Abstract
There are a number of hypophosphatemic disorders due to renal phosphate wasting that cannot be explained by elevated levels of parathyroid hormone. The circulating factors responsible for the phosphaturia have been designated as phosphatonins. Studies of patients with tumor-induced osteomalacia and other genetic diseases of phosphate metabolism have resulted in the identification of a number of hormones that regulate phosphate homeostasis, including matrix extracellular phosphoglycoprotein (MEPE), secreted frizzled-related protein 4 (sFRP-4), dentin matrix protein 1 (DMP1), fibroblast growth factor 7 (FGF7), fibroblast growth factor 23 (FGF23), and Klotho. Our understanding of the actions of these hypophosphatemic peptides has been enhanced by studies in mice either overexpressing or not expressing these hormones. This review focuses on FGF23 since its regulation is disordered in diseases that affect children, such as X-linked hypophosphatemia, autosomal dominant and recessive hypophosphatemic rickets as well as chronic kidney disease. Recent studies have shown that FGF23 is unique among the FGFs in its requirement for Klotho for receptor activation. Here, we also discuss new potentially clinically important data pointing to the receptor(s) that mediate the binding and action of FGF23 and Klotho.
Keywords: Hypophosphatemia, Klotho, Proximal tubule, Sodium phosphate cotransporter
Introduction
An adult ingests about 1.5 g of phosphorus per day, of which two-thirds is absorbed by the intestine by both a transcellular and paracellular route. An adult must excrete the absorbed phosphate to remain in phosphate balance; however, a child is in positive phosphate balance for growth. Phosphate plays an important and necessary role in nucleotide generation, including ATP, in the formation of 2,3-diphosphoglycerate for facilitating oxygen release from hemoglobin, in the formation of phospholipids, and in the regulation of many proteins. It is also a structural element for bone, functions as a urinary buffer, and is necessary for virtually every enzymatic process. For these reasons, the serum phosphorus level is very tightly regulated. The regulation of phosphate has traditionally been thought to be mediated by parathyroid hormone (PTH), which acts on bone, causing bone resorption, and on the kidney, causing phosphaturia and increased levels of circulating 1,25 (OH)2 vitamin D3, which is turn predominantly acts on the intestine to increase calcium and phosphate absorption.
About 90% of the plasma phosphate is filtered by the glomerulus, of which approximately 15% is excreted in the urine when the dietary intake is normal. With hypophosphatemia that is not due to renal wasting, less than 10% of the filtered phosphate is excreted in the urine. Approximately 90% of the filtered phosphate is reabsorbed by the proximal tubule; the rest is reabsorbed distally by an as yet unknown distal mechanism. Luminal phosphate is absorbed from the glomerular ultrafiltrate by an electrogenic phosphate transporter designated as NaPi-2a (sodium-dependent phosphate transporter 2a) that transports three sodium ions into the proximal tubule cell for each phosphate ion and an electroneutral transporter designated as NaPi-2c that transports two sodium ions for each phosphate [1]. The driving force for apical phosphate transport is the low intracellular sodium mediated by the basolateral Na+/K+-ATPase. Phosphate exits the cell across the basolateral membrane by an as yet to be characterized transporter.
Both the NaPi-2a and NaPi-2c transporters are concurrently regulated and increase in abundance on the apical membrane with dietary phosphate deprivation and decrease when a high phosphate diet is ingested [2–5]. Parathyroid hormone reduces the abundance of NaPi-2a on the brush border membrane, resulting in a decrease in phosphate transport and phosphaturia [6–8]. The effect of dietary phosphate on NaPi-2a abundance has been observed in thyroparathyroidectomized animals, indicating that it is independent of the action of PTH [9]. Administration of a high phosphate diet or high amounts of PTH results in a rapid decrease in brush border membrane NaPi-2a abundance and internalization into lysosomes [5, 10, 11]. Thus, the regulation of NaPi-2a likely does not involve recycling, but rather synthesis and insertion and endocytic vesicle removal and degradation. On the other hand, decrease in the expression of NaPi-2c following the ingestion of a high phosphate diet is slower than that of NaPi-2a, and immunohistochemistry studies have shown that NaPi-2c is translocated to the subapical compartment of the proximal tubule [5]. Acute administration of PTH results in the decreased expression of NaPi-2c on brush border membrane, similar to that of NaPi-2a, albeit relatively slowly. Moreover, intact microtubules are essential for the internalization of NaPi-2c, unlike NaPi-2a [11]. The mechanisms of intracellular degradation of NaPi-2c are not well known, unlike those for NaPi-2a.
While 1,25 (OH)2 vitamin D3 and PTH actions can explain most of the physiological and pathophysiological processes of phosphate metabolism, there are several disorders, many of which affect children, that cannot be explained by the dysregulation of these hormones alone. The unknown factors responsible for hypophosphatemia and phosphaturia were therefore designated as phosphatonins.
Many phosphatonins, including matrix extracellular phosphoglycoprotein (MEPE), secreted frizzled-related protein 4 (sFRP-4), dentin matrix protein 1 (DMP1), fibroblast growth factor 7 (FGF7), FGF23, and Klotho, have been identified. MEPE, sFRP-4, DMP1, FGF7, and FGF23 mRNAs have been found to be highly expressed in tumors associated with tumor-induced osteomalacia, a condition characterized by low serum phosphorus levels [12, 13]. The administration of sFRP-4 to rats results in a decrease in serum phosphorus levels and an increase in the fractional excretion of phosphate (FEPO4) [14]. However, sFRP-4 levels are not affected by serum phosphorus levels in patients with chronic kidney disease, nor does sFRP-4 appear to play a role in the hypophosphatemia seen in patients after renal transplant [15]. FGF7 inhibits phosphate transport in vitro, but its role in hypophosphatemic conditions has not been examined [12]. MEPE also causes phosphaturia when administered to rats, with no observable change in the glomerular filtration rate [16]. Unlike sFRP-4, serum MEPE levels correlate with serum phosphorus and PTH levels, which is consistent with MEPE having a role in the regulation of phosphorus metabolism [17]. In addition, high serum MEPE levels have been measured in patients with X-linked hypophosphatemia [18]. Inactivating mutations in DMP1 result in autosomal recessive hypophosphatemic rickets [19]. Patients with this disorder and DMP1 null mice have very high levels of FGF23 [19]. Although studies on the regulation of phosphate homeostasis by these phosphatonins are relatively recent, a considerable body of knowledge has been compiled on the regulation of phosphorus by FGF23 and Klotho. This review focuses on the hypophosphatemic and hyperphosphatemic disorders that are due to the dysregulation of Klotho and FGF23. We also discuss how Klotho and FGF23 interact to regulate phosphate transport and serum phosphorus levels.
Role of FGF23 and Klotho on proximal tubule phosphate transport
Substantive data show that elevated serum levels of FGF23 result in low levels of 1,25 (OH)2 vitamin D3, hypophosphatemia, and increased renal phosphate wasting. The implantation of Chinese hamster ovary cells that express high levels of FGF23 into nude mice results in hypophosphatemia due to renal phosphate wasting as well as low serum levels of 1,25 (OH)2 vitamin D3 [20, 21]. Similarly, transgenic mice that overexpress FGF23 have low serum phosphate, low serum 1,25 (OH)2 vitamin D3 levels, and decreased renal expression of NaPi-2a as well as resultant renal phosphate wasting [22, 23]. These mice demonstrate severe rickets comparable to that of Hyp mice [23]. The FGF23 effect on 1,25 (OH)2 vitamin D3 is mediated by a reduction in 1α-hydroxylase and increased levels of 25-hydroxyvitamin D-24-hydroxylase, resulting in decreased synthesis and increased inactivation of 1,25 (OH)2 vitamin D3 [24, 25]. The injection of FGF23 into mice results in a reduction in renal NaPi-2a mRNA and protein abundance that is concordant with the observed phosphaturia and hypophosphatemia [24, 26]. The effect of FGF23 is not mediated by changes in serum PTH levels [24, 26] and was seen in mice that had a parathyroidectomy [24]. Indeed, FGF23 has been shown to lower PTH levels that would, in and of itself, act to reduce phosphate excretion [27]. The effect of FGF23 to lower serum phosphate levels and reduce renal NaPi-2a abundance in vitamin D receptor null mice demonstrates that FGF23’s action on NaPi-2a is independent of its effect on vitamin D [28].
Further evidence for the importance of FGF23 on the regulation of phosphate transport comes from experiments where FGF23 levels are reduced. The injection of anti-FGF23 neutralizing antibodies into mice and rats resulted in increased serum phosphorus levels and a concomitant increase in renal phosphate absorption due to increased renal NaPi-2a protein abundance [29]. These neutralizing antibodies caused an increase in 1α-hydroxylase and a decrease in 25-hydroxyvitamin D-24-hydroxylase mRNA abundance, resulting in an increase in serum 1,25 (OH)2 vitamin D3 levels [29]. Mice with a targeted ablation of FGF23 have hyperphosphatemia due to increased renal phosphate absorption, increased levels of 1,25 (OH)2 vitamin D3, abnormal bone formation, and poor growth [30, 31]. Importantly, these mice have a shortened life span and vascular calcifications. Placing these mice on a low phosphorus diet, but not a low vitamin D diet, has been shown to prevent the hyperphosphatemia and vascular calcification and to result in a prolonged life [31]. In addition, deletion of the 1α-hydroxylase gene or deletion of the vitamin D receptor in the Fgf23−/− mice also resulted in amelioration of vascular calcifications and a prolonged life span [32–34]. To examine whether FGF23 directly affected the inhibition of phosphate transport, FGF23 was added to OK cells, which have characteristics of proximal tubule cells. One research group found that FGF23 did directly inhibit phosphate transport [35], while another group failed to find a direct inhibition of FGF23 on OK cell transport [20, 36]. However, in the presence of heparin, which has been shown to enhance the binding of other FGFs to FGF receptors [37, 38], FGF23 has been found to inhibit phosphate transport in OK cells via a MAPK pathway [39]. We have previously demonstrated that in tubules perfused in vitro, FGF23 had an effect on the basolateral membrane in the presence but not in the absence of heparin [40].
Studies examining the factors that regulate FGF23 are often difficult to interpret since there are changes in serum levels of calcium, phosphate, 1,25 (OH)2 vitamin D3, and parathyroid hormone with manipulation of any one of these factors [41]. In vivo studies on rodents have shown that serum FGF23 levels and FGF23 mRNA levels in the bone increase with the administration of 1,25 (OH)2 vitamin D3 in wild-type mice, mice that have lack parathyroid glands (Gcm2 mice), Hyp mice (murine model for X-linked hypophosphatemic rickets), and wild-type rats [42, 43]. In addition, the administration of 1,25 (OH)2 vitamin D3 to rats that had a thyroparathyroidectomy or 5/6 nephrectomy also increases serum FGF23 levels [43], suggesting that the increase in FGF23 levels is independent of PTH. Moreover, vitamin D receptor null mice (VDR−/− mice) have low serum FGF23 levels, reiterating the importance of 1,25 (OH)2 vitamin D3 in the regulation of FGF23 [28, 43, 44]. An in vitro study by Liu et al. [42] demonstrated that the addition of 1,25 (OH)2 vitamin D3 to osteoblasts in culture increased FGF23 mRNA levels and that 1,25 (OH)2 vitamin D3 increased the promoter activity of the FGF23 gene via a vitamin D response element in the promoter region of FGF23. Thus, FGF23 promoter activity is stimulated by 1,25 (OH)2 vitamin D3 [42]. The FGF23 promoter was found to be mildly suppressed by PTH and not affected by calcium and phosphate levels [42]. Another study using a smaller part of the FGF23 promoter showed that the predominant regulator of FGF23 gene expression is 1,25 (OH)2 vitamin D3 [45]. Phosphorus increased promoter activity in a manner that was additive to the effect of 1,25 (OH)2 vitamin D3, but there was no effect of calcium [43]. These data clearly show that 1,25 (OH)2 vitamin D3 regulates serum FGF23 levels and FGF23 mRNA levels in the bone. However, this is likely to counterbalance the increase in phosphate absorption from the gut by 1,25 (OH)2 vitamin D3 as FGF23 causes urinary phosphate loss.
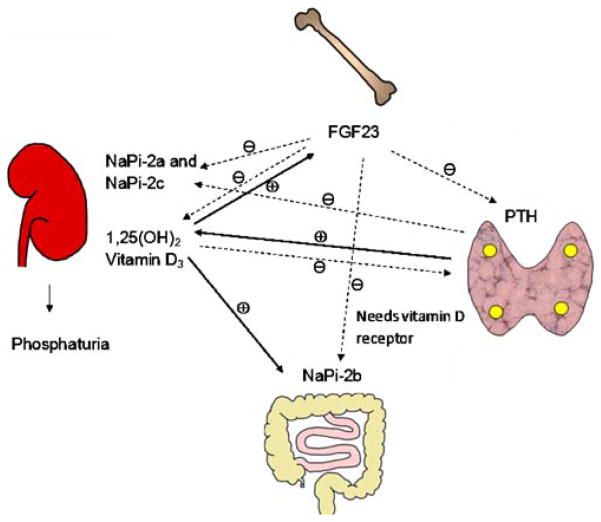
There is also significant evidence that FGF23 is regulated by serum phosphate levels. In mice, dietary phosphate intake is reflected by changes in serum phosphate levels [46]. A high phosphate diet increases FGF23 levels, whereas a low phosphate diet causes a reduction in FGF23 levels [44–46]. These dietary phosphate-induced changes in FGF23 levels is also seen in rats with chronic renal failure secondary to 5/6 renal ablation [43]. Serum phosphorus-mediated changes in serum FGF23 levels are paralleled by changes in mouse bone FGF23 mRNA abundance [46]. A similar dietary effect of phosphate on serum FGF23 has been confirmed in humans [47, 48]. To tease out whether changes in dietary phosphate content can cause a change in serum FGF23 levels independent of 1,25 (OH)2 vitamin D3, VDR−/− mice were fed a diet high in calcium and phosphate; this normalized the serum levels of phosphate as compared to those of wild-type mice. At baseline, VDR−/− mice have undetectable levels of FGF23, but when serum phosphate levels were normalized by diet, serum FGF23 levels increased [44]. This result suggests that serum phosphorus levels can regulate serum FGF23 levels independent of the actions of 1,25 (OH)2 vitamin D3 [44]. The complex interaction between the bone, where FGF23 is primarily synthesized, gastrointestinal tract, kidney, and parathyroid gland is shown in Fig. 1.
Fig. 1.
Fibroblast growth factor 23 (FGF23)–kidney–gastrointestinal tract–parathyroid axis. This figure demonstrates the complex interaction between the various hormones that play a role in phosphate homeostasis. FGF23 is primarily produced in the bone, which decreases the expression of an electrogenic phosphate transporter (sodium-dependent phosphate transporter 2a, NaPi-2a) and an electro-neutral phosphate transporter (NaPi-2c) on the apical surface of the proximal tubule, causing phosphaturia. FGF23 also decreases the serum levels of 1,25(OH)2 vitamin D3. Decreased 1,25(OH)2 vitamin D3 levels in turn decreases the gastrointestinal absorption of phosphorus. 1,25(OH)2 Vitamin D3 stimulates FGF23 production, which in turn decreases 1,25(OH)2 vitamin D3. FGF23 directly or indirectly decreases NaPi-2b expression in the small intestine. Serum parathyroid hormone (PTH) stimulates the synthesis of 1,25(OH)2 vitamin D3, which in turn decreases PTH levels as a negative feedback mechanism. PTH also decreases NaPi-2a and NaPi-2c expression. FGF23 decreases PTH secretion. The solid lines indicate positive regulation, and the dotted lines indicate negative regulation
What is known thus far is that FGF23 is a phosphaturic hormone causing both hypophosphatemia and low serum levels of 1,25 (OH)2 vitamin D3. There is also evidence that both phosphorus and 1,25 (OH)2 vitamin D3 regulate FGF23 levels in the serum. However, many questions still remain unanswered. Is a cofactor essential for the interaction of FGF23 with its receptor? If so, is heparin that cofactor, or is there another cofactor that determines the specificity of the actions of FGF23. As will become clear below, it is likely that the proximal tubule synthesizes its cofactor in order to facilitate binding to the apical membrane receptors.
There are several lines of evidence suggesting that the actions of FGF23 and Klotho may be linked. Both Klotho null mice and FGF23 null mice age prematurely, have a shortened life span [30, 33, 49, 50], have high serum phosphate levels, high levels of NaPi-2a on the renal brush border, and high 1,25 (OH)2 vitamin D3 levels, and develop ectopic calcifications, including vascular calcifications [30, 33, 49–51]. The FGF23 levels in Klotho null mice are 150-fold higher than those in control mice, yet these significantly higher levels of FGF23 do not correct the hyperphosphatemia [51]. Injection of an anti-Klotho anti-body into wild-type mice results in an increase in 1,25 (OH)2 vitamin D3, serum FGF23 and serum phosphorus levels, and in renal brush border membrane NaPi-2a expression [52]. This is consistent with the absence of an effect of circulating FGF23 as is seen with the administration of neutralizing FGF23 antibodies [29]. Transgenic mice that overexpress FGF23 and are Klotho-deficient have a Klotho-deficient phenotype, which is consistent with the requirement for Klotho for the action of FGF23 [53]. Furthermore, Klotho/FGF23 double knock out mice have a phenotype that is not different than either knockout alone. The administration of FGF23 to the Klotho/FGF23 double knock out mice did not reduce the elevated serum levels of phosphorus. These results provide compelling evidence that Klotho is necessary for the action of FGF23 in vivo [51, 54].
Klotho protein has been localized to the distal convoluted tubule, parathyroid gland, choroid plexus, pituitary gland and in the reproductive organs [55]. The addition of Klotho protein to either the apical or basolateral side of the proximal tubule-like cells in culture results in a decrease in phosphate transport [56]. Many questions remain unanswered about Klotho. How does Klotho protein affect proximal tubule phosphate transport? Does Klotho protein get filtered or secreted into the lumen of the proximal tubule to exert an effect on the apical membrane? How does Klotho protein act to affect phosphate transport? How can Klotho and FGF23 affect phosphate transport independently of each other in vitro, while the loss of function of either protein results hyperphosphatemia in vivo?
FGF23 and Klotho receptor binding and signal transduction
Fibroblast growth factors mediate their action by binding to one of four receptor kinases, designated as FGFR1–4 [57, 58]. Alternative splicing of the third immunoglobulin-like portion results in “b” and “c” isoforms of FGF receptors 1, 2, and 3 [57, 58]. Of these four receptors, only FGFR1, FGFR3, and FGFR4 are present on the proximal tubule [26]. Unlike other FGFs, FGF23 does not bind to FGF receptors even in the presence of heparin [52].
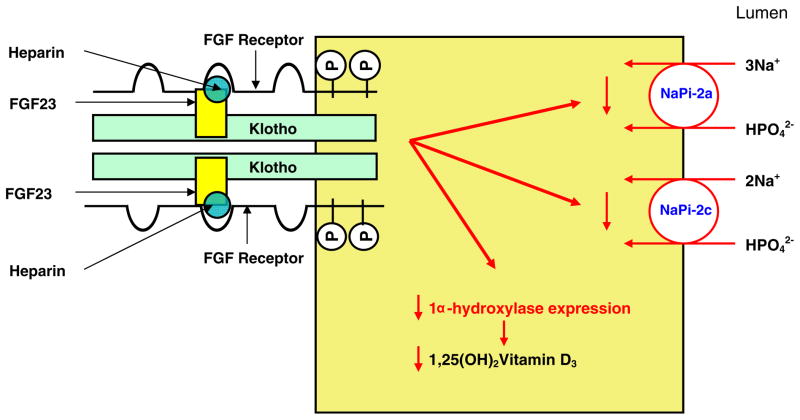
The receptor for FGF23 remained enigmatic until recently when the similarity in the phenotype between FGF23 and Klotho null mice provided a clue as to the mechanism of action [52, 59]. While FGF23 does not bind to any FGF receptor, Klotho binds to all of them [59]. In the presence of Klotho, FGF23 binds to FGFR1c, FGFR3c, and FGFR4 [59]. Heparin stabilizes the binding of FGF23 to the Klotho FGFR1 complex [52]. The binding of FGF23 to the Klotho–FGF receptor complex results in FGF receptor phosphorylation and activation of the extracellular signal regulated kinase (ERK) pathway [52, 59]. The above studies were all performed in vitro using co-immunoprecipitation and immunoblotting techniques. The hypothesized interaction between FGF23, the FGF receptor, Klotho, and heparin is shown in Fig. 2.
Fig. 2.
FGF23–Klotho binding to the FGF receptor and schematic representation of the actions of FGF23 in the proximal tubular cell. This figure shows the interaction between the FGF receptor (FGFR), FGF23, Klotho, and heparin on the surface of the cell. FGF23 binds to the FGFR–Klotho complex with heparin stabilizing this complex. Activation of FGFR results in the activation of intracellular signaling pathways, which in turn decreases the expression of NaPi-2a, NaPi-2c, and 1α-hydroxylase. This results in decreased phosphate absorption from the proximal tubule and decreased synthesis of 1,25(OH)2 vitamin D3
The receptors that mediate the actions of FGF23 in vivo are uncertain at present. If one receptor mediates the action of FGF23, then compared to the controls the absence of that receptor would be expected to result in hyperphosphatemia, an increase in renal brush border membrane NaPi-2a protein abundance, an increase in serum 1,25 (OH)2 vitamin D3 levels, and an elevation in serum FGF23 levels. This is not observed in FGFR3, FGFR4, and FGFR1 null mice, where the FGFR1 receptor is ablated in the nephron [26, 60]. Administration of FGF23 to FGFR3 and FGFR4 null mice results in a reduction in serum phosphorus levels, brush border membrane NaPi-2a protein abundance, and 1,25 (OH)2 vitamin D3 levels; these results are consistent with none of these receptors playing a primary role in the action of FGF23 [26]. The administration of FGF23 to mice in which FGFR1 is deleted along the nephron failed to decrease serum phosphorus levels or brush border membrane NaPi-2a protein abundance, which is consistent with FGFR1 being the predominant FGFR receptor in vivo [26]. Hyp mice, which have elevated levels of FGF23 and low 1,25 (OH)2 vitamin D3 and phosphorus levels, did not have a correction in the metabolic abnormalities seen in Hyp mice when crossed to either FGFR3 or FGFR4 null mice [60]. While it appears that FGF23 cannot regulate phosphate homeostasis without Klotho, the reverse is not the case. Klotho can bind to FGF receptors without FGF23 [59]. Klotho can inhibit phosphate transport in proximal tubule cells in vivo and can reduce serum phosphorus levels by promoting a phosphaturia in vivo [56]. Klotho has a direct effect on the lumen and bath of the proximal tubule to reduce NaPi-2a expression [56]. The implication of this is unclear, and future studies will shed more information.
Human disorders with altered serum FGF23 and Klotho levels
FGF23 is a 251-amino acid protein (approx. 32 kDa) with an N terminal signal peptide and a novel C-terminal end [61]. It is cleaved between arginine179 and serine180, resulting in an N-terminal fragment (18 kDa) and a C-terminal fragment (12 kDa). The amino acid sequence adjacent to the cleavage site is Arg176-X-X-Arg179, which is recognized by a subtilisin-like proprotein convertase, furin [20]. Inhibitors of proprotein convertase have been shown to inhibit the proteolytic cleavage of FGF23 [62]. The FGF receptor (FGFR) binding domain is in the N-terminal fragment, and the Klotho binding domain lies in the C-terminal [29]. FGF23 is predominantly expressed in the bone (osteocytes/osteoblasts), but it is also expressed in smaller amounts in other organs, including the brain, heart, thymus, muscle, and spleen [63–65].
Conditions of FGF23 excess
Autosomal dominant hypophosphatemic rickets
FGF23 was first identified as a gene causing human disease in autosomal dominant hypophosphatemic rickets (ADHR) [63]. ADHR was first described in 1971 as a phosphate wasting disease with an autosomal dominant inheritance [66]. In ADHR, there is a missense mutation in either the Arg176 or Arg179 site, resulting in a FGF23 protein that is not identified or cleaved by furin [21, 67, 68]. This results in high serum levels of full-length FGF23 protein, causing the phenotype of hypophosphatemia, renal phosphate wasting, inappropriately low or normal levels of 1,25 (OH)2 vitamin D3 along with skeletal defects that include rickets/osteomalacia, fractures, and dental abscess.
A study of a large kindred with ADHR revealed that ADHR has incomplete penetrance, with variable disease severity and symptoms depending upon the age at presentation [69]. The group of patients who developed the disease as children presented with hypophosphatemia, weakness, bone pain, short stature, rickets, and deformity of their lower extremities. The other group of patients, who developed the disease as adults or adolescents, presented with hypophosphatemia, muscle weakness, bone pain, and pseudofractures, but there was no evidence of rickets or bone deformity. Interestingly, the latter group of patients were all females and in the majority of cases, pregnancy was the precipitating factor resulting in the onset of symptoms. Additionally, some of the patients who presented as children showed a reduction in renal phosphate wasting and an improvement in their symptoms upon entering puberty [69, 70]. All of the patients described in this kindred had normal PTH and alkaline phosphatase levels. Serum FGF23 levels have also been shown to vary with the disease severity [71].
Tumor-induced osteomalacia
Tumor-induced osteomalacia (TIO) is an acquired paraneo-plastic disorder characterized by tumors that are mainly of mesenchymal origin and which produce excessive amounts of phosphaturic peptides [20, 72–75]. Patients with TIO share a similar phenotype with ADHR patients and present with fatigue, muscle weakness, bone pain, and fractures. Patients with TIO have hypophosphatemia, phosphaturia, inappropriately low levels of 1,25 (OH)2 vitamin D3, and increased serum alkaline phosphatase with evidence of osteomalacia on bone biopsy [76].
The initial identification of a circulating phosphaturic substance different from PTH was from a patient with TIO [74]. The tumor cells from the patient were grown in vitro, and the growth media induced a decrease in sodium-dependent phosphate transport in opossum kidney cells (proximal tubular cell line) [74]. Thus, the term “phosphatonin” was coined [73]. In addition, implantation of the tumor cells into mice resulted in hypophosphatemia [77]. Further analysis of the tumoral tissue using different techniques revealed increased gene expression of FGF23, MEPE, DMP1, and sFRP-4 [13, 20, 75, 78]. Importantly, these tumors have also been shown to secrete excessive levels of FGF23 [20, 68, 72, 75], MEPE [79], FGF7 [12] and sFRP-4 [80]. However, an in vivo study using recombinant cells secreting the different peptides, including DMP1, FGF23, and MEPE, showed that mice implanted with cells secreting FGF23 only showed hypophosphatemia.
Tumors causing TIO are usually benign and of mesenchymal origin, and the majority can be classified as phosphaturic mesenchymal tumors with a mixed connective tissue variant [81, 82]. These tumors are small and thus difficult to localize. When these tumors are localized and removed, the serum FGF23 levels fall rapidly. After tumor removal, the disease phenotype reverses [72]. Even though these tumors are benign, they can recur [74].
Autosomal recessive hypophosphatemic rickets
Autosomal recessive hypophosphatemic rickets (ARHR) shares a similar phenotype with ADHR [83, 84]. Patients with ARHR have hypophosphatemia, phosphaturia, low or inappropriately normal levels of 1,25 (OH)2 vitamin D3, high serum alkaline phosphatase, and evidence of skeletal defects (rickets/osteomalacia). The genetic defect for ARHR has recently been identified and shown to be due to a homozygous inactivating mutation of the gene encoding for dentin matrix protein 1 (DMP1) [19, 85]. DMP1 belongs to the SIBLING protein family (small Integrin-binding ligand N-linked glycoprotein protein) and is a bone- and teeth-specific protein produced by osteocytes/osteoblasts [86, 87]. Patients with ARHR and DMP1 null mice (mouse model for ARHR) have elevated serum levels of FGF23 [19, 85]. It is still unclear as to how a mutation of the DMP1 gene results in elevated levels of FGF23. More confirmatory evidence linking DMP1 and FGF23 come from a study in which Fgf23−/− mice were cross bred with Dmp1−/− mice. Dmp1−/− mice have hypophosphatemia, low or inappropriately normal levels of 1,25 (OH)2 vitamin D3, and evidence of rickets/osteomalacia. On the contrary, Fgf23−/− mice have hyperphosphatemia, hypercalcemia, increased levels of 1,25 (OH)2 vitamin D3 along with growth retardation and shortened life span. The phenotype of the double Fgf23−/−/Dmp1−/− mice was found to be similar to that of the Fgf23−/− mouse, indicating that DMP1 is important in the regulation of FGF23 production by the bone and that FGF23 is downstream of DMP1. This result also explains the lack of hypophosphatemia seen in mice implanted with recombinant cells secreting DMP1 even though the DMP1 gene is highly expressed in the tumors of TIO [20].
Fibrous dysplasia
Fibrous dysplasia (FD) is characterized by fibrous skeletal lesions and mineralization defects of the bone. It may occur in monostotic (single bone lesion) or polyostotic (multiple bone lesions) forms. Patients with McCune Albright syndrome (MAS) have cutaneous hyperpigmented patches, precocious puberty, and polyostotic fibrous dysplasia. Some of the patients with MAS develop the hypophosphatemic phenotype, similar to that of ARHR and ADHR patients [88, 89]. Patients with the hypophosphatemic phenotype have high levels of FGF23, which correlates with the number of skeletal fibrous dysplastic lesions. MAS is due to an activating mutation in GNAS-1, which encodes for the alpha subunit of stimulatory G protein [90]. How this activating mutation in GNAS-1 results in high FGF23 levels is unclear [90–93].
X-linked hypophosphatemic rickets
X-linked hypophosphatemia is the most common form of inherited rickets, with an incidence of 1:20,000 [94]. Patients with this disorder have severe hypophosphatemia due to renal phosphate wasting that is not due to hyperparathyroidism, and vitamin D levels are inappropriately normal or low for their level of hypophosphatemia. These abnormal vitamin D levels are due to both a low 25 (OH)D-1α-hydroxylase activity [95–98] and as well as increased C-24 oxidation of 1,25 (OH)2 vitamin D, which results in the inactivation of 1,25 (OH)2 vitamin D [98]. Patients with this disorder present with bone pain, dental abscesses, and rickets that are refractory to treatment with physiologic replacement doses of vitamin D, and they have a marked short stature.
Patients with X-linked hypophosphatemia have a mutation in the PHEX gene, which is an endopeptidase [94, 99, 100]. The Hyp mouse (mouse model of X-linked hypophosphatemic rickets) has a 3′ deletion in the PHEX gene [101]. Patients with X-linked hypophosphatemia and the Hyp mouse have elevated levels of FGF23 [72, 102, 103]. While it originally seemed logical that FGF23 would be a substrate for the endopeptidase and that loss of activity would result in high serum FGF23 levels [35], it does not appear that the endopeptidase acts on FGF23 [62, 64, 104]. Thus, it remains an enigma as to how a mutation in the PHEX gene results in an increase in FGF23 levels. Furthermore, when Fgf23 null mice were cross-bred with Hyp mice, the resulting phenotype was that of the Fgf 23 null mouse with hyperphosphatemia rather than the hypophosphatemia seen in Hyp mice, suggesting that FGF23 is downstream of PHEX and that FGF23 is essential for causing the Hyp phenotype [49].
To summarize, the conditions described above have increased serum levels of FGF23, and they are characterized by hypophosphatemia and decreased serum levels of 1,25 (OH)2 vitamin D3. The mechanisms behind the increased FGF23 levels are different in each of these conditions. Mutations of PHEX, DMP-1, and GNAS-1 function via a common pathway to increase FGF23 levels—but the mechanism is as yet unknown. Future studies will hopefully reveal the precise mechanisms for the increase in FGF23 in the differently inherited hypophosphatemic disorders.
Conditions of FGF23 deficiency
Conditions of FGF23 deficiency result in hyperphosphatemia and increased serum levels of 1,25 (OH)2 vitamin D3, and they have been described in both humans and in animal models. In humans, familial tumoral calcinosis is an autosomal recessive condition characterized by painful depositions of calcium and phosphate in the joints and soft tissues [105–107]. Mutations in GALNT3 and in the FGF23 gene have been identified to be the cause for this disease. GALNT3 encodes for the enzyme UDP-N-acetyl-alpha-D-galactosamine; polypeptide N-acetylgalactosaminyl transferase 3 is responsible for O-linked glycosylation of FGF23. The inactivating mutation of GALNT3 protein prevents O-glycosylation of FGF23, resulting in the rapid degradation of the FGF23 protein [107–110]. Therefore, in patients with GALNT3 mutation, intact FGF23 levels are low, but the C-terminal fragment of FGF23 is elevated [108]. Inactivating mutations of FGF23 have also been shown to cause tumoral calcinosis [111, 112]. A mutation in a highly conserved site of FGF23 (serine71) has been reported in some patients with tumoral calcinosis [112, 113]. This mutation results in a FGF23 protein that either is not secreted in its intact form or is prone to early degradation. Similarly, in mice, deletion of FGF23 results in hyperphosphatemia, hypercalcemia, increased serum levels of 1,25 (OH)2 vitamin D3, vascular calcifications, growth retardation, infertility, and a shortened life span [33, 49, 114].
Human diseases associated with the dysregulation of Klotho
Human diseases involving the dysregulation of Klotho have also been described. A homozygous missense mutation in the Klotho gene causing decreased Klotho levels results in tumoral calcinosis analogous to the tumoral calcinosis seen in patients with decreased FGF23 levels [105]. On the other hand, a translocation of the Klotho gene causing increased Klotho levels results in hypophosphatemic rickets similar to that seen in patients with increased serum FGF23 levels [115].
FGF23 in chronic kidney disease and end stage renal disease
Disturbances in phosphate homeostasis are also seen in patients with chronic kidney disease (CKD) and end stage renal disease (ESRD). The FGF23 levels in CKD patients are higher than those in control patients, and the reason for this is likely multi-factorial. Hyperphosphatemia is a stimulus for increased FGF23 levels. The administration of 1,25 (OH)2 vitamin D3 analogs can in itself cause an increase in FGF23 levels. Moreover, increased FGF23 levels can also represent a decrease in the clearance of FGF23 [48, 116–118]. Serum FGF23 levels have been found to be higher in hemodialysis patients who died than in those who survived, even when the serum phosphorus levels were comparable in the two groups [119]. Thus, elevated FGF23 levels are associated with an increased risk of mortality in patients on hemodialysis. This association is not found when the serum phosphorus levels are higher than 5.5 mg/dl. High FGF23 levels have recently been associated with an increased risk of secondary hyperparathyroidism in dialysis patients [120]. It is unclear if the association of increased FGF23 levels and mortality in hemodialysis patients is just an association or due to direct harmful effects of FGF23.
Acknowledgments
This work was supported by NIH grants DK41612, DK065842, and DK078596 to M.B, T32 DK07257 (Peter Igarashi and M.B.), and the O’Brien Center P30DK079328 (Peter Igarashi, PI).
Contributor Information
Jyothsna Gattineni, Department of Pediatrics, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas 75390-9063 TX, USA.
Michel Baum, Email: Michel.Baum@UTSouthwestern.edu, Department of Pediatrics, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas 75390-9063 TX, USA. Department of Internal Medicine, University of Texas Southwestern Medical Center at Dallas, Dallas 75235-9063 TX, USA.
References
- 1.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006;70:1548–1559. doi: 10.1038/sj.ki.5001813. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Lotscher M, Sorribas V, Custer M, Arar M, Kaissling B, Murer H, Biber J. Cellular mechanisms of acute and chronic adaptation of rat renal P(I) transporter to alterations in dietary P(I) Am J Physiol. 1994;267:F900–F908. doi: 10.1152/ajprenal.1994.267.5.F900. [DOI] [PubMed] [Google Scholar]
- 3.Murer H, Forster I, Hernando N, Lambert G, Traebert M, Biber J. Posttranscriptional regulation of the proximal tubule NaPi-II transporter in response to PTH and dietary P(I) Am J Physiol. 1999;277:F676–F684. doi: 10.1152/ajprenal.1999.277.5.F676. [DOI] [PubMed] [Google Scholar]
- 4.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/Pi cotransporter. J Biol Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 5.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K. Internalization of renal type IIc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol. 2005;288:F587–F596. doi: 10.1152/ajprenal.00097.2004. [DOI] [PubMed] [Google Scholar]
- 6.Hernando N, Forgo J, Biber J, Murer H. PTH-Induced downregulation of the type IIa Na/P(i)-cotransporter is independent of known endocytic motifs. J Am Soc Nephrol. 2000;11:1961–1968. doi: 10.1681/ASN.V11111961. [DOI] [PubMed] [Google Scholar]
- 7.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol. 2004;287:F896–F906. doi: 10.1152/ajprenal.00160.2004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol. 1999;276:F711–F719. doi: 10.1152/ajprenal.1999.276.5.F711. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi F, Morita K, Katai K, Segawa H, Fujioka A, Kouda T, Tatsumi S, Nii T, Taketani Y, Haga H, Hisano S, Fukui Y, Miyamoto KI, Takeda E. Effects of dietary Pi on the renal Na+-dependent Pi transporter NaPi-2 in thyroparathyroidectomized rats. Biochem J. 1998;333:175–181. doi: 10.1042/bj3330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J. Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int. 1998;54:1224–1232. doi: 10.1046/j.1523-1755.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- 11.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol. 2007;292:F395–F403. doi: 10.1152/ajprenal.00100.2006. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R. Fibroblast growth factor 7: An inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab. 2005;90:1012–1020. doi: 10.1210/jc.2004-0357. [DOI] [PubMed] [Google Scholar]
- 13.de Beur SMJ, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, Manavalan P, Petroziello J, Madden SL, Cho JY, Kumar R, Levine MA, Schiavi SC. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 14.Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, Jan de Beur SM, Schiavi SC, Kumar R. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pande S, Ritter CS, Rothstein M, Wiesen K, Vassiliadis J, Kumar R, Schiavi SC, Slatapolsky E, Brown AJ. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol. 2006;104:23–32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobbie H, Unwin RJ, Faria NJR, Shirley DG. Matrix extracellular phosphoglycoprotein causes phosphaturia in rats by inhibiting tubular phosphate reabsorption. Nephrol Dial Transplant. 2008;23:730–733. doi: 10.1093/ndt/gfm535. [DOI] [PubMed] [Google Scholar]
- 17.Jain A, Fedarko NS, Collins MT, Gelman R, Ankrom MA, Tayback M, Fisher LW. Serum levels of matrix extracellular phosphoglycoprotein (MEPE) in normal humans correlate with serum phosphorus, parathyroid hormone and bone mineral density. J Clin Endocrinol Metab. 2004;89:4158–4161. doi: 10.1210/jc.2003-032031. [DOI] [PubMed] [Google Scholar]
- 18.Bresler D, Bruder J, Mohnike K, Fraser WD, Rowe PS. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–R9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng JQ, Ward LM, Liu SG, Lu YB, Xie YX, Yuan BZ, Yu XJ, Rauch F, Davis SI, Zhang SB, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 22.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing Fibroblast Growth Factor 23 under the control of the {alpha}1(I) collagen promoter exhibit growth retardation, osteomalacia and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 24.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 25.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 26.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Kakitani M, Hasegawa H, Yamazaki Y, Ohguma A, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of FGF-23 causes hyperphosphatemia, increased 1, 25-dihydroxyvitamin D level and severe growth retardation. J Bone Miner Res. 2002;17:S168. [Google Scholar]
- 31.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1, 25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 32.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Razzaque MS, Sitara D, Taguchi T, St Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitara D, Razzaque MS, St Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature. 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 38.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc Natl Acad Sci USA. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita T, Hasegawa H, Yamazaki Y, Kawata T, Urakawa I, Shimada T, Takeuchi Y, Fujita T, Fukumoto S, Nagano N. Involvement of FGF-23 in abnormal vitamin D and mineral metabolism associated with renal insufficiency. J Am Soc Nephrol. 2002;13:577A. [Google Scholar]
- 40.Baum M, Schiavi S, Dwarakanath V, Quigley R. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules 2. Kidney Int. 2005;68:1148–1153. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 43.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1{alpha}, 25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 44.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating FGF23 concentrations. Bone. 2005;36:971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K. Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab. 2005;288:E1101–E1109. doi: 10.1152/ajpendo.00502.2004. [DOI] [PubMed] [Google Scholar]
- 46.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 47.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 48.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 49.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 51.Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 52.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. [Google Scholar]
- 53.Bai X, Dinghong Q, Miao D, Goltzman D, Karaplis AC. Klotho ablation converts the biochemical and skeletal alterations in FGF23 (R176Q) transgenic mice to a Klotho-deficient phenotype. Am J Physiol Endocrinol Metab. 2009;296:E79–E88. doi: 10.1152/ajpendo.90539.2008. [DOI] [PubMed] [Google Scholar]
- 54.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for Klotho-dependent, fibroblast growth factor 23 (FGF23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 56.Hu MC, Zhang J, Rosenblatt KP, Baum M, Kuro-o M, Moe O. Klotho inhibition of Na-phosphate transporter (Na/Pi2a) in OK cells. J Am Soc Nephrol. 2006;17:354A–355A. [Google Scholar]
- 57.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 62.Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35:455–462. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 63.The ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 65.Mirams M, Robinson BG, Mason RS, Nelson AE. Bone as a source of FGF23: regulation by phosphate? Bone. 2004;35:1192–1199. doi: 10.1016/j.bone.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Bianchine JW, Stambler AA, Harrison HE. Familial hypophosphatemic rickets showing autosomal dominant inheritance. Birth Defects Orig Arctic Ser. 1971;7:287–295. [PubMed] [Google Scholar]
- 67.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 68.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 69.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–681. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- 70.Econs MJ, McEnery PT, Lennon F, Speer MC. Autosomal dominant hypophosphatemic rickets is linked to chromosome 12p13. J Clin Invest. 1997;100:2653–2657. doi: 10.1172/JCI119809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22:520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 72.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 73.Econs MJ, Drezner MK. Tumor-induced osteomalacia–unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- 74.Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, Kumar R. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med. 1994;330:1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- 75.White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endorinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- 76.Gore MO, Welch BJ, Geng W, Kabbani W, Maalouf NM, Zerwekh JE, Moe OW, Sakhaee K. Renal phosphate wasting due to tumor-induced osteomalacia: a frequently delayed diagnosis. Kidney Int. 2008 doi: 10.1038/ki.2008.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chalew SA, Lovchik JC, Brown CM, Sun CC. Hypophosphatemia induced in mice by transplantation of a tumor-derived cell line from a patient with oncogenic rickets. J Pediatr Endocrinol Metab. 1996;9:593–597. doi: 10.1515/jpem.1996.9.6.593. [DOI] [PubMed] [Google Scholar]
- 78.Toyosawa S, Tomita Y, Kishino M, Hashimoto J, Ueda T, Tsujimura T, Aozasa K, Ijuhin N, Komori T. Expression of dentin matrix protein 1 in tumors causing oncogenic osteomalacia. Mod Pathol. 2004;17:573–578. doi: 10.1038/modpathol.3800084. [DOI] [PubMed] [Google Scholar]
- 79.Rowe PSN, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 80.Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, de Beur SMJ, Schiavi SC, Kumar R. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, Econs MJ, Inwards CY, Jan de Beur SM, Mentzel T, Montgomery E, Michal M, Miettinen M, Mills SE, Reith JD, O’Connell JX, Rosenberg AE, Rubin BP, Sweet DE, Vinh TN, Wold LE, Wehrli BM, White KE, Zaino RJ, Weiss SW. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Jan de Beur SM. Tumor-induced osteomalacia. JAMA. 2005;294:1260–1267. doi: 10.1001/jama.294.10.1260. [DOI] [PubMed] [Google Scholar]
- 83.Perry W, Stamp TC. Hereditary hypophosphataemic rickets with autosomal recessive inheritance and severe osteo-sclerosis. A report of two cases. J Bone Joint Surg Br. 1978;60-B:430–434. doi: 10.1302/0301-620X.60B3.681423. [DOI] [PubMed] [Google Scholar]
- 84.Scriver CR, Reade T, Halal F, Costa T, Cole DE. Autosomal hypophosphataemic bone disease responds to 1,25-(OH)2D3. Arch Dis Child. 1981;56:203–207. doi: 10.1136/adc.56.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611–620. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- 87.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 88.Imel EA, Econs MJ. Fibrous dysplasia, phosphate wasting and fibroblast growth factor 23. Pediatr Endocrinol Rev. 2007;4(Suppl 4):434–439. [PubMed] [Google Scholar]
- 89.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 90.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 91.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Robey PG. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci USA. 1992;89:5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shenker A, Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney CM, Van Wyk JJ, Merino MJ, Feuillan PP, Spiegel AM. Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr. 1993;123:509–518. doi: 10.1016/s0022-3476(05)80943-6. [DOI] [PubMed] [Google Scholar]
- 94.Tenenhouse HS, Econs MJ. Mendelian hypophosphate-mias. In: Scriver CR, Beaudet AL, Sly WS, Valley D, editors. The metabolic basis of inherited disease. McGraw-Hill; New York: 2001. pp. 5039–5067. [Google Scholar]
- 95.Scriver CR, Reade TM, DeLuca HF, Hamstra AJ. Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med. 1978;299:976–979. doi: 10.1056/NEJM197811022991803. [DOI] [PubMed] [Google Scholar]
- 96.Drezner MK, Lyles KW, Haussler MR, Harrelson JM. Evaluation of a role for 1,25-dihydroxyvitamin D3 in the pathogenesis and treatment of X-linked hypophosphatemic rickets and osteomalacia. J Clin Invest. 1980;66:1020–1032. doi: 10.1172/JCI109930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fukase M, Avioli LV, Birge SJ, Chase LR. Abnormal regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity by calcium and calcitonin in renal cortex from hypophosphatemic (Hyp) mice. Endocrinology. 1984;114:1203–1207. doi: 10.1210/endo-114-4-1203. [DOI] [PubMed] [Google Scholar]
- 98.Tenenhouse HS, Jones G. Abnormal regulation of renal vitamin D catabolism by dietary phosphate in murine X-linked hypophosphatemic rickets. J Clin Invest. 1990;85:1450–1455. doi: 10.1172/JCI114590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997;60:790–797. [PMC free article] [PubMed] [Google Scholar]
- 100.The HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 101.Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–1209. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 103.Weber TJ, Liu SG, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 104.Guo R, Liu S, Spurney RF, Quarles LD. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab. 2001;281:E837–E847. doi: 10.1152/ajpendo.2001.281.4.E837. [DOI] [PubMed] [Google Scholar]
- 105.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lyles KW, Burkes EJ, Ellis GJ, Lucas KJ, Dolan EA, Drezner MK. Genetic transmission of tumoral calcinosis: autosomal dominant with variable clinical expressivity. J Clin Endocrinol Metab. 1985;60:1093–1096. doi: 10.1210/jcem-60-6-1093. [DOI] [PubMed] [Google Scholar]
- 107.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 108.Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, Draman MS, Conlon N, Jain A, Fedarko NS, Dasgupta B, White KE. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab. 2006;91:4037–4042. doi: 10.1210/jc.2006-0305. [DOI] [PubMed] [Google Scholar]
- 109.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 110.Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab. 2005;90:2420–2423. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- 111.Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:5523–5527. doi: 10.1210/jc.2005-0301. [DOI] [PubMed] [Google Scholar]
- 112.Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 113.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 114.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 117.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 118.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 119.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]