Abstract
Adult stem cell-derived smooth muscle cells (SMC) may be a promising source of cells for applications in regenerative medicine, including cardiovascular tissue engineering. Primary SMC from native vessels may have limited proliferative capacity and reduced collagen production when sourced from elderly donors, who are the patients in need of vascular grafts due to coronary disease or peripheral arterial disease. Our recent work showed that the ability of human bone marrow-derived mesenchymal stem cells (hMSCs) to differentiate into SMC was modulated by various growth factors, matrix proteins, and mechanical forces. In addition, the components of the culture medium play a very important role in SMC differentiation from hMSCs. In this chapter, we will summarize our experience with the impact of various factors on SMC differentiation from hMSCs. Based upon our findings regarding growth factors, cyclic strain and matrix proteins, a two-phase vessel regeneration culture protocol including a 4-week proliferation phase and a 4-week differentiation phase was developed to optimize proliferation and SMC differentiation of hMSCs consecutively.
Keywords: Mesenchymal stem cells, Smooth muscle cells, Small-diameter vessel, Differentiation
1. Introduction
Bone marrow-derived mesenchymal stem cells (hMSCs) have become an attractive cell source in tissue engineering because they are relatively easy to obtain, autologous in nature, and have the potential to differentiate into diverse cell types, such as adipogenic, osteogenic, chondrogenic, and myogenic lineages. Beyond these studies of differentiation potential of MSCs, the application of bone marrow-derived stem cells in vitro and in vivo for vascular engineering is just emerging (1–8).
We hypothesized that the local environment (9) and the resident cellular population in the intact or regenerating vascular wall should be involved in directing the differentiation of hMSCs toward a smooth muscle cell (SMC) phenotype. Indeed, bone marrow-derived MSCs have been shown to differentiate into SMCs in response to transforming growth factor β (TGF-β) (10), mechanical stress (11), direct contact with vascular endothelial cells (12), and interaction with endothelial cell matrix (13). We have found that growth factors that are elaborated by platelets and vascular cells after vessel injury (PDGF, TGF-β1, and bFGF), extracellular proteins found in native vessel wall, and cyclic mechanical strain all impact SMC differentiation from hMSCs (14). In addition, a novel two-phase vessel engineering protocol was developed and is described here. The novel protocol is based upon our original vascular tissue engineering protocol (15, 16), but is modified in order to promote hMSC proliferation and subsequent SMC differentiation (14) in the regenerating blood vessel.
2. Materials
2.1. Isolation and Cell Culture of hMSCs
25 ml fresh unprocessed human bone marrow (Lonza, Basel, Switzerland).
Ficoll-Paque Plus density media (StemCell Technologies, Vancouver, BC, Canada) stored at 4°C.
Dulbecco’s phosphate buffered saline (D-PBS; Invitrogen, Carlsbad, CA, USA).
Basal hMSC culture medium: Dulbecco’s Modified Eagle’s Medium (DMEM), low-glucose (Invitrogen, stored at 4°C, avoid direct light) containing 10% selected lot of fetal bovine serum (selected lot of FBS; Hyclone, South Logan, UT, USA) and 1% penicillin-streptomycin-glutamate (Invitrogen). The screening of the FBS lot was based on the support of cell proliferation of hMSC.
2.2. Flow Cytometry
Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human IgGs: CD14 (Abcam Inc., Cambridge, MA, USA); CD45 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and CD34 (Miltenyi Biotec Inc., Auburn, CA, USA).
SH2 and SH3 hybridoma (American Type Culture Collection, ATCC, Manassas, VA, USA) cultured in ATCC Hybri-Care Medium plus 20% FBS (ATCC). Supernatant was obtained with a series dilution where the optimal dilution provided the brightest fluorescence and highest dilution of the supernatant.
Isotype control for each primary antibody: For CD14- and CD45-IgG1-FITC antibody, isotype control was mouse IgG1-FITC (Santa Cruz Biotechnology); For CD34-IgG2a-FITC antibody, isotype control was purchased from Miltenyi Biotech. For SH2 and SH3, the isotype controls were goat-anti-mouse IgG1 and IgG2b-FITC, respectively (Santa Cruz Biotechnology).
Fixative: 4% paraformaldehyde (Boston BioProducts, Boston, MA, USA) stored at 4°C.
FACStar flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
2.3. Immunofluorescence Staining of SMC Markers
Primary antibodies: 1:100 dilution for mouse anti-human monoclonal SMA and calponin, 1:50 dilution for smooth muscle myosin heavy chain (SM-MHC) antibodies (Dako, Copenhagen, Denmark) in dilution buffer.
Secondary antibody: 1:2,000 dilution for FITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) in dilution buffer.
Vectashield Mounting Medium for Fluorescence with 4′6-diamidine-2-phenylindole (DAPI) kit (Vector Laboratories, Inc., Burlingame, CA, USA).
2.4. Western Blot for SMC Markers
Tris-buffered saline (TBS) buffer: Dilute in deionized water from 10× TBS (Boston BioProducts) containing 0.5 M Tris and 1.5 M NaCl (pH 7.4).
TBS/Tween-20 buffer (TBST): Dilute from 10× TBST buffer (Boston BioProducts) containing TBS buffer with 0.5% Tween-20.
Blocking buffer: 5% nonfat dry milk (Bio-Rad, Hercules, CA, USA) in TBST buffer stored at 4°C.
Antibody dilution buffer: Dilute blocking buffer to 1% with TBST buffer
Primary antibodies for SMC markers: 1:100 dilution for mouse anti-human SMA and calponin, 1:50 dilution for mouse anti-human smooth muscle myosin heavy chain (SM-MHC) antibodies (Dako, Copenhagen, Denmark) in dilution buffer freshly prepared before Western blot (see Note 1).
Mouse anti-human β-actin (Sigma) 1:5,000 dilution before use.
Secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) 1:2,000 diluted in dilution buffer before use.
Polyvinylidene difluoride membranes (Millipore, Bedford, MA).
SuperSignal West Pico Chemiluminescent detection system (Pierce, Rockford, IL).
Restore™ Western Blot Stripping Buffer (Pierce).
RIPA buffer with Triton (Boston BioProducts, Worcester, MA) consisting of 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Tritonx 100, 0.5% sodium deoxycholate, and 0.1% SDS.
2.5. Effect of Various Soluble Factors on hMSCs
Human coronary artery SMCs (CASMC, Lonza).
Smooth muscle growth medium (SmBM) supplemented with SmGM-2 SingleQuots (Lonza).
Preparation of growth factors (see Note 2): stock solution for TGFβ1 (R&D Systems, Inc., Minneapolis, MN, USA) was prepared by dissolving 2 μg of TGFβ1 in 400 μl dilution buffer (1% BSA in PBS, store at 4°C), resulting in a final concentration of 5 μg/ml. Aliquot 100 μl/tube and store at −70°C. PDGF-BB stock solution (10 μg/ml) was made by dissolving 50 μg PDGF-BB (R&D Systems, Inc.) in 5 ml dilution buffer. Aliquot 500 μl/tube and store at −70°C. PDGF-CC stock solution (50 μg/ml) was made by dissolving 25 μg PDGF-CC (R&D Systems, Inc.) in 500 μl dilution buffer. Aliquot 100 μl/tube and store at −70°C. bFGF stock solution (50 μg/ml) was made by dissolving 25 μg bFGF (R&D Systems, Inc.) in 500 μl dilution buffer. Aliquot 100 μl/tube and store at −70°C.
2.6. Effect of Various Matrix Proteins on hMSCs
6-well plates untreated or coated with collagen type I, IV, elastin, fibronectin, and laminin (Flexcell International, Hillsborough, NC, USA).
2.7. Effect of Cyclic Strain on hMSCs
Flexcell 4000T unit (Flexcell International): The strain unit is a computer-regulated device that applies cyclic tensile strain to the cell culture through regulated vacuum pressure on the bottom culture plates with a flexible membrane that is untreated or pretreated with fibronectin or type I collagen. The strain causes the flexible plate to stretch across a cylindrical loading post to provide equibiaxial strain to the cells.
2.8. Engineered Vessel Culture Bioreactor
The flow system consists of two tubing sets – large and small (Ark-Plas Products, Flippin, AR, USA) connected by a series of different connectors (Cole-Parmer, Vernon Hills, IL, USA). Please see Fig. 1 for details of measurement and connection between the tubes with connectors. The fat tubing in the large tubing set is L/S 18. The rest of the tubes are L/S 16. There is a Y connector for the small tubing set. For the large tubing, there are one Y-connector, one pair of locked connectors (male and female), and two thick connectors.
Bioreactor hand-blown by an expert glass-blower. Dimension and measurement of the bioreactor is illustrated in Fig. 2.
Stir bar (Fisher Scientific, Pittsburgh, PA, USA).
Bioreactor lids were made by slicing a silicone stopper (Cole-Parmer) into half horizontally and four holes were created on the lid with a cork borer (use borer #2, 6 mm diameter, Cole-Parmer) for gas exchange via PTFE filters (Cole-Parmer).
PGA mesh purchased from Concordia Medical, Coventry Rhode Island. Keep dry in a sealed bag.
Silicone tubing (Saint-Gobain Performance Plastics) cleaned with dH2O (Sigma) and let dry.
Dexon 6.0 suture: 18 in. in length, Dexon S uncoated braided synthetic absorbable polyglycolic acid suture from United States Surgical Corporation (US Surgical, Norwalk, CT, USA).
Prepare 1 M NaOH solution by dissolving 40 g NaOH (Sigma) in 1 liter dH2O.
Three 1 liter beakers (Fisher Scientific) filled with dH2O.
Kimwipes (Kimberly-Clark, Neenah, WI, USA).
Dacron sleeves: “Cooley Veri-Soft Woven Vascular Graft” (Boston Scientific, Natick, MA, USA).
Prolene 4.0 suture from US Surgical Corporation.
Petri dishes: Large (100 mm diameter) and small (60 mm diameter) (BD Biosciences).
Sterile tissue culture water (Sigma).
5 or 10 ml serological pipettes (BD Biosciences).
Flow equipment consisting of a PBS bag (Miltenyi Biotech), a pressure transducer and a 3-way stopcock (Edwards Lifesciences, Irvine, CA, USA).
Two gallons of fresh 190-proof ethanol (Fisher Scientific).
Tools for attaching the PGA mesh: Two sets of forceps, scissors, and hemostat forceps (VWR Scientific, West Chester, PA, USA).
Alcohol wipes (Fisher Scientific).
Walrus tubing (Arrow International, Inc., Reading, PA, USA).
PBS w/fungizone: Use 5 ml fungizone (Sigma) per 500 ml PBS.
Fibronectin: Prepared fresh on the launch day by dissolving 5 mg fibronectin (BD Biosciences) in 10 ml sterile Ca2+ and Mg2+-free PBS to make a concentration of 0.5 mg/ml. 100 μl of fibronectin is needed to coat PGA mesh (approximate 10 cm2 surface area) with fibronectin at 5 μg/cm2.
10, 20, and 60 ml syringes (BD Biosciences).
Injection port with luer lock (Baxter Healthcare, Deerfield, IL, USA).
Parafilm (Pechiney Plastic Packaging, Menasha, WI, USA).
Tube clamp (Small Parts, Inc., Miramar, FL, USA).
100× pen/strep solution (Invitrogen), aliquot 5 ml/tube, store at 4°C.
Blue Nalgene 0.20 μm filter (Pall Corporation, Ann Arbor, MI, USA).
Needles: Pink (18G1/2) and green (21G1/2) (BD Biosciences).
Easy-load Masterflex pump, Model 7518-10 (Cole-Parmer).
Pressure monitor for intravenous or invasive blood pressure (Abbott Labs, Abbott Park, IL, USA).
Proliferation medium (14): Each 500 ml of medium consists of DMEM (low-glucose) (Invitrogen) plus 50 ml (10%) selected lot of FBS (Hyclone), 5 ml 1% penicillin-streptomycin-glutamate (Invitrogen), vitamin C (25 mg/5 ml PBS or DMEM), 1.5 μg CuSO4, 25 mg Proline and Glycine, 10 mg Alanine (5 ml standard solution), 5 ml 1 M HEPES solution, and 10 ng/ml PDGF-BB (R&D Systems).
Differentiation medium (14): Prepare proliferation medium with the substitution of PDGF-BB with 1 ng/ml TGFβ1 (R&D Systems).
3% acetic acid with methylene blue (StemCell Technologies, Inc., Vancouver, BC, Canada).
Quick Start Bradford Dye Reagents (Life Science, Hercules, CA).
Image J software (National Institutes of Health, Bethesda, MD).
Fig. 1.
Measurement of the large and small tubing for the flow system.
Fig. 2.
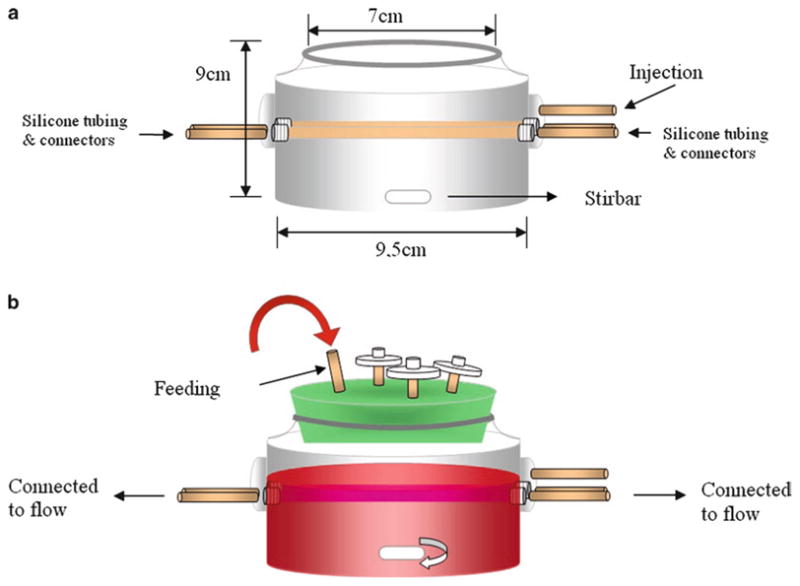
General schematic of the bioreactor/mesh setup before (a) and after (b) the assembly on the launch day.
3. Methods
3.1. Isolation and Cell Culture of hMSCs
Slowly load fresh unprocessed human bone marrow onto Ficoll-Paque Plus and fractionate in a centrifuge at room temperature for 30 min at 1,200 × g (14) with no brake (see Note 3).
Remove the mononuclear cell layer at the interface and wash once with D-PBS and plate in a 25 cm2 flasks in basal culture medium.
Maintain cultures at 37°C in a humidified atmosphere con- and provide complete medium changes twice taining 5% CO2 per week.
Upon reaching 80–90% confluence, primary cultures were detached using trypsin, and harvested cells were replated into basal culture medium in 75 cm2 flasks (for protein analyses) or in chamber slides (for histochemical analysis).
3.2. Flow Cytometry
Obtain harvested hMSCs and resuspend cells at 107 cells per 100 μl in D-PBS buffer.
Stain 100 μl aliquots of cells with Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human IgGs (20 μl of 100 μg/ml CD14, 10 μl of 200 μg/ml CD45, and 10 μl for CD34) or properly matched isotype IgG controls at 4°C for 1 h in the dark. For SH2 and SH3 staining, 100 μl aliquots of cells were incubated with 20 μl of supernatant from SH2 and SH3 hybridoma cell culture.
After staining, fix the cells in 4% paraformaldehyde for 5 min at room temperature.
Quantitative flow cytometry was performed on a FACStar flow cytometer as per the manufacturer’s instructions.
3.3. Immunofluorescence Staining of SMC Markers
Perform immunofluorescence staining for SMC markers as described previously (14).
Incubate paraformaldehyde-fixed cells in chamber slides with mouse anti-human SMA (1:100) or calponin (1:50) monoclonal antibodies in 10% BSA buffer for 1 h at room temperature.
Wash chamber slides 3 times with PBS and incubate with the secondary antibody (FITC-conjugated goat anti-mouse IgG) in 10% BSA buffer for 30 min at room temperature.
Wash the chamber slides with cell culture grade water for 3 times and then remove the gaskets with a tweezer.
Stain nuclei with DAPI using Vectashield Mounting Medium for Fluorescence with DAPI kit with one drop of the mounting medium on top of the slide and cover with a coverslip before visualization.
3.4. Western Blot for SMC Markers
3.4.1. hMSC Cell Detachment and Cell Lysate Preparation
Wash a 75 cm2 flask containing hMSCs twice with calcium-magnesium-free D-PBS and add 0.5 ml Trypsin-EDTA to each well.
Incubate at 37°C for no more than 5 min.
Shake the plate gently to detach the cells from the plate.
Inactivate the trypsin by adding 0.5 ml basal culture medium containing 10% FBS to each well.
Harvest the detached cells in a 15 ml conical tube.
To determine cell number, ensure cell solution is thoroughly mixed and then mix 20 μl of cell suspension with 80 μl (1:5 dilution) or 180 μl (1:10 dilution) 3% acetic acid with methylene blue in a 1 ml eppendorf tube. Load approximately 10 μl of the mixture to each side of a hemocytometer and determine cell concentration.
Wash cells twice with D-PBS and remove the supernatant.
Resuspend the cell pellet in RIPA buffer (approximately 5 times of the volume of the cell pellet) containing Triton X-100 and protease inhibitor cocktail and thoroughly homogenize by repetitive pipetting and vortexing on ice.
After homogenization, centrifuge the cell lysate at 14,000 × g in the cold room at 4°C for 30 min.
Transfer the supernatant to a clean eppendorf test tube and remove 25 μl of lysate for protein quantification. Mix the remaining protein lysate with ¼ volume of Laemmli’s SDS-sample buffer (4×, on-reducing, Boston BioProducts) and boil at 100°C for 10 min followed by a quick chill on ice for 5 min and store at −70°C.
Perform protein quantification using the Quick Start Bradford Dye Reagents as per the manufacturer’s instructions.
3.4.2. Western Blot Analysis of SMC-Specific Markers
Separate 25 microgram of protein lysate on a 10% SDS-PAGE gel and transfer to polyvinylidene difluoride membranes.
Develop the membrane using the SuperSignal West Pico Chemiluminescent detection system by mixing 1 ml of Reagent A and B each in a conical tube and immediately apply to the membrane. Incubate for 5 min at room temperature in the darkness.
Develop the film after exposing the membrane to a Kodak film on a cassette in the dark room and incubate for 1–5 min depending on the intensity of the signal.
After visualization, strip the membrane with 12–15 ml Restore™ Western Blot Stripping Buffer at room temperature for 5–15 min.
Wash the blot with TBS buffer for 5 min and incubate at 4°C overnight in blocking buffer.
Reprobe the blot for β-actin, which serves as an equal-loading control.
Quantify Western blots using Image J (National Institutes of Health, Bethesda, MD).
Present results as relative density after correction with β-actin.
3.5. Effect of Various Soluble Factors on hMSCs
Seed harvested hMSCs in 6-well plates at 5.6 × 103 cells/cm2 in DMEM plus 5% FBS medium with one of the following supplements: 0 (control), 0.01, 0.1, 1, or 10 ng/ml transforming growth factor β1, 10 ng/ml PDGF-BB, PDGF-CC, bFGF or 50 μg/ml ascorbic acid.
Culture CASMCs as positive controls in SmBM supplemented with SmGM-2 SingleQuots as per the manufacturer’s instructions.
Change culture medium on day 4 after seeding, and harvest cells after 7 days of treatment for SMC differentiation analysis.
3.6. Effect of Various Matrix Proteins on hMSCs
Seed harvested hMSCs at 2 × 103 cells/cm2 in DMEM plus 10% FBS medium on 6-well plates untreated or coated with collagen type I, IV, elastin, fibronectin, and laminin.
After 7 days, detach the cells with trypsin and enumerate with 3% acetic acid with methylene blue on a hemocytometer (see Subheading 3.4.1, steps 1–6).
Lyse the remaining cells with RIPA buffer containing Triton X-100 and protease inhibitor cocktail (see Subheading 3.4.1, steps 7–10).
Protein lysates were quantified by the Bradford assay and stored at −70°C until needed for SMC marker western blot analysis (see Subheading 3.4.2).
3.7. Effect of Cyclic Strain on hMSCs
Seed hMSCs at 2 × 104 cells/ml in DMEM plus 10% FBS in a Flexcell 4000T unit and subject the seeded cells to cyclic strain in the presence or absence of 10 ng/ml PDGF-BB.
Subject hMSCs to equibiaxial cyclic strain for 5 days at a frequency of 0.5 Hz, resulting in 8–12% substrate elongation.
Prepare unstrained controls in an identical manner and culture on unstrained untreated or collagen I or fibronectin-coated flexible plates for 5 days.
3.8. Engineered Vessel Culture in a Bioreactor
3.8.1. Bioreactor Preparation the Day Before Initiating the Culture
Autoclave the following components: Bioreactor with a stir-bar, a lid with a feeding tube, flow system (two tubing sets – large and small), connectors, Pasteur pipettes, media cap, and injection tubes (see Figs. 1 and 2).
Cut PGA to 1.1 × ~8 cm (depending on bioreactor size).
Sew the PGA mesh around clean silicone tubing starting with three surgical knots followed by single stitches looped within each other.
Treat the surface of a PGA tube in 1 M NaOH for 1–2 min and rinse in three separate dH2O baths. Pat dry with Kimwipes between each dip in water baths and dry the PGA tube in a laminar flow hood with the blower on and the UV light off.
Cut the Dacron into 1 cm stubs and sew the small pieces of Dacron onto each end of the PGA mesh with an overlap of 2–3 mm. Be careful not to puncture the silicone tubing.
Thread suture to edge of Dacron and prepare one surgical knot (two windings) and one single knot.
Soak the sewn mesh on silicone tubing, tools, and wire in an ethanol bath for 20–30 min.
Flush the tubing with ethanol by lifting one side of tube up (observe air bubbles leaving).
Set-up the bioreactor (see Fig. 2a) by feeding the silicone tubing through the side arms using thin wire to pull it through.
Pull the bioreactor out of the ethanol bath (keep mesh submerged in ethanol), and attach vessels to the glass bioreactor by tightening Dacron over the glass lip with Prolene sutures already set (surgical knot + 2 single knots).
Connect one side of the bioreactor side arms to connectors via silicone tubing. Pull the other side of the silicone tubing with enough tension (about one finger width) and insert remaining two connectors into silicone tubing.
Reinsert the bioreactor into the ethanol bath and flush with ethanol by gently pulling connectors out of the side arms.
Flip the bioreactor over and allow soaking for 10 min.
Flip bioreactor right-side up and soak in the ethanol bath for an additional 10 min.
Drain all ethanol.
Set up three large Petri dishes (tops or bottoms) in series, and place the bioreactor in the center dish.
Flush bioreactor (mesh included) with tissue culture water using a 5 or 10 ml pipette. Also flush the tissue culture water into the silicone tubing.
Thoroughly drain all excess water into the Petri dishes on either side of the bioreactor.
Dry the bioreactor overnight in the hood with the blower on and the UV light off.
3.8.2. Day 1 Bioreactor Culture Set-Up (see Note 4)
Coat each of the PGA tubes with 100 μl 0.5 mg/ml fibronectin freshly made by dissolving 5 mg fibronectin in 10 ml sterile Ca2+ and Mg2+-free PBS. Allow to air dry for at least 45 min at room temperature.
Place a sterile Petri dish over the opening of the bioreactor.
Assemble the flow system to the bioreactor (see Fig. 3).
Wipe the connectors first with alcohol wipes. Attach flow system to all four arms. Close off the system as soon as possible.
Attach the injection port to the third, unused arm of bioreactor.
Remove walrus tubing and tie off the blue end as close to the Y-junction as possible. Pull the tube clamp in place to ensure no liquid transfer to this part of the tube.
Remove the PBS bag and attach the red end of the Walrus tubing to the far end of the PBS bag. Make sure to wipe insertion port with alcohol wipe first.
Attach the white end tube of the walrus tubing to one side of flow system.
Insert a 3-way stopcock into the flow system.
Remove pressure transducer from the package and connect to a 3-way stopcock.
Attach the other end of the pressure transducer to the middle opening of the PBS bag.
Parafilm everything including the connections between the tubings and between the connector and the tubings or between the connectors and the bioreactor. Spray system with ethanol.
Use a 60 ml syringe to inject about 350 ml of PBS with fungizone solution into the PBS bag through an open port.
Squeeze the bag to flush the system.
Move the stop cocks to allow for full movement of liquid. Check inside bioreactor to ensure there is no leaking.
Spray the entire system with 70% ethanol.
Trypsinize hMSCs, enumerate and centrifuge.
While waiting for the centrifugation, assemble the bioreactor lid by attaching the injection port to the feeding tube.
Attach the three PTFE 0.20 μm filters to each of the three air ports. Be careful not to expose or touch the bottom of the lid during this process.
Parafilm the injection port.
Resuspend one confluent 75 cm2 flask (5–8 × 106 cells) into 1.25 ml and seed onto one vessel. Make sure cell suspension has been introduced to the Dacron-mesh junction as well as the bottom of the mesh (see Note 5).
Insert the lid into the glass bioreactor, making sure that the feeding tube does not touch the seeded mesh.
Parafilm the lid and the lip of the glass bioreactor.
Place the bioreactor in the incubator on its side and rotate every 5 min for 25–30 min.
Fill the bioreactor with culture medium (above Dacron, below the third arm) with a pump (see Fig. 2b).
Fig. 3.
Flow system set-up.
3.8.3. Day 2 Pen/Strep Feeding
Draw up 5 ml of pen/strep into a syringe, then attach a blue Nalgene 0.20 μm filter to the syringe.
Attach a green needle (21G) to a 20 ml syringe.
Wipe the injection port with alcohol wipe before and after every syringe injection.
Withdraw about 20 ml medium slowly from feeding tube with the 20 ml syringe.
Replace the 20 ml syringe with 10 ml syringe with pen/strep and inject the pen/strep into the bioreactor.
Reinject the culture medium.
3.8.4. Weekly Feeding Regimen
Starting with the first feeding on day 7, feed every 7 days.
For each medium change, remove about 200 ml (or 50% of the total volume) of medium and replace with 200 ml fresh medium.
Dissolve vitamin C into 5 ml sterile PBS and supplement the bioreactor 3 times per week (see Note 6) following the same protocol used for pen/strep feeding (see Subheading 3.8.2).
3.8.5. Turning on the Pump
After 4 weeks of culture under static conditions, change from proliferation medium to differentiation medium (14) (see Note 7). And turn on the pump (Day 29).
Gradually increase the speed with a target of 160 beats per minute.
Make sure there are no leaks.
Tighten all the injection ports as they tend to loosen at 37°C.
Record pressures daily throughout the culture.
3.8.6. Termination of the Culture
After 8 weeks, a complete vessel can be formed via endothelialization by seeding human endothelial or HUVEC cells into the lumen of the SMC vessel wall (14).
When the culture is complete (with or without endothelialization), the culture is terminated when the pump is shut down and the vessel is taken out of the bioreactor for further analysis (i.e., mechanical testing, immunohistochemical staining, or molecular analysis). See Fig. 4 as an example bioreactor in the incubator and a close-up view of an engineered vessel in the bioreactor at the completion of culture.
Fig. 4.
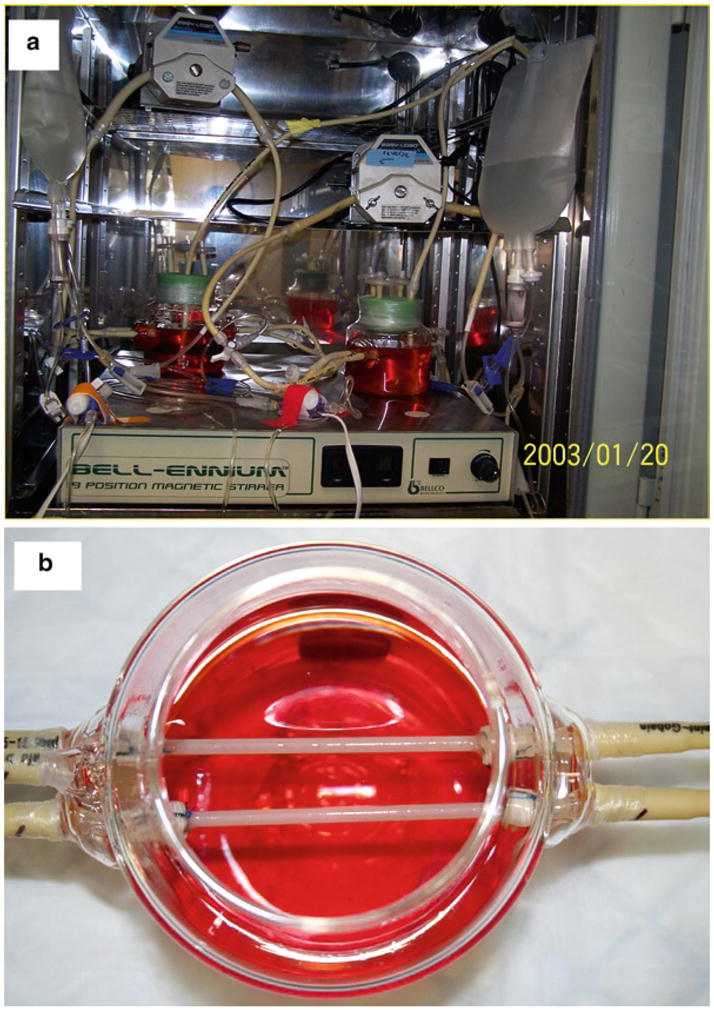
Pictures of two glass bioreactors in an incubator (a) and two engineered vessels (b).
Acknowledgments
The authors are grateful for Drs. Caroline Rhim and Shannon L. M. Dahl for their contribution to the development and optimization of the bioreactor setup protocol. This work is funded by National Institute of Health RO1HL083895 and HL063766 (both to LEN).
Footnotes
Diluted primary SMA and calponin antibodies can be recycled by collecting the solution in 15 ml conical tube and stored at 4°C for up to 1 week. Also because of different molecular weight of SMA and calponin, you can run both antibodies nicely on one blot simultaneously.
Preparation of growth factors: Dilute the various growth factors in 1% BSA in PBS to minimize adsorption of growth factors onto the wall of plastic eppendorf tubes.
Isolation of hMSC from fresh bone marrow step should be done promptly. Any delay in the processing of bone marrow will decrease the yield of hMSCs. Usually hMSC account for approximately 0.1–1 per 106 total bone marrow mononuclear cells obtained after the Ficoll-Paque gradient separation step.
On the launch day, before start, make sure the sterile stirbar is in the bioreactor. Make sure not to hover over the bioreactor to decrease risk of contamination. Cut up plenty of parafilm and soak (with paper removed) in an ethanol bath (large Petri dish works well).
The key to seeding the cells onto the PGA mesh is the volume of the cell suspension. When the PGA mesh is precoated with fibronectin, the total volume of cell suspension should be around 1–1.25 ml depending on the length of the PGA tube. Extra volume will cause dripping of the cell suspension off the PGA mesh to the bottom of the bioreactor.
Vitamin C is a very important cofactor for collagen synthesis. Because of its unstable nature, it is freshly made and added 3 times per week to the culture medium to promote collagen synthesis, which significantly affects vessel strength.
The whole 8-week vessel culture period is divided into 4-week of proliferation phase and 4-week of differentiation phase. During the proliferation phase, PDGF-BB, a potent mitogen for hMSCs (11), is supplemented in the culture medium to promote proliferation of hMSCs. Pulsatile flow and cyclic strain provided via the pump are not started until week 5 during the differentiation phase. In addition, PDGF-BB is replaced by TGFβ1 to promote SMC differentiation from week 5 onward.
Disclosure
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Kaushal S, Amiel GE, Guleserian KJ, Sharpira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadner A, Heorstrup SP, Zund G, Eid K, Maurus C, Melinitchouk S, Grunenfelder J, Turina MI. A new source for cardiovascular tissue engineering: Human bone marrow stromal cells. Eur J Cardiothorac Surg. 2002;21:1055–1060. doi: 10.1016/s1010-7940(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Hoerstrup SP, Kadner A, Melnitchouk S, Trojan A, Eid K, Tracy J, Sodian R, Visjager JF, Kolb SA, Grunenfelder J, Zund G, Turina MI. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106:I143–I150. [PubMed] [Google Scholar]
- 4.Perry TE, Kaushal S, Sutherland FW, Guleserian KJ, Bischoff J, Sacks M, Mayer JE. Bone marrow as a cell source for tissue engineering heart valves. Ann Thorac Surg. 2003;75:761–767. doi: 10.1016/s0003-4975(02)03776-1. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FW, Schoen FJ, Mayer JE, Bischoff J. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–H487. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 7.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, Park HY, Jang Y, Cahng BC, Choi CY, Hwang KC, Kim BS. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–515. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618–628. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Gong Z, Calkins G, Cheng E, Krause D, Niklason LE. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:319–330. doi: 10.1089/ten.tea.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Tsai S, Kato K, Yamanouchi D, Wang C, Rafii S, Liu B, Kent KC. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J Biol Chem. 2009;284:17564–17574. doi: 10.1074/jbc.M109.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, Saito M, Kawakami M. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32:1238–1245. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36:714–727. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Lozito TP, Kuo CK, Taboas JM, Tuan RS. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107:714–722. doi: 10.1002/jcb.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 16.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, Conroy N, Jones R, Vasanawala A, Sanzgiri S, Langer RL. Morphologic and mechanical characteristics of bovine engineered arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]




