Abstract
Interference is a critical problem for memory systems and a primary cause of retrieval failure. One strategy for minimizing interference is to associate the items to be remembered with the context in which they were learned. For example, human subjects who learn two lists of words in separate contexts experience less interference and better recall than subjects who learn both lists in the same context. The hippocampus has long been known to be involved in processing contextual information and recent studies have shown that hippocampal neurons exhibit context-unique firing patterns that could serve as a neural representation of the context. These observations suggest that hippocampal context processing may play a critical role in overcoming interference. To test this hypothesis, we adapted the context based list learning procedure for use with rats. Control rats and rats given temporary lesions of the hippocampus were trained on two lists of eight odor pairs, either in the same context or in different contexts. In order to induce interference, some of the odors appeared on both lists with their predictive value reversed. As with human subjects, rats that learned the two lists in different contexts performed significantly better than rats that learned the lists in the same context. However, hippocampal lesions completely abolished this contextual learning advantage. We also trained rats on a low interference version of the task by using lists that did not contain any common items. Interestingly, rats with hippocampal lesions were entirely unimpaired when the learning situation did not involve high levels of interference. These findings are consistent with the idea that the hippocampus encodes contexts and further suggest that hippocampal context coding is beneficial because it provides a means of overcoming interference.
Keywords: hippocampus, context, interference
Introduction
Interference is a critical problem for memory systems and a primary cause of memory retrieval failure. Interference occurs when subjects must retrieve a particular target item from among many competing targets and is particularly acute for high volume memory systems that contain many similar targets. One strategy that the brain uses for coping with interference is to associate the items to be remembered with the context in which they were learned. This strategy automatically promotes the retrieval of the appropriate memories whenever subjects return to the context and helps to minimize interference from similar memories that belong to other contexts. For example, memory retrieval is enhanced when subjects are tested in the learning context (Baddely, 1987; Godden and Baddely, 1975) and revisiting a familiar context can evoke memories that are relevant to that context (Smith, 1988). In classic experiments on interference (e.g. Bilodeau and Schlosberg, 1951), subjects were trained on one list of paired associates, then given training on a second list in either the same or a different context. The subjects were then tested for recall of the items on the first list. Subjects that learned the two lists in different contexts experienced less interference and better recall than subjects that learned both lists in the same context, indicating that contextual associations help to mitigate interference.
Since the 1970s the hippocampus has been known to be involved in processing contextual information (for reviews see Anagnostaras et al., 2001; Hirsh, 1974; Maren, 2001; Myers and Gluck, 1994; Winocur and Olds, 1978). Hippocampal lesions impair conditioned fear responses to contextual stimuli (Kim and Fanselow, 1992; Phillips and LeDoux, 1992) and lesions of the hippocampus or entorhinal cortex render subjects insensitive to changes in the context (Freeman et al., 1997; Penick and Solomon, 1991). Hippocampal lesions also impair tasks that require subjects to discriminate novel conjunctions of objects and the context where they are presented (Eacott and Norman, 2004; Good et al., 2007). Fornix lesions impair the ability to learn two different discrimination tasks that take place in separate contexts and they disrupt context specific firing patterns in downstream brain structures (Smith et al., 2004). The well known spatial representations generated by the hippocampus are also consistent with a hippocampal role in representing contexts (Mizumori et al., 1999; Nadel et al., 1985).
There is also an extensive literature on the role of the hippocampus in minimizing interference (Shapiro and Olton, 1994). Computational models have suggested that the hippocampus plays a critical role in reducing interference (McClelland et al., 1995) and much work has focused on pattern separation or decorrelation of overlapping memories in the hippocampus as a means of reducing interference (Colgin et al., 2008; Leutgeb et al., 2007; O'Reilly and McClelland, 1994). However, experimental results have been mixed. Although some studies have reported hippocampal lesion induced impairments in tasks thought to involve interference, including concurrent discrimination learning (Moss et al., 1981), reversal learning (Berger and Orr, 1983), and negative transfer tests (Winocur, 1979), others have not (Eichenbaum et al., 1986; Gilbert and Kesner, 2003; Jarrard, 1995).
To date, no studies have specifically sought to determine whether hippocampal context processing provides a means of overcoming interference. The present study was designed to address this issue by adapting the context-based list learning task previously used with human subjects for use in rats so that we could assess the effects of hippocampal lesions. We trained the rats on two lists of odor pairs, either in the same or different contexts and we induced temporary lesions of the hippocampus during learning of the second list of odors. We also explicitly manipulated the amount of interference by including overlapping items on the two lists in a high interference condition and no overlapping items in a low interference condition.
Materials and Methods
Subjects, Surgical Procedures and Infusions
Subjects were 48 adult male Long-Evans rats (Charles River Laboratories, Wilmington, MA). Guide cannula (Plastics One, Roanoke, VA) were stereotaxically positioned just above the target location so that the infusion cannula, which protruded 1.0 mm beyond the tip of the guide cannula, would be positioned in CA1 (3.6 mm posterior and 2.6 mm lateral to Bregma, 2.2 mm ventral to the cortical surface). The rats were given an antibiotic (5 mg/kg Baytril) and an analgesic (5 mg/kg ketoprofen). All procedures complied with guidelines established by the Cornell University Animal Care and Use Committee. After one week for recovery from surgery, the rats were placed on a restricted feeding regimen (80–85% of free feeding weight) and they began training.
Temporary lesions were induced with the GABAA agonist muscimol. Thirty minutes prior to the relevant training sessions, muscimol (0.5 μl of a solution containing 1 μg/μl of muscimol) or saline solution was infused into each hemisphere. The cannulae were left in place for one minute after the infusions. Previous studies have shown these infusions to be sufficient to induce learning impairments (Holt and Maren, 1999; Smith and Mizumori, 2006a).
Apparatus and General Training Procedures
These experiments made use of the well known digging task used to study olfactory memory (Eichenbaum, 1998), in which rats are trained to dig in cups of odorized bedding material to retrieve buried food rewards (45 mg sucrose pellets, Bioserve, Inc., Frenchtown, NJ). All of the rats were first trained on one list of odor pairs. They were then given either muscimol or saline infusions and training on a second list of odors either in the same context or a different context. Thus, the experimental manipulations took place during training on the second list in a 2X2 design with lesion condition (saline or muscimol) and context condition (same or different) as factors.
The two contexts differed along the following dimensions: color of the chamber (white or black), color of the curtains surrounding the training area (black or white), substrate in the chamber (uncovered Plexiglass floor or a black rubber mat), the 65 dB continuous background masking noise (white noise or pink noise) and the ambient odor left by wiping out the chamber with baby wipes prior to each training session (unscented or scented, Rite Aid, Inc). Additionally, the rats were transported in covered cages to the experimental area by different methods in the two contexts (via a cart or carried by hand).
The rats were trained in Plexiglas chambers (45 cm wide X 60 cm long X 40 cm deep) equipped with a removable divider, which separated the odor presentation area from an area where the rats waited during the intertrial interval. Odor cues were presented in ceramic dessert cups (8.25cm in diameter, 4.5cm deep). The digging cups fit into circular cutouts cemented to the floor of the chamber to discourage the rats from moving the cups or tipping them over. Training was carried out in a circular area (2.7m in diameter) enclosed by curtains.
Thirty-two pure odorants served as cues. The amount of each odorant was calculated so that they produced an equivalent vapor phase partial pressure when mixed with 50 ml of mineral oil (Cleland et al., 2002). 10 ml of each odorant solution was then mixed with 2 liters of corncob bedding material and stored in covered containers. The odors included: propyl butyrate, citronellal, ethyl isovalerate, furfuryl proprionate, n-butyl glycidyl ether, methyl salicylate, n-amyl acetate, ethyl butyrate, propionic acid, benzaldehyde, 1-octanol, pentanol, trans-2-hexenyl acetate, propenoic acid, heptanol, ethyl valerate, 1,8-cineole, anisole, 5-methylfurfural, ethyl acetate, (+/−) limonene, methyl butyrate, 2-phenylethanol, 1-butanol, methyl 2-furoate, butyl butyrate, cis-3-hexenyl acetate, pentyl butyrate, benzyl benzoate, 2-furyl methyl ketone, 1-nonanol, and butyl pentanoate.
Experiment 1: High Interference Lists
Prior to training, the rats were given a 15 min session of acclimation to each of the two contexts. The rats were then shaped to dig in the cups of bedding to retrieve rewards. After the rats had learned to reliably retrieve the rewards, they began training on the first of two lists of odor pairs. Each list contained 8 odor pairs (16 individual odors). The two odor cups comprising each pair were always presented together. Within each odor pair, one odor was always rewarded and the other was not. The predictive value of the odors (rewarded or non-rewarded) was counterbalanced across subjects and their locations (left or right side of the chamber) were randomized. The daily training sessions consisted of 64 trials (8 trials with each odor pair, presented in an unpredictable sequence).
At the start of each trial, the experimenter placed the two cups containing the odorized bedding into the chamber and removed the divider so that the rat could approach the cups and dig until he retrieved the reward. A digging response was recorded if the rat displaced any of the bedding, except for incidental displacement (e.g. stepping into the cup while walking over it). After consuming the reward, the rat was returned to the waiting area for an intertrial interval of approximately 15 seconds while the experimenter prepared the cups for the next trial. The rats were given daily training sessions on list 1 until they reached a behavioral criterion of 90% correct choices on two consecutive sessions.
After reaching the criterion, the rats were assigned to groups and given 5 training sessions on a second list of 8 odor pairs. Thirty min before each of the first three training sessions of list 2, the rats were given muscimol or saline infusions. No infusions were given during the final two sessions. The rats were trained in either the same context where they learned list 1 or in a different context, yielding a 2X2 design with the following groups, saline-different, saline-same, muscimol-different and muscimol-same. Each of the training sessions for list 2 was carried out in the same manner as the list 1 training sessions (64 trials, 8 with each odor pair). In order to induce high levels of interference between the two lists, each of the new odor pairs for list 2 consisted of a novel odor and an odor which had previously been presented in list 1. Of the 8 odors taken from list 1, half had been rewarded previously and half had not. For example, if the first two odor pairs on list 1 were A+/B- and C+/D-, the first two odor pairs on list 2 would be X+/A- and D+/Y-. This ensured that the rats could not adopt a strategy of simply approaching the novel odor (or avoiding the familiar odor) within each new odor pair.
Experiment 2: Low Interference Lists
In order to determine whether the interference arising from overlapping items on the two lists was an important contributing factor for the lesion induced impairment seen in the different context condition of experiment 1 (see Results), a second experiment was conducted. For this experiment, rats were trained the same manner as rats in the different context condition of experiment 1, except that second list did not have any overlapping items. After completing training on list 1, the rats were assigned to one of 2 groups (saline or muscimol) and they were trained on a second list in a new context, as described above. However, the second list of 8 odor pairs was constructed from 16 novel odors. Since there were no overlapping odors on the two lists, this condition should involve less interference. Thus, these rats were given training that was identical in every respect to the different context condition above, except for the amount of interference present in the learning situation. A finding in which hippocampal lesions have a greater impact in the high interference condition would indicate that hippocampal context processing is advantageous because it helps subjects to overcome interference.
Pellet Detection Test
Pilot data indicated that the rats could not smell the buried rewards. Nevertheless, a subset of the rats (n=11) were tested to ensure that the rats were not able to directly detect the pellets. After the completion of training, the rats were given a session consisting of 64 trials (8 trials with each rewarded odor from list 2). On each trial, the rats were presented with two cups containing the same odor. However, only one of the cups was baited. If the rats could directly detect the pellets, they would be expected to perform better than chance (50%). The rats chose the baited cup 49.86% (±1.60) of the time, which did not differ significantly from chance performance (t(10)=0.09, p=.93). Even after three repeated sessions, none of the rats performed better than 52% correct.
Histology
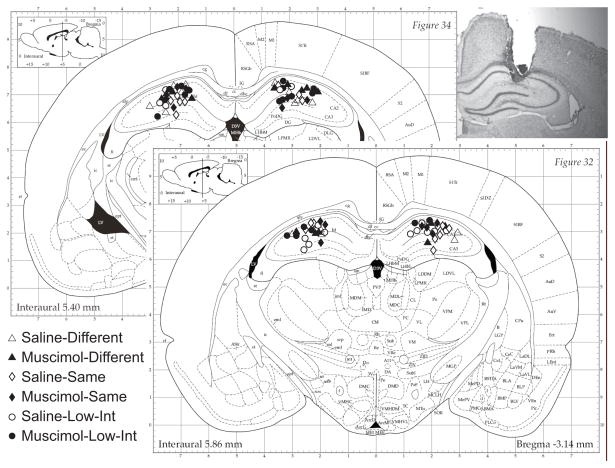
After the completion of all training, the rats were deeply anesthetized with Nembutal, transcardially perfused with 10% formalin and their brains were removed, sectioned at 40 microns, mounted on slides and stained with cresyl violet in order to identify the infusion locations. Figure 1 shows the location of the infusion cannula.
Figure 1.
Location of infusions for subjects in the four groups of experiment 1 and the two groups of experiment 2 (see key) are shown on figures adapted from Paxinos and Watson (1998). A typical cannula track is shown in the inset photo.
Results
Experiment 1
List 1
All of the rats were trained until they reached a behavioral criterion on list 1 before they began training on list 2. The rats took an average of 4.25 ±0.17 (Mean ± SEM) training sessions to reach this criterion. The average percent correct for the final day of training was 96.78 ±0.45% correct. There were no differences between groups with respect to performance on the final day of list 1 (F[3,28]=0.49, p=.69).
The Effects of Hippocampal Lesions in the High Interference Condition
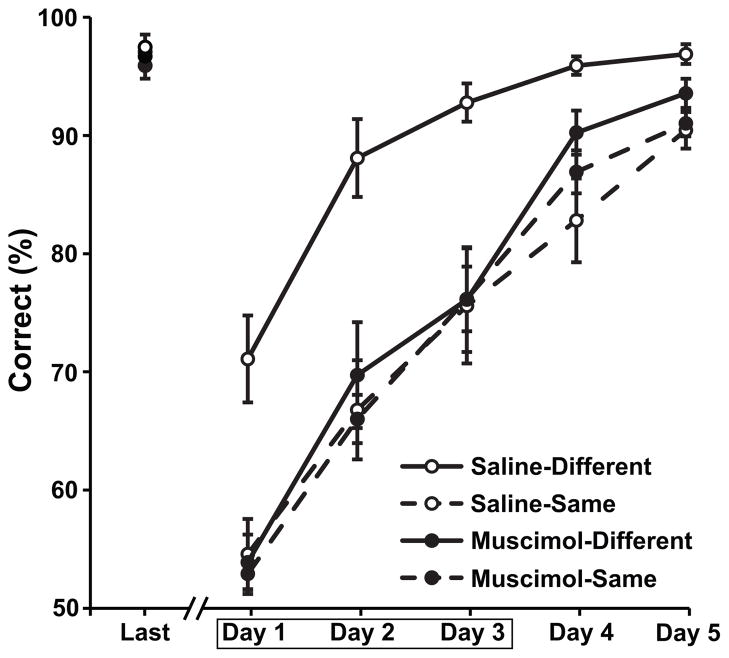
In order to assess the effects of the context manipulation and the hippocampal lesions, the percentage of trials with a correct response for each session of list 2 was submitted to a 2-way repeated measures ANOVA with lesion condition (saline or muscimol) and context condition (same or different context) as the between subjects factors and training session (5 levels) as the within subjects factor (Fig. 2). This analysis revealed a significant interaction the context and lesion condition factors (F[1,28]=7.44, p<.05). Planned comparisons indicated that control rats that learned the second list in a new context performed significantly better than rats that learned the two lists in the same context(t (14)=4.58, p<.001). However, muscimol lesions completely abolished this contextual learning advantage. Rats with lesions that were trained in different contexts performed no better than control rats that were trained in the same context (t(14)=0.54, p=.48).
Figure 2.
Average percent correct choices are shown for saline control rats (open circles) and muscimol rats (filled circles) and for the different context (solid lines) and same context conditions (dashed lines). Performance data are shown for the final session of list 1 training (Last) and the five training sessions of list 2. Muscimol or saline infusions were given prior to the first three training sessions of list 2, indicated by the box.
Although they were delayed relative to rats that learned the two lists in different contexts, rats in the same context condition clearly learned the second list, eventually performing at better than 90% correct during the final training session. Interestingly, hippocampal lesions did not impair learning in the same context condition (t(14)=0.18, p=.86). This result indicates that the hippocampal lesions did not impair the basic elements of task performance (e.g. the digging response, memory for multiple odors and their association with reward, etc.).
The analysis also revealed a significant interaction of the lesion and training session factors (F[4,112]=4.36, p<.005). This was likely attributable to the experimental design, which involved saline or muscimol infusions during the first 3 sessions but not during the last 2 sessions. Therefore, we decomposed this interaction by performed two additional repeated measures ANOVAs, one to compare the performance of control and lesion subjects across the first three (infusion) sessions of list 2 and a second to compare performance during the final two (no infusion) sessions. These analyses showed that controls performed significantly better than muscimol rats during the infusion sessions (main effect of the lesion condition, F[1,30]=6.54, p<.05). However, performance of the muscimol rats caught up with that of controls during the final two training sessions when no infusions were given (F[1,30]=0.21, p=.65).
Experiment 2
The Effects of Hippocampal Lesions in the Low Interference Condition
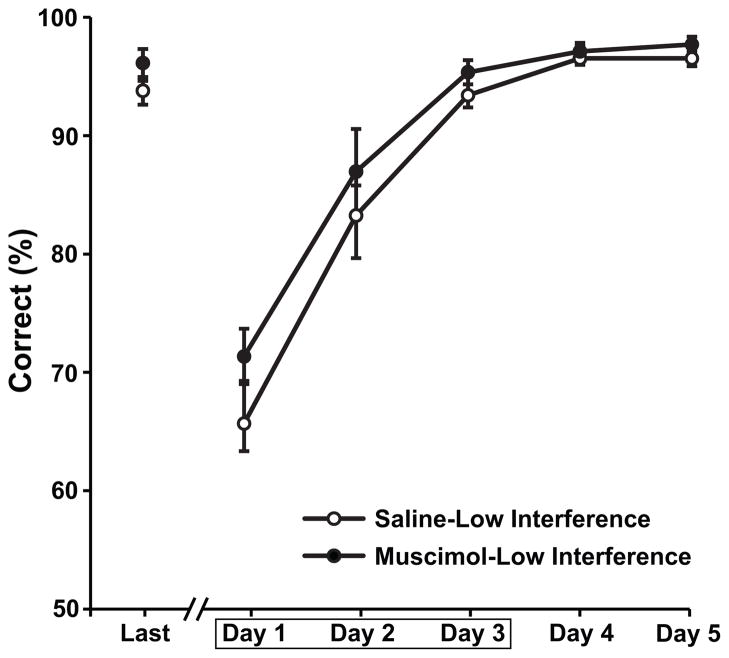
Hippocampal lesions had no effect on performance in the low interference condition (Fig. 3). A repeated measures ANOVA with lesion condition (saline or muscimol) as a between subjects factor and training session (5 levels) as a within subjects factor showed no effect of the lesions on the percent correct (F[1,14]=1.58, p=.23) and no interaction of the lesion condition and training session factors (F[4,56]=0.72, p=.55).
Figure 3.
Average percent correct choices are shown for rats in the low interference condition with no overlapping items on the two lists. Performance data are shown for saline control rats (open circles) and muscimol rats (filled circles, notations as in Fig 1). All rats were trained on the two lists in different contexts.
Overall Results
Assessment of Interference in Each Condition
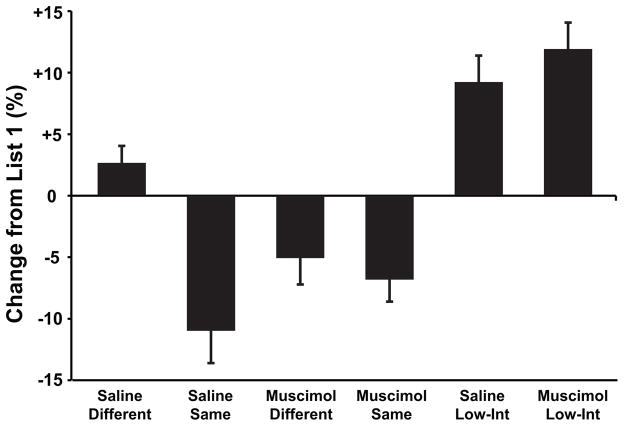
If proactive interference occurred, performance should decline when subjects had to learn a second list after having learned the first list. Conversely, if no interference occurred, then performance on list 2 should be as good as (or better than) performance on list 1. The change in performance across lists (average percent correct on list 2 minus the average percent correct on list 1) is illustrated in figure 4. We assessed the role of interference in the above-described results by comparing performance on list 1 and list 2 for each of the experimental groups. In the low interference condition, rats performed significantly better on the second list, as compared to performance on the first list (saline-low interference: t(7)=4.28, p<.005; muscimol-low interference: t(7)=5.53, p<.001), confirming that training on non- overlapping lists did not produce interference. Instead, the performance of these rats on list 2 was facilitated by prior training (positive transfer). In contrast, rats that were trained on high interference (overlapping) lists in the same context showed a significant decline in performance from list 1 to list 2 (saline-same: t(7)=4.20, p<.005; muscimol-same: t(7)=3.81, p<.01), suggesting that interference was a significant problem for rats that learned the two lists in the same context. However, control rats that were trained in different contexts (saline-different) did not show evidence of interference. For these rats, performance did not decline from list 1 to list 2 (t(7)=1.89, p=.10). This result indicates that that the improved performance resulting from training in different contexts was, in fact, due to a reduction in interference. Interestingly, rats with hippocampal lesions that were trained on the two lists in different contexts also exhibited a decline in performance (Musc-Diff: t(7)=2.36, p<.05), suggesting that these rats also experienced substantial interference even though they were trained on the two lists in separate contexts.
Figure 4.
Change in performance from list 1 to list 2, computed as the average percent correct on list 2 minus the average percent correct on list 1, is shown for each of the experimental groups. Facilitation is indicated by better performance on list 2 than on list 1 (positive values) while interference is indicated by worse performance on list 2 (negative values).
Discussion
Just as with human subjects (Bilodeau and Schlosberg, 1951), rats that learned the two lists in different contexts performed significantly better than rats that learned the two lists in the same context. However, hippocampal lesions completely abolished this contextual advantage, indicating a critical role for the hippocampus in using contextual information to enhance memory performance. Indeed, rats with hippocampal lesions in the different context condition were indistinguishable from rats that learned the two lists in the same context. The lesion induced impairment was present during the first three days of training, when infusions were given, but performance caught up with controls during the final two training sessions when no infusions were given.
Interestingly, the lesions did not cause an impairment in all of the conditions. The lesions had no effect on performance in the same context condition, indicating that the hippocampus was not needed for remembering multiple odors and their association with reward or with the ability to acquire a complex set of new associations on the second list after learning the first list. Instead, the hippocampus was only needed when subjects could use contextual information to enhance task performance. Additionally, the hippocampus was not needed when interference was low. Rats in the low interference condition (experiment 2) were given training that was identical to the different context condition of experiment 1, except that the two lists of odors had no overlapping items and therefore less interference (Fig. 4), and the rats with lesions were entirely unimpaired. Thus, the lesion induced impairment was remarkably specific. The hippocampus was only engaged when there was substantial potential for interference and when contextual information was useful for overcoming the interference. Thus, the present results are consistent with the idea that the hippocampus encodes contexts and further suggests that hippocampal context coding is important because it provides a means of minimizing interference.
The present results suggest that the hippocampus plays a critical role in memory when interference is high. Indeed, high levels of interference are characteristic of many of the tasks that are sensitive to hippocampal damage. For example, complex olfactory memory tasks such as transitive inference (Dusek and Eichenbaum, 1997), transverse patterning (Dusek and Eichenbaum, 1998) and cue sequence learning (Agster et al., 2002; Fortin et al., 2002) all require subjects to select a cue that has previously been rewarded on some trials but not on others, resulting in interference. Findings such as these have suggested that hippocampal processing characteristically promotes the flexible expression of memory. The present results clearly support this notion and suggest that a critical feature of this flexibility is the ability to retrieve the appropriate memories and behavioral responses without interference from competing memories.
Interference is also probably a key factor in the well documented spatial navigation deficits seen after hippocampal damage. For example, radial maze performance requires that subjects remember which arms they have visited on the current trail, without interference from memories of previous trials. Variants of the Morris water maze task that involve repositioning the hidden platform at the start of each session have similar potential for interference from previous sessions. Delayed spatial alternation tasks also require rats to choose from among responses (e.g. turn left or right) that have been equally rewarded on previous trials.
These considerations suggest that interference is a critical aspect of hippocampal function. However, the present results suggest that interference is only part of the story. Hippocampal lesions had no effect on performance in the same context condition, even though there was substantial interference, indicating that the presence of interference, by itself, was not sufficient to recruit the hippocampus. It was only when contextual information was needed for overcoming interference that the hippocampus was needed. Consistent with this idea, contextually cued conditional discrimination tasks require that subjects learn one discrimination in one context and the reversal of that discrimination in another context. The reversal induces interference which can only be resolved through the use of contextual information and this task is sensitive to hippocampal damage (Rajji et al., 2006; Smith et al., 2004). At present, it is not clear why some high interference tasks that do not have an explicit contextual learning requirement depend on the hippocampus. However, contextual information can play an important role in memory, even when the context is incidental to the experimental design (Smith and Vela, 2001), and the present results suggest that both interference and a contextual component are important for recruiting the hippocampus.
The critical involvement of the hippocampus in contextual learning and memory functions has been well documented (for reviews see Anagnostaras et al., 2001; Maren, 2001; Myers and Gluck, 1994). A common finding in these studies is that subjects seem to be unable to identify or represent contexts. Neurophysiological studies have begun to identify hippocampal firing patterns which could serve as a neural representation of the context (Smith and Mizumori, 2006b). Hippocampal neurons have long been known to generate new spatial firing patterns when subjects encounter a new environment (Muller and Kubie, 1987). More recently, studies have shown that hippocampal neurons also differentiate contexts that are defined by more abstract features of the context, such as the task demands (Markus et al., 1995; Smith and Mizumori, 2006b), behavioral strategies (Eschenko and Mizumori, 2007) and motivational state (Kennedy and Shapiro, 2009). Moreover, these firing patterns have now been shown to include a variety of non-spatial responses, including responses to various task stimuli such as cues and rewards and firing during intertrial delay periods (Gill et al., 2010). These firing patterns are very distinct for each context and highly reproducible each time the subject re-enters the context. We have suggested that these context-unique hippocampal firing patterns could serve as a neural representation of the context and that the output of these hippocampal context representations could promote the retrieval of context appropriate memories and behaviors (Smith, 2008). The present results support this hypothesis and suggest a model for how contextual information facilitates memory retrieval.
When a subject encounters a new context, a unique hippocampal context code is automatically generated. With experience, this context code becomes associated with the stimuli, events and behaviors that occur in that context. In the present study, the memory for a particular odor and its association with reward is presumably established and stored in extra-hippocampal circuitry (since memory for individual odors and their association with reward does not depend on the hippocampus). With experience the hippocampal context code becomes associated with the memory representation of the odor. When the rat is later returned to the context, the hippocampal context code is re-expressed and this primes the retrieval of the odor memory. If the subject is then asked to learn a new list of odors in a different context, a new context code is generated and it quickly becomes associated with the new odors. Because the two context codes are very different and each context code primes a different set of odor memories, retrieval is not hampered by interference. However, if the subject needs to retrieve a new set of odor memories within the same context, as in the same context condition of the present study, the same hippocampal context code continues to prime the old odor memories, resulting in significant interference. As a result, learning is delayed until the rat forms new associations over the course of many repetitions. Since rats with lesions don’t have hippocampal context representations, they are left with only the intrinsic (hippocampal independent) strength of the odor memories. As a result, they perseverate on the previously rewarded odors and cannot prime the new odor memories, resulting in very slow learning.
The present results join an extensive body of data that suggests that the hippocampus is critical for processing contextual information and also for coping with interference. Although these ideas have been around since the 1970s, the precise relationship between hippocampal function, context and interference has been unclear. Our findings suggest that hippocampal context coding is adaptive because it provides an important mechanism for overcoming interference and that contextual priming of memories allows subjects to express situationally appropriate behavior.
Acknowledgments
This work was supported by NIH grant MH083809 to D. Smith. We would like to thank C. Linster and T. Cleland for their advice and assistance with the odor stimuli and training procedures used in this experiment.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience. 2002;22(13):5760–8. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Baddely A. Memory and Context. In: Gregory RL, editor. The Oxford Companion to the Mind. Oxford: Oxford University Press; 1987. pp. 20–22. [Google Scholar]
- Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behavioral Brain Research. 1983;8(1):49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- Bilodeau IM, Schlosberg H. Similarity in stimulating conditions as a variable in retroactive inhibition. J Exp Psychol. 1951;41(3):199–204. doi: 10.1037/h0056809. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–31. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31(9):469–77. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(13):7109–14. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and transverse patterning guided by olfactory cues. Behavioral Neuroscience. 1998;112(4):762–71. doi: 10.1037//0735-7044.112.4.762. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24(8):1948–53. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Using olfaction to study memory. Ann N Y Acad Sci. 1998;855:657–69. doi: 10.1111/j.1749-6632.1998.tb10642.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fagan A, Cohen NJ. Normal olfactory discrimination learning set and facilitation of reversal learning after medial-temporal damage in rats: implications for an account of preserved learning abilities in amnesia. J Neurosci. 1986;6(7):1876–84. doi: 10.1523/JNEUROSCI.06-07-01876.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Mizumori SJ. Memory influences on hippocampal and striatal neural codes: effects of a shift between task rules. Neurobiol Learn Mem. 2007;87(4):495–509. doi: 10.1016/j.nlm.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Weible A, Rossi J, Gabriel M. Lesions of the entorhinal cortex disrupt behavioral and neuronal responses to context change during extinction of discriminative avoidance behavior. Experimental Brain Research. 1997;115(3):445–57. doi: 10.1007/pl00005714. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learn Mem. 2003;10(6):525–30. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PR, Mizumori SJY, Smith DM. Hippocampal Episode Fields Develop With Learning. Hippocampus. 2010 doi: 10.1002/hipo.20832. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden D, Baddely A. Context-dependent memory in two natural environments: On land and underwater. Brittish Journal of Psychology. 1975;66:325–331. [Google Scholar]
- Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci. 2007;121(1):218–23. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behavioral Biology. 1974;12(4):421–44. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. Journal of Neuroscience. 1999;19(20):9054–62. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behav Brain Res. 1995;71(1–2):1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106(26):10805–10. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. Journal of Neuroscience. 1995;15(11):7079–94. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102(3):419–57. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S. Hippocampal representational organization and spatial context. Hippocampus. 1999;9(4):444–51. doi: 10.1002/(SICI)1098-1063(1999)9:4<444::AID-HIPO10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Moss M, Mahut H, Zola-Morgan S. Concurrent discrimination learning of monkeys after hippocampal, entorhinal, or fornix lesions. Journal of Neuroscience. 1981;1(3):227–40. doi: 10.1523/JNEUROSCI.01-03-00227.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–68. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Gluck M. Context, conditioning, and hippocampal rerepresentation in animal learning. Behavioral Neuroscience. 1994;108(5):835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam P, Tomie A, editors. Context and Learning. Hillsdale NJ: Erlbaump ; 1985. pp. 385–406. [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4(6):661–82. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Penick S, Solomon PR. Hippocampus, context, and conditioning. Behavioral Neuroscience. 1991;105(5):611–7. doi: 10.1037//0735-7044.105.5.611. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26(3):908–15. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Olton DS. Hippocampal Function and Interference. In: Schacter D, Tulving E, editors. Memory Systems. Cambridge: MIT Press; 1994. pp. 87–117. [Google Scholar]
- Smith DM. The hippocampus, context processing and episodic memory. In: Huston JP, Dere Ekrem, Easton Alexander, Nadel Lynn, Huston Joseph P, editors. Handbook of Behavioral Neuroscience,Vol 18, Handbook of Episodic Memory. The Netherlands: Elsevier; 2008. pp. 465–481. [Google Scholar]
- Smith DM, Mizumori SJY. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006a;16(9):716–29. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY. Learning-Related Development of Context-Specific Neuronal Responses to Places and Events: The Hippocampal Role in Context Processing. Journal of Neuroscience. 2006b;26(12):3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix Lesions Impair Context-Related Cingulothalamic Neuronal Patterns and Concurrent Discrimination Learning. Behavioral Neuroscience. 2004;118(6):1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Smith SM. Environmental context-dependent memory. In: Davies G, Thomson DM, editors. Memory in context: context in memory. New York: John Wiley and Sons, Ltd; 1988. pp. 13–34. [Google Scholar]
- Smith SM, Vela E. Environmental context-dependent memory: a review and meta- analysis. Psychonomic Bulletin and Review. 2001;8(2):203–220. doi: 10.3758/bf03196157. [DOI] [PubMed] [Google Scholar]
- Winocur G. Effects of interference on discrimination learning and recall by rats with hippocampal lesions. Physiol Behav. 1979;22(2):339–45. doi: 10.1016/0031-9384(79)90096-9. [DOI] [PubMed] [Google Scholar]
- Winocur G, Olds J. Effects of context manipulation on memory and reversal learning in rats with hippocampal lesions. Journal of Comparative & Physiological Psychology. 1978;92(2):312–21. doi: 10.1037/h0077468. [DOI] [PubMed] [Google Scholar]