Abstract
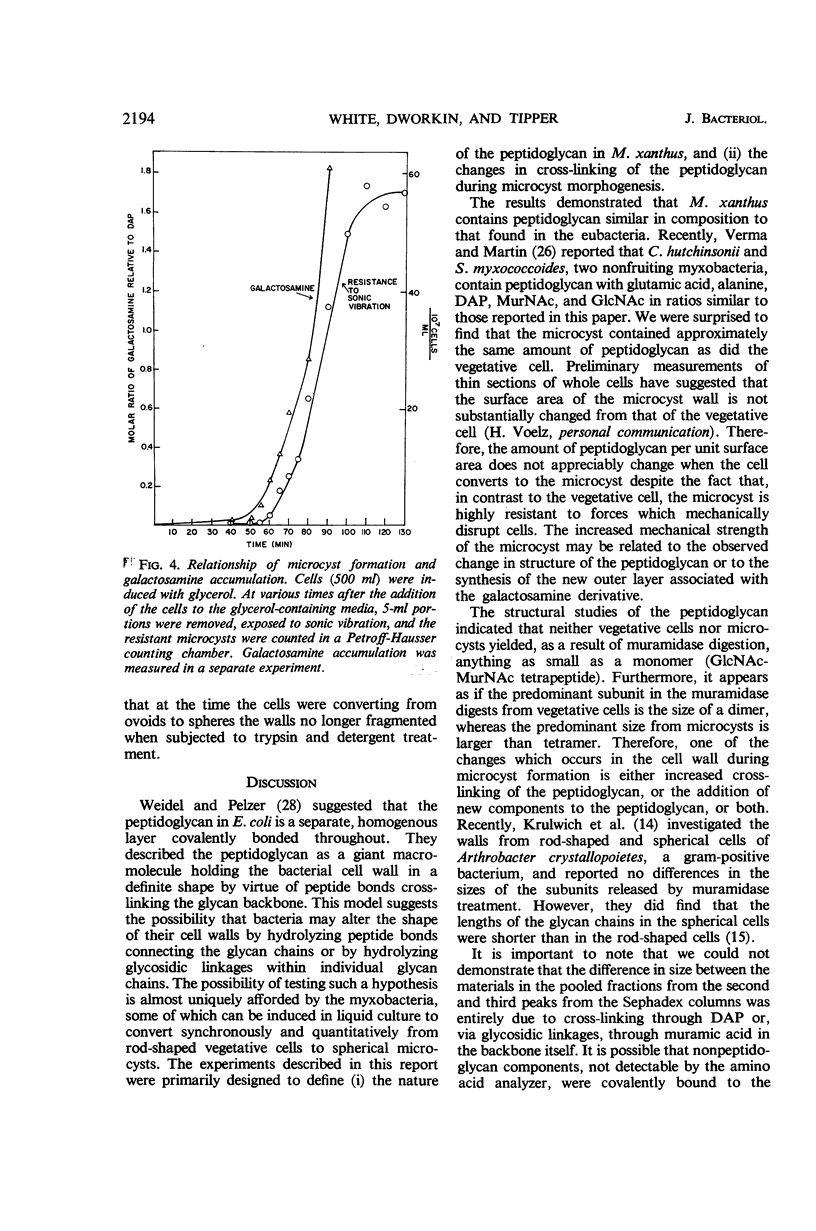
The chemical nature and distribution of the peptidoglycan in Myxococcus xanthus at various stages of the cellular life cycle were investigated. Vegetative cells and microcysts contained approximately 0.6% by weight of peptidoglycan. The overall composition of the peptidoglycan was similar in both cell types and was approximately 1 glutamic acid, 1 diaminopimelic acid, 1.7 alanine, 0.75 N-acetylglucosamine, and 0.75 N-acetylmuramic acid. (We have assumed that all the hexosamines are N-acetylated.) The sizes of the subunits (estimated by gel filtration) solubilized by muramidases were considerably larger (tetramer and oligomer) in the microcysts than in the vegetative cells (mostly dimer). There was a transient decrease in cross-linking (measured as an increase in the amount of free amino group of diaminopimelic acid) during the stage of microcyst formation when the cells converted from ovoids to spheres. At the same time, there occurred a large and rapid increase in a galactosamine derivative which may have reflected the synthesis of capsular material. Immediately prior to this period of morphogenesis, the cells became resistant to penicillin but remained sensitive to d-cycloserine. The walls of vegetative cells were completely disaggregated by trypsin and sodium lauryl sulfate, suggesting a discontinuous peptidoglycan layer. This was no longer apparent after the ovoid-sphere stage of microcyst formation. The relationship to morphogenesis of the chemical changes in the cell wall is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J. C., POWELSON D. M. Microcyst of Myxococcus xanthus. Chemical composition of the wall. J Bacteriol. 1961 May;81:780–785. doi: 10.1128/jb.81.5.780-785.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. A bacteriophage for Myxococcus xanthus: isolation, characterization and relation of infectivity to host morphogenesis. J Bacteriol. 1966 Mar;91(3):1305–1313. doi: 10.1128/jb.91.3.1305-1313.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUMPTON M. J. Identification of amino sugars. Biochem J. 1959 Jul;72:479–486. doi: 10.1042/bj0720479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. NUTRITIONAL REGU.ATION OF MORPHOGENESIS IN MYXOCOCCUS XANTHUS. J Bacteriol. 1963 Jul;86:67–72. doi: 10.1128/jb.86.1.67-72.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- GRULA M. M., GRULA E. A. ACTION OF CYCLOSERINE ON A SPECIES OF ERWINIA WITH REFERENCE TO CELL DIVISION. Can J Microbiol. 1965 Jun;11:453–461. doi: 10.1139/m65-060. [DOI] [PubMed] [Google Scholar]
- Grula E. A., Smith G. L., Grula M. M. Cell division in a species of Erwinia. 8. Amino acid composition of the mucopeptide in dividing and non-dividing cells. Can J Microbiol. 1965 Aug;11(4):605–610. doi: 10.1139/m65-080. [DOI] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. The cell wall of Myxococcus xanthus. Biochim Biophys Acta. 1958 Jul;29(1):1–7. doi: 10.1016/0006-3002(58)90138-0. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R., ROGERS H. J. The products of the partial acid hydrolysis of the mucopeptide from cell walls of Micrococcus lysodeikticus. Biochem J. 1959 Aug;72:647–654. doi: 10.1042/bj0720647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Pickering B. T. Components of the cell wall of Clostridium welchii (type A). Biochem J. 1966 Aug;100(2):430–440. doi: 10.1042/bj1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E. Glucosamine values of muramic acid and other amino-sugars by the Elson and Morgan method. Nature. 1960 Jul 2;187:38–40. doi: 10.1038/187038a0. [DOI] [PubMed] [Google Scholar]
- Sadler W., Dworkin M. Induction of cellular morphogenesis in Myxococcus xanthus. II. Macromolecular synthesis and mechanism of inducer action. J Bacteriol. 1966 Apr;91(4):1520–1525. doi: 10.1128/jb.91.4.1520-1525.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of the murein. Biochem Biophys Res Commun. 1967 Sep 27;28(6):965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma J. P., Martin H. H. Uber die Oberflächenstruktur von Myxobakterien. I. Chemie und Morphologie der Zellwände von Cytophaga hutchinsonii und Sporocytophaga myxococcoides. Arch Mikrobiol. 1967;59(4):355–380. [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weinbaum G. Characteristics of cell walls from morphological variants of Escherichia coli. J Gen Microbiol. 1966 Jan;42(1):83–92. doi: 10.1099/00221287-42-1-83. [DOI] [PubMed] [Google Scholar]
- Weinbaum G., Rich R., Fischman D. A. Enzyme-induced formation of spheres from cells and envelopes of Escherichia coli. J Bacteriol. 1967 May;93(5):1693–1698. doi: 10.1128/jb.93.5.1693-1698.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]



