Abstract
The matrix metalloproteinase (MMP) family is involved in the breakdown of extracellular matrix in normal physiological processes, as well as in the disease processes such as arthritis and cancer metastasis. In the present study, dieckol was obtained with high yield from marine brown alga Ecklonia cava (EC), and its effect was assessed on the expression of MMP-2 and -9 and morphological changes in human fibrosarcoma cell line (HT1080). Dieckol inhibited the expression of MMP-2 and -9 in a dose-dependent manner and also suppressed the cell invasion and the cytomorphology in 3D culture system on HT1080 cells. Moreover, dieckol may influence nuclear factor kappa B (NF-κB) pathway without obvious influence on activator protein-1 (AP-1) pathway and tissue inhibitor of metalloproteinases (TIMPs). In conclusion, dieckol could significantly suppress MMP-2 and -9 expression and alter cytomorphology of HT1080 cell line via NF-κB pathway.
1. Introduction
The past few decades of cancer research has revealed that matrix metalloproteinases (MMPs) play a significant role in a variety of pathologic conditions. Especially in malignant tumors, the activities of MMPs are deregulated and their expressions are often associated with poor prognosis. Among all the MMPs, MMP-2 and -9 have demonstrated to play a major role in the establishment of metastasis, which substantially increases in majority of malignant tumors. Therefore, inhibition of MMP-2 and -9 is thought to have a therapeutic benefit for cancer [1–8].
Recently, significant achievements have been made in synthetic bioactive applications. However, natural compounds and their derivatives are still known as the richest source of bioactive compounds with huge pharmaceutical application potential [9]. Dieckol (Figure 1) is a phloroglucinal derivative isolated from marine brown alga Ecklonia cava (EC) with a variety of biological functions in vitro and in vivo, for example, antioxidant, antitumor, antihuman immunodeficiency virus (-HIV), and anti-inflammatory activities. For instance, dieckol is a novel safe phloroglucinal derivative which inhibited the cytopathic effects of HIV-1 including HIV-1-induced syncytia formation, lytic effects, and viral p24 antigen production, as well as exhibited reverse transcriptase enzymes inhibitory and HIV-1 entry activity in addition to its other biological properties [10, 11].
Figure 1.
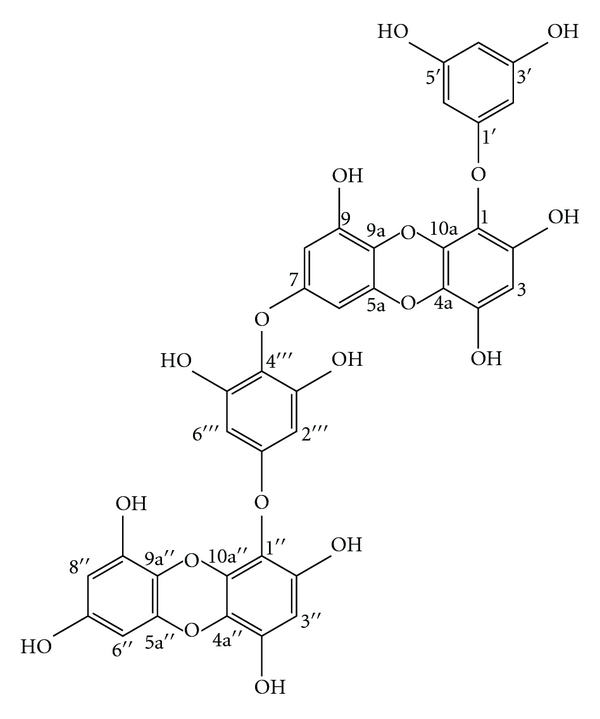
Chemical structure of dieckol.
In the present study, effect of dieckol on the expression of MMP-2 and -9, cytotoxicity, and cellular invasiveness was evaluated in HT1080 cells. The selectivity of this cell line was based on the extensive studies performed previously on MMPs expression [12]. In addition, the mechanism of dieckol regulating MMP-2 and -9 via the influence on nuclear factor kappa B (NF-κB) pathway has also been investigated.
2. Materials and Methods
2.1. General Materials
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a JEOL JNM-ECP 400 NMR spectrometer (JEOL, Japan), using DMSO-d 6 solvent peak (2.50 ppm in 1H and 39.5 ppm in 13C NMR) as an internal reference standard. For some signals, the chemical shifts approximated at the third decimal place. This is to distinguish between signals of very close value, but which could nevertheless be clearly differentiated by visual inspection of the spectra. MS spectra were obtained on a JEOL JMS-700 spectrometer (JEOL, Japan). Extraction of EC was performed using extraction unit (Dongwon Scientific Co., Republic of Korea). Column chromatography was carried out by silica gel 60 (230–400 mesh, Merck, Germany) and Sephadex LH-20 (Sigma, St. Louis, Mo). Thin-layer chromatography (TLC) was run on precoated Merck Kieselgel 60 F254 plates (0.25 mm), and the spots on the TLC plate were detected under UV lamp (254 and 365 nm) using CHCl3/MeOH/H2O/acetic acid (65 : 25 : 4 : 3, v/v/v/v) as a development solvent system. Vanillin-H2SO4 was employed as the detecting agent for phenolic compounds. All the solvent and chemicals used in this study were of reagent grade from commercial sources (Duksan Pure Chemicals Co., Ltd., Republic of Korea).
2.2. Extraction, Isolation, Purification, and Elucidation of Dieckol
Ecklonia cava was collected from Jeju Island coast of Republic of Korea, washed three times with water to remove salt, and subjected to lyophilization. The lyophilized EC was ground into powder before extraction. The dried EC powder (10 kg) was extracted by stirring extraction unit with MeOH (3 × 5 L) for 10 days at room temperature (25°C). The extract (273 g) was suspended in water and partitioned with n-hexane (35.92 g), CH2Cl2 (20.49 g), EtOAc (24.87 g), and n-BuOH (106 g) in a sequence. The EtOAc fraction (24.87 g) was subjected to a silica gel flash chromatography eluted with Hexane/EtOAc/MeOH/CH2Cl2 (gradient) to yield ten subfractions (F1–F10). The F5 subfraction was further purified by Sephadex LH-20 with MeOH obtained dieckol (58.30 mg).
2.3. Cell Culture and Cell Viability Assay
Human fibrosarcoma cells (HT1080) from ATCC were cultured in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 U/mL penicillin-streptomycin, and incubated at 5% CO2 and 37°C humidified atmosphere. For experiments, cells were passaged at least for 5 times and detached with Trypsin-EDTA. For cell viability assay, cells were seeded in 96-well plates at a density of 1 × 104 cells/well in 200 μL DMEM containing 10% fetal bovine serum. After 24 hours, the medium was removed and the cells were incubated for 48 h with DMEM containing 1% FBS in the absence or presence of various concentrations of dieckol. Forty-eight hours later, 100 μL 3-(4,5)-dimethylthiahiazol (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) (0.5 mg/mL final concentration) was added to each well and incubated for another 4 hours at 37°C in 5% CO2. Finally, DMSO (150 μL) was added to solubilize the formazan salt formed and amount of formazan salt was determined by measuring the OD at 540 nm using GENios microplate reader (Tecan Austria GmbH, Austria). Relative cell viability was determined by the amount of MTT converted into formazan salt. Viability of cells was quantified as a percentage compared to the control and dose response curves were developed. The data were expressed as mean (±SD) from three independent experiments.
2.4. In Vitro Invasion Assay
An in vitro invasion assay was performed using a 24-well transwell unit (8 μm pore size) with polyvinylpyrrolidone-free polycarbonate filters coated with 500 μg/mL of Matrigel and placed in transwell chambers. The coated filters were washed thoroughly in PBS and dried immediately before use. Cells were placed in the upper part of the transwell plate and allowed cell attachement for 24 hours, then incubated with dieckol for 36 hours at 37°C. The cells that invaded the lower surface of the membrane were fixed with methanol and stained with 0.5% crystal violet for 10 min. Finally, we determined invasive phenotypes by counting the cells that migrated to the lower side of the filter using Leica DM6000B microscopy at 200 × (Leica Microsystems Wetzlar GmbH, Germany) in at least 5 bright fields [13].
2.5. Three-Dimensional (3D) Culture of HT1080 Cell Line
The cells behavior and morphology in 3D culture system is quite different from that observed in the 2D system [14]. The 3D culture model was established as described previously [15]. Briefly, HT1080 cells (1.5 × 103) were suspended with a neutralized solution of type I collagen (1 mg/mL) (Sigma, St. Louis, Mo) and 1/5 volume of a 5 × DMEM. The cell suspension containing various final concentrations of dieckol was added to 24-well plates and kept at 37°C until gelled. The plates were then incubated at 37°C for 24 hours. The results were observed under microscope at 200 × (Leica Microsystems Wetzlar GmbH, Germany).
2.6. Gelatin Zymography
For gelatin zymography, HT1080 cells were seeded in 24-well plates using serum-free media and pretreated with different concentrations of dieckol for 1 hour. MMP expression was stimulated by 12-O-tetradecanoylphorbol 13-acetate (PMA) (10 ng/mL) and incubation was continued for 48 hours. After incubation, conditioned media were collected and their protein contents were determined by the Bradford method [16]. After normalizing the protein content, equal amounts of proteins were electrophoresed under nonreducing conditions on 10% polyacrylamide gels containing 1.5 mg/mL gelatin. Following electrophoresis, polyacrylamide gels were washed with 50 mM Tris-HCl (pH 7.5) containing 2.5% Triton X-100 to remove sodium dodecyl sulfate. Gels were then incubated overnight at 37°C in a developing buffer containing 10 mM CaCl2, 50 mM Tris-HCl, and 150 mM NaCl to digest gelatin by MMPs. Areas of gelatin hydrolyzed by MMPs were visualized as clear zones against blue background by Coomassie blue staining and the intensities of the bands were estimated by densitometry (Multi Gauge V3.0 software, Fujifilm Life Science, Japan) [12].
2.7. Reverse Transcription-PCR
Total RNA from each sample was isolated by TRIzol reagent and subjected for reverse transcription-PCR (RT-PCR). For RT-PCR analysis, total RNA was reverse-transcribed to synthesize cDNA using a commercial kit (TaKaRa RNA PCR kit (AMV)) according to the manufacturer's protocol. PCR was then carried out in 50 μL of reaction volumes containing RNA PCR buffer, 2.5 mM MgCl2, 0.2 μM of each primer, and 2.5 units of TaKaRa Taq polymerase. Samples were predenatured at 94°C for 4 min, followed by amplification at 94°C for 1 min, at 55°C for 30 s, and at 72°C for 1 min for 25 cycles, followed by a final 10 min extension step at 72°C. The primers for GAPDH were 5′-GTCAACGGATTTGGTCGTATT-3′′ and 5′-AGTCTTCTGGGTGGCAGTGAT-3′ with an expected amplified product of 300 bp. The MMP-2 primers were 5′-CTCAGATCCGTGGTGAGATCT-3′ and 5′-CT TTGGTTCTCCAGCTTCAGG-3 with an expected amplified product of 496 bp. The MMP-9 primers were 5′-ATCCAGTTTGGTGTCGCGGAGC-3′ and 5′-GAAGGGGAAGACGCACA GCT-3′ with an expected amplified product of 552 bp.
2.8. Extraction of Nuclear and Plasma Proteins and Western Blot Analysis
HT1080 cells were pretreated without or with different concentrations of dieckol for 1 hour followed by the stimulation with PMA, and the incubation was continued for another 48 hours. For separate extraction of nuclear and cytoplasm proteins, CelLytic NuCLEAR Extraction kit (S26-36-23, Sigma-Aldrich Co., Mo, USA) was used by following manufacturer's instructions. The cells were lysed with 0.5 mL of lysis buffer (500 μL hypotonic lysis buffer, 5 μL 0.1 M dithiothreitol (DTT), and 5 μL protease inhibitor cocktail, proteasome inhibitor, MG132) for 15 min on the ice. Igepal CA-630 solution (4 μL) was added and vortexed for 20 s. nuclei were separated by centrifugation at 10,000 × g for 10 min and supernatant (cytoplasm protein) was collected. Precipitated nuclei were lysed with 100 μL of extraction buffer mix (98 μL of extraction buffer, 1 μL of 0.1 M, DTT and 1 μL of protease inhibitor cocktail) for 10 min and nuclei protein were collected by centrifugation at 12,000 × g for 10 min. Proteins were separated by 12% SDS-PAGE and electrotransferred onto PVDF membranes (Millipore, Bedford, Mass). After blocking with 5% skim milk in PBST (PBS, pH 7.6, containing 0.2% Tween-20), the membranes incubated with primary antibody (Santa Cruz Biotechnology, Inc. us). for 1 hours, washed with 0.2% Tween-20 in PBS, and then incubated with horseradish peroxidase-linked secondary antibody (Santa Cruz Biotechnology, Inc., Calif, USA). The reactive signals were visualized by ECL kit (PE Applied Biosystems).
2.9. Statistical Analysis
All the experiments were repeated at least three times. All results were expressed as the mean of three replicates determination and standard deviation (SD). Statistical comparisons were made with Student's t-test and P values <0.05 were considered to be significant.
3. Results
3.1. Cell Viability Assay
Cell viability assay was introduced for determining whether dieckol can have toxic effect on HT1080 cells at high concentration. Comparison of cell growth over 48 h with various dieckol concentrations (0–250 μM) were showed in Figure 2. The values for all concentrations were similar with control (dieckol 0 μM) indicating that dieckol does not affect cell viability below the concentrations of 200 μM.
Figure 2.
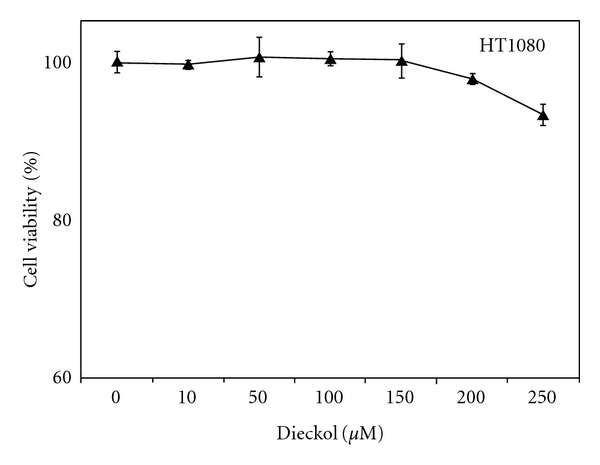
Effect of dieckol on the viability of HT1080 cells. Data represented as mean ± SD of three independent experiments.
3.2. Transwell Invasion Assay
Although no cytotoxic effect was observed even below 200 μM concentration of dieckol on HT1080 cell line, ideally, 0 to 100 μM of dieckol was used in the cell invasion and the subsequent experiments. To investigate whether dieckol inhibits tumor invasion, Matrigel invasion assays were performed for dieckol-treated HT1080 cells. It was observed that dieckol treatment reduced the cell invasion, and 100 μM of dieckol concentration significantly blocked tumor invasion (Figure 3(a)) [13, 17].
Figure 3.
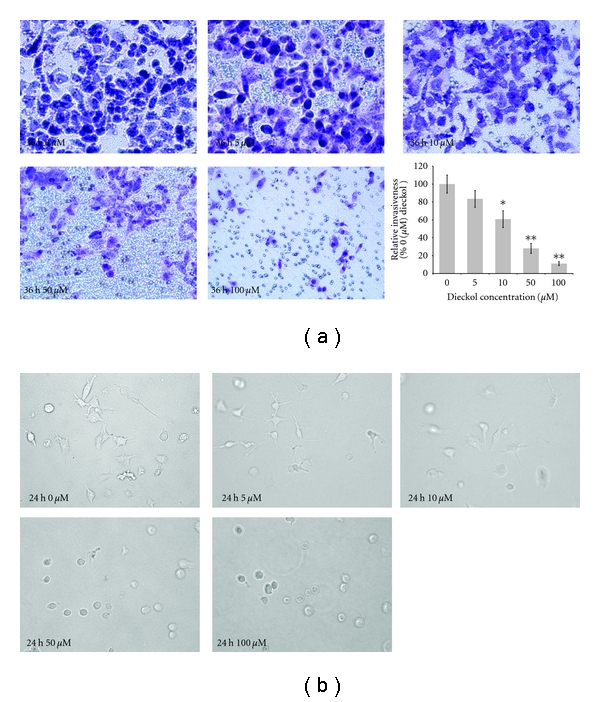
Effect of dieckol on invasion in HT1080 cells. (a) Microphotographs of filters of the Matrigel chamber invasion assay. Inset (a) represents the conclusive data of cellular invasion. (b) Dieckol Effect on the morphology of HT1080 cells in three-dimensional (3D) cultures system. The data presented are mean ± SD of three independent experiments. Values are compared with the 0 μM dieckol, which are significant at *P < 0.05 and **P < 0.01.
3.3. Effects of Dieckol on the 3D Culture in HT1080 Cell Line
To investigate the effect of dieckol on the 3D culture system of HT1080 cells, same density of cells were seeded into 3D collagen gel with various final concentrations of the dieckol. After 36 h, the control (without dieckol) stretched out in the gel and formed many branched and elongated structures in type I collagen matrix (Figure 3(b)). In contrast, the branches were reduced in the cells treated with 5 μM concentration of dieckol. As the concentration increased to 100 μM, these branches disappeared and cells changed to spherical shape.
3.4. Effects of Dieckol on the MMP-2, -9 Transcription and Expression Levels in HT1080 Cell Line
Gelatin zymography analysis was performed, for investigating whether dieckol could inhibit protein levels of MMP-2 and MMP-9. As shown in Figure 4(a), protein expressions for MMP-2 and MMP-9 were significantly inhibited by dieckol in a dose-dependent manner. In addition, results from the RT-PCR and western blot revealed that the expression of MMP-2 and -9 was inhibited at transcription levels by dieckol, more or less similar manner with zymography analysis (Figures 4(b) and 4(c)).
Figure 4.
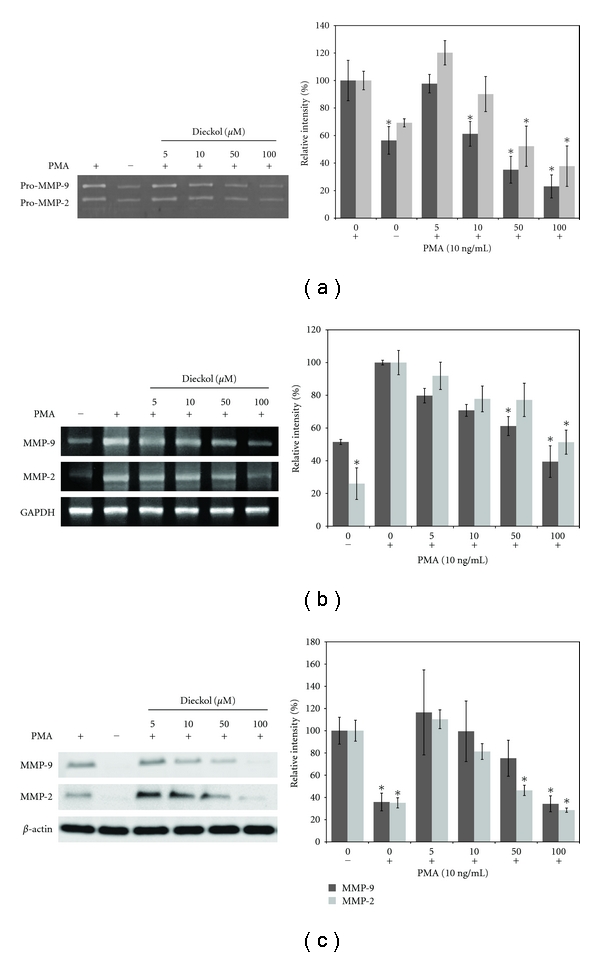
Effects of dieckol on the MMP expression. (a) Expression of MMP-2, -9 in dieckol-treated HT1080 cells by gelatin zymography. (b) mRNA transcription levels of MMP-2, -9 in HT1080 cells by RT-PCR analysis. (c) MMP-2, -9 protein expressions in the dieckol-treated HT1080 cells by western blot analysis. The columns presented in (a), (b), and (c) were the representative of three independent experiments. *, P < 0.05, Compared with the 0 μM dieckol with PMA 10 ng/mL.
3.5. Effects of Dieckol Influenced AP-1, NF-κB, and IκB Expressions on HT1080 Cell Line
Western blot studies were carried out to assay the downregulation effects of dieckol on the possible mechanism of expression of activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB). Meanwhile, the tissue inhibitor of metalloproteinases (TIMP) was been checked by western blot. As shown in Figure 5(b), dieckol did not exhibit a clear influence of activator protein-1 (AP-1) (c-jun) expression and mitogen-activated protein kinase (MAPK) (ERK, JNK, p38), which mediated the AP-1. The NF-κB transcription family proteins consist of several protein subunits, and it was clear that the level of NF-κB (p65 and p50) was decreased in a dose-dependent manner. The p-IκB-α expression was increased significantly, when treated with high concentration of dieckol (100 μM) (Figure 5(a)). As observed in Figure 5(c), there is no distinct change in the expressions of TIMP-1 and TIMP-2 when treated with different concentrations of dieckol.
Figure 5.
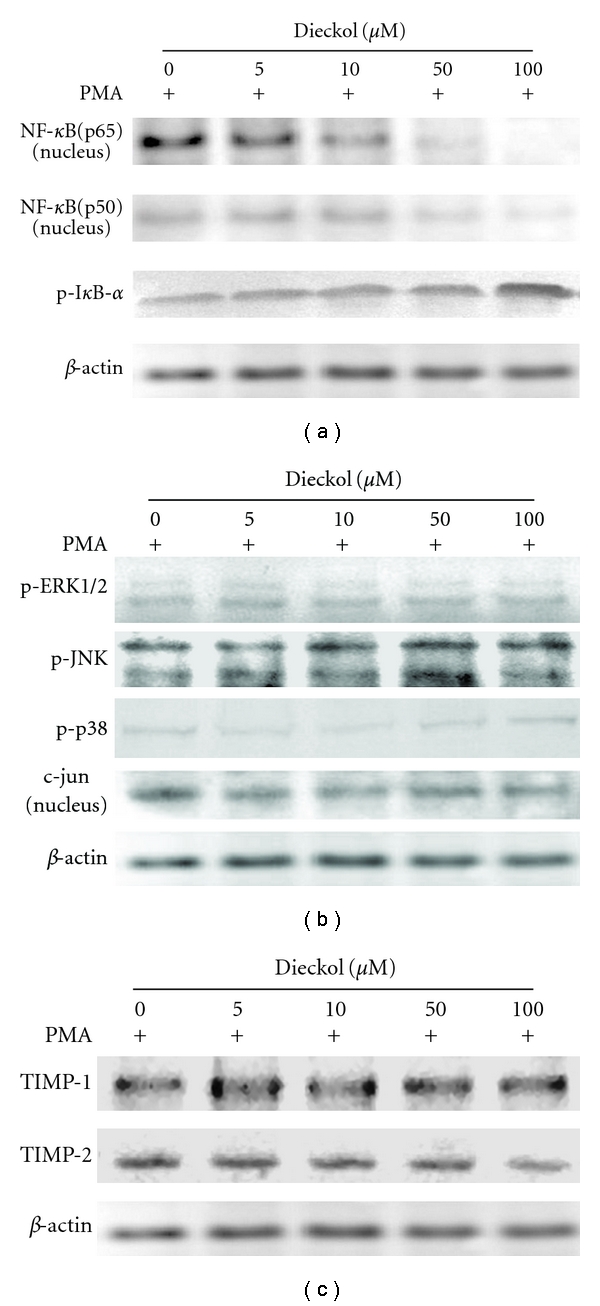
Effect of dieckol on the NF-κB and AP-1 cell signaling pathway and TIMP-1, -2 expressions in HT1080 cells. (a) Nucleus NF-κB (p65 and p50) and IκB (p-IκB-α) protein expressions in the dieckol treated HT1080 cells. (b) (c-jun), MAPK pathway (ERK, JNK, p38). (c) Western blot analysis of TIMP-1 and TIMP-2.
4. Discussion
MMPs, a family of zinc-dependent endopeptidases, participate in many physiological and pathological processes. An imbalance between the inhibition and activation of MMPs is related to some diseases such as osteoarthritis, rheumatoid arthritis, tumor metastasis, cardiovascular diseases and congestive heart failure [18–20]. Finding MMP inhibitors (MMPIs) for treatment of these prevalent diseases has been an important aspect of present day cancer research [21]. MMPIs regulate MMPs at several biochemical pathways, but most of them directly inhibit the activity of enzyme. Till now, MMPIs entering clinical trial are only of synthetic origin (organic compounds) and have failed to enter the pharmaceutical market owing to their side effects [22]. The search for novel and natural compounds has led to the discovery of oceans as the treasure house of substances with amazing pharmacological potential. Therefore, discovering the ideal MMPIs from marine natural sources is feasible and more advantageous [23].
Ecklonia cava (a marine alga), one of the marine floral members, produces dieckol which has been well studied previously to inhibit the expression of various MMPs [12, 24]. In this study, dieckol did not show any cytotoxic effect on HT1080 cells at the concentrations below 200 μM. However, dieckol influenced the cell mobility as measured by transwell invasion assay; it also suppressed HT1080 cells crossing the matrix gel in a dose-dependent manner. In addition, we have examined the effect of dieckol on the organization of HT1080 cells in a 3D culture environment. In vivo, cells experience a 3D environment and are surrounded by other cells and ECM. The traditional 2D monolayer culture system cannot emulate the complexities of the 3D tissue microenvironment and will always represent a suboptimal milieu for studying physiological ECM interactions with connective tissue cells [25, 26]. MMPs also affect cell morphology in 3D culture environment. MMPs cleave the matrix proteins fibronectin, vitronectin, and collagen I into smaller fragments. The cleaved ECM fragments then facilitate and accelerate cancer cell adhesion and invasion [15]. HT1080 cells formed the stellate structures in the 3D culture environment, and these structures migrated into the collagen matrix cleaving the ECM with the help of the MMPs. However, dieckol treatment significantly reduced the number and length of stellate structures. The cultured HT1080 cells were treated with dieckol with selected concentrations (0–200 μM) and the expression of MMP-2, -9 was induced by PMA. Several studies have shown that PMA can induce expression of MMP-2, -9 [27]. Dieckol inhibited the expression of MMP-2, -9 in a dose-dependent manner, according to the results of RT-PCR, western blot and zymography assays.
AP-1 and NF-κB are major transcription factors that regulate MMP-2, -9 expression [28–30]. Members of the MAPK superfamily (ERK1/2, JNKs, and p38 kinase) are known to regulate AP-1 transactivation by increasing the level of AP-1 components or altering the phosphorylation of their subunits (c-jun, c-fos) and then regulate MMPs expression and activity in various cell types [31]. NF-κB, in its inactive form, it is sequestered in the cytoplasm, bound by members of the inhibitor of kappa B (IκB) family of inhibitor proteins (including IκBa, IκBb, IκBg, and IκBe). Various stimuli that activate NF-κB result in phosphorylation of IκB, is followed by its ubiquitination and subsequent degradation [32, 33]. Dieckol suppress NF-κB (p65 and p50) expression in a dose-dependent manner. However, dieckol did not show any clear effect on AP-1 and ERK1/2, JNKs, and p38 kinase expressions. It has been found that p-IκB-α expression increased when treated with high concentration of dieckol (100 μM), but not in low concentration [34]. It may suggest that the inhibitor of kappa B family could be upregulated by high concentration of dieckol, and this may be another reason for NF-κB suppression by dieckol. Western blot resulted from TIMP-1 and TIMP-2 depicted that TIMP have not been influenced by different concentrations of dieckol. It is indicating that inhibition of MMP-2, -9 activity by dieckol is not because of the TIMP at the test concentrations.
At the promoter site of MMP-2 and -9 genes, several putative binding sites for transcription factors are present, which regulate gene expression. This promoter contains an AP-1 binding consensus site at −79 upstream from the starter site and further upstream, there is a cluster of regulatory elements including another AP-1 binding site called AP-2 and NF-κB binding site [35]. It is commonly known that MMP-9 transcription is mainly regulated via AP-1 [36, 37]. In addition to this, under some inflammatory and other pathology conditions, MMP-2, -9 transcription can be regulated via NF-κB signaling pathways. Interestingly, dieckol inhibited NF-κB expression with a similar dose-dependent manner as observed for MMP-2, -9 expressions. However, it did not significantly influence the expression of AP-1. This mode of action may be contributed to the unique chemical structure of dieckol [38–40]. The reasons could be discussed based on the structural features of the isolated dieckol and the structure-activity relationship could be described due to its unique skeleton. For example, the number of hydroxyl groups present in its chemical structure probably plays an important role because of the higher polymerization of phloroglucinal units and on the other hand, the O-bridge linkages (ether linkage) among phloroglucinals donate more free anions to attract responsible receptors.
5. Conclusion
Dieckol was isolated from an edible marine brown alga Ecklonia cava has been characterized according to the MS and NMR data. It acts as an inhibitor of MMP-2, -9 expressions by the downregulation of NF-κB pathway without significant influence on AP-1, MAPK pathway. Furthermore, the modulation of MMP-2 and -9 expressions could be one reason for the suppression of invasiveness and 3D culturing of HT1080 cell line. Hence, this study suggested that dieckol could be an effective candidate for the suppression of cancer invasion.
Acknowledgment
This paper was supported by a grant from Marine Bioprocess Research Center of the Marine Biotechnology program funded by the Ministry of Land, Transport and Maritime, Republic of Korea.
References
- 1.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. Journal of Cell Science. 2002;115(19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 2.Tanimura S, Kadomoto R, Tanaka T, Zhang YJ, Kouno I, Kohno M. Suppression of tumor cell invasiveness by hydrolyzable tannins (plant polyphenols) via the inhibition of matrix metalloproteinase-2/-9 activity. Biochemical and Biophysical Research Communications. 2005;330(4):1306–1313. doi: 10.1016/j.bbrc.2005.03.116. [DOI] [PubMed] [Google Scholar]
- 3.McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Molecular Medicine Today. 2000;6(4):149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 4.Ashley RA. Clinical trials of a matrix metalloproteinase inhibitor in human periodontal disease. Annals of the New York Academy of Sciences. 1999;878:335–346. doi: 10.1111/j.1749-6632.1999.tb07693.x. [DOI] [PubMed] [Google Scholar]
- 5.Kang HS, Chung HY, Kim JY, Son BW, Jung HA, Choi JS. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Archives of Pharmacal Research. 2004;27(2):194–198. doi: 10.1007/BF02980106. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Banerji A, Frei E, Chatterjee A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sciences. 2008;82(9-10):467–476. doi: 10.1016/j.lfs.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB Journal. 1999;13(8):781–792. [PubMed] [Google Scholar]
- 8.Le QT, Li Y, Qian ZJ, Kim MM, Kim SK. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochemistry. 2009;44(2):168–176. [Google Scholar]
- 9.McChesney JD, Venkataraman SK, Henri JT. Plant natural products: back to the future or into extinction? Phytochemistry. 2007;68(14):2015–2022. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, Kim SK. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava . Bioorganic and Medicinal Chemistry. 2008;16(17):7921–7926. doi: 10.1016/j.bmc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 11.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathology and Oncology Research. 2001;7(1):14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 12.Kim MM, Ta QV, Mendis E, et al. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sciences. 2006;79(15):1436–1443. doi: 10.1016/j.lfs.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Pasco S, Brassart B, Ramont L, Maquart FX, Monboisse JC. Control of melanoma cell invasion by type IV collagen. Cancer Detection and Prevention. 2005;29(3):260–266. doi: 10.1016/j.cdp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 15.Frisk T, Rydholm S, Liebmann T, Andersson Svahn H, Stemme G, Brismar H. A microfluidic device for parallel 3-D cell cultures in asymmetric environments. Electrophoresis. 2007;28(24):4705–4712. doi: 10.1002/elps.200700342. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee KJ, Kim JY, Choi JH, et al. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum . Food and Chemical Toxicology. 2006;44(11):1890–1896. doi: 10.1016/j.fct.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG, Coker ML, Krombach SR, et al. Matrix metalloproteinase inhibition during the development of congestive heart failure: effects on left ventricular dimensions and function. Circulation Research. 1999;85(4):364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 19.Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Annals of the Rheumatic Diseases. 2000;59(6):455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stricklin GP, Welgus HG. Human skin fibroblasts collagenase inhibitor. Purification and biochemical characterization. Journal of Biological Chemistry. 1983;258(20):12252–12258. [PubMed] [Google Scholar]
- 21.Zhang C, Kim SK. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: the current situation and future prospects. Marine Drugs. 2009;7(2):71–84. doi: 10.3390/md7020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nature Reviews Cancer. 2002;2(9):657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 23.Mayer AMS, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. International Journal of Cancer. 2003;105(3):291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- 24.Kim SO, Choi YH. The ethyl alcohol extract of Hizikia fusiforme inhibits matrix metalloproteinase activity and regulates tight junction related protein expression in Hep3B human hepatocarcinoma cells. Journal of Medicinal Food. 2010;13(1):31–38. doi: 10.1089/jmf.2009.1233. [DOI] [PubMed] [Google Scholar]
- 25.Edelman DB, Keefer EW. A cultural renaissance: in vitro cell biology embraces three-dimensional context. Experimental Neurology. 2005;192(1):1–6. doi: 10.1016/j.expneurol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 27.Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-κB pathway. Journal of Biological Chemistry. 2002;277(33):30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- 28.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1β . Journal of Biological Chemistry. 2004;279(38):39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 29.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB Journal. 1999;13(8):781–792. [PubMed] [Google Scholar]
- 30.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-κB. Biochemical Pharmacology. 2004;68(2):361–371. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Jin UH, Suh SJ, Hyen WC, et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. Journal of Cellular Biochemistry. 2008;104(1):15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, Baltimore D. Activation in vitro of NF-κB by phosphorylation of its inhibitor IκB. Nature. 1990;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 33.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κb. Nature. 1993;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 34.Wisniewski P, Ellert-Miklaszewska A, Kwiatkowska A, Kaminska B. Non-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFκB-TIMP-2 pathway. Cellular Signalling. 2010;22(2):212–220. doi: 10.1016/j.cellsig.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Moon SK, Kim HM, Kim CH. PTEN induces G1 cell cycle arrest and inhibits MMP-9 expression via the regulation of NF-κB and AP-1 in vascular smooth muscle cells. Archives of Biochemistry and Biophysics. 2004;421(2):267–276. doi: 10.1016/j.abb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Shim JS, Choi EJ, Lee CW, Kim HS, Hwang JK. Matrix metalloproteinase-1 inhibitory activity of kaempferia pandurata roxb. Journal of Medicinal Food. 2009;12(3):601–607. doi: 10.1089/jmf.2007.1041. [DOI] [PubMed] [Google Scholar]
- 37.Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-κB binding to the promoter as a consequence of IκBα-induced block of p65/p50 nuclear translocation. Journal of Biological Chemistry. 2001;276(2):1164–1172. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Jung E, Lee J, et al. Emodin inhibits TNF α-induced MMP-1 expression through suppression of activator protein-1 (AP-1) Life Sciences. 2006;79(26):2480–2485. doi: 10.1016/j.lfs.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Matsunaga N, Chikaraishi Y, Shimazawa M, Yokota S, Hara H. Vaccinium myrtillus (bilberry) extracts reduce angiogenesis in vitro and in vivo . Evidence-Based Complementary and Alternative Medicine. 2010;7(1):47–56. doi: 10.1093/ecam/nem151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper EL. Drug discovery, CAM and natural products. Evidence-Based Complementary and Alternative Medicine. 2004;1(3):215–217. doi: 10.1093/ecam/neh032. [DOI] [PMC free article] [PubMed] [Google Scholar]


