Abstract
Nocardia is one of five main pathogens that infect chronic granulomatous disease (CGD) patients. Despite aggressive antimicrobial therapy, medical treatment is not always successful and surgical resection of infected tissue has been intermittently required. We present two CGD patients with severe Nocardia pneumonia whose pulmonary status worsened despite appropriate antimicrobials, but then improved clinically and radiographically with the addition of corticosteroids.
Keywords: Chronic Granulomatous Disease, Nocardia pneumonia
Introduction
Chronic granulomatous disease (CGD) is a rare genetic disease of the phagocyte NADPH oxidase that impairs production of superoxide and its metabolites, allowing bacterial and fungal infections and exuberant granuloma formation. The organisms that characteristically cause infection in CGD are distinctive and important to recognize: Staphylococcus aureus, Serratia marcescens, Burkholderia cepacia complex, Nocardia species and Aspergillus species1,2. CGD is also characterized by significant granulomatous complications of the gastrointestinal and genitourinary tracts, which frequently require treatment with immunosuppressant agents, typically corticosteroids, 1–2.
Nocardia infections have been irregularly reported among different CGD cohorts, with surprisingly few identified outside of North America3. Nocardia species are found worldwide in water, soil, dust, and decomposing organic matter. We report two boys with X-linked CGD and severe multi-lobar Nocardia pneumonia whose intense inflammatory responses despite antimicrobial therapy responded to the addition of corticosteroids.
Cases
Patient 1, a 17-year-old Caucasian boy with X-linked CGD (missense mutation in gp91phox), was hospitalized with 4 days of fever, mild cough, nausea, chills and myalgias. Previous infections included cervical lymphadenitis at 4 and 8 years, S. aureus liver abscess at 6 years, and Aspergillus fumigatus pneumonia at 9 years. Pulmonary infiltrates without diagnosis occurred at 10 and 12 years. For CGD related colitis, he received low dose prednisone from six to 10 years of age. His last episode of colitis was at 15 years. Adherence to prophylaxis with trimethoprim/sulfamethoxazole (TMP/SMX), itraconazole, and interferon gamma was poor.
On admission, he was febrile (39°C) and chest CT scan showed consolidations in the right middle and lower lungs with necrosis but without effusion (Figure 1A). The remainder of the physical examination was normal, except for weight (47.4 Kg, <3rd percentile for age), and height 163.2 cm (just above 3rd percentile). Laboratory studies showed an elevated white blood cell count (WBC) of 25,500 cells/ml, an elevated erythrocyte sedimentation rate of 61mm/h, and an elevated C-reactive protein (CRP) of 28.9 mg/L. Nocardia farcinica grew from needle aspirate of the lung as well as from sputum. Microbiologic diagnosis was confirmed by genomic sequencing.
Figure 1.
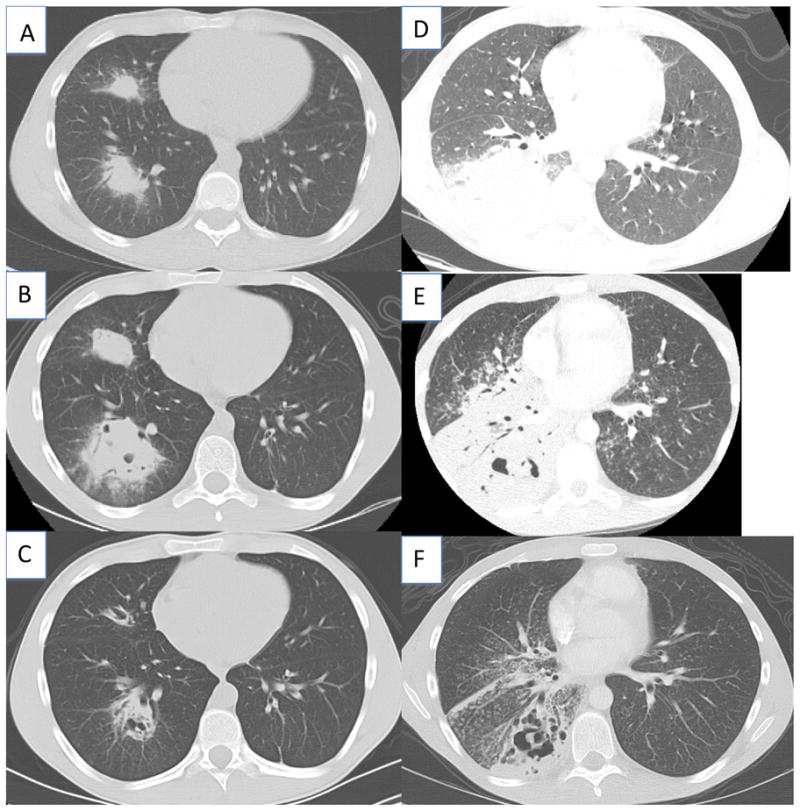
Chest CT findings at the initiation of antimicrobial therapy for Patient 1 (A), 2 weeks after starting antibiotics (B), and one week after initiation of corticosteroids (C). Chest CT for patient 2 at the initiation of antimicrobial therapy for Patient 2 (D), 3 weeks after starting antimicrobials (E), and 2 weeks after initiation of corticosteroids (F).
He was treated initially with intravenous TMP/SMX and levofloxacin. His symptoms improved, but after about 2 weeks on treatment fever recurred and worsened (peaks around 40°C) despite intensified Nocardia coverage with linezolid, meropenem, and amikacin. Voriconazole was added for possible occult co-infection with mold. Repeat CT chest showed marked worsening of the lesions with cavitation (Figure 1B). Brain MRI, echocardiogram, bone scan and abdominal CT scan were unremarkable. Because his inflammation was profound and incapacitating and the only infection identified was the Nocardia for which he was receiving broad coverage, methylprednisolone 0.8 mg/kg/day was added intravenously. He had a rapid clinical improvement, along with decreases in white blood cell count and inflammatory markers.
After one week of intravenous corticosteroids CT showed improvement (Figure 1C). Corticosteroids were transitioned to oral prednisone and tapered to 0.6mg/kg/day. Ten days later, fever recurred with worsened infiltrates. A repeat needle biopsy showed only chronic inflammation without organisms; all cultures were negative.
Intravenous methylprednisolone 0.8 mg/kg/day was restarted with rapid chest CT improvement. After a slower wean of intravenous corticosteroids over 4 weeks to 0.4mg/kg/day, followed by transition to oral prednisone (0.4mg/kg/day), he was discharged home. A slow taper of oral corticosteroids during the next 7 months, was accompanied by continued posaconazole, levofloxacin, and TMP/SMX. He had complete resolution and has had no relapse.
Patient 2 is a 21-year-old man with X-linked CGD receiving no prophylaxis who presented with fever and cough. Chest radiograph and CT scan showed multilobar pneumonia and enlarged mediastinal lymph nodes. Bronchoscopy cultures grew Nocardia cyriacigeorgica; intravenous imipenem and TMP/SMX were begun (Figure 1D). Hypoxemia required intubation and ventilation. Linezolid and tobramycin were added to the TMP/SMX and imipenem based on antibiotic susceptibilities; voriconazole was added for possible mold co-infection. After extubation fevers returned and chest imaging showed increased consolidation in the right lung base, cavitations in the left upper lobe, miliary nodules and infiltrates bilaterally (Figure 1E). Intravenous methylprednisone (0.7 mg/kg/d) was started; he defervesced in 24 hours and his chest radiograph improved.
On transfer to the NIH Clinical Center oral TMP/SMX, linezolid (with pyridoxine), meropenem and voriconazole were continued (Figure 1F). Corticosteroids were weaned during three weeks, but, at 0.25mg/kg of prednisone, inflammatory markers increased and the infiltrates worsened. A fine needle lung biopsy showed no organisms and cultures were negative. Corticosteroids were increased to 0.34 mg/kg, and symptoms and inflammatory markers resolved. He was discharged after 2 months of hospitalization markedly improved to receive oral TMP/SMX, linezolid (with pyridoxine to prevent linezolid-induced neuropathy), voriconazole. A slow taper of oral prednisone over several months was planned.
Discussion
The immune dysregulation of CGD results in infection susceptibility and exuberant granuloma. Corticosteroids have been frequently used to control presumably non-infectious granulomatous complications, such as inflammatory gastrointestinal and genitourinary lesions1–2. Corticosteroids have also been used in addition to antimicrobials to treat refractory infections in CGD. For instance, fulminant mulch pneumonitis” occurs after inhalation of Aspergillus in decaying organic matter, causing a diffuse, severe hypersensitivity-like reaction with fever and hypoxemia 4. Treatment with corticosteroids in addition to antifungals can be life saving. Other reports have included successful treatment of inoperable liver abscesses (one without a pathogen identified, others with S. aureus), combined Bukholderia cepacia and Aspergillus pneumonia, and a pneumonia with effusion with several bacterial isolates5–7.
Severe Nocardia pneumonia in CGD has often resulted in major pulmonary surgery3. In one report of 29 Nocardia infections in CGD, 7 (25%) required surgery in addition to antibiotics, either with lung resection, empyema drainage, or bone or skin debridement. Pulmonary resection was an unattractive option for our patients with multilobar disease, and is a difficult option in CGD patients who have already had recurrent lung infections with associated damage and dysfunction.
Corticosteroids have been a beneficial adjunct to specific therapy in other (non-CGD) infections characterized by organ damage due to intense inflammation. Tuberculous meningitis, Haemophilus influenzae meningitis and Pneumocystis jiroveci pneumonia are well-accepted examples8. In addition, corticosteroids have been advantageous in severe immune reconstitution syndromes, such as those seen after initiation of HAART (highly active antiretroviral therapy) in HIV. Cryptococcus and Mycobacteria species, which cause granulomatous inflammation, are frequent inducers of this syndrome, and corticosteroids may cause improvement when added to specific therapy9.
Because corticosteroids are capable of suppressing symptomatic inflammation, it is essential to be certain that all infections are adequately covered prior to steroid initiation. Corticosteroid treatment without optimal antimicrobial coverage can have disastrous consequences in CGD10,11. In our patients, repeat cultures were obtained before initiation of corticosteroids and antimicrobial coverage was maximized. In particular for Nocardia infections, species determination can guide antibiotic selection, as in vitro Nocardia susceptibility testing is often problematic. Co-infection with Nocardia and mold is common, so concomitant antifungal treatment is prudent. Disseminated infection may complicate severe Nocardia infection, and brain MRI, should be performed even if neurologic symptoms are absent. Neither of our patients had extra-pulmonary disease, so the use of adjunctive corticosteroids in that setting is unexplored.
Nocardia is one of the five typical infections in North American CGD and its treatment can be difficult despite appropriate antimicrobials. Neither of our patients was receiving appropriate prophylactic TMP/SMX at the time of infection, which might have prevented their infections. TMP/SMX prophylaxis has been shown to significantly decrease bacterial infections in CGD12; however, pulmonary Nocardia infections without dissemination have occurred despite prophylaxis3. These two cases of CGD Nocardia pneumonia had worsening symptoms on aggressive therapy and had dramatic improvement with the addition of corticosteroids, which required very slow tapers. Both patients avoided lung surgery and have returned to normal pulmonary function. Controlled prospective experience, including animal models, is necessary to determine the safe and proper roles for adjunctive corticosteroids in CGD.
Acknowledgments
This research was supported by the Division of Intramural Research of the NIAID, NIH, Bethesda, MD 20892. The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government.
References
- 1.Holland SM. Chronic granulomatous disease. Clinical Reviews in Allergy and Immunology. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 2.Winkelstein JA, Marino MC, Johnston RB, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dorman SE, Guide SV, Conville PS, et al. Nocardia infection in chronic granulomatous disease. Clinical Infectious Diseases. 2002;35:390–4. doi: 10.1086/341416. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui S, Anderson VL, Hilligoss DM, et al. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clinical Infectious Diseases. 2007;45:673–81. doi: 10.1086/520985. [DOI] [PubMed] [Google Scholar]
- 5.Narita M, Shibata M, Tagashi T, et al. Steroid therapy for bronchopneumonia in chronic granulomatous disease. Acta Paediatr Jpn. 1991;33:181–5. doi: 10.1111/j.1442-200x.1991.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 6.Okano M, Yamada M, Ohtsu M, et al. Successful treatment with methylprednisolone pulse therapy for a life threatening pulmonary insufficiency in a patient with chronic granulomatous disease following pulmonary invasive Aspergillosis and Burkholderia cepacia infection. Respiration. 1999;66:551–554. doi: 10.1159/000029435. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki-Nakashimada MA, Stiehm ER, Pietropaolo-Cienfuegos D, et al. Corticosteroid therapy for refractory infections in chronic granulomatous disease: case report and review of the literature. Annals of Allergy and Asthma Immunology. 2006;97:257–61. doi: 10.1016/S1081-1206(10)60023-3. [DOI] [PubMed] [Google Scholar]
- 8.McGee S, Hirschmann J. Use of corticosteroids in treating infectious diseases. Arch Intern Med. 2008;168:1034–46. doi: 10.1001/archinte.168.10.1034. [DOI] [PubMed] [Google Scholar]
- 9.Shelburne SA, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS rev. 2003;5:67–79. [PubMed] [Google Scholar]
- 10.Kelly JK, Pinto AR, Whitelaw WA, et al. Fatal Aspergillus pneumonia in chronic granulomatous disease. Am J Clin Pathol. 1986;86:235–40. doi: 10.1093/ajcp/86.2.235. [DOI] [PubMed] [Google Scholar]
- 11.Trawick D, Kotch A, Matthay R, et al. Eosinophilic pneumonia as a presentation of occult chronic granulomatous disease. Eur Respir J. 1997;10:2166–70. doi: 10.1183/09031936.97.10092166. [DOI] [PubMed] [Google Scholar]
- 12.Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprim-sulfamethoxasole prophylaxis in the management of chronic granulomatous disease. The Journal of Infectious Diseases. 1990;162:723–6. doi: 10.1093/infdis/162.3.723. [DOI] [PubMed] [Google Scholar]


