Abstract
It is widely believed that signature patterns of microtubule polarity orientation within axons and dendrites underlie compositional and morphological differences that distinguish these neuronal processes from one another. Axons of vertebrate neurons display uniformly plus-end-distal microtubules, whereas their dendrites display non-uniformly oriented microtubules. Fly axons also display uniformly plus-end-distal microtubules, but their dendritic microtubules are nearly uniformly minus-end-distal. Discussed in this article are the history of these findings, their implications for the regulation of neuronal polarity across the animal kingdom, and potential mechanisms by which neurons establish the distinct microtubule polarity patterns that define axons and dendrites.
Keywords: microtubule, neuron, axon, dendrite, microtubule polarity, neuronal polarity, +tips, cytoplasmic dynein, kinesin
Introduction
Microtubules are intrinsically polar cytoskeletal filaments (Alberts, 2008). They consist of a “plus end” favored for assembly/disassembly and a “minus end” which is less favored for these dynamics. The structural polarity of the microtubule results from the fact that the tubulin subunits that comprise the microtubule are heterodimers of alpha and beta tubulin. The polarity of the microtubule exists not only at the two ends of the filament, but all along the length of its lattice. This is critical for the movement along the microtubule of molecular motor proteins, which are enzymes that walk specifically toward either the plus end or the minus end of the microtubule. Minus ends of microtubules are often, but not always, attached to structures from which the microtubule is nucleated, while plus ends of microtubules interact with a wide variety of specialized proteins. In living cells, microtubules are organized relative to their intrinsic polarity into characteristic patterns of microtubule polarity orientation. These microtubule polarity patterns dictate the distribution of both ends of the microtubule, and hence the locations where in the cell microtubule assembly/disassembly occur, as well as where plus-end-associated proteins can interact with other cellular structures. In addition, the polarity patterns of microtubules direct motor-driven traffic within the cytoplasm, and hence establish asymmetric distributions of various organelles. For all of these reasons, the characteristic polarity patterns of microtubules in various types of cells are instrumental for establishing and maintaining the structural and compositional polarity of each cell type.
Of all cell types in nature, neurons are arguably the one whose polarity and specific morphology are most intimately related to the unique functions it must perform. A typical vertebrate neuron consists of a small rounded cell body that may be located in the brain, the spinal cord, or a peripheral ganglion, and two distinct types of processes that extend from the cell body. While there is some variability among the types of neurons that comprise the nervous system, a typical vertebrate neuron consists of one axon and several dendrites. The axon is specialized to transmit information over potentially very long distances, while the dendrites are specialized to receive and process incoming information. As such, they are morphologically very different. Axons are effectively unlimited in their growth potential, and can exceed even a meter in length in large animals. Dendrites, by comparison, are generally shorter and stouter than axons, and are usually highly branched to create enormous receptive fields upon which axons of other neurons can synapse. Compositionally, axons and dendrites are also very different; for example, dendrites are rich in ribosomes and Golgi outposts, while axons contain few or no ribosomes and no Golgi outposts. Collectively, these various differences between axons and dendrites are sometimes referred to as key features of “neuronal polarity,” and the study of neuronal polarity has become one of the most exciting and complex areas of neuroscience and cell biology.
One of the most pivotal findings in this arena came in 1981, when the laboratories of Steven Heidemann and Paul Burton independently reported that the microtubules within axons are uniformly oriented with their plus ends directed away from the cell body (Burton and Paige, 1981; Heidemann et al., 1981). This was extraordinarily interesting because it had already been reported by others that microtubules in the axon are “free” at both ends; they are not attached at their minus ends to any known structure (such as the centrosome) that could be responsible for organizing them (Lyser, 1964; Tennyson, 1965; Lyser, 1968). In fact, the microtubules in the axon were shown to be a variety of lengths, some exceeding 100 microns in long axons, but with many others as short as 1 micron or even shorter (Bray and Bunge, 1981; Yu and Baas, 1994). With growing knowledge of microtubule-based motor proteins in subsequent years, knowing the polarity pattern of axonal microtubules became an instrumental piece of information for understanding how motors direct organelle traffic. According to the polarity orientation of microtubules in the axon, the anterograde transport of organelles would require molecular motors that move toward plus ends of microtubules, while the retrograde transport of organelles would require molecular motors that move toward minus ends of microtubules.
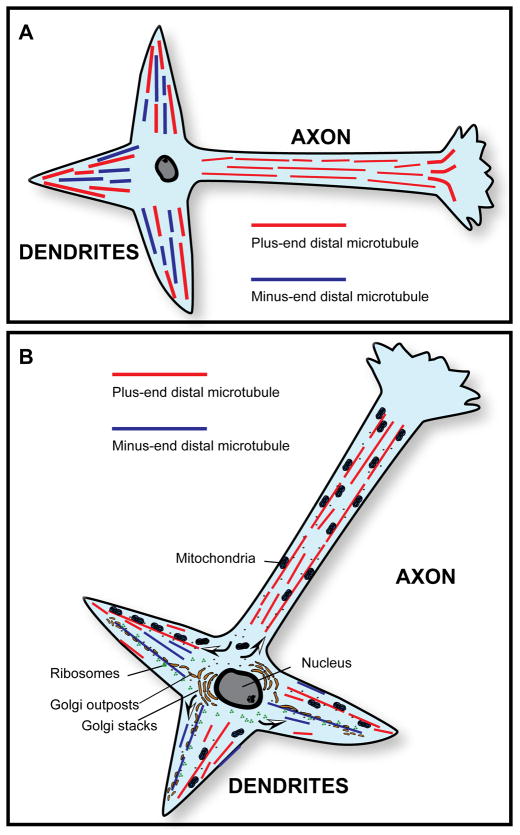
With the discovery made on axons, there was growing belief that uniformly plus-ends-out orientation was a “default” organization for microtubules, and that cells would find one way or another to organize their microtubules with this pattern. The classic exception, of course, was the mitotic spindle, in which the centrosome duplicated to form two foci for minus ends of microtubules that subsequently moved apart into daughter cells. The region between the two duplicated centrosomes consisted of overlapping microtubules of opposite orientation, and the spindle stood at the time as the only known example of a non-uniformly oriented microtubule array in nature (Telzer and Haimo, 1981; McIntosh and Euteneuer, 1984). Since then, other exceptions to the plus-ends-out rule have emerged such as in the case of Xenopus oocytes, which display minus-ends of microtubules outward, toward the cell cortex (Pfeiffer and Gard, 1999). With regard to neurons, in the late 1980’s, studies were reported using cultures of hippocampal neurons showing that dendrites of vertebrate neurons contain non-uniformly oriented microtubules (Baas et al., 1988). Microtubules were found to be roughly half plus-end-distal and roughly half minus-end-distal. The same result was reported in studies on frog mitral dendrites (Burton, 1988). These results (shown schematically in figure 1A) demonstrated that axons and dendrites have fundamentally different patterns of microtubule polarity orientation, prompting the idea that these distinct patterns might underlie many, most, or even all of the morphological and compositional differences that distinguish the two types of processes from one another (figure 1B).
Figure 1.
A, Microtubule polarity patterns in typical vertebrate axons and dendrites. Axons contain uniformly oriented microtubules (plus-end-distal), while dendrites contain non-uniformly oriented microtubules. B, Microtubule polarity patterns as the basis for asymmetric organelle distribution in vertebrate neurons. A simple yet attractive model for the asymmetric distribution of organelles in the vertebrate neuron posits that axons and dendrites contain those organelles that are transported from the cell body toward plus ends of microtubules (such as mitochondria) but only dendrites contain those organelles that are transported from the cell body toward minus ends of microtubules (such as Golgi outposts and ribosomes). Arrows in B show the direction of organelle movement.
It is certainly fair to say, however, that the diversity of dendrites (or even axons) that were analyzed for microtubule polarity orientation was quite small, leaving the door open to more complexity than these initial studies suggested. Also, these studies were all performed using one sampling technique that, like any other technique, has potential strengths and weaknesses. In addition, mechanistic studies in the 1990’s to determine how the patterns are established were fairly limited. Recently, however, there has been a resurgence of interest and studies using newer techniques and a wider variety of organisms, including flies. A wealth of information is emerging that sheds new light and suggests exciting new ideas for how the microtubule polarity patterns of axons and dendrites are established and regulated, and how they impact the composition, morphology, and identity of each type of process. The purposes of the present article are to present an historical perspective on microtubule polarity orientation in neurons, to discuss the exciting new findings that have been emerging recently, and to ponder the questions that remain in this important arena.
The hooking technique for assessing microtubule polarity orientation
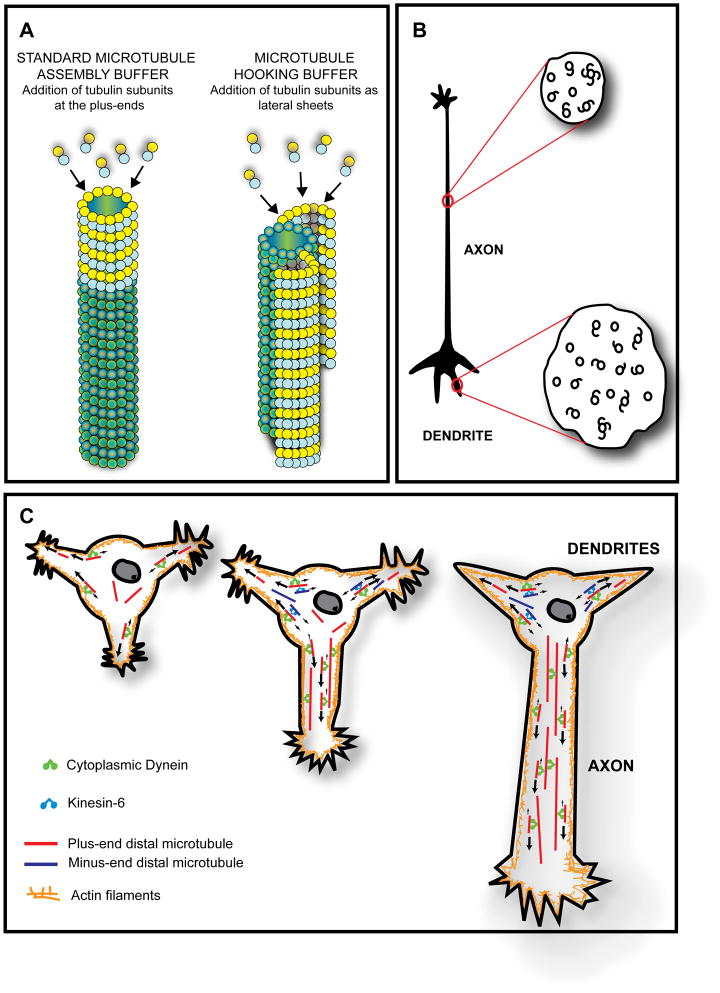
The original technique for assessing microtubule polarity orientation in cells, developed by Heidemann and McIntosh (Heidemann and McIntosh, 1980), is called hooking. These workers fortuitously discovered that if they allow microtubule assembly to occur in the presence of a rather unique microtubule assembly buffer, the tubulin subunits preferentially add along the sides of existing microtubules rather than at their ends. The newly added tubulin forms a protofilament sheet that curves around to close on the existing microtubule in a fashion similar to the nine microtubule doublets in a cilium or flagellum. If the reaction is stopped early enough, however, by introducing fixative, the curved sheet stopped short of closing, and appears, if viewed in cross-section, as a hooked appendage on the microtubule (see figure 2A). Using electron microscopy, looking down on the microtubule, a clockwise hook corresponds to the plus end of the microtubule, while a counterclockwise hook corresponds to the minus end of the microtubule (as shown schematically in figure 2B). This correspondence was shown using microtubule arrays with polarity orientations already known on the basis of assembly dynamics. The technique was believed to have a small amount of inaccuracy, because microtubule arrays of known orientation produced common curvature of hooking of anywhere from 90–100%. Around the same time as the hooking method was used, another method was also used to reveal microtubule polarity orientation by decorating the microtubules with exogenously added dynein (Telzer and Haimo, 1981), but to the best of our knowledge, this approach has never been used on neurons.
Figure 2.
A and B, The hooking procedure for ascertaining microtubule polarity orientation. In this technique, microtubule assembly is allowed to occur in the presence of a rather unique microtubule assembly buffer that promotes tubulin subunit addition preferentially along the sides of existing microtubules rather than at their ends. A, The newly added tubulin forms a protofilament sheet that curves around the existing microtubule. If the reaction is stopped early enough, the curved sheet stops short of closing, and appears, if viewed in cross-section, as a hooked appendage on the microtubule. B, A clockwise hook corresponds to the plus end of the microtubule facing the observer, while a counterclockwise hook corresponds to the minus end of the microtubule facing the observer. Thus, in cross-sections of axons, virtually all hooks are oriented in the same direction, while in the case of dendrites, there are clockwise hooks on some microtubules and counterclockwise hooks on other microtubules. In using this approach to assess microtubule polarity orientation in cells, it is necessary to include a detergent in the hooking mixture in order for the exogenous tubulin subunits to enter the cell. C, Model for establishing and maintaining microtubule polarity patterns in vertebrate axons and dendrites based on cytoplasmic dynein and kinesin-6. It has been proposed that cytoplasmic dynein drives microtubules from the cell body into developing axons and dendrites with plus-end-leading. Then, kinesin-6 transports microtubules with minus-ends-leading specifically into the developing dendrites but not into the developing axon. Axonal microtubule polarity orientation thus remains uniformly plus-end-distal, while dendritic microtubule polarity orientation becomes non-uniform (i.e., mixed). The first neuron shown in the figure is a hippocampal neuron with multiple immature processes. The middle neuron shows the next stage of development wherein one of the immature processes has begun to differentiate into the axon. The third neuron shows later yet in development when the axon has further elongated and the remaining immature processes have become dendrites. As shown schematically, cytoplasmic dynein can transport microtubules with their plus-ends-leading either against other (longer) microtubules or against the actin cytoskeleton, whereas kinesin-6 transports microtubules with their minus-ends-leading exclusively against plus-end-distal microtubules.
For use on tissue or cell cultures, the procedure for hooking was to introduce a detergent together with the unique microtubule assembly buffer, including exogenous tubulin that had been purified from bovine or porcine brain. After allowing a period of time for the assembly to occur, the tissue was fixed and prepared for electron microscopy. The samples needed to be sectioned in cross-section relative to the long axis of the microtubules, and it was necessary at each step (sectioning, picking up sections, introducing the sections into the electron microscope, photographing them, and printing the negatives on paper) to keep track of orientation and potential flipping that would make a counterclockwise hook appear clockwise, and vice versa. Microtubules were then scored as hooked clockwise, hooked counterclockwise, hooked ambiguous, or unhooked (figure 2B). Ambiguous hooks were those that fully closed, or hooks on hooks, or microtubules with two hooks with opposite curvature. Generally speaking, a hooking frequency of about 30–60% was considered good, given that higher percentages of hooking gave too much ambiguity. The technique was tedious, time-consuming and technically difficult, but it gave reliable data (Heidemann, 1991). Not surprisingly, given all of this, only a few laboratories employed this technique, and hence the number of studies assessing microtubule polarity orientation was fairly limited.
The first studies using microtubule hooking on axons were done on isolated cat sciatic nerve (Heidemann et al., 1981) and isolated frog olfactory nerve (Burton and Paige, 1981). Uniformly plus-end distal microtubule polarity orientation was then observed, using the hooking technique, on the axons of cultured chick dorsal root ganglion neurons (Baas et al., 1987a), the axons of cultured rat hippocampal neurons (Baas et al., 1988; Baas et al., 1989) and the axons of cultured rat sympathetic neurons (Baas and Ahmad, 1992). Entirely similar results were also obtained on squid and lobster axons (Viancour and Forman, 1987), as well as on the unipolar axon-like processes extended by certain kinds of sensory neurons (Topp et al., 1994). In all of these studies, the microtubules were interpreted as uniformly oriented because the percentage of the hooks was over 90% and generally even above 95%, although the formal possibility existed that there may be a small number of microtubules of the opposite orientation. The studies on cultured neurons included relatively short rapidly growing axons, indicating that the characteristic microtubule polarity pattern is established as the axon forms sooner rather than later in maturation.
Microtubule polarity orientation in vertebrate dendrites
Prior to the work on the dendrites of cultured hippocampal neurons and frog mitral dendrites mentioned above, there had been studies on the specialized dendrites of frog primary olfactory neurons (Burton, 1985) and in teleost retinal cone cells (Troutt and Burnside, 1988; Troutt and Burnside, 1988). These studies demonstrated uniformly oriented microtubules of opposite orientation to those observed in axons (i.e., uniformly minus-end-distal). The presence of centrosomes in the distal regions of these specialized dendrites, which is not at all typical of dendrites, called into question whether this finding was broadly applicable. Even so, it was curious that an elongated process with the properties of a dendrite could have microtubules oriented completely opposite to those in the axon. The subsequent studies on the more typical dendrites of cultured hippocampal neurons and frog mitral dendrites [and also the dendrites of cultured rat sympathetic neurons (Baas et al., 1991a)] suggested that the presence of high numbers of minus-end-distal microtubules is, indeed, a general characteristic of dendrites. However, it now seemed that a mixed (non-uniform) array containing microtubules of both orientations was more the norm.
Although hooking was the only technique used at this time to assess polarity orientation of microtubules in axons and dendrites, another approach provided supportive data. In this approach, the more newly assembled regions of microtubules were distinguished from the older regions of the microtubules using immunostaining for tyrosinated tubulin, or immunostaining for biotin-labeled tubulin that had been introduced into the neuron by microinjection. In the case of the axon, the newly assembled regions of microtubules were all directed away from the cell body (Baas and Black, 1990; Baas and Ahmad, 1992; Brown et al., 1993; Yu et al., 1996; Slaughter et al., 1997), while in dendrites, the newly assembled regions were directed both toward and away from the cell body (Wang et al., 1996). These studies were conducted on cultured rat sympathetic neurons.
After the initial results on rat hippocampal dendrites were reported, the authors of this work set forth to determine the developmental sequence of events by which the distinct polarity patterns of axonal and dendritic microtubules are established (Baas et al., 1989). Shortly after plating, cultured rat hippocampal neurons extend several essentially identical immature processes, after which one of these (and apparently it can be any of them) starts growing rapidly and becomes the axon. Then, several days later, the other immature processes that did not become the axon start to differentiate into dendrites (Dotti and Banker, 1987; Dotti et al., 1988). It was found, with the hooking procedure, that the immature processes have uniformly plus-end-distal microtubules, indicating that when dendrites develop, the plus-end-distal microtubules are present first, and then the minus-end-distal microtubules are subsequently added (figure 2C). In addition, it was found that, at least in the case of the still-growing dendrites of cultured neurons, the thinner more distal regions of the dendrites contained a predominance of plus-end-distal microtubules, with mixed orientations predominating in the thicker proximal and middle regions of the dendrite (Baas et al., 1989).
Are microtubule polarity patterns the basis of neuronal polarity?
In the early 1980’s, Gary Banker coined the term “neuronal polarity,” and jump-started a field that has since expanded greatly. There are now studies of “polarity genes” and pathways (Nishimura et al., 2004; Shi et al., 2004; Siegrist and Doe, 2005; Horiguchi et al., 2006), as well as studies on the domains of particular motor proteins (Saito et al., 1997; Marszalek et al., 1999; Guillaud et al., 2003; Nakata and Hirokawa, 2003; Hirokawa and Takemura, 2004; Jacobson et al., 2006) that might tell them where to go in the neuron. When the distinct polarity patterns of axonal and dendritic microtubules were first discovered, it was proposed that most or all aspects of neuronal polarity may be secondary to the establishment of the unique polarity patterns of axons and dendrites (Baas et al., 1988; Black and Baas, 1989). Now, over two decades later, this idea seems overly simplistic, but still has merit. The idea (shown schematically in figure 1B) was that organelles from the cell body that move along microtubules toward their plus ends would appear in both axons and dendrites, while organelles from the cell body that move toward minus ends of microtubules would only appear in dendrites. The best example of this is Golgi, which is completely absent from axons, but appears as outposts and sometimes even as stacks in larger dendrites. This makes good sense because Golgi is known to move along microtubules toward their minus ends via forces generated by cytoplasmic dynein (Corthesy-Theulaz et al., 1992; Fath et al., 1994). Another example, although more speculative, was ribosomes. Ribosomes are abundant in dendrites but far less so in axons, and one study on insect ovaries had demonstrated the movement of ribosomes specifically toward minus ends of microtubules (Stebbings and Hunt, 1983). Ribosomes are theoretically small enough to diffuse (Baas et al., 1987b), but would presumably not accumulate in axons because they would be transported back into the cell body if they engaged the plus-end-distal microtubules via a minus-end-directed motor protein. More work needs to be done on ribosomes, as it is possible that their final distribution also involves docking or immobilization once they reach their appropriate locales.
The polarity patterns of microtubules in axons and dendrites might also be responsible for their distinctive morphologies. This idea is supported by the fact that the thick tapering morphology of dendrites begins to appear at the same point in development as the minus-end-distal microtubules begin to appear (Baas et al., 1989). The idea is that the uniform orientation of axonal microtubules creates a unidirectional vector for the steady addition of new membrane components to the growing tip of the axon, and this enables the axon to remain thin and keep growing until it reaches a target. By contrast, these membrane components, by virtue of moving toward plus ends of microtubules, do not have a unidirectional vector in dendrites and hence dendrites are not designed to stay thin and keep growing longer but rather to stop growing without reaching a target and then grow in diameter rather than length. Interestingly, microtubules are transiently found to grow into dendritic spines with their plus-end distal (Hu et al., 2008; Jaworski et al., 2009). This is thought to be important for the shaping of the spine head and also ensuring delivery of synaptic cargoes important for neurotransmission.
There are features of neuronal polarity that cannot be easily explained with this model. For example, the model does not explain why there are some cytoplasmic constituents in axons but not dendrites, or how microtubule-associated proteins become compartmentalized differently in each type of process (Dehmelt and Halpain, 2005). It also does not explain why a typical neuron has a single axon but multiple dendrites. Clearly the business of establishing and maintaining neuronal polarity is not simple, and involves a cadre of complex mechanisms and pathways. Even so, two studies seem to support the idea that other features of neuronal polarity are secondary to the distinct polarity patterns of axons and dendrites. In one study, performed on cultured sympathetic neurons, when minus-end-distal microtubules were experimentally induced to move back into the cell body, the dendrites became axons, as assessed by morphological and compositional criteria (Yu et al., 2000). In the other study, cortical neurons were very gently dissociated such that a large dendrite was retained at the time of plating; this large dendrite gradually became an axon during which time its non-uniform microtubule polarity orientation converted to uniformly plus-end-distal (Takahashi et al., 2007). Thus, the possibility exists that the distinct microtubule polarity patterns of axons and dendrites are upstream to chains of events leading to features of neuronal polarity that may not seem obviously related to microtubule polarity orientation.
A few studies conducted in ensuing years challenged the idea that all of the information that directs organelle traffic in vertebrate neurons results from available motors moving toward their preferred end of the microtubule, with the polarity patterns of dendrites and axons being all determinative. These studies suggested the existence of “smart motors,” specifically kinesins that move cargo into dendrites but not axons, along plus-end-distal microtubules (Saito et al., 1997; Marszalek et al., 1999; Guillaud et al., 2003). Recently, however, a detailed experimental study provided strong support for the original idea, at least with regard to cytoplasmic dynein (Kapitein et al., 2010). In these studies, a novel tracking assay was established that permitted the authors to visualize the transport of structures associated with cytopasmic dynein or with conventional kinesin. The results demonstrated that cytoplasmic dynein transports cargoes selectively into dendrites in a manner consistent with their mixed microtubule polarity pattern. In addition, cargoes normally transported specifically into axons were re-directed into dendrites when they were experimentally bound to cytoplasmic dynein. Finally, in this same study, a modeling approach demonstrated that bi-directional transport on non-uniformly oriented microtubules is sufficient to explain a normal dendritic population of continuously renewing vesicular organelles.
Establishing the microtubule polarity patterns of vertebrate neurons
Perhaps the most perplexing question is how the distinct polarity patterns of axonal and dendritic microtubules are established. One idea put forth in the 1990s was that these microtubule polarity patterns occurred secondarily to the differential enrichment of certain fibrous microtubule-associated proteins (MAPs) in each type of process. This idea had some appeal because it was known that neurons compartmentalize isoforms of tau and MAP2 (Dehmelt and Halpain 2005), but it was unclear why they do this. However, the idea was also somewhat shaky because there was no evidence that tau or MAP2 bundled microtubules with any polarity preference, and the timetable of MAP2 compartmentalization in neurons did not seem to occur quite soon enough to be the cause of the appearance of minus-end-distal microtubules. Moreover, in experimental studies, when either tau or MAP2 was ectopically expressed in Sf9 cells, the processes extended by these cells displayed microtubules of uniformly plus-end-distal orientation, regardless of which MAP was expressed (Baas et al., 1991b; Chen et al., 1992). Thus, the MAP-based idea was dismissed. Another idea was that the polarity orientation of the microtubules was determined by molecular motor proteins that transported the microtubules into each type of process from the cell body (Baas and Yu, 1996). This idea arose at a time when the issue of microtubule transport in neurons was highly controversial (Baas and Brown, 1997; Hirokawa et al., 1997) and yet, the logic appeared right, because a growing body of evidence about motor proteins demonstrated their ability to sort/organize microtubules relative to their polarity (Heald et al., 1996; Nedelec et al., 1997; Walczak et al., 1998).
To test whether microtubule polarity orientation is established by the transport of microtubules, rather than anything relating to their assembly properties, researchers in the 1990s took advantage of the anti-microtubule drug called vinblastine. If neurons were cultured in the presence of low concentrations of this drug, the assembly of the microtubules was damped so that changes in their organization could only be attributed to the movement of the already assembled microtubules. If low levels of vinblastine were added to the culture before axonal outgrowth, axons still grew, albeit slower, and the cell body was gradually depleted of microtubules, suggesting a transfer of the microtubules from the cell body into the axon (Baas and Ahmad, 1993). The microtubules that accumulated in the axon were of uniformly plus-end-distal orientation. If the drug was added after axonal differentiation but prior to dendritic differentiation, dendrites still formed. They were shorter than normal, but the non-uniform microtubule polarity pattern still arose (Sharp et al., 1995). These results suggested that it is the manner by which the microtubules are transported into these processes from the cell body that establishes their distinct patterns of orientation in axons and dendrites.
The question then became: what are the relevant molecular motor proteins and how are they regulated to elicit these polarity patterns? In terms of the axon, an attractive idea was that cytoplasmic dynein is the workhorse motor for transporting microtubules with their plus ends leading (Vallee and Bloom, 1991; Dillman et al., 1996; Ahmad et al., 1998). To function in this manner, cytoplasmic dynein must be anchored against a structure with more resistance to movement than the microtubule undergoing motion. This could be another microtubule, or a non-microtubule structure such as the actin cytoskeleton (see later). If this scenario is correct, presumably cytoplasmic dynein would also be responsible for transporting plus-end-distal microtubules into dendrites, or at least nascent dendrites. Then, a different motor, presumably a kinesin, would transport microtubules with minus-ends-leading into dendrites but not axons (see figure 2C). In a spate of papers, evidence was shown that the relevant kinesin is kinesin-6 (also called CHO1/MKLP1 or kif23), a motor known to be important for mitosis and documented to transport the minus-ends of microtubules toward the plus ends of other microtubules (Nislow et al., 1992). First, it was reported that ectopic expression of kinesin-6 in Sf9 cells caused them to generate dendrite-like processes with non-uniformly oriented microtubules (Sharp et al., 1996), a feat that ectopic expression of MAP2 could not accomplish (Chen et al., 1992). Then it was shown that kinesin-6 is expressed in developing neurons, in a developmental and spatial pattern consistent with its proposed role in dendritic development (Sharp et al., 1997a). When kinesin-6 was depleted by antisense oligonucleotides, dendrites failed to develop (Sharp et al., 1997a), and when kinesin-6 was depleted after dendrites had developed, the minus-end-distal microtubules in dendrites were chased back into the cell body (Yu et al., 2000). The latter result presumably owed to cytoplasmic dynein, transporting the minus-end-distal microtubules with their plus-ends-leading in the reverse direction from that of their entry into the dendrite.
These studies on kinesin-6 were conducted with antisense oligonucleotides before the advent of more reliable RNAi approaches such as siRNA or shRNA. This is a cause for some concern because a plethora of earlier reports on phenotypes resulting from antisense oligonucleotides to various fibrous MAPs were called into question by recent studies using siRNA to knock down these proteins (Qiang et al., 2006). This is not to say antisense oligonucleotides can never give reliable results, and in the case of the kinesin-6 studies on dendrites, there are additional reasons to believe the results are valid. As noted above, the results fit the expectations based on the known properties of kinesin-6. Entirely similar results were obtained on podocytes, which were discovered to have non-uniform microtubule polarity orientation that also became uniform when treated with kinesin-6 antisense oligonucleotides (Kobayashi et al., 1998). In addition, and perhaps most convincingly, kinesin-6 has now been functionally dissected in molecular studies (completely independent of any knockdown approach), with solid evidence that it is indeed targeted to dendrites but not axons (Xu et al., 2006). Even so, the kinesin-6 story on vertebrate dendrites is far from complete, and awaits future chapters using more contemporary approaches.
Live-cell imaging of microtubule polarity orientation
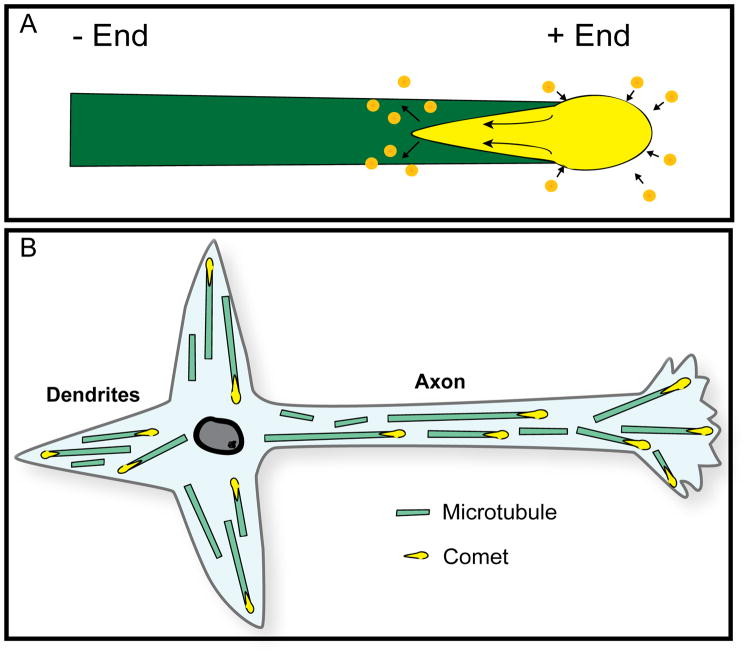
In the mid-1990s, a new technique emerged for visualizing microtubule dynamics in living cells. This technique was based on the discovery of a new category of microtubule-interacting proteins called +tips (Schroer, 2001; Carvalho et al., 2003; Mimori-Kiyosue and Tsukita, 2003). The +tips associate with the plus ends of microtubules as they assemble. The association is transient, however, and exists only in the vicinity of the plus end. Therefore, as the microtubule continues to assemble, the +tip molecules lose association with the older region of the microtubule while continuing to associate with the plus end. When GFP fusions of +tips are expressed in cells, they appear as comet-shaped fluorescence at the plus ends of rapidly assembling microtubules (see figure 3A for schematic illustration). The greatest intensity of fluorescence is toward the plus end of the microtubule, while the comet tail (representing the gradual loss of +tip association with the microtubule) is directed toward the minus end of the microtubule (Akhmanova and Steinmetz, 2008). In live-cell imaging, the shape of the comet is sometimes ambiguous, but usually not needed to reveal microtubule polarity orientation, because the direction of the assembly is sufficient to reveal which end is the plus end. The theoretical exception to this would be a microtubule moving with minus-end-leading while assembling from the plus end, but such a scenario would be rare and would require that the movement be notably faster than the assembly in order to give a deceptive appearance regarding microtubule polarity orientation. A more significant limitation of this technique is that it only reveals the polarity orientation of microtubules that are in the process of undergoing rapid bouts of assembly (i.e., there would be no comets at the tips of microtubules undergoing disassembly or remaining the same length during the time of observation). Hence, it is conceivable that the more stable microtubules not undergoing bouts of assembly have different orientations than those that are undergoing bouts of assembly. However, to date, this does not appear to have presented a problem, with the +tip approach providing generally similar results as the hooking procedure in the cell types that have been examined with both techniques.
Figure 3. The +tip approach for ascertaining microtubule polarity orientation.
This technique takes advantages of the properties of +tips, such as EB1 or EB3, which associate with the plus ends of microtubules as they assemble. The association is transient and exists only in the vicinity of the plus end. Therefore, as the microtubule continues to assemble, the +tip molecules lose association with the older region of the microtubule while continuing to associate with the plus end. A, When GFP fusions of +tips are expressed in cells, they appear as comet-shaped fluorescence at the plus ends of the microtubules. The greatest intensity of fluorescence is toward the plus end of the microtubule, while the comet tail (representing the gradual loss of +tip association with the microtubule) is directed toward the minus end of the microtubule (see arrows). B, Uniform and non-uniform microtubule polarity patterns in vertebrate neurons as revealed by the +tip “comet” approach.
The most prominent early paper to be published using the +tip approach on neurons was from the laboratory of Niels Galjart (Stepanova et al., 2003). In this work, the authors used a GFP fusion for EB3, a neuron-specific member of the EB (“end binding”) group of +tips. Other workers have used GFP fusions of EB1 (Ma et al., 2004), the EB family member more widely expressed across cell types, as well as other fluorescent tags such as mcherry (Qiang et al., 2010). In the Stepanova paper, results were reported on cultured rodent hippocampal neurons that are generally similar to those obtained with the hooking procedure. Axons and immature processes displayed predominantly plus-end-distal microtubules, as did distal regions of dendrites, while the main bodies of the dendrites displayed mixed orientations of microtubules (figure 3B). The notable difference observed in this and subsequent studies of this kind (Hasaka et al., 2004) was that minus-end-distal microtubules were not completely absent from minor processes or axons, suggesting that the small numbers of oppositely curved hooks in the early studies may have accurately depicted the orientation of those microtubules, rather than being “noise” in the experimental protocol. As a matter of general principle, it appears from these studies that minus-end-distal microtubules are less frequently observed when the axon is longer and more differentiated, and more often observed when the axon is early in development, in a more plastic phase of differentiation, or in more plastic regions of the axon such as growth cones or sites of nascent branch formation. For example, there was a notable uptick in the number of minus-end-distal microtubules in axons treated with bFGF, a potent promoter of interstitial branch formation, and in the regions of the axon that gave rise to new branches (Qiang et al., 2010). In another study on dendrites of cultured neurons, using the +tip approach, the proportion of minus-end-distal microtubules was somewhat lower than observed with the hooking procedure (Kollins et al., 2009). Collectively, these results confirm the general patterns indicated by the earlier hooking studies, but also indicate more plasticity and variability in the patterns than originally believed.
Testing the cytoplasmic dynein hypothesis for axonal microtubules
The emergence of the +tip approach for visualizing microtubule polarity orientation together with effective new RNAi approaches have provided some new information relevant to the hypothesis that microtubule transport by cytoplasmic dynein establishes the uniformly plus-end-distal polarity pattern of axonal microtubules. An idea originally proposed by the laboratory of Kevin Pfister and then pursued by others is that the forces for dynein-driven microtubule transport in the axon are generated against the actin cytoskeleton (Ahmad et al., 1998; Myers et al., 2006). Notably, however, when axons were allowed to grow in the presence of drugs that prevent the assembly of actin filaments, the microtubules were still uniformly plus-end-distal (Hasaka et al., 2004). This and other experiments led to the proposal that the dynein-driven transport of plus-end-distal microtubules could occur via forces generated either against the actin cytoskeleton or against other microtubules (Hasaka et al., 2004; Myers et al., 2006). However, it was then reported that microtubules are also uniformly oriented in axons allowed to grow from neurons in which 85% of the cytoplasmic dynein (heavy chain) had been depleted by siRNA (He et al., 2005; Ahmad et al., 2006). One possibility from these observations is that there is at least one redundant motor that can compensate for the absence or diminution of cytoplasmic dynein. An attractive candidate in this regard would be a member of the kinesin-14 family, an unusual group of kinesins that moves toward the minus end of the microtubule like cytoplasmic dynein. In fact, overexpression of kinesin-14 in Sf9 cells causes them to grow long thin axon-like processes with uniformly oriented microtubules (Sharp et al., 1997b). Another possibility is that, at least in the very young neurons thus far studied, there is such an overwhelming amount of cytoplasmic dynein, that even a small fraction of the total is sufficient to orient microtubules properly. In fact, potentially supporting this idea, it was reported that a greater depletion of cytoplasmic dynein resulted in much shorter axons with microtubules organized in a highly abnormal splayed criss-crossing pattern (Ahmad et al., 2006).
There are other compelling reasons to favor the dynein-based hypothesis for axonal microtubule polarity orientation. For example, experimental data have clearly demonstrated the capacity of cytoplasmic dynein to transport microtubules with plus-ends-leading from the cell body outward and into developing processes (Ahmad et al., 1998; Dehmelt et al., 2006), and in a recent study on Drosophila, it was shown that knockdowns of cytoplasmic dynein result in the appearance of minus-end-distal microtubules in axons (Zheng et al., 2008). The latter study suggests that cytoplasmic dynein is also important for preserving the uniform microtubule pattern of axonal microtubules by preventing minus-end-distal microtubules from accumulating in axons. Presumably cytoplasmic dynein would normally safeguard the uniform polarity orientation of axonal microtubules by transporting minus-end-distal microtubules back to the cell body, with plus ends leading. Presumably, in the case of vertebrate dendrites, kinesin-6 is important for preventing cytoplasmic dynein from doing this (Yu et al., 2000).
Microtubule polarity orientation in fly neurons
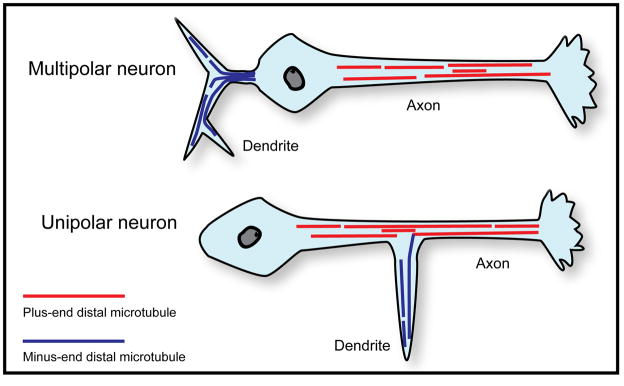
A dramatic and unexpected finding has now been reported on microtubule polarity orientation in the neurons of Drosophila. These findings not only indicate a significant difference among dendrites of the animal kingdom, but also shed new light on what it means to be a dendrite. These studies, by Melissa Rolls and colleagues, used GFP-tagged EB1 to visualize microtubules in neurons in vivo in the fly. They found that axons and dendrites both have microtubule arrays of uniform or nearly uniform polarity orientation. However, while axonal microtubules were uniformly plus end distal, dendritic microtubules were of the opposite orientation, nearly all minus-end-distal (Rolls et al., 2007; Stone et al., 2008). Unlike the specialized dendrites discussed earlier, these dendrites were of the more generic type in the fly, which do not have centrosomes or any identified nucleating structures in their distal regions. Interestingly, fly dendrites are generally shorter and have thinner diameters than vertebrate ones and can extend either from the cell body or from the axon, giving rise to multipolar and unipolar morphologies respectively (figure 4). These results, even more so than the results on vertebrate neurons, show a stunning difference in microtubule organization in each type of process that is undoubtedly at the heart of what makes axons and dendrites so different from one another. The potential concern, that perhaps the fly dendrites contain robust populations minus-ends-distal microtubules that are too stable to show assembly excursions with the EB1 approach, was considered by the Rolls laboratory, but dismissed on the basis of additional experiments designed to seek out such hypothetical microtubules. Moreover, in axotomy studies wherein the axon was removed near the cell body, it was determined that one of the dendrites undergoes a reversal in microtubule polarity orientation over about a day’s time, in order to become the neuron’s new axon (Stone et al., 2010). Thus, there is little doubt that “uniform but opposite” microtubule polarity patterns are the characteristic patterns in these invertebrate insect axons and dendrites (see figure 4).
Figure 4. Microtubule polarity patterns in fly axons and dendrites.
Drosophila neurons can be multipolar, with an axon and dendrites extending from the cell body, or unipolar, with dendrites extending out of the axon. Axons of Drosophila neurons contain uniformly plus-end-distal microtubules, while the dendrites of these neurons contain nearly uniform (90–95%) minus-end-distal microtubules.
Prior to these results, it was generally concluded that non-uniform orientation of microtubules was the quintessential or signature microtubule polarity pattern that “makes a dendrite a dendrite” (Alberts, 2008). On the basis of the fly results, it seems more reasonable to conclude that it is the presence of abundant minus-end-distal microtubules that makes a dendrite a dendrite, and that the presence of high numbers of plus-end-distal microtubules in dendrites is the flexible “option” taken by some branches of the animal kingdom. It may be that, in order for a more complex nervous system to function, there are some elements of intracellular traffic for which it makes more sense to have plus-end-distal microtubule tracks, than to work out a way by which motors can transport appropriate and sufficient cargo toward minus ends. In Drosophila, there is a growing abundance of data implicating cytoplasmic dynein as the major motor for transporting cargo into dendrites [see for example (Liu et al., 2000)], which makes sense on the basis of their pattern of microtubule polarity orientation. While still in progress, the emerging results on Drosophila are a fascinating new twist on the microtubule polarity story that should provide great insight in coming years on the mechanisms of neuronal polarity as well as intracellular traffic in neurons.
With regard to the mechanisms that establish microtubule polarity patterns in neurons, the findings on Drosophila also introduce a dilemma. In the case of vertebrate dendrites, the kinesin-6 model makes sense because kinesin-6 would transport minus-end-distal microtubules against the plus-end-distal microtubules in the nascent dendrites and then presumably lock them into place via the continued generation of forces between oppositely oriented microtubules. In the case of fly dendrites, there are some plus-end-distal microtubules, but not many; generally lower than 10%. Thus, the same mechanism may not pertain to fly dendrites, or perhaps the entire model for all dendrites needs to be re-evaluated in light of these new findings. Another possibility is that fly dendrites actually do undergo a developmental stage in which they contain mixed orientation of microtubules, but the plus-end-distal microtubules are then cleared as the dendrites continue to mature. In fact, new unpublished data from the Rolls laboratory supports this latter possibility (M.M. Rolls, personal communication). In addition, in the axotomy studies mentioned above, there is a transient period in which the dendrite being converted to an axon displayed mixed orientation of microtubules (Stone et al., 2010), possibly recapitulating an earlier developmental stage. There is also a notable change in microtubule activity in the cell body after axotomy that might suggest a reversion to a more plastic stage of development involving a variety of factors, potentially including motor proteins. At present, the Rolls laboratory is investigating an exciting array of microtubule-related proteins as potential players in establishing and maintaining microtubule polarity patterns in fly axons and dendrites, including kinesins and +tips. In parallel, other laboratories focused on vertebrate cell migration and axonal guidance studies are pursuing a similar list of molecules including LIS1 (Kholmanskikh et al., 2006), CLASP (Lee et al., 2004), APC (Zhou et al., 2004) and drebrin (Geraldo et al., 2008), many of which act as signaling cues between microtubules and actin filaments during neuronal polarization.
Microtubule polarity determination by SHG microscopy
Second harmonic generation (SHG) microscopy is a method that has been used recently to noninvasively assess microtubule polarity patterns in axons and dendrites of brain tissue. The method does not reveal the actual orientation of the microtubule, but it is able to distinguish uniformly oriented microtubule arrays from non-uniformly oriented arrays. It is still not fully known just how closely the SHG signal corresponds to the proportion of commonly oriented microtubules. However, in studies on developing vertebrate neurons, the correspondence of the signal and known microtubule polarity patterns from the hooking approach seems quite good, with the SHG signal detected in axons and immature processes but not dendrites (Dombeck et al., 2003). Given these results in the first SHG report, it is fascinating that in the second report, the polarity orientation of microtubules was estimated to be greater than 80% common polarity in the dendrites of mature hippocampal CA1 and cortical layer V pyramidal neurons (Kwan et al., 2008). These observations suggest that as these particular dendrites mature, there is a shift toward greater uniformity of microtubule polarity orientation, although it remains unknown whether the shift is toward a preponderance of plus-end-distal or minus-end-distal microtubules. The SHG approach holds great promise for generating abundant data on a variety of different types of neurons in their normal environment within the intact nervous system. Another approach that holds some promise in this regard is cryo-electron microscopy, which can reveal microtubule orientation on the basis of the skew of the native protofilament sheets the comprise the microtubules (Chretien et al., 1996). These interesting and potentially powerful methods have not been used extensively, but perhaps will be given new attention now that it is becoming clearer that a simple microtubule polarity scenario of uniformity in axons and non-uniformity in dendrites is incomplete.
Concluding Remarks
Three decades have passed since axons were initially reported to have uniformly plus-end-distal microtubules. Since then, the work of several laboratories has fortified interest in the microtubule polarity patterns of the neuron, and their fundamental importance to neuronal polarity. Perhaps the most crucial discovery was the finding that dendrites are very different from axons with regard to their pattern of microtubule polarity orientation. The development of powerful new methods for evaluating microtubule polarity orientation has opened the door for rapid progress to be made in expanding the base of knowledge on how these patterns manifest across different types of neurons and across the animal kingdom. Greater sophistication in cellular, molecular, and contemporary imaging techniques in the 21st century bodes well for elucidating the mechanisms by which these microtubule polarity patterns are established, regulated, and maintained.
Acknowledgments
The research program of PWB has been supported in recent years by the National Institutes of Health, the National Science Foundation, the Hereditary Spastic Paraplegia Foundation, the Alzheimer’s Association, and the Christopher and Dana Reeve Foundation. SH is funded by a postdoctoral fellowship from the Craig H. Neilsen Foundation. We thank Melissa Rolls for helpful discussions and comments on the manuscript.
References
- Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FJ, He Y, Myers KA, Hasaka TP, Francis F, Black MM, Baas PW. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. doi: 10.1111/j.1600-0854.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Alberts B. Molecular biology of the cell. Vol. 16. New York: Garland Science; 2008. pp. 965–1052. [Google Scholar]
- Baas PW, Ahmad FJ. The plus ends of stable microtubules are the exclusive nucleating structures for microtubules in the axon. J Cell Biol. 1992;116:1231–1241. doi: 10.1083/jcb.116.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The transport properties of axonal microtubules establish their polarity orientation. J Cell Biol. 1993;120:1427–1437. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Brown A. Slow axonal transport: the polymer transport model. Trends Cell Biol. 1997;7:380–384. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Pienkowski TP, Kosik KS. Processes induced by tau expression in Sf9 cells have an axon-like microtubule organization. J Cell Biol. 1991b;115:1333–1344. doi: 10.1083/jcb.115.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Sinclair GI, Heidemann SR. Role of microtubules in the cytoplasmic compartmentation of neurons. Brain Res. 1987b;420:73–81. doi: 10.1016/0006-8993(87)90241-1. [DOI] [PubMed] [Google Scholar]
- Baas PW, Slaughter T, Brown A, Black MM. Microtubule dynamics in axons and dendrites. J Neurosci Res. 1991a;30:134–153. doi: 10.1002/jnr.490300115. [DOI] [PubMed] [Google Scholar]
- Baas PW, White LA, Heidemann SR. Microtubule polarity reversal accompanies regrowth of amputated neurites. Proc Natl Acad Sci U S A. 1987a;84:5272–5276. doi: 10.1073/pnas.84.15.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Yu W. A composite model for establishing the microtubule arrays of the neuron. Mol Neurobiol. 1996;12:145–161. doi: 10.1007/BF02740651. [DOI] [PubMed] [Google Scholar]
- Black MM, Baas PW. The basis of polarity in neurons. Trends Neurosci. 1989;12:211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Bray D, Bunge MB. Serial analysis of microtubules in cultured rat sensory axons. J Neurocytol. 1981;10:589–605. doi: 10.1007/BF01262592. [DOI] [PubMed] [Google Scholar]
- Brown A, Li Y, Slaughter T, Black MM. Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J Cell Sci. 1993;104 (Pt 2):339–352. doi: 10.1242/jcs.104.2.339. [DOI] [PubMed] [Google Scholar]
- Burton PR. Ultrastructure of the olfactory neuron of the bullfrog: the dendrite and its microtubules. J Comp Neurol. 1985;242:147–160. doi: 10.1002/cne.902420202. [DOI] [PubMed] [Google Scholar]
- Burton PR. Dendrites of mitral cell neurons contain microtubules of opposite polarity. Brain Res. 1988;473:107–115. doi: 10.1016/0006-8993(88)90321-6. [DOI] [PubMed] [Google Scholar]
- Burton PR, Paige JL. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci U S A. 1981;78:3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P, Tirnauer JS, Pellman D. Surfing on microtubule ends. Trends Cell Biol. 2003;13:229–237. doi: 10.1016/s0962-8924(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Chretien D, Kenney JM, Fuller SD, Wade RH. Determination of microtubule polarity by cryo-electron microscopy. Structure. 1996;4:1031–1040. doi: 10.1016/s0969-2126(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Corthesy-Theulaz I, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Nalbant P, Steffen W, Halpain S. A microtubule-based, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain Cell Biol. 2006;35:39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- Dillman JF, 3rd, Dabney LP, Pfister KK. Cytoplasmic dynein is associated with slow axonal transport. Proc Natl Acad Sci U S A. 1996;93:141–144. doi: 10.1073/pnas.93.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Kasischke KA, Vishwasrao HD, Ingelsson M, Hyman BT, Webb WW. Uniform polarity microtubule assemblies imaged in native brain tissue by second-harmonic generation microscopy. Proc Natl Acad Sci U S A. 2003;100:7081–7086. doi: 10.1073/pnas.0731953100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess DR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23:131–140. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasaka TP, Myers KA, Baas PW. Role of actin filaments in the axonal transport of microtubules. J Neurosci. 2004;24:11291–11301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heidemann SR. Microtubule polarity determination based on formation of protofilament hooks. Methods Enzymol. 1991;196:469–477. doi: 10.1016/0076-6879(91)96040-x. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Landers JM, Hamborg MA. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann SR, McIntosh JR. Visualization of the structural polarity of microtubules. Nature. 1980;286:517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Funakoshi ST, Takeda S. Slow axonal transport: the subunit transport model. Trends Cell Biol. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res. 2004;301:50–59. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Hanada T, Fukui Y, Chishti AH. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol. 2006;174:425–436. doi: 10.1083/jcb.200604031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Reiser J, Kriz W, Kuriyama R, Mundel P. Nonuniform microtubular polarity established by CHO1/MKLP1 motor protein is necessary for process formation of podocytes. J Cell Biol. 1998;143:1961–1970. doi: 10.1083/jcb.143.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins KM, Bell RL, Butts M, Withers GS. Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural Dev. 2009;4:26. doi: 10.1186/1749-8104-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan AC, Dombeck DA, Webb WW. Polarized microtubule arrays in apical dendrites and axons. Proc Natl Acad Sci U S A. 2008;105:11370–11375. doi: 10.1073/pnas.0805199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Engel U, Rusch J, Scherrer S, Sheard K, Van Vactor D. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 2004;42:913–926. doi: 10.1016/j.neuron.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Liu Z, Steward R, Luo L. Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat Cell Biol. 2000;2:776–783. doi: 10.1038/35041011. [DOI] [PubMed] [Google Scholar]
- Lyser KM. Early Differentiation of Motor Neuroblasts in the Chick Embryo as Studied by Electron Microscopy. I. General Aspects. Dev Biol. 1964;10:433–466. doi: 10.1016/0012-1606(64)90054-5. [DOI] [PubMed] [Google Scholar]
- Lyser KM. Early differentiation of motor neuroblasts in the chick embryo as studied by electron microscopy. II. Microtubules and neurofilaments. Dev Biol. 1968;17:117–142. doi: 10.1016/0012-1606(68)90057-2. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shakiryanova D, Vardya I, Popov SV. Quantitative analysis of microtubule transport in growing nerve processes. Curr Biol. 2004;14:725–730. doi: 10.1016/j.cub.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Weiner JA, Farlow SJ, Chun J, Goldstein LS. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. J Cell Biol. 1999;145:469–479. doi: 10.1083/jcb.145.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, Euteneuer U. Tubulin hooks as probes for microtubule polarity: an analysis of the method and an evaluation of data on microtubule polarity in the mitotic spindle. J Cell Biol. 1984;98:525–533. doi: 10.1083/jcb.98.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Tsukita S. “Search-and-capture” of microtubules through plus-end-binding proteins (+TIPs) J Biochem. 2003;134:321–326. doi: 10.1093/jb/mvg148. [DOI] [PubMed] [Google Scholar]
- Myers KA, He Y, Hasaka TP, Baas PW. Microtubule transport in the axon: Re-thinking a potential role for the actin cytoskeleton. Neuroscientist. 2006;12:107–118. doi: 10.1177/1073858405283428. [DOI] [PubMed] [Google Scholar]
- Myers KA, Tint I, Nadar CV, He Y, Black MM, Baas PW. Antagonistic forces generated by cytoplasmic dynein and myosin-II during growth cone turning and axonal retraction. Traffic. 2006;7:1333–1351. doi: 10.1111/j.1600-0854.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec FJ, Surrey T, Maggs AC, Leibler S. Self-organization of microtubules and motors. Nature. 1997;389:305–308. doi: 10.1038/38532. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–334. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer DC, Gard DL. Microtubules in Xenopus oocytes are oriented with their minus-ends towards the cortex. Cell Motil Cytoskeleton. 1999;44:34–43. doi: 10.1002/(SICI)1097-0169(199909)44:1<34::AID-CM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Yu W, Liu M, Solowska JM, Baas PW. Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Mol Biol Cell. 2010;21:334–344. doi: 10.1091/mbc.E09-09-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Schroer TA. Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr Opin Cell Biol. 2001;13:92–96. doi: 10.1016/s0955-0674(00)00179-4. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Kuriyama R, Baas PW. Expression of a kinesin-related motor protein induces Sf9 cells to form dendrite-like processes with nonuniform microtubule polarity orientation. J Neurosci. 1996;16:4370–4375. doi: 10.1523/JNEUROSCI.16-14-04370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW. Identification of a microtubule-associated motor protein essential for dendritic differentiation. J Cell Biol. 1997a;138:833–843. doi: 10.1083/jcb.138.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Kuriyama R, Essner R, Baas PW. Expression of a minus-end-directed motor protein induces Sf9 cells to form axon-like processes with uniform microtubule polarity orientation. J Cell Sci. 1997b;110 (Pt 19):2373–2380. doi: 10.1242/jcs.110.19.2373. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Yu W, Baas PW. Transport of dendritic microtubules establishes their nonuniform polarity orientation. J Cell Biol. 1995;130:93–103. doi: 10.1083/jcb.130.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Slaughter T, Wang J, Black MM. Microtubule transport from the cell body into the axons of growing neurons. J Neurosci. 1997;17:5807–5819. doi: 10.1523/JNEUROSCI.17-15-05807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings H, Hunt C. Microtubule polarity in the nutritive tubes of insect ovarioles. Cell Tissue Res. 1983;233:133–141. doi: 10.1007/BF00222238. [DOI] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell. 2010;21:767–777. doi: 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Yu W, Baas PW, Kawai-Hirai R, Hayashi K. Rearrangement of microtubule polarity orientation during conversion of dendrites to axons in cultured pyramidal neurons. Cell Motil Cytoskeleton. 2007;64:347–359. doi: 10.1002/cm.20188. [DOI] [PubMed] [Google Scholar]
- Telzer BR, Haimo LT. Decoration of spindle microtubules with Dynein: evidence for uniform polarity. J Cell Biol. 1981;89:373–378. doi: 10.1083/jcb.89.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennyson VM. Electron microscopic study of the developing neuroblast of the dorsal root ganglion of the rabbit embryo. J Comp Neurol. 1965;124:267–317. doi: 10.1002/cne.901240302. [DOI] [PubMed] [Google Scholar]
- Topp KS, Meade LB, LaVail JH. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J Neurosci. 1994;14:318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt LL, Burnside B. Microtubule polarity and distribution in teleost photoreceptors. J Neurosci. 1988;8:2371–2380. doi: 10.1523/JNEUROSCI.08-07-02371.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt LL, Burnside B. The unusual microtubule polarity in teleost retinal pigment epithelial cells. J Cell Biol. 1988;107:1461–1464. doi: 10.1083/jcb.107.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Bloom GS. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- Viancour TA, Forman DS. Polarity orientations of microtubules in squid and lobster axons. J Neurocytol. 1987;16:69–75. doi: 10.1007/BF02456698. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Vernos I, Mitchison TJ, Karsenti E, Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Yu W, Baas PW, Black MM. Microtubule assembly in growing dendrites. JNeurosci. 1996;16:6065–6078. doi: 10.1523/JNEUROSCI.16-19-06065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, He C, Zhang Z, Chen Y. MKLP1 requires specific domains for its dendritictargeting. J Cell Sci. 2006;119:452–458. doi: 10.1242/jcs.02750. [DOI] [PubMed] [Google Scholar]
- Yu W, Baas PW. Changes in microtubule number and length during axon differentiation. JNeurosci. 1994;14:2818–2829. doi: 10.1523/JNEUROSCI.14-05-02818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Cook C, Sauter C, Kuriyama R, Kaplan PL, Baas PW. Depletion of a microtubule-associated motor protein induces the loss of dendritic identity. J Neurosci. 2000;20:5782–5791. doi: 10.1523/JNEUROSCI.20-15-05782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Schwei MJ, Baas PW. Microtubule transport and assembly during axon growth. J Cell Biol. 1996;133:151–157. doi: 10.1083/jcb.133.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]