake (ml/24 h), or preference (ratio of ethanol over total fluid intake) ± SEM at each drinking session. **p < 0.01 and ***p < 0.001 compared with the first drinking session (one-way repeated measures ANOVA followed by Newman–Keuls post hoc test) n = 12.
The robust escalation in ethanol intake was paralleled by a significant increase in preference for ethanol over time [F(19,11) = 7.7, p < 0.0001, n = 12, Fig. 1B]. The significant increase in preference for ethanol was induced not only by a significant increase in ethanol intake over time [F(19,11) = 12, p < 0.0001, n = 12, Fig. 1C], but also by a significant decrease in water intake on the days when ethanol was present [F(19,11) = 16, p < 0.0001, n = 12, Fig. 1C]. Notably, there was no difference in the total fluid intake (ml) on the ethanol days (ethanol + water) compared with the water days (t = 1.2, n.s., Fig. 1D).
Experiment 2—Effect of a Prolonged Period of Abstinence (40 days) From Ethanol on Subsequent Ethanol Consumption
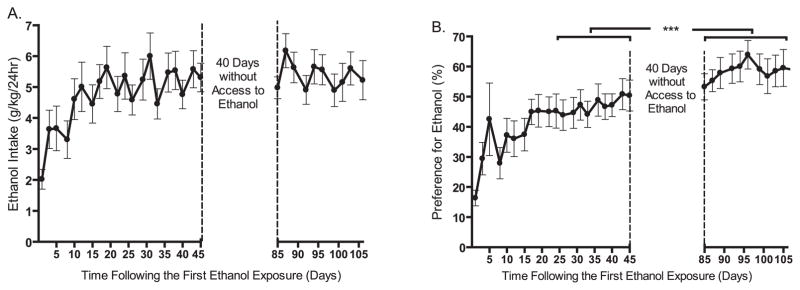
When the Long–Evans rats in Experiment 1 had completed 20 drinking sessions, the access to ethanol was withheld for a 40-day period to determine if the high ethanol consumption levels would be maintained following a prolonged period of abstinence. There was a slight but not significant difference in mean ethanol consumption (g/kg/24 h) between the 10 drinking sessions before and after the 40-day period (before: 5.2 ± 0.6; after 5.4 ± 0.6 g/kg/24 h, respectively, t = 0.59, n.s., n = 12, Fig. 2A). However, there was a significant increase in the preference for ethanol over water following the 40-day period (before: 47 ± 5; after: 57 ± 5%, respectively, t = 8.3, p < 0.0001, n = 12, Fig. 2B).
Fig. 2.
High levels of ethanol intake are maintained and ethanol preference is increased following a prolonged (40 days) period of abstinence from ethanol. Mean ethanol consumption (A) and preference for ethanol (B) were compared for the 10 drinking sessions before and after a 40-day-long ethanol deprivation period (paired Student’s t-test). The values are expressed as mean ethanol consumed (g/kg/24 h) and preference for ethanol (%) ± SEM, respectively, ***p < 0.001 compared as described in figure, n = 12.
Experiment 3—The Effect of Acamprosate and Naltrexone on Ethanol Consumption Using the Intermittent-Access 20% Ethanol Drinking Paradigm
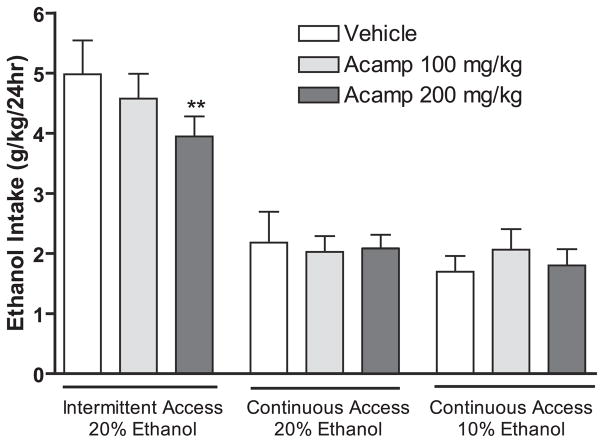
Acamprosate (100 and 200 mg/kg i.p.) treatment had an overall main effect on ethanol consumption [F(2,11) = 5.8, p < 0.01, n = 12, Fig. 3] and preference for ethanol [F(2,11) = 3.6, p < 0.05, n = 12, data not shown] at the 24-hour time point using the intermittent-access 20% ethanol drinking paradigm. Post hoc analysis revealed that the dose of 200 mg/kg significantly reduced the ethanol consumption (Fig. 3) and preference for ethanol (data not shown) compared with vehicle. In contrast, acamprosate treatment had no overall main effect on ethanol consumption in the groups given continuous-access of 10 or 20% ethanol, respectively [10% ethanol: F(2,11) = 2.9, n.s, n = 12; 20% ethanol: F(2,6) = 2.4, n.s, n = 7, Fig. 3] at the 24-hour time point. Acamprosate treatment did not decrease ethanol consumption at the 30 minute time point or water intake at any time point in any of the examined drinking paradigms (data not shown).
Fig. 3.
Acamprosate (200 mg/kg i.p.) decreases ethanol consumption using the intermittent-access 20% ethanol but not the continuous-access 10 or 20% ethanol drinking paradigms in Long–Evans rats. Data from the 3 drinking paradigms were analyzed separately (one-way repeated measures ANOVA within each group followed by Newman–Keuls post hoc test). The values are expressed as mean ethanol consumed (g/kg/24 h) ± SEM, **p < 0.01 compared with vehicle within treatment group, n = 7 to 12 per group. Acamp, Acamprosate.
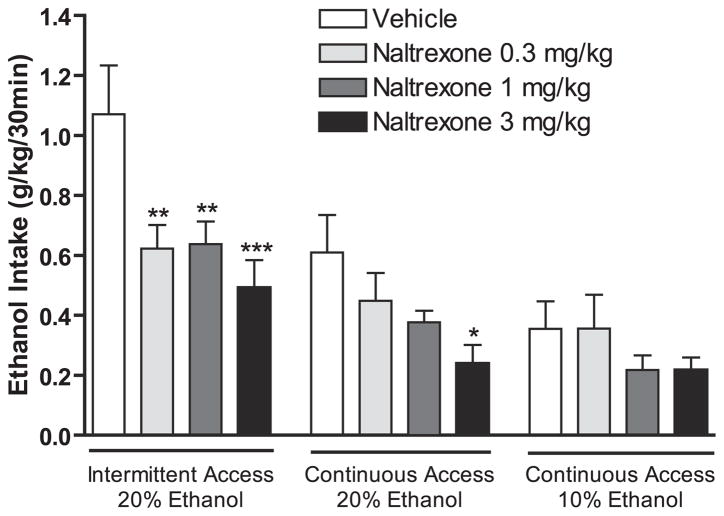
Naltrexone (0.3, 1, and 3 mg/kg s.c.) had an overall effect on ethanol consumption in the intermittent-access 20% ethanol paradigm [F(3,11) = 6.8, p < 0.001]. Post hoc analysis revealed that all doses significantly reduced ethanol consumption compared with vehicle (Fig. 4). There was also an overall effect on ethanol consumption in the continuous-access 20% ethanol group following naltrexone administration [F(2,6) = 3.7, p < 0.05]. Post hoc analysis showed that only the highest dose of naltrexone reduced the ethanol intake compared with vehicle (Fig. 4). Furthermore, there was an overall effect on the ethanol consumption following administration of naltrexone in the continuous-access 10% ethanol drinking group [F(3,8) = 3.0, p < 0.05]. Post hoc analysis revealed that all doses decreased the ethanol intake (Fig. 4). In addition, naltrexone significantly decreased the water intake at the 30 minute time point in all investigated drinking paradigms (data not shown). Naltrexone treatment did not decrease ethanol consumption at the 24-hour time point in any of the drinking paradigms (data not shown).
Fig. 4.
Low doses of naltrexone decrease ethanol consumption using the intermittent 20% ethanol drinking paradigm in Long–Evans rats when compared with continuous 10 or 20% ethanol access. Data from the 3 drinking paradigms were analyzed separately (one-way repeated measures ANOVA within each group followed by Newman–Keuls post hoc test). The values are expressed as mean ethanol consumed (g/kg/30 min) ± SEM; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with vehicle within treatment group, n = 7 to 12 per group.
Experiment 4—Ethanol Consumption in Wistar and P-Rats Using the Intermittent-Access 20% Ethanol Drinking Paradigm
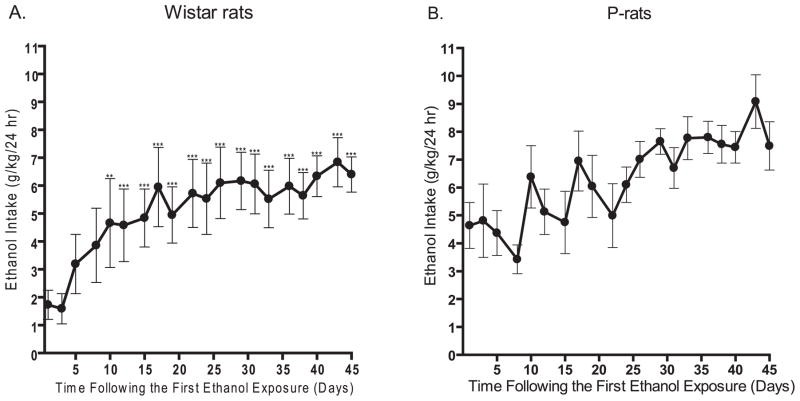
The intermittent-access 20% ethanol 2-bottle-choice drinking paradigm resulted in a steady escalation in ethanol consumption in Wistar rats. There was an overall effect on the ethanol intake (g/kg/24 h) over time [F(19,10) = 21, p < 0.0001, n = 11, Fig. 5A]. Post hoc analysis revealed that the ethanol consumed during each of the drinking sessions from number 5 through 20 were significantly higher than the ethanol consumed during the first drinking session (Fig. 5A). Post hoc analysis further revealed that following the sixth drinking session there was no significant difference in the ethanol intake between any of the subsequent drinking sessions. Thus, the Wistar rats reached a stable baseline consumption of 5.8 ± 0.8 g/kg/24 h following the sixth exposure of ethanol. The mean baseline consumption for each rat ranged from 1.2 to 9.7 g/kg/24 h with 45% of the rats reaching a baseline above the group mean.
Fig. 5.
High ethanol consumption in Wistar and P-rats using the intermittent-access 20% ethanol drinking paradigm. (A) Intermittent-access to 20% ethanol induced high levels of ethanol consumption in Wistar rats (n = 11), reaching a baseline consumption of 5.8 ± 0.8 g/kg/24 h and (B) P-rats (n = 6) initiate drinking at a high level and show a nonsignificant trend to increase their consumption of ethanol, baseline consumption: 6.3 ± 0.8 g/kg/24 h. The values are expressed as mean ethanol intake (g/kg/24 h) ± SEM at each drinking session. **p < 0.01 and ***p < 0.001 compared with the first drinking session (one-way repeated measures ANOVA followed by Newman–Keuls post hoc test).
We then measured ethanol intake in the P-rats using the intermittent-access 20% ethanol 2-bottle-choice drinking paradigm. There was an overall main effect on the ethanol intake [F(19,5) = 2.7, p < 0.001, n=6, Fig. 5B], however, post hoc analysis failed to reveal any significant difference in ethanol intake at any drinking session compared with the first drinking session (mean ethanol consumption throughout the experiment: 6.3 ± 0.8 g/kg/24 h, Fig. 5B). The mean consumption for each rat ranged from 5.2 to 7.5 g/kg/24 h with 50% of the rats reaching a consumption above the group mean. Furthermore, the ethanol intake on the first ethanol drinking occasion (g/kg/24 h) was higher in P-rats compared with both Long–Evans and Wistar rats [F(2,26) = 9.2, p < 0.001; Figs 1A and 5(A and B), respectively].
Experiment 5—Blood Ethanol Concentrations Obtained Following Voluntary Oral Ethanol Consumption Using the Intermittent-Access 20% Ethanol Drinking Paradigm
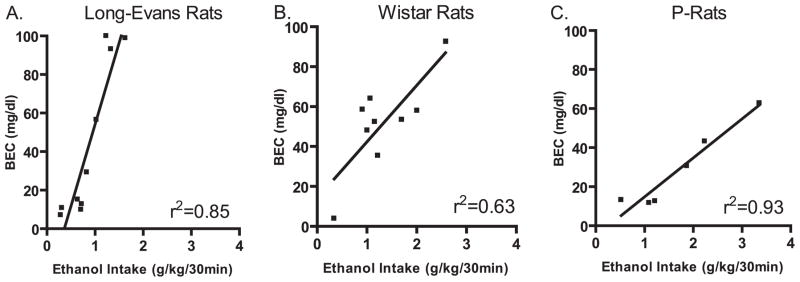
The amount of ethanol consumed after 30 minutes significantly correlated with the measured BECs in all strains (Long–Evans: r2 = 0.85, n = 10; Wistar: r2 = 0.63, n = 10; and P-rats: r2 = 0.93, n=6, Fig. 6). In Long–Evans rats, the BECs ranged from 10 to 100 mg/dl with 30% of them being above 93 mg/dl (Fig. 6A). In Wistar and P-rats, the BECs ranged from 4 to 93 mg/dl and 11 to 63 mg/dl, respectively (Fig. 6B and C). Although there was a trend toward higher ethanol intake in the Wistar and P-rats compared with Long–Evans rats, there was no overall main effect on the ethanol intake during the 30 drinking session that proceeded the BEC measurements [F(2.22) = 3.0, n.s. Long–Evans: 0.87 ± 0.14; Wistar 1.3 ± 0.2; and P-rats 1.7 ± 0.4 g/kg/30 min, respectively).
Fig. 6.
Blood ethanol concentrations (BECs; mg/dl) obtained following 30 minutes of voluntary oral ethanol consumption using the intermittent-access 20% ethanol drinking paradigm. The blood sample for BEC analysis was collected approximately 45 minutes into the dark cycle. The amount of ethanol consumed after 30 minutes of a drinking session significantly correlated with the measured BECs (linear regression): (A) Long–Evans: r2 = 0.85, n = 10; (B) Wistar: r2 = 0.63, n = 10; (C) P-rats: r2 = 0.93, n = 6.
DISCUSSION
In this study, we show that ethanol-naïve Long–Evans and Wistar rats given intermittent-access to 20% ethanol in a 2-bottle-choice drinking paradigm consume high levels of ethanol, equivalent to levels obtained in selectively in-bred alcohol preferring rats (Bell et al., 2006; Dyr and Kostowski, 2000; Myers et al., 1998). In Long–Evans and Wistar rats, the repeated cycles of excessive drinking and abstinence produced robust high levels of ethanol consumption that were maintained over a long period of time and induced long-lasting changes in ethanol drinking behaviors.
Rats in the intermittent-access 20% ethanol paradigm consumed ethanol that reached pharmacologically relevant BECs (Bell et al., 2006; Myers et al., 1998). In fact, 40% of the Long–Evans rats in the intermittent-access group reached BECs seen in rat strains selectively bred for alcohol preference (Bell et al., 2006; Myers et al., 1998). Interestingly, the Wistar rats and the P-rats needed to consume higher amounts of ethanol than the Long–Evans rats to reach the same BECs. This discrepancy may potentially be due to higher rates of ethanol metabolism or a differential thermic response to ethanol (which could affect blood samples collected from the tail vein as the tail is the major thermoregulatory device in the rat) but these possibilities remain to be investigated.
Both Long–Evans and Wistar rats will voluntarily consume excessive amounts of ethanol using the intermittent-access 20% ethanol drinking paradigm (Figs 1 and 5). This is in line with previous studies showing that rats tend to prefer a 20% ethanol solution over a weaker ethanol solution (Amit et al., 1970; Mormede et al., 2004; Myers et al., 1998). The intermittent-access 20% ethanol drinking paradigm, unlike continuous-access drinking paradigms, consists of repeated cycles of excessive drinking and abstinence. The established ethanol deprivation effect (ADE) is defined as a temporary increase in the voluntary intake over baseline drinking when ethanol is reinstated after a period of deprivation (Sinclair and Senter, 1968). It is possible that the initial escalation in ethanol consumption when given intermittent-access to 20% ethanol is driven by repeated ADEs. Furthermore, it is possible that the escalation in ethanol consumption, during the first 2 weeks of intermittent-access to 20% ethanol, induces neuroadaptive changes that lead to the sustained high amounts of baseline ethanol consumption following the escalation phase, however, this remains to be determined.
Following the 40-day period of abstinence from ethanol, the level of ethanol intake returned to same high level as that measured before the period of abstinence and there was a significant increase in the preference for ethanol over water. This suggests that intermittent-access to 20% ethanol induces long-lasting changes that are maintained even following long periods of abstinence from ethanol. The lack of an ADE following the 40-day abstinent period was surprising. However, it may be possible that the length of the deprivation period, 40 days, was too long and this may have resulted in a reduced ADE when compared with that obtained in other studies (Rodd-Henricks et al., 2000).
In this study, acamprosate significantly reduced ethanol consumption only in high ethanol consuming rats using the intermittent-access 20% ethanol drinking paradigm and lacked effect in any of the other drinking paradigms. This is similar to that seen in patients, where acamprosate is most effective in decreasing alcohol intake in individuals that are consuming high amounts of alcohol (Mann et al., 2004; Verheul et al., 2005). We also show that the decrease in ethanol consumption following acute administration of a low dose of naltrexone (0.3 mg/kg s.c.) was significant in animals exposed to ethanol using the intermittent-access 20% drinking paradigm. These results suggests that the repeated cycles of excessive drinking and abstinence may induce changes in the endogenous opioid and glutamatergic systems, rendering the rats more sensitive to naltrexone and acamprosate but this remains to be investigated.
One caveat in the experimental design of this study is the difference in body weight between the intermittent- and continuous- access groups at the time of acamprosate and naltrexone administration. However, all rats were adult when ethanol was first introduced, thus it is unlikely that the increased efficacy of both acamprosate and naltrexone following intermittent-access to 20% ethanol compared with continuous-access to ethanol, was affected by developmental issues.
The current study presents an ethanol drinking paradigm that appears to turn a standard laboratory rat into a high ethanol consuming rat. The intermittent-access 20% ethanol paradigm produces very robust and reproducible levels of high voluntarily ethanol consumption in Long–Evans and Wistar rats that are maintained over a long period of time, up to 8 months (data not shown). This drinking paradigm also induces high ethanol consumption in P-rats. However, the escalation of drinking is not as pronounced as that obtained using standard laboratory rats. This is possibly related to the higher initial ethanol intake in the P-rats compared with both Long–Evans and Wistar rats.
The use of rat strains selectively bred for high ethanol consumption allows the opportunity to study animals that are preselected for high ethanol consumption. In contrast, the intermittent-access 20% ethanol 2-bottle-choice paradigm will facilitate the study of the effects of high ethanol consumption in standard laboratory rats. Furthermore, the intermittentaccess 20% ethanol 2-bottle-choice drinking paradigms will also facilitate studies examining several stages of ethanol consumption including escalation, maintenance and deprivation.
The combination of voluntary oral ethanol consumption followed by abstinence that is utilized in the intermittent-access 20% ethanol drinking paradigm, provides a unique opportunity to investigate neurochemical and behavioral changes induced by high ethanol consumption over time. This elegant and straight forward method can easily be replicated in any laboratory and promises to be a useful preclinical tool for modeling some aspects of AUD.
Acknowledgments
This work was supported by funding from the State of California for Medical Research through UCSF to SEB and Department of Defense Grants W81XWH-06-1-0240 and W81XWH-07-1-0075 to SEB, and by The Foundation BLANCEFLOR Boncompagni-Ludovisi, née Bildt, The Sweden-America Foundation and The Swedish Brain Foundation awarded to PS, by AA010983 awarded to LJC, and by AA015512-02 that supports the P-rats at Indiana University. We thank Tiffany Ho and Haley Pierson for excellent technical assistance with the behavioral experiments.
References
- Amit Z, Stern MH, Wise RA. Alcohol preference in the laboratory rat induced by hypothalamic stimulation. Psychopharmacologia. 1970;17:367–377. doi: 10.1007/BF00403808. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Myers RD. Selection of a single ethanol test solution in free-choice studies with animals. Q J Stud Alcohol. 1968;29:446–448. [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Dyr W, Kostowski W. Animal model of ethanol abuse: rats selectively bred for high and low voluntary alcohol intake. Acta Pol Pharm. 2000;57(Suppl):90–92. [PubMed] [Google Scholar]
- Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science. 1968;159:739–741. doi: 10.1126/science.159.3816.739. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Badia-Elder N. Alterations in taste reactivity to alcohol in rats given continuous alcohol access followed by abstinence. Alcohol Clin Exp Res. 1994;18:555–559. doi: 10.1111/j.1530-0277.1994.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol. 1987;(Suppl 1):91–96. [PubMed] [Google Scholar]
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol. 1983;5:171–178. [PubMed] [Google Scholar]
- Meisch RA, Thompson T. Ethanol intake during schedule-induced polydipsia. Physiol Behav. 1972;8:471–475. doi: 10.1016/0031-9384(72)90331-9. [DOI] [PubMed] [Google Scholar]
- Mormede P, Colas A, Jones BC. High ethanol preferring rats fail to show dependence following short- or long-term ethanol exposure. Alcohol Alcohol. 2004;39:183–189. doi: 10.1093/alcalc/agh037. [DOI] [PubMed] [Google Scholar]
- Myers RD, Robinson DE, West MW, Biggs TA, McMillen BA. Genetics of alcoholism: rapid development of a new high-ethanol-preferring (HEP) strain of female and male rats. Alcohol. 1998;16:343–357. doi: 10.1016/s0741-8329(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav. 1976;16:693–698. doi: 10.1016/0031-9384(76)90238-9. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000;24:747–753. [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Hyytia P, Nurmi M. The limited access paradigm: description of one method. Alcohol. 1992;9:441–444. doi: 10.1016/0741-8329(92)90045-c. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Tomie A, di Poce J, Derenzo CC, Pohorecky LA. Autoshaping of ethanol drinking: an animal model of binge drinking. Alcohol Alcohol. 2002;37:138–146. doi: 10.1093/alcalc/37.2.138. [DOI] [PubMed] [Google Scholar]
- Tomie A, Miller WC, Dranoff E, Pohorecky LA. Intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol Alcohol. 2006;41:225–230. doi: 10.1093/alcalc/agl002. [DOI] [PubMed] [Google Scholar]
- Verheul R, Lehert P, Geerlings PJ, Koeter MW, van den Brink W. Predictors of acamprosate efficacy: results from a pooled analysis of seven European trials including 1485 alcohol-dependent patients. Psychopharmacology (Berl) 2005;178:167–173. doi: 10.1007/s00213-004-1991-7. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiol Behav. 1972;9:737–740. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]