Abstract
Previous studies have demonstrated that following stroke, motor impairment can occur ipsilateral to the lesion. Such impairments have provided insight into the contributions of each hemisphere to movement control, showing that left and right hemisphere damage produce different effects on movement: Left hemisphere damage produces deficits in specifying features of movement trajectory, while right hemisphere damage produces deficits in achieving an accurate and stable final position. We now propose that left and right hemisphere damage should also produce different deficits in the adaptation of trajectory and position. To test this idea, we examined adaptation to visuomotor rotations in the ipsilesional arms of hemiparetic stroke patients with left (LHD) and right hemisphere damage (RHD). We found that LHD interfered with adaptation of initial direction, but not with the ability to adapt the final position of the limb. In contrast, RHD interfered with online corrections to the final position during the course of adaptation. These findings support our hypothesis that the control of trajectory and steady-state position may be lateralized to the left and right hemispheres, respectively.
Keywords: Lateralization, Stroke, Reaching
1. INTRODUCTION
Hemispheric specialization for controlling different aspects of behavior has been studied in a variety of behavioral systems, such as perception, memory, and language, primarily in the context of split-brain patients and patients with unilateral brain damage (Gazzaniga, 2000; Milner, 1971). However, the degree to which lateralization of motor function reflects hemispheric specialization remains controversial. Primary motor and premotor regions in both hemispheres are recruited in the planning and execution of unilateral movement, as supported by functional imaging studies (Dassonville et al., 1997; Kawashima et al., 1993; Kim et al., 1993). Behavioral work further suggests that both hemispheres contribute differently to the control of movement. Based on studies in which dominant arm advantages were observed in right-handers for coordinating intersegmental dynamics (Bagesteiro and Sainburg, 2002; Sainburg and Kalakanis, 2000), Sainburg (2002) proposed that the processes underlying control of limb trajectory may be specialized to the (left) hemisphere. Additional studies have shown a nondominant arm advantage for spatial accuracy relative to the dominant arm (Guiard et al., 1983; Lenhard and Hoffmann, 2007), despite a relative disadvantage for coordination (Bagesteiro and Sainburg, 2002; Hore et al., 1996; Sainburg and Kalakanis, 2000). These findings suggest that the control of limb position may be lateralized to the nondominant (right) hemisphere (Duff and Sainburg, 2007; Schabowsky et al., 2007). Moreover, previous research in animals (Friel et al., 2007; Kurtzer et al., 2005) and humans (Dizio and Lackner, 1995; Sainburg et al., 1999; Scheidt and Ghez, 2007) has demonstrated that the control of steady-state limb posture is dissociable and separate from the control of limb trajectory.
The contribution of both hemispheres to unilateral movement has implications for patients with hemisphere damage following stroke. Specifically, not only are motor deficits present in the arm contralateral to the damaged hemisphere, but deficits have also been revealed in the arm ipsilateral to the damaged hemisphere. In fact, a number of previous studies have documented ipsilesional motor deficits using clinical measures, such as finger-tapping and manual dexterity (Desrosiers et al., 1996; Haaland and Delaney, 1981; Jones et al., 1989; Sunderland et al., 1999). Motor performance of the ipsilesional arm may also be impaired, based on reports of changes in kinematic measures, such as peak velocity and time spent in deceleration, following stroke (Fisk and Goodale, 1988; Haaland and Harrington, 1989a; Haaland et al., 2004; Yarosh et al., 2004). The work of Haaland and colleagues (Haaland and Flaherty, 1984; Haaland and Harrington, 1989a, 1989b, 1994; Haaland et al., 2004) and others (Fisk and Goodale, 1988; Hermsdorfer et al., 1999a; Hermsdorfer et al., 1999b; Winstein and Pohl, 1995) has shown that these deficits are hemisphere-specific. These studies showed impairment of the acceleration phase following left but not right hemisphere damage, yet impairment of the deceleration phase following right but not left hemisphere damage; such findings occurred in studies examining discrete reaching movements, as well as reciprocal finger tapping. Collectively, such findings have been explained in the context of the open-loop/closed-loop model of motor lateralization, which hypothesizes that the left hemisphere is specialized for open-loop control, reflecting movement planning, while the right hemisphere is specialized for closed-loop control, reflecting online error corrections. This model predicts impaired open- or closed-loop processing following left or right hemisphere damage, respectively. However, in a recent test of this hypothesis, results from ipsilesional arm performance in response to unexpected target displacements (Haaland et al., 2004) failed to support this prediction, given that patients with right hemisphere damage corrected for these target jumps as well as control subjects did.
An alternative model of motor lateralization has emerged from interlimb differences in performance in healthy right-handers. Sainburg (2002) proposed the dynamic-dominance hypothesis of hemispheric specialization in which control of limb trajectory is specialized within the left (dominant) hemisphere, while control of steady-state limb posture is specialized within the right (nondominant) hemisphere (Sainburg, 2002, 2005). Efficient control of limb trajectory has been shown to depend on neural processes that predict and exploit dynamic interactions during voluntary movement, such as object interaction forces, gravitational loads, and intersegmental interaction torques (Gribble and Ostry, 1999; Lackner and Dizio, 1994; Sainburg et al., 1999; Shadmehr and Mussa-Ivaldi, 1994; Smith and Zernicke, 1987). Previous studies have suggested that the mechanisms underlying the coordination of intersegmental dynamic interactions are specialized to the left hemisphere (Sainburg, 2002), based on a right (dominant) arm advantage for coordination during tasks such as reaching and throwing in right-handed adults (Bagesteiro and Sainburg, 2002; Hore et al., 2005; Hore et al., 1999; Sainburg and Kalakanis, 2000). In addition, the neural mechanisms that underlie control of steady-state limb position may be specialized to the right hemisphere, accounting for the observed left (nondominant) arm advantage for achieving and maintaining limb configurations during tasks such as load compensation and proprioceptive matching (Bagesteiro and Sainburg, 2003; Goble and Brown, 2008; Goble et al., 2006).
We recently tested this hypothesis more directly by comparing ipsilesional arm performance of right-handed patients with left or right hemisphere damage to the performance of healthy right-handed adults during targeted single-joint (Schaefer et al., 2007) and multijoint (Schaefer et al., 2006) reaching movements. In both cases, we found that ipsilesional motor deficits were hemisphere-specific in nature, and reflected predictable changes in performance measures. During multijoint reaching, patients with left hemisphere damage tended to produce highly curved handpaths that were associated with inefficient coordination of intersegmental dynamics (Schaefer et al., 2006). These changes in trajectory following left hemisphere damage were direction-dependent and were associated with large errors in the initial direction of movement, but were not accompanied by large final position errors, relative to the target’s location. Patients with right hemisphere damage, in contrast, produced large final position errors, but maintained the ability to make straight, coordinated movements regardless of direction, providing preliminary support for the specialized roles of each hemispheres in controlling movement of each arm.
If this model of hemispheric specialization of motor control is valid, it should predict hemisphere-dependent patterns of motor adaptation as well. Because motor adaptation is thought to rely on the evaluation, or “costing,” of movement-related errors (Pouget and Snyder, 2000; Rumelhart et al., 1986; Wolpert et al., 2001), we hypothesize that each hemisphere might employ different cost functions that rely on different error signals during performance and adaptation to novel task conditions. We now use a task that introduces visual errors by dissociating the displayed and actual location of the hand. By analyzing how subjects respond to these errors, we can deduce whether trajectory- or position-based error information is used to adapt reaching behavior over time. If the left hemisphere is specialized for controlling trajectory, then patients with damage to the right hemisphere (i.e. intact left hemisphere) should improve initial trajectories but not final positions when exposed to novel visuomotor rotations. In addition, if the right hemisphere is specialized for controlling position, then patients with damage to the left hemisphere (i.e. intact right hemisphere) should improve final positions but not initial trajectories.
2. RESULTS
Subject characteristics
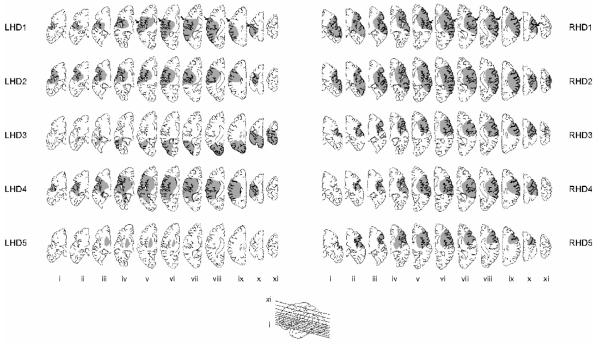
Age and education were similar across the four groups (age: p = .85; education: p = .75). The two stroke groups, comprised of patients with left hemisphere damage (LHD) or right hemisphere damage (RHD), were similar in number of years post stroke (p = .69), limb apraxia (p = .82), language comprehension (? 2 =2.5, p=.11), or lesion volume (p = .27). The RHD group demonstrated greater involvement in several areas, as evidenced by damage to somatomotor cortex on 7 of 11 axial slices in all 5 RHD patients and only 3 LHD patients (Fig. 1). The damaged areas were similar between the two groups, except that dorsolateral prefrontal damage was present in 4 RHD patients and none of the LHD patients (Table 1). Degree of hemiparesis was significantly greater in the RHD group based on the Upper-extremity motor subscore of the Fugl-Meyer Motor Assessment (p <. 05), and marginally so based on contralesional grip strength (p = .11).
Figure 1.
Lesion locations were traced on 11 axial slices (see inset for slice level) from MRI or CT scans for each LHD (1-5) and RHD (1-5) patient. Slices are displayed left-to-right from inferior to superior (i-xi) for both groups of patients. Arrows in top row indicate location of central sulcus.
Table 1.
Summary of participant information.
| Subject | Sex | Age (yrs) |
Education (yrs) |
Post- stroke (yrs)a |
Lesion volume (cm3)b |
UE Fugl- Meyerc |
Language abilityd |
Limb apraxiae |
Grip strength rightff |
Grip strength leftf |
Lesion locationg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LHD1 | M | 42 | 14 | 7.0 | 147 | 22 | 46 | 10 | 0 | 51 | SMC, IC, BG, PC |
| LHD2 | M | 45 | 17 | 5.1 | 153 | 61 | 80 | 14 | 27 | 48 | SMC, IC, BG, PC |
| LHD3 | M | 64 | 18 | 9.8 | 231 | 7 | 36 | 10 | 0 | 47 | SMC, IC, BG, PC |
| LHD4 | M | 76 | 12 | 12.0 | 114 | 33 | 80 | 11 | 9 | 46 | SMC, PC |
| LHD5 | M | 69 | 18 | 1.0 | 14 | 52 | 80 | 13 | 20 | 43 | IC, BG |
|
| |||||||||||
| mean ± SD |
59.2
± 15.0 |
15.8 ± 2.7 |
7.0 ±
4.3 |
131.8 ±
78.6 |
35.0 ±
21.9 |
64.4 ± 21.7 |
11.6 ±
1.2 |
11.2 ±
12.1 |
47.0 ± 2.9 |
||
|
| |||||||||||
| RHD1 | M | 49 | 14 | 19.5 | 245 | 4 | 80 | 11 | 24 | 0 | SMC, IC, BG, PC, DLPF |
| RHD2 | M | 55 | 16 | 5.9 | 283 | 6 | 80 | 11 | 36 | 0 | SMC, IC, BG, PC, DLPF |
| RHD3 | F | 58 | 16 | 9.0 | 137 | 6 | 80 | 11 | 33 | 0 | SMC, IC, BG, PC, DLPF |
| RHD4 | M | 61 | 12 | 3.7 | 159 | 2 | 80 | 12 | 58 | 0 | SMC, IC, BG, PC |
| RHD5 | M | 63 | 18 | 3.8 | 119 | 5 | 80 | 12 | 38 | 0 | SMC, IC, BG, DLPF |
|
| |||||||||||
| mean ± SD |
57.2
± 5.5 |
15.2 ± 2.3 |
8.4 ±
6.6 |
188.6 ±
71.5 |
4.6 ±
1.7 |
80.0 ± 0 |
11.4 ±
0.5 |
37.8 ±
12.5 |
0 ± 0 | ||
Years post-stroke are calculated as time elapsed between incidence of stroke and day of data collection.
Lesion volume is computed from MRI using a computer algorithm.
Maximum score on the upper-extremity (UE) motor subscore of the Fugl-Meyer Motor Assessment is 66.
Language ability was assess using the Western Aphasia Battery.
Apraxia is designated as mean number correct out of 15 items using a validated apraxia battery.
Grip strength from dynamometer are expressed as standardized t scores.
SMC=Somatomotor cortex [Brodmann Areas (BA) 4, 6, and/or 3,1,2]; IC=Internal Capsule; BG=Basal Ganglia; PC=Parietal Cortex [BA=39, 40, and/or 7]; DLPF=Dorsolateral Prefrontal cortex [BA 8, 9, and/or 46]
Baseline session
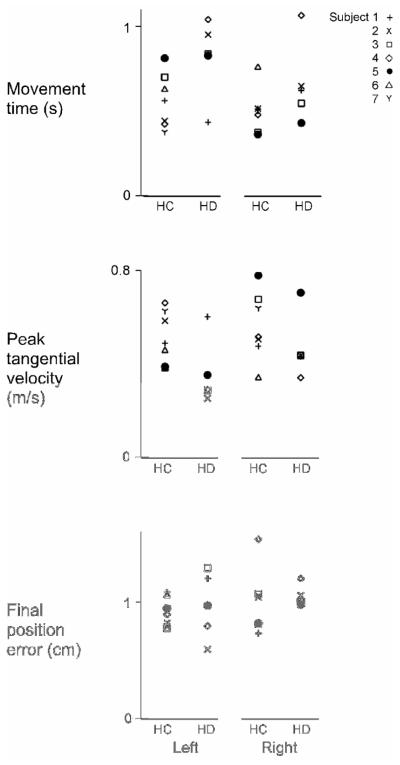
Figure 2 shows the mean movement time, peak tangential velocity, and final position error from the last cycle of the baseline session for each subject in each group. This illustrates that despite within-group variability of lesion location and volume, there did not appear to be direct relationship between patients’ lesion characteristics and their baseline performance of the task within the LHD or RHD groups. In fact, the degree of overlap demonstrates that within groups, baseline performance was consistent.
Figure 2.
Mean movement time, peak tangential velocity, and (absolute) final position error of the last cycle of baseline trials are displayed for each subject (1-7) in the LHC and RHC groups and each patient (1-5) in the LHD and RHD groups.
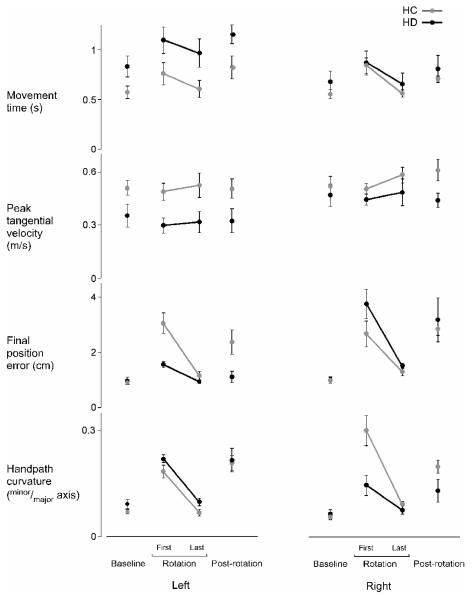
There were, however, differences in baseline performance between groups (Fig. 3, “Baseline”). There was a main effect of lesion status (HC or HD) on movement time (F1,20 = 5.83; p < .05), with longer movement times for both hemisphere damage (HD) groups relative to healthy controls (HC) (p < .05), regardless of lesion side. However, there was no main effect of lesion status on peak tangential velocity of movement (F1,20 = 3.40; p = .08), final position error (F1,20 = 0.57; p = .46), or handpath curvature (F1,20 = 2.78; p = .11) in the final cycle of baseline reaches. There were also no main effects of arm (L or R) or interaction effects of arm and lesion status on the speed (F1,20 = 1.16; p = .30; F1,20 = 0.89; p = .36, respectively) or accuracy (F1,20 = 0.65; p = .43; F1,20 = 0.0056; p = .94, respectively) of movement in the final baseline cycle. There was no interaction effect of arm and lesion status on handpath curvature (F1,20 = 0.39; p = .53), but there was a main effect of arm (F1,20 = 6.25; p < .05). Post-hoc analysis determined that curvature of handpath was larger in the left arm (p < .05), which is consistent with our previous findings in healthy young adults (Bagesteiro and Sainburg, 2002).
Figure 3.
Mean movement times, peak tangential velocities, final position errors, and handpath curvatures of the last cycle of baseline trials, first and last cycles of rotation trials, and first cycle of post-rotation trials are displayed for the left and right arms of healthy control groups (LHC,RHC; gray) and the ipsilesional arms of left and right hemisphere damage groups (LHD,RHD; black). Bars indicate standard error of mean.
Rotation session: Adaptation of final position and initial trajectory
As shown in Figure 3, all subject groups except the LHD group had significantly larger final position errors in the first cycle of trials under the rotated condition, relative to their baseline performances. To assess the degree to which each group was initially perturbed by the rotation, we compared the last baseline cycle to the first rotation cycle. Our ANOVA revealed a significant 3-way interaction including arm (L or R), lesion status (HC or HD), and cycle (last baseline and first rotation) for final position error (F1,20 = 8.55; p < .01). Post-hoc analysis confirmed that all groups showed significant increases in final position error (p < .05), except the LHD group (p > .05). Therefore, even when initially exposed to the visuomotor rotation (first cycle), the LHD group appeared to correct for the dissociation between visual and actual hand location more effectively than all other groups in order to accurately reach the target.
To quantify adaptation of steady-state position when exposed to a visuomotor rotation, we compared mean final position errors made during the first (cycle 1) and last (cycle 26) cycles of the rotation session for each group (Fig. 3, “Rotation”). Our ANOVA revealed a significant 3-way interaction including arm, lesion status, and cycle (first rotation and last rotation) for final position error (F1,20 = 5.91; p < .05). Post-hoc analyses confirmed that 3 of the 4 groups improved the accuracy of their movements by the end of the rotation session (p < .05), except the LHD group (p > .05). However, the LHD group’s final position errors were small during the first cycle of the rotation, and remained small throughout the rotation session (Fig. 3), such that there was no significant difference between errors during the first and last cycles of the session (p > .05).
It is unlikely that these differences in final position accuracy are directly related to movement time or speed. Our ANOVA revealed no significant interactions for movement time; there was, however, a main effect of cycle (F1,20 = 22.26; p < .0001) and of lesion status (F1,20 = 4.90; p < .05). Post-hoc tests revealed that movement time was longer during the first cycle than in the last cycle for all groups (p < .05) (Fig. 3). Our ANOVA also revealed no significant interactions for peak tangential velocity, but there was a main effect of lesion status (F1,20 = 8.73; p < .01). Post-hoc analysis revealed that peak velocity was lower for the HD groups than the HC groups (p < .05).
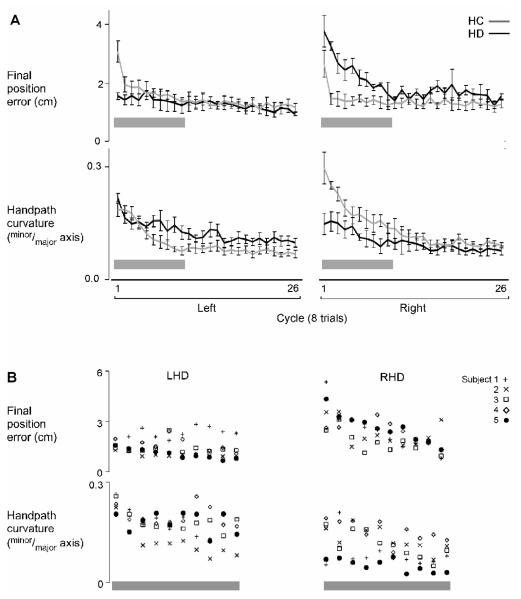
Although there were decreases in final position error over time, Figure 4A shows that such improvement in final position occurred at different rates for different groups. The LHC and RHC groups adapted final position rapidly, showing large decreases in final position error within the second and third cycles of the rotation session. However, while the RHD group improved the accuracy of their movements to the same degree as the RHC group by the end of the session, they took much longer to adapt final position (Fig. 4A). This effect was consistent across patients within each HD group. Figure 4B shows the mean final position error (top) and handpath curvature (bottom) for each cycle of movements for each LHD and RHD patient over the first 10 cycles. Gray horizontal bars in Figure 4A indicate this time frame.
Figure 4.
A) Mean final position error (top) and handpath curvature (bottom) of each cycle of the rotation session (cycles 1-26) is displayed for the left and right arms of healthy control groups (LHC,RHC; gray) and the ipsilesional arms of left and right hemisphere damage groups (LHD,RHD; black). The LHC and LHD groups are overlapped on the left; the RHC and RHD groups are overlapped on the right. Bars indicate standard error of mean. Horizontal gray bars denote first 10 cycles. B) Mean final position error (top) and handpath curvature (bottom) of each cycle of the rotation session for the first 10 cycles (gray bar) is displayed for the ipsilesional arms of each left and right hemisphere damage patient (LHD,RHD).
When initially exposed to a novel visuomotor rotation, subjects typically correct online to some degree for this rotation, thereby curving the trajectory of their hand in order to move the displaced cursor to the target (Krakauer et al., 1999; Sainburg, 2002). Thus, in order to quantify the degree of online correction, we compared mean handpath curvatures made during the first (cycle 1) and last (cycle 26) cycles of the rotation session for each group (see Fig. 3). Our ANOVA revealed a significant 3-way interaction including arm, lesion status, and cycle (first rotation and last rotation) for handpath curvature (F1,20 = 6.74; p < .05). Post-hoc analyses confirmed that 3 of the 4 groups reduced the curvature of their handpaths (i.e. straighter handpaths) over the course of adaptation (p < .05), except the RHD group (p > .05). However, handpath curvature for the RHD group was low even during initial exposure to the rotation, and remained relatively low throughout the rotation session (Fig. 4,), such that there was no significant difference between curvatures of the first and last cycles of the session (p > .05).
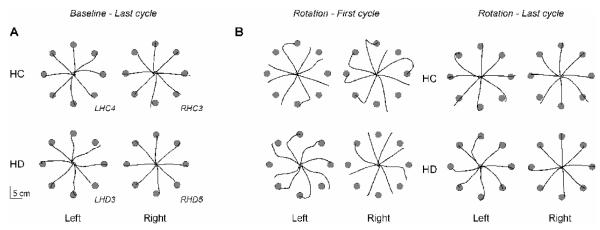
This effect is illustrated in the handpaths of representative subjects (Fig. 5). The last cycle of baseline trials are shown in Figure 5A; the first and last cycles of rotation trials are shown in Figure 5B. In each cycle, the RHD patient moved the cursor straight (minimal curvature), and did not direct the displaced cursor back to the target, resulting in large final position errors in the first cycle. However, by the last rotation cycle, these straight cursor trajectories were appropriately directed toward the target, much like those of the RHC subject (Fig. 5).
Figure 5.
Cursor trajectories. A) The last 8 trials (last cycle) of the baseline session are shown for a representative subject from each group (LHC4, RHC3, LHD3, RHD5). B) The first 8 trials (first cycle) and last 8 trials (last cycle) of the rotation session are shown for these representative subjects.
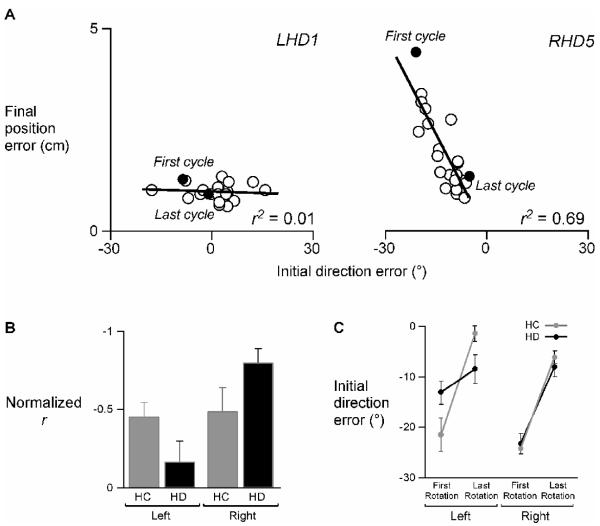
The consistent “straightness” of the RHD group’s trajectories and the gradual reduction of final position error over the course of the rotation session suggests that the accuracy in final position of their movements was, in part, related to the accuracy in initial movement direction of their movements. Figure 6A illustrates the relationship between initial trajectory and final accuracy in a representative LHD and RHD patient. Each point is the mean of one cycle; the first and last cycles are labeled and denoted in solid black dots. The RHD patient initiated the first cycle of movements −30° relative to the straight-line path required for moving the visual cursor into the target, but did not appear to effectively correct online, resulting in large final position errors in the first cycle. It appeared, though, that over the course of the rotation session, this patient gradually reduced final position error by reducing the initial direction error (i.e. changing initial movement direction) over time; by the last rotation cycle, the initial direction error was near zero, indicating adaptation of the hand’s initial trajectory to the visuomotor rotation. Not only was initial direction error near zero, but final position error was also significantly reduced. However, although the LHD patient initiated movements over a range of direction errors, final position error was consistently low and showed little change between the first and last cycle of movements. This trend was significant across subjects, as shown in Figure 6B, such that the relationship between final position error and initial direction error was stronger for the RHD group, and a weaker for the LHD group, as compared to the HC groups. Two-way ANOVA of the normalized r values revealed a significant interaction between arm and lesion status for this relationship (F1, 20 = 5.53; p < .05). In other words, the RHD group appeared to improve final position accuracy by changing their initial trajectories, whereas the LHD group appeared to maintain accuracy irrespective of how their movements were initiated.
Figure 6.
A) Mean final position error of each cycle of the rotation session is plotted as a function of mean initial direction error of each cycle of the rotation session for representative LHD and RHD patients (LHD1 and RHD5). Each dot represents one cycle; the solid black dots indicate the first and last cycle of the rotation session. Corresponding r2 values are displayed in the bottom right corner of each scatterplot. B) Mean normalized r (Fisher z-score) of mean final position error vs. mean initial direction error is displayed for the left and right arms of healthy control groups (LHC,RHC; gray) and the ipsilesional arms of left and right hemisphere damage groups (LHD,RHD; black). Bars indicate standard error of mean. C) Mean initial direction error (baseline subtracted out) at peak acceleration of the first and last cycle of the rotation session is displayed for the left and right arms of healthy control groups (LHC,RHC; gray) and the ipsilesional arms of left and right hemisphere damage groups (LHD,RHD; black). Bars indicate standard error of mean.
Figure 6C shows mean initial direction error of the hand’s trajectory during the first and last cycle of the rotation session across subjects for each group, thereby reflecting the degree of its adaptation. Our ANOVA revealed a significant 3-way interaction including arm, lesion status, and cycle (first or last) for initial direction error (F1,20 = 10.67; p < .01). Post-hoc analysis revealed that initial direction error was significantly different in the first and last rotation cycle for all groups (p < .05), except the LHD group. Initial direction error was near −30° during the first cycle for 3 of the 4 groups, except for the LHD group, whose initial direction errors were closer to −15° (Fig. 6C). The fact that the LHD group did not make a 30° initial error in the opposite direction in response to the 30° rotation of the cursor during the very first cycle of movement in the rotation session indicates that these patients did not initiate their movements in the direction of the target’s location. Moreover, the persistence of initial direction error between the first and last rotation cycles for the LHD group indicates lack of adaptation of initial trajectory direction over time. Despite this lack of adaptation, however, the LHD group was consistently accurate, suggesting that these patients relied on online corrective mechanisms within each trial. In fact, the LHD group was the only group to not show large after-effects in final position error immediately following removal of the rotation (Fig. 3, “Post-rotation”). Overall, final position error of the LHD group changed very little between sessions and during adaptation, relative to other groups.
3. DISCUSSION
Previous studies have used exposure to novel visuomotor transformations to demonstrate the separate control of limb trajectory and steady-state position in young adults (Scheidt and Ghez, 2007), and have suggested that such processes are lateralized across the two hemisphere control systems (Sainburg and Wang, 2002; Wang and Sainburg, 2004). Each system might employ different cost functions to modify task performance under novel conditions, which might result in the adaptation of different aspects of performance during motor learning in healthy adults. In the current study, we predicted that during exposure to novel visuomotor rotations, patients with damage to the left hemisphere (i.e. intact right hemisphere) should correct final position errors but not initial direction errors, while patients with damage to the right hemisphere (i.e. intact left hemisphere) should correct initial direction errors but not final position errors during adaptation. We found that although patients with left hemisphere damage (LHD) did not effectively adapt the initiation of their handpaths to the visuomotor rotation across trials, they did effectively correct within each trial for the rotation, resulting in accurate final positions that were maintained across all experimental sessions. In contrast, while patients with right hemisphere damage (RHD) demonstrated adaptation of both initial trajectory and final position during the rotation session, they did not appear to correct online for errors in initial trajectory, resulting in large errors in final position when initially exposed to the rotation. Over time, though, these patients improved the final position accuracy of their movements by correctly adapting their initial trajectories across trials to compensate for the rotation.
Lateralization of trajectory and posture control mechanisms
Our current findings support that independent neural mechanisms may control trajectory and posture for a single limb, as reported by other recent studies (Friel et al., 2007; Kurtzer et al., 2005; Scheidt and Ghez, 2007). Not only do these processes appear to be separate, but they may also be lateralized across the hemispheres. Sainburg and colleagues (Bagesteiro and Sainburg, 2002, 2003; Sainburg, 2002; Sainburg and Kalakanis, 2000; Sainburg and Schaefer, 2004; Sainburg and Wang, 2002) and others (Schabowsky et al., 2007) have reported interlimb differences in the trajectories and positional accuracies in healthy young adults. However, these results from patients with unilateral brain damage offer more direct evidence that interlimb differences in perfeomance may arise from interhemispheric differences in control, irrespective of hand preference-related practice effects.
Interhemispheric differences in adaptation strategies
Previous studies in stroke patients have demonstrated the presence of ipsilesional motor deficits (Baskett et al., 1996; Chestnut and Haaland, 2008; Desrosiers et al., 1996; Jones et al., 1989; Kim et al., 2003). Studies have also shown that the nature of such deficits is hemisphere-dependent, such that different aspects of movement are impaired following left or right hemisphere damage (Fisk and Goodale, 1988; Haaland et al., 2004; Schaefer et al., 2007; Yelnik et al., 1996). Previously, ipsilesional deficits have been attributed to the lateralization of how movements are controlled. Feedforward control and feedback processing were thought be specialized to the left and right hemispheres, respectively (Haaland and Harrington, 1989a,1989b, 1994; Winstein and Pohl, 1995). However, recent findings from Haaland et al. (2004) failed to support a right hemisphere specialization for closed-loop processing, given that patients with right hemisphere damage performed as well as control subjects did when they were given visual feedback. Our model of lateralization extends this hypothesis to the lateralization of what may be controlled: trajectory and position (Sainburg, 2002). Theoretically, trajectory control relies on planning mechanisms that predict task-specific dynamics (Lackner and Dizio, 1994; Sainburg et al., 1999; Shadmehr and Mussa-Ivaldi, 1994; Smith and Zernicke, 1987). If the left hemisphere is specialized for such control, patients with damage to the left hemisphere (LHD) should show impaired adaptation of initial trajectory. We found that while LHD patients did not significantly adapt the initial direction of their movements over time, they were relatively unperturbed by the initial exposure to the rotation., Such findings suggest that the LHD group did not use trajectory errors from previous trials to plan subsequent movements, nor did they rely on visual information of initial conditions to plan a single movement. Rather, our data suggest that the LHD group relied on proprioceptive feedback to correct online, resulting in small positional errors regardless of which direction their movements were initiated. We attribute this strategy to the contribution of the intact right hemisphere in these patients. Impairment of predictive inter-trial trajectory adaptation, but not online intra-trial correction, following LHD strongly supports a left hemisphere specialization for a dynamics-based planning of trajectory, and maintains a right hemisphere specialization not for planning, but for online control of final position.
We also found that while the RHD group did show substantial reductions in final position error over time, they did so at a slower rate than their control group. They maintained straight trajectories and adapted the initial direction of their trajectories to account for the rotation. Thus, rather than correcting errors within a trial, the RHD group appeared to improve final position between trials by adapting initial trajectory. We attribute the use of this strategy by the RHD group to the contribution of the intact left hemisphere in these patients. As mentioned above, we hypothesized that the left hemisphere is specialized for predicting and accounting for the effects of intersegmental dynamics, which are necessary for adapting one’s movements in novel environments (Lackner and Dizio, 1994; Sainburg et al., 1999; Shadmehr and Mussa-Ivaldi, 1994; Smith and Zernicke, 1987). Our data suggest that damage to the left hemisphere interferes with these processes, while damage to right hemisphere does not. Moreover, our data also suggest that damage to the right hemisphere interferes with within-movement feedback processes that are necessary for error-based corrections, while damage to the left hemisphere does not.
The current study is consistent with previous work suggesting the separate contributions of within-trial feedback control and across-trial adaptive control to motor adaptation (Shabbott and Sainburg, 2009; Smith and Shadmehr, 2005; Taylor and Thoroughman, 2007). Shabbott and Sainburg (2009) demonstrated that while healthy adults adapt to visuomotor rotations across trials based on the angle of rotation, they employ a different strategy when perturbed within a trial by the same visuomotor rotation. Instead of correcting for the angle of rotation, subjects correct for the linear displacement of the cursor’s location from the finger’s location. Smith and Shadmehr (2005) have suggested that corrective and adaptive mechanisms involve separate motor areas, based on differential impairment in patients with Huntington’s disease or cerebellar degeneration, respectively. Although other regions of the basal ganglia may play a role in visuomotor adaptation (Contreras-Vidal and Buchs, 2003; Seidler et al. 2006), it is unlikely that these specific subcortical lesions following stroke would specifically impair adaptation of trajectory or position. Our findings do not preclude the role of some subcortical structures in movement correction and adaptation, but they do support the role of left and right sensorimotor areas in such processes. We are also not suggesting that motor learning itself is lateralized to the left sensorimotor regions, as previous studies have shown preserved motor adaptation in patients with either left or right sensorimotor damage (Frassinetti et al., 2002; Luaute et al., 2006; Tilikete et al., 2001; Winstein et al., 1999). We do, however, propose that the predictive control mechanisms employed during the planning and execution of a single movement, or during the adaptation of subsequent movements, are specialized to the left hemisphere, as supported by our work in young healthy adults (Bagesteiro and Sainburg, 2002, 2003; Sainburg, 2002, 2005; Sainburg and Kalakanis, 2000; Sainburg and Schaefer, 2004; Sainburg and Wang, 2002) and chronic stroke patients (Schaefer et al., 2007).
The present findings suggest that the left and right hemispheres employ different cost functions to execute and/or modify task performance under novel conditions. Both hemisphere/arm systems appeared to have access to trajectory and position information, but each system used such information differently, depending on what each hemisphere is specialized for controlling. The present study provides further support that each intact hemisphere uses information related to initial trajectory or final position in order modify its ipsilesional arm performance, based on its motor specialization.
4. EXPERIMENTAL PROCEDURE
Participants
Ten right-handed hemiparetic stroke patients and 14 right-handed healthy control subjects (Table 1) were examined after obtaining approval from the Human Research and Review Committee of the University of New Mexico School of Medicine and informed consent from each participant, according to the Declaration of Helsinki. Handedness was determined by the Edinburgh Inventory (Oldfield, 1971). All subjects were screened and excluded based on history of 1) substance abuse and/or psychiatric diagnosis, 2) non-stroke neurological diseases for the stroke patients and all neurological diagnoses for the control subjects, and 3) peripheral movement restrictions, such as neuropathy or orthopedic disorders. Five stroke patients had left hemisphere damage (LHD), and 5 patients had right hemisphere damage (RHD). All stroke patients completed the experiment with their ipsilesional arm. All stroke patients were hemiparetic in the contralesional arm, as defined by a contralesional grip strength at least 1.5 standard deviations below normal and at least 1.5 standard deviations less than ipsilesional grip strength using a hand dynamometer. Additional measures of hemiparesis (Upper-extremity motor subscore of the Fugl-Meyer Motor Assessment) (Fugl-Meyer et al., 1975), language comprehension (Kertesz, 1982), and limb apraxia (Haaland and Flaherty, 1984) were also used. Fourteen age- and education-matched healthy control subjects completed the experiment with their left arm (LHC: n = 7; male = 7; age (mean ± SD) = 61 ± 5.5 yrs) or right arm (RHC: n = 7; male = 6, female = 1; age (mean ± SD) = 59 ± 8.8 yrs).
Lesion analysis
MRIs (Phillips Edge 1.5 tesla machine) were obtained in 7 of 10 stroke patients (4 LHD, 3 RHD), unless there were medical contraindications. In 3 cases (1 LHD, 2 RHD), CTs (Siemens, Sensation 16 or Picker, PQ 6000 scanners) were obtained. MRI slice thickness was 5 mm with a slice gap of 1.5 or 2 mm, and CT slice thickness was 10 mm with no gap between slices. A board-certified neurologist, who was blinded to the behavioral characteristics of the patients, outlined the area of damage for each patient on 11 standardized horizontal sections derived from the DeArmond atlas (DeArmond et al., 1989) using T1 weighted MRI images for anatomical detail and T2 weighted images to specify borders of the damaged tissue (see Fig. 1). These tracings were retraced on a digitizing tablet for input into a computer program that used an algorithm to calculate lesion volume and location within each hemisphere (Frey et al., 1987).
Experimental setup
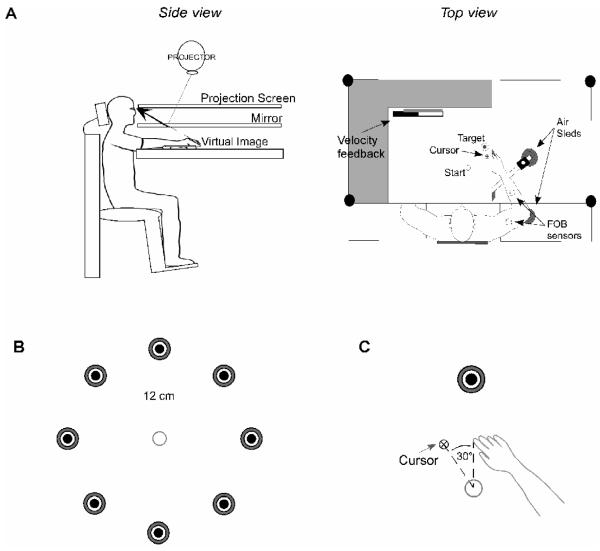
Figure 7A illustrates the experimental setup. Participants sat facing a projection screen with either their left or right arm supported over a horizontal surface by an air-jet system to reduce the effects of friction and gravity. The arm was positioned just below shoulder height. The start circle, a target, and a cursor that represented finger position were projected on a horizontal back-projection screen positioned above the arm, with a horizontal mirror positioned below this screen. The mirror reflected the visual display to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display assured that this projection was veridical at the start of each experiment.
Figure 7.
A) Side and top view of experimental apparatus are shown. B) Experimental task required movement of cursor from start circle to 1 of 8 target circles located 12 cm from a constant starting position. All targets were presented in the ipsilateral hemispace. C) A schematic of the relationship between the display cursor and the actual location of the fingertip during the rotation session is shown.
All joints distal to the elbow were immobilized using an adjustable brace. Position and orientation of the segments proximal and distal to the elbow joint were sampled using a Flock of Birds (FoB) ® (Ascension Technology, Burlington, VT) magnetic six-degree-of-freedom (6-DOF) movement recording system. A single sensor was attached to the upper arm segment via an adjustable plastic cuff, while another sensor was fixed to the air sled where the forearm was fitted. The sensors were positioned approximately at the center of each arm segment. The positions of the following three bony landmarks were digitized using a stylus that was rigidly attached to a FoB sensor: 1) index finger tip; 2) the lateral epicondyle of the humerus; 3) the acromion, directly posterior to the acromio-clavicular joint. These positions were relative to the sensors attached to each arm segment, thereby remaining constant throughout the experimental session. Our custom software used the FoB sensor data to compute the three-dimensional (3D) position of the index finger tip. Because the table surface defined our X-Y plane, perpendicular axis displacement was constant; thus, we used the recorded X-Y coordinates of the fingertip to project a cursor onto the screen. Screen redrawing occurred fast enough to maintain the cursor centered on the fingertip throughout the sampled arm movements. Digital data were collected at 103 Hz using a Macintosh computer, which controlled the sensors through separated serial ports, and stored on disk for further analysis. Custom computer algorithms for experiment control and data analysis were written in REAL BASIC™ (REAL Software, Inc.), C and IgorPro™ (Wavemetric, Inc.).
Experimental task
All subjects participated in three sessions: a baseline session, a rotation session, and a post-rotation session. In each session, subjects were instructed to move the displayed cursor from the start circle to one of 8 targets (3 cm in diameter) using a single, uncorrected rapid motion. All 8 targets were projected in the ipsilesional hemispace, and were oriented radially at a distance of 12 cm from a constant start position (Fig. 1C). For analysis purposes, we considered each session as cycles of movement, with a single cycle defined as a full series of movement to each of 8 targets. The baseline session had 120 trials (15 cycles), while the rotation session and the post-rotation session had 208 trials each (26 cycles). During the baseline session, the relationship between the finger and the cursor remained veridical; this session was used to familiarize subjects to the experimental setup and to establish baseline performance measures. During the rotation session, the position of the cursor was rotated 30° counterclockwise (−30°) relative to the start circle (Fig. 1D); this session was used to examine adaptation to novel visuomotor transformations in stroke patients using their ipsilesional arm and in healthy control subjects. During the post-rotation session, the visuomotor rotation was removed and the relationship between the finger and the cursor was again veridical; this session was used to examine negative “aftereffects” (i.e. movements in the opposite direction of the rotation) that are typically present following visuomotor adaptation and are thought to reflect the modification of a motor representation (Ghahramani et al., 1996; Ghilardi et al., 1995; Scheidt and Ghez, 2007; Shadmehr and Brashers-Krug, 1997; Weiner et al., 1983).
The cursor, which was relative to the real-time position of the index fingertip (veridical or rotated), and the start circle were displayed on the screen prior to each trial. The target did not appear until after the subjects had held the cursor within the starting circle (for 200 milliseconds) to trigger the audiovisual ‘go’ signal; the target for that trial then appeared. Visual feedback (i.e. cursor) was provided throughout the entire experiment. Knowledge of performance (i.e. the produced handpath) was also provided at the end of the movement. Subjects received knowledge of results in the form of a numerical score at the end of each trial to maintain motivation, based on the location of the index finger relative to the target at movement end. The 8 targets were presented in a pseudorandom order once over each cycle, such that no single target was presented consecutively.
Kinematic data
The 3D position of the index finger, elbow point, and shoulder point were calculated from sensor position and orientation data. Then, joint angles were calculated from these data. All kinematic data were low-pass filtered at 8 Hz (3rd order, dual-pass Butterworth), and differentiated to yield tangential velocity and acceleration values. Movement start was determined by identifying the time of peak velocity and searching backward in time for the first minimum in velocity (acceleration cross-zero) below 6% of peak tangential velocity of the index finger tip, or for zero velocity, whichever was identified first. Movement end was similarly determined by searching forward in time from peak velocity to find the first minimum in velocity (acceleration cross-zero) below 6% of peak tangential velocity, thereby excluding any small, corrective submovements.
Dependent measures
The following measures were calculated for each trial: movement time, peak tangential velocity, final position error, handpath curvature, and initial direction error. Movement time was defined as the elapsed time from movement start to movement end. Peak tangential velocity was defined as the absolute maximum tangential velocity between movement start and movement end. Final position error was calculated as the absolute value of the distance from the center of the displayed cursor at movement end to the center of the target. In baseline and post-rotation sessions, this corresponded to the location of the fingertip, but not in the rotation session. Handpath curvature was calculated as the minor axis divided by the major axis of the hand path. The major axis was defined as the largest distance between any two points in the handpath, while the minor axis was defined as the largest distance, perpendicular to the major axis (Bagesteiro and Sainburg, 2002; Sainburg et al., 1993). This measure quantifies the degree to which the handpath is linear (=0) or curved (>0), and considers the hand’s trajectory over the entire course of a movement. Initial direction error, however, considers the hand’s trajectory in the initial phase of movement. During reaching, subjects make relatively straight handpaths from the start location to the target (Hollerbach and Flash, 1982; Morasso, 1981). Deviations from this straight-line path early in the hand’s trajectory have been characterized as errors in initial direction (Sainburg and Kalakanis, 2000); we quantified initial direction errors in the hand’s trajectory as the angle between the target line (from the start circle in order to move rotated cursor straight to the target) and the handpath line at the time of peak tangential acceleration (~ 100 ms). This was done to compute the difference between the target direction and the actual trajectory direction during the earliest phase of motion. Initial direction error was measured in a right-arm coordinate system, such that positive values indicate hand paths that were directed lateral (clockwise: CW) to the target line, whereas negative values indicate hand paths that were directed medial (counter-clockwise: CCW) to the target line. This angle describes the difference between the target direction and the actual movement direction during the earliest phase of motion. To account for baseline interlimb differences in this measure (Sainburg and Kalakanis, 2000), we subtracted the mean initial direction error of the final cycle (i.e. last 8 reaches) of the baseline session from the mean of each rotation session cycle for each subject. Although initial direction error is signed (CW or CCW), we averaged these values within each cycle to characterize the overall performance on a cycle-by-cycle, rather than trial-by-trial, basis. We considered this early kinematic measure as a means of quantifying the planning of task-related motor output.
Statistical analysis
We subjected the means of dependent measures to a 3-way repeated-measure ANOVA, with arm (L = left or R = right) and lesion status (HC = healthy control or HD = hemisphere damage) as between-subject factors, and cycle (first or last) as the within-subject factor. We did not, for this study, consider target as a within-subject factor. Although limb dynamics are direction-dependent, our task was designed to examine how the presence and side of unilateralbrain damage would affect the use of either trajectory- or position-based error information in correcting for and/or adapting to novel visuomotor transformations. Therefore, our analyses for this study focused on changes in kinematics of each subject group during the rotation session, while other work has investigated the effect of movement direction on movement coordination in LHD and RHD patients (Schaefer et al. 2006).
To quantify the degree to which initial exposure to the visuomotor rotation affected the performance of each group (LHC, RHC, LHD, or RHD), we compared each group’s performance from the last baseline cycle to the first rotation cycle. To quantify changes in each group’s performance over time, or adaptation, we compared performance from the first rotation cycle to the last rotation cycle. Based upon our hypothesis, we predicted significant 3-way interactions to reflect performance differences in initial exposure to rotation and degree of adaptation of the hand’s initial trajectory and finalposition as a function of hemisphere of damage. Mean data were subjected to 3-way, repeated measures ANOVA in JMP® statistical software (SAS®). When warranted, post-hoc analyses were performed using Student’s t-test or Tukey HSD. To test for differences in baseline performance, two-way ANOVAs were used to test whether arm (L or R) and lesion status (HC or HD) had any significant effect on the dependent measures of the last cycle of the baseline session. We also conducted simple linear regression analysis for select sets of data using JMP® statistical software (SAS®).
ACKNOWLEDGEMENTS
This research was supported by Department of Veterans Affairs, Merit Review Grant, and the National Institutes of Health, National Institute for Child Health and Human Development (#RO1HD39311); National Institute on Aging training grant, Interdisciplinary Training in Gerontology (#T32AG00048). This project was also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. Further acknowledgments are to 1) Jennifer Hogan, Rena Singleton, and Lee Stapp for data collection, 2) Dr. Robert Knight for MRI tracings and neuroanatomical consultation, 3) Dr. Alvaro Magalhaes for clinical neuroradiological consultation, and 4) Drs. John Adair and Sally Harris, as well as HealthSouth Rehabilitation Hospital and Lovelace Medical Center, for patient referral.
Glossary
- LHD
left hemisphere damage
- RHD
right hemisphere damage
- LHC
left healthy control
- RHC
right healthy control
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J. Neurophysiol. 2002;88:2408–2421. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J. Neurophysiol. 2003;90:1503–1513. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskett JJ, Marshall HJ, Broad JB, Owen PH, Green G. The good side after stroke: ipsilateral sensory-motor function needs careful assessment. Age Ageing. 1996;25:239–244. doi: 10.1093/ageing/25.3.239. [DOI] [PubMed] [Google Scholar]
- Chestnut C, Haaland KY. Functional significance of ipsilesional motor deficits after unilateral stroke. Arch. Phys. Med. Rehabil. 2008;89:62–68. doi: 10.1016/j.apmr.2007.08.125. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Buch ER. Effects of Parkinson’s disease on visuomotor adaptation. Exp Brain Res. 2003;150:25–32. doi: 10.1007/s00221-003-1403-y. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond S, Fusco M, Dewey M. Structure of the human brain: a photographic atlas. 3rd ed Oxford University Press; New York: 1989. [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 1996;27:1564–1570. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J. Neurophysiol. 1995;74:1787–1792. doi: 10.1152/jn.1995.74.4.1787. [DOI] [PubMed] [Google Scholar]
- Duff SV, Sainburg RL. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp. Brain Res. 2007;179:551–561. doi: 10.1007/s00221-006-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JD, Goodale MA. The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp. Brain Res. 1988;72:425–435. doi: 10.1007/BF00250264. [DOI] [PubMed] [Google Scholar]
- Frassinetti F, Angeli V, Meneghello F, Avanzi S, Ladavas E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain. 2002;125:608–623. doi: 10.1093/brain/awf056. [DOI] [PubMed] [Google Scholar]
- Frey R, Woods D, Knight R, Scabini D, Clayworth C. Defining functional areas with averaged CT scans. Soc. Neurosci. 1987;13:1266. [Google Scholar]
- Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually guided locomotion. J. Neurophysiol. 2007;97:3396–3406. doi: 10.1152/jn.00750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Ghahramani Z, Wolpert DM, Jordan MI. Generalization to local remappings of the visuomotor coordinate transformation. J. Neurosci. 1996;16:7085–7096. doi: 10.1523/JNEUROSCI.16-21-07085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi MF, Gordon J, Ghez C. Learning a visuomotor transformation in a local area of work space produces directional biases in other areas. J. Neurophysiol. 1995;73:2535–2539. doi: 10.1152/jn.1995.73.6.2535. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Upper limb asymmetries in the matching of proprioceptive versus visual targets. J. Neurophysiol. 2008;99:3063–3074. doi: 10.1152/jn.90259.2008. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp. Brain Res. 2006;168:307–311. doi: 10.1007/s00221-005-0280-y. [DOI] [PubMed] [Google Scholar]
- Gribble P, Ostry D. Compensation for interaction torques during single- and multijoint limb movement. J. Neurophys. 1999;82:2310–2326. doi: 10.1152/jn.1999.82.5.2310. [DOI] [PubMed] [Google Scholar]
- Guiard Y, Diaz G, Beaubaton D. Left-hand advantage in right-handers for spatial constant error: preliminary evidence in a unimanual ballistic aimed movement. Neuropsychologia. 1983;21:111–115. doi: 10.1016/0028-3932(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Delaney HD. Motor deficits after left or right hemisphere damage due to stroke or tumor. Neuropsychologia. 1981;19:17–27. doi: 10.1016/0028-3932(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984;3:370–384. doi: 10.1016/0278-2626(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989a;27:961–969. doi: 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. The role of the hemispheres in closed loop movements. Brain Cogn. 1989b;9:158–180. doi: 10.1016/0278-2626(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Limb-sequencing deficits after left but not right hemisphere damage. Brain Cogn. 1994;24:104–122. doi: 10.1006/brcg.1994.1006. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–1158. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Laimgruber K, Kerkhoff G, Mai N, Goldenberg G. Effects of unilateral brain damage on grip selection, coordination, and kinematics of ipsilesional prehension. Exp. Brain Res. 1999a;128:41–51. doi: 10.1007/s002210050815. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Ulrich S, Marquardt C, Goldenberg G, Mai N. Prehension with the ipsilesional hand after unilateral brain damage. Cortex. 1999b;35:139–161. doi: 10.1016/s0010-9452(08)70791-3. [DOI] [PubMed] [Google Scholar]
- Hollerbach MJ, Flash T. Dynamic interactions between limb segments during planar arm movement. Biol. Cybern. 1982;44:67–77. doi: 10.1007/BF00353957. [DOI] [PubMed] [Google Scholar]
- Hore J, O’Brien M, Watts S. Control of joint rotations in overarm throws of different speeds made by dominant and nondominant arms. J. Neurophysiol. 2005;94:3975–3986. doi: 10.1152/jn.00327.2005. [DOI] [PubMed] [Google Scholar]
- Hore J, Watts S, Tweed D. Prediction and compensation by an internal model for back forces during finger opening in an overarm throw. J. Neurophysiol. 1999;82:1187–1197. doi: 10.1152/jn.1999.82.3.1187. [DOI] [PubMed] [Google Scholar]
- Hore J, Watts S, Tweed D, Miller B. Overarm throws with the nondominant arm: kinematics of accuracy. J. Neurophysiol. 1996;76:3693–3704. doi: 10.1152/jn.1996.76.6.3693. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112(Pt 1):113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, Fukuda H. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993;623:33–40. doi: 10.1016/0006-8993(93)90006-9. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. The Psychological Corporation; New York: 1982. [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Kim SH, Pohl PS, Luchies CW, Stylianou AP, Won Y. Ipsilateral deficits of targeted movements after stroke. Arch. Phys. Med. Rehabil. 2003;84:719–724. doi: 10.1016/s0003-9993(02)04973-0. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat. Neurosci. 1999;2:1026–1031. doi: 10.1038/14826. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat. Neurosci. 2005;8:498–504. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Dizio P. Rapid adaptation to Coriolis force perturbations of arm trajectory. J. Neurophysiol. 1994;72:299–313. doi: 10.1152/jn.1994.72.1.299. [DOI] [PubMed] [Google Scholar]
- Lenhard A, Hoffmann J. Constant error in aiming movements without visual feedback is higher in the preferred hand. Laterality. 2007;12:227–238. doi: 10.1080/13576500701203891. [DOI] [PubMed] [Google Scholar]
- Luaute J, Michel C, Rode G, Pisella L, Jacquin-Courtois S, Costes N, Cotton F, le Bars D, Boisson D, Halligan P, Rossetti Y. Functional anatomy of the therapeutic effects of prism adaptation on left neglect. Neurology. 2006;66:1859–1867. doi: 10.1212/01.wnl.0000219614.33171.01. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. Br. Med. Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Morasso P. Spatial control of arm movements. Exp. Brain Res. 1981;42:223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pouget A, Snyder LH. Computational approaches to sensorimotor transformations. Nat. Neurosci. 2000;3(Suppl):1192–1198. doi: 10.1038/81469. [DOI] [PubMed] [Google Scholar]
- Rumelhart DE, Hinton GE, Williams RJ. Learning representations by back-propagating errors. Nature. 1986;323:533–536. [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc. Sport Sci. Rev. 2005;33:206–213. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J. Neurophysiol. 1999;81:1045–1056. doi: 10.1152/jn.1999.81.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J. Neurophysiol. 2000;83:2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J. Neurophysiol. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY. Interlimb differences in control of movement extent. J. Neurophysiol. 2004;92:1374–1383. doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp. Brain Res. 2002;145:437–447. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabowsky CN, Hidler JM, Lum PS. Greater reliance on impedance control in the nondominant arm compared with the dominant arm when adapting to a novel dynamic environment. Exp. Brain Res. 2007;182:567–577. doi: 10.1007/s00221-007-1017-x. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Left but not right hemisphere produces ipsilesional deficis in intersegmental coordination. Abstr. - Soc. Neurosci. 2006;181 [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Ghez C. Separate adaptive mechanisms for controlling trajectory and final position in reaching. J. Neurophysiol. 2007;98:3600–13. doi: 10.1152/jn.00121.2007. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–55. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- Shabbott BA, Sainburg RL. On-line corrections for visuomotor errors. Exp Brain Res. Mar. 2009;14 doi: 10.1007/s00221-009-1749-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J. Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Zernicke RF. Predictions for neural control based on limb dynamics. Trends in Neuroscience. 1987;10:123–128. [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J. Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30:949–955. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. J. Neurophysiol. 2007;98:317–326. doi: 10.1152/jn.01070.2006. [DOI] [PubMed] [Google Scholar]
- Tilikete C, Rode G, Rossetti Y, Pichon J, Boisson D. Prism adaptation to rightward optical deviation improves postural imbalance in left-hemiparetic patients. Curr Biol. 2001;11:524–8. doi: 10.1016/s0960-9822(01)00151-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Limitations in interlimb transfer of visuomotor rotations. Exp. Brain Res. 2004;155:1–8. doi: 10.1007/s00221-003-1691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Merians AS, Sullivan KJ. Motor learning after unilateral brain damage. Neuropsychologia. 1999;37:975–987. doi: 10.1016/s0028-3932(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp. Brain Res. 1995;105:163–174. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn. Sci. 2001;5:487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J. Neurophysiol. 2004;92:3276–3285. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]
- Yelnik A, Bonan I, Debray M, Lo E, Gelbert F, Bussel B. Changes in the execution of a complex manual task after ipsilateral ischemic cerebral hemispheric stroke. Arch. Phys. Med. Rehabil. 1996;77:806–810. doi: 10.1016/s0003-9993(96)90261-0. [DOI] [PubMed] [Google Scholar]