Abstract
Please cite this paper as: Ambrose CS et al. (2011) The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza and Other Respiratory Viruses 5(2), 67–75.
In the United States, two types of vaccines are recommended for the prevention of influenza: an intranasal live attenuated influenza vaccine (LAIV) for eligible individuals aged 2–49 years and unadjuvanted injectable trivalent inactivated vaccines (TIV) for eligible individuals aged ≥6 months. Several recent studies have compared the efficacy of the 2 vaccines in children and adults. In children 6 months to 18 years of age, each of the four comparative studies of LAIV and TIV demonstrated that LAIV was more protective. In individuals 17–49 years of age, most comparative studies have demonstrated that LAIV and TIV were similarly efficacious or that TIV was more efficacious. However, LAIV was shown to be more protective than TIV in new military recruits of all ages, and placebo‐controlled studies in adults in 1997–1998 suggested that LAIV was more protective against the mismatched A/H3N2 strain. The relative efficacy of LAIV and TIV among young adults may vary depending on the specific population and the antigenic match between the vaccines and circulating strains. In adults 60 years of age and older, limited data suggest that the two vaccines are similarly effective. In children and adults, studies also suggest that the relative efficacy of LAIV versus TIV may increase when measured against more severe illness. Additional research comparing LAIV and TIV is needed in adults and would also be valuable in older children and adolescents. Studies should examine the role of pre‐existing immunity as well as vaccine impact on influenza illness of varying severity.
Keywords: Adults, inactivated influenza vaccine, influenza, live attenuated influenza vaccine, pediatrics
Introduction
Vaccination is universally accepted as the most effective strategy for the prevention of influenza. 1 In the United States, two types of vaccines are available: a live, attenuated, intranasal influenza vaccine (LAIV) and unadjuvanted trivalent inactivated influenza vaccines (TIV) for intramuscular administration. LAIV is approved for eligible individuals 2–49 years of age. TIV indications have no upper age limit; lower age limits vary by manufacturer, with some approved for children as young as 6 months. 1
Understanding the relative efficacy of influenza vaccines in various populations is critical to their optimal use. The first priority must be to ensure that as many individuals as possible are vaccinated. For this reason, the availability of both LAIV and TIV is valuable because of individual preferences regarding administration route, side effects, and other factors. However, a secondary objective should be to direct individuals to the vaccine that will provide them and potentially their contacts with optimal protection against influenza. The efficacy of influenza vaccines can vary with the age of the vaccine recipient and possibly other factors. This review summarizes recent studies comparing the relative efficacy of LAIV and TIV in children and adults to facilitate provider and policymaker decisions regarding optimal vaccination strategies for the prevention of influenza.
Relative efficacy of LAIV and TIV in children aged 6 months–18 years
Studies directly comparing LAIV and TIV against natural infection with influenza
The relative protection provided by LAIV and TIV in children was first evaluated in 2 studies conducted in 2002–2003 in Europe and Israel (Figure 1, Table 1, [link]). 2 , 3 Ashkenazi et al. 2 compared 2 doses of LAIV or TIV in a multinational, randomized, open‐label study in 2187 children 6–71 months of age with a history of recurrent respiratory tract infections (RTIs). RTIs included, but were not limited to, common colds, acute otitis media, bronchitis, pneumonia, and bronchiolitis; recurrence was defined as ≥2 practitioner‐attended RTIs in the previous 12 months. Treatment groups were well matched with respect to baseline characteristics, including the proportion of children with a history of wheezing in the prior 12 months (34–36%) or asthma (23%). There were 53% (95% CI: 22, 72) fewer culture‐confirmed influenza cases caused by vaccine‐matched strains among recipients of LAIV compared with recipients of TIV (24/1050 versus 50/1035, respectively). In a post hoc analysis, efficacy was shown to be consistent across age groups. 4 In the study’s evaluation of health outcomes related to all‐cause respiratory illness (i.e. influenza and non‐influenza), LAIV recipients reported 9% (95% CI: 2, 16) fewer health care provider visit days and 16% (95% CI: 10, 22) fewer missed days from school or child care compared with TIV recipients. In addition to the greater reduction in influenza in LAIV recipients, the severity of breakthrough influenza illness was reduced among LAIV recipients. In post hoc analyses, LAIV recipients with breakthrough influenza were more frequently afebrile (LAIV, 26%; TIV, 5%; P = 0·005), missed 1·5 (95% CI: 2·9, 0·2) fewer days of school or daycare (LAIV, 1·6 days; TIV, 3·1 days; P = 0·025), and had less antibiotic use (LAIV, 17%; TIV, 33%; P = 0·14).
Figure 1.
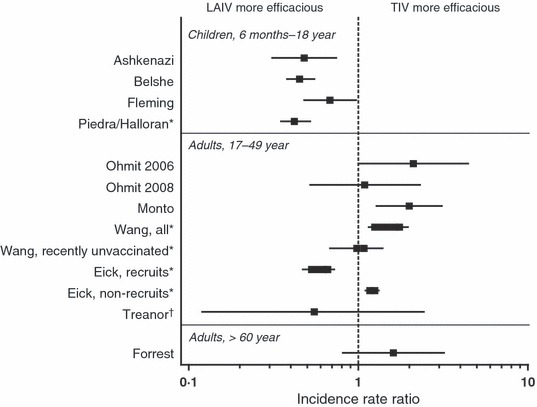
Incidence Rate Ratios from Studies Directly Comparing LAIV and TIV in Children and Adults. LAIV, live attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine. Halloran et al., Wang et al., and Eick et al. should be interpreted with caution as the non‐randomized LAIV and TIV groups differ in baseline characteristics. For Wang et al. and Eick et al., a range across study years and cohorts is displayed for the point estimate; published incidence rate ratios for Wang were inverted to represent LAIV/TIV. *Effectiveness studies, †Challenge study. Data sources: Ashkenazi et al. 2 ; Belshe et al. 5 ; Fleming et al. 3 ; Halloran et al. 8 ; Ohmit et al. 11 ; Ohmit et al. 12 ; Monto et al. 10 ; Wang et al. 13 ; Eick et al. 14 ; Treanor et al. 9 ; Forrest et al. 17 .
Table 1.
Summary of studies directly comparing the efficacy of LAIV and TIV in children and adults
| Study, Year | Subject age | Sample size | Design | Endpoint | Incidence regardless of antigenic match (LAIV versus TIV) | Predominant Strains | Absolute Efficacy LAIV, % (95% CI) | Absolute Efficacy TIV, % (95% CI) | Incidence Rate Ratio (LAIV/TIV) (95% CI) | Illness Severity in Vaccinated Individuals |
|---|---|---|---|---|---|---|---|---|---|---|
| Children 6 months to 18 years of age | ||||||||||
| Ashkenazi et al., 2 2002–2003 | 6–71 months | 2187 | Prospective, randomized, open‐label | CCI | 2·8% versus 5·8% | Matched B | NA | NA | 0·48 (0·31, 0·74) | Less fever and fewer missed school/daycare days among LAIV breakthrough cases, with trend toward less antibiotic use |
| Belshe et al., 5 2004–2005 | 6–59 months | 8352 | Prospective, randomized, double‐blind | Culture‐confirmed modified CDC‐ILI | 3·9% versus 8·6% | Mismatched A/H3N2 | NA | NA | 0·45 (0·38, 0·55) | Less fever among LAIV breakthrough cases |
| Fleming et al., 3 2002–2003 | 6–17 years | 2229 | Prospective, randomized, open‐label | CCI | 4·5% versus 6·6% | Matched B | NA | NA | 0·68 (0·48, 0·97) | No differences seen |
| Piedra/Halloran et al., 8 2003–2004 | 5–18 years | 6403 | Prospective, non‐randomized, open‐label | MAARI | 2·81 versus 6·64 per 1000 child‐days | Mismatched A/H3N2 | 56 (32, 75) | No effectiveness observed | 0·42 (0·35, 0·52)† | Not reported |
| Adults 17–49 years of age | ||||||||||
| Ohmit et al., 11 2004–2005 | 18–46years | 1247 | Prospective, randomized, open‐label* | Culture‐ or PCR‐confirmed illness | 4·0% versus 1·9% | Mismatched A/H3N2 | 48 (−7, 74) | 75 (42, 90) | 2·11 (1·00, 4·44) | Not reported |
| Ohmit et al., 12 2005–2006 | 18–48 years | 2058 | Prospective, randomized, open‐label* | Culture‐ or PCR‐confirmed illness | 1·6% versus 1·5% | Matched A/H3N2 | 8 (−194, 67) | 16 (−171, 70) | 1·09 (0·52, 2·31) | Not reported |
| Monto et al., 2007–2008 [10] | 18–49 years | 1952 | Prospective, randomized, open‐label* | Culture‐ or PCR‐confirmed illness | 6·9% versus 3·4% | Matched A/H3N2 | 36 (0, 59) | 68 (46, 81) | 2·00 (1·28, 3·11) | Not reported |
| Wang et al., 13 2004–2007†,‡ | 17–49 years | >1 million per season | Retrospective, non‐randomized | ICD‐9 code for pneumonia or influenza | NA | Not reported | NA | NA | All: 1·25–1·75 (1·15, 1·96) Recently unvaccinated: 0·98–1·08 (0·68, 1·39) | NA |
| Eick et al., 14 2005–2007†,‡,§ | 17–49 years | >750 000 per season | Retrospective, non‐randomized | ICD‐9 code for ILI | NA | Not reported | NA | NA | Recruits: 0·53–0·66 (0·47, 0·69) Non‐recruits: 1·17–1·25 (1·15, 1·27) | NA |
| Challenge study: Treanor et al., 9 1995–1996 | 18–45 years | 92 | Prospective, randomized, double‐blind, wild‐type challenge | CCI | 6·9% versus 12·5% | Matched A/H1N1, A/H3N2, B | 85 (28, 100) | 71 (2, 97) | 0·55 (0·12, 2·43) | Trend toward less severe symptoms among LAIV vaccinees |
| Adults ≥60 years of age | ||||||||||
| Forrest et al., 17 | >60 years | 3009 | Prospective, randomized, ouble‐blind | CCI | 1·4% versus 0·9% | Matched A/H1N1, matched A/H3N2, mismatched B | NA | NA | 1·61 (0·81, 3·20) | Trend toward less feverishness and fever among LAIV breakthrough cases |
CCI, culture‐confirmed illness; ILI, influenza‐like illness; LAIV, live attenuated influenza vaccine; MAARI, medically attended acute respiratory illness; TIV, trivalent inactivated influenza vaccine.
*Double‐blind for vaccine versus placebo, but open‐label for nasal spray versus injection.
†Should be interpreted with caution as the non‐randomized LAIV and TIV groups differed in baseline characteristics.
‡Range across study years is displayed; published incidence rate ratios for Wang were inverted to represent LAIV/TIV.
§Continuous cohort data are displayed.
Concurrently, Fleming et al. 3 evaluated a single dose of LAIV or TIV in a randomized, multinational, open‐label trial in 2229 children 6–17 years of age with a prior clinical diagnosis of asthma (Figure 1, Table 1, [link]). Children with serious chronic disease, altered immune function, and those on immunosuppressive therapy, including high‐dose systemic corticosteroids (≥2 mg/kg per days or ≥20 mg/days of prednisolone or its equivalent), were excluded. However, in each treatment group, current inhaled steroid use was reported in 69% of participants and 43% had a history of systemic steroid treatment.. LAIV recipients experienced 35% (95% CI: 4, 56) fewer cases of influenza caused by matched strains than TIV recipients (46/1109 versus 70/1102, respectively). The relative efficacy of LAIV versus TIV was similar for children 6–11 and 12–17 years of age. 4 Unlike the observations by Ashkenazi et al. 2 , there were no significant differences for other outcome measures (e.g. health care provider visits, medication use, and days missed from school or work) or in illness severity between LAIV and TIV recipients who developed breakthrough influenza.
In 2004–2005, Belshe et al. 5 compared LAIV and TIV in a multinational, randomized, double‐blind study in 8352 children 6–59 months of age (Figure 1, Table 1, [link]). Children without prior influenza vaccination were given two doses of vaccine and those previously vaccinated were given one dose. Study groups were well matched with respect to demographic characteristics, including history of prior influenza vaccination (22–23%), history of wheezing (21–22%), recurrent wheezing (6–7%), and asthma (4%). The primary endpoint was the incidence of culture‐confirmed modified Centers for Disease Control and Prevention (CDC) influenza‐like illness (ILI), defined as fever plus ≥1 other symptom of cough, sore throat, or runny nose/nasal congestion. There were 45% (95% CI: 22, 61) fewer cases of influenza caused by matched strains in LAIV recipients than TIV recipients (53/3916 versus 93/3936, respectively), and 58% (95% CI: 47, 67) fewer cases caused by mismatched strains (102/3916 versus 245/3936, respectively). Efficacy was consistent across age groups. 4 Similar to the observation by Ashkenazi et al. 2 , breakthrough illness was less severe among LAIV recipients than TIV recipients. In a post hoc analysis of any symptomatic influenza illness (regardless of whether fever was present), more LAIV recipients with breakthrough influenza were afebrile (22% versus 12%, respectively; P = 0·001) 6 .
Studies comparing LAIV and TIV against influenza‐like illness
A large, open‐label, non‐randomized trial was conducted by Piedra et al. 7 in the United States during the 2003–2004 influenza season (Figure 1, Table 1, [link]). The study demonstrated that LAIV was effective in preventing medically attended, acute respiratory illness among children 5–18 years of age when the mismatched A/Fujian/411/02 (H3N2) virus predominated, 7 whereas no effectiveness was seen for TIV. LAIV efficacy against influenza was estimated at 56% (95% CI: 32, 75), and efficacy was similar for children 5–9 and 10–18 years of age. 7 , 8 However, the direct comparison of LAIV and TIV in this study should be interpreted with caution as the non‐randomized LAIV and TIV groups differed in their baseline characteristics.
Relative efficacy of LAIV and TIV in individuals aged 17–49 years
Studies comparing LAIV and TIV against experimental challenge with influenza
The relative protection provided by LAIV and TIV in adults was first evaluated by Treanor et al. 9 in a placebo‐controlled, double‐blind wild‐type challenge study in 92 adult volunteers 18–45 years of age (Figure 1, Table 1). Subjects with baseline serum hemagglutination‐inhibition (HAI) antibody titers of ≤1:8 to the vaccine strains were randomized to receive LAIV, TIV, or placebo and challenged intranasally with 1 vaccine‐like wild‐type virus (A/H1N1, A/H3N2, or B) approximately 28 days later. The primary objective was to evaluate protection against documented influenza, defined as viral shedding (evaluated daily for 7 days after challenge) and/or ≥4‐fold increase in HAI titer (28 days after challenge) in the presence of respiratory symptoms. Laboratory‐documented influenza illness occurred in 45% (14/31) of placebo recipients following wild‐type virus challenge, compared with 6·9% (2/29) and 12·5% (4/32) of those given LAIV and TIV, respectively (P = 0·001 for LAIV versus placebo; P = 0·006 for TIV versus placebo; P = 0·67 for LAIV versus TIV). Protective efficacy was 85% (95% CI: 28, 100) for LAIV and 71% (95% CI: 2, 97) for TIV. There were trends toward less severe illness among LAIV recipients compared with TIV recipients and less severe illness in both vaccinated groups compared with placebo recipients. LAIV vaccinees had a lower mean symptom score than TIV and placebo recipients (mean ± SE: 2·7 ± 1·3, 5·7 ± 1·3, 9·2 ± 1·3, respectively; P = 0·002 for LAIV versus placebo, P = 0·12 for TIV versus placebo, P = 0·24 for LAIV versus TIV). Similarly, 28% of LAIV and 38% of TIV recipients experienced respiratory symptoms on consecutive days, compared with 61% of placebo recipients (P = 0·009 for LAIV versus placebo, P = 0·035 for TIV versus placebo).
Studies comparing LAIV and TIV against natural infection with influenza
During the 2004–2005, 2005–2006, and 2007–2008 influenza seasons, University of Michigan researchers conducted a multiyear study on state campuses to compare the efficacy of a single dose of LAIV or TIV in healthy adults 18–49 years of age (Figure 1, Table 1). 10 , 11 , 12 Each season, only 43%, 30%, and 22% of participants were ≥25 years of age, respectively. The studies were randomized, placebo‐controlled, and double‐blind for vaccine versus placebo but open‐label for nasal spray versus injection. During 2004–2005, the predominant circulating strain was a drifted A/H3N2 strain. Depending on whether culture, PCR, or both were used to detect influenza, the observed efficacies were 48–57% for LAIV and 74–77% for TIV; the difference between LAIV and TIV was not statistically significant. 11 During 2005–2006, the influenza attack rate observed in the placebo group was much lower than in 2004–2005 (1·8% versus 7·8%), rendering the trial underpowered to detect vaccine efficacy. 12 Depending on the detection method, the absolute efficacies ranged from 8–61% for LAIV and 16–23% for TIV and were statistically similar. During 2007–2008, when there was significant influenza activity caused predominantly by a minor antigenic variant A/H3N2 strain, 10 the absolute efficacy was 36–51% for LAIV and 68–73% for TIV. The relative efficacy of TIV versus LAIV was 45% (95% CI: 3, 69) using viral culture and 50% (95% CI: 20, 69) using PCR. No analysis of illness severity among breakthrough cases was reported for any study season.
Studies comparing LAIV and TIV against influenza‐like illness
Since 2004, the United States military has used large amounts of LAIV and TIV with the goal of vaccinating all personnel annually; LAIV use increased from 34% of vaccinations in 2004–2005 to 48% in 2006–2007 (Figure 1, Table 1). 13 The extensive use of both vaccines has enabled retrospective, non‐randomized, observational effectiveness studies of ILI using US military databases. As noted for Piedra et al. 7 , the direct comparison of LAIV and TIV in these studies should be interpreted with caution as the non‐randomized LAIV and TIV groups may have differed in their baseline characteristics. Wang et al. 13 conducted a retrospective cohort study for non‐recruit service members 17–49 years of age who received LAIV or TIV during 2004–2005, 2005–2006, or 2006–2007. A multivariate Poisson regression model and propensity‐based matching were used to control for covariates such as age, sex, service branch, medical encounter history, and immunization history. Because laboratory confirmation of influenza was not available, the outcome of interest was the first medical encounter with a diagnosis code associated with pneumonia or influenza. The incidence rate was highest for the unimmunized groups and lower in the TIV cohort than in the LAIV cohort during each season. Adjusted incidence rate ratios (IRRs) for LAIV‐vaccinated versus TIV‐vaccinated subjects ranged from 1·25 to 1·75. Among individuals for whom there was no database record of influenza vaccination in the previous 1 or 2 seasons since 2004–2005, ILI incidence was similar among the LAIV and TIV cohorts.
A subsequent analysis by the same investigative group evaluated protection by LAIV and TIV in military recruits and non‐recruits during 2005–2006 and 2006–2007 (Figure 1, Table 1). 14 Similar to Wang et al. 13 , an ICD‐9 code definition of ILI was used. However, the definitions differ, with Eick et al. 14 using diagnoses demonstrated to be associated in the US military population with culture‐confirmed influenza; diagnoses attributed to other pathogens were excluded. In contrast to Wang et al. 13 , the regression model included geographic region as a confounding variable, multiple ILI events were included per individuals, and results were calculated by age category. Among non‐recruits, TIV recipients had a lower incidence of ILI; adjusted IRRs for LAIV versus TIV were 1·17 and 1·25–1·33 in 2005–2006 and 2006–2007, respectively. When analyzed by age, IRRs were 1·42–1·53 among 17‐ to 19‐year‐olds and steadily decreased in older cohorts to 1·0–1·1 among 40‐ to 49‐year‐olds. Among recruits, disease incidence rates were 2–16 times higher than among non‐recruits. The LAIV cohort had a lower incidence of ILI during both seasons with adjusted IRRs of 0·49–0·78 and 0·53–0·82 for 2005–2006 and 2006–2007, respectively. Adjusted IRRs were not reported by age group for recruits, but crude incidence rates suggest that IRRs were similar in recruits of all ages.
Studies conducted in the same influenza season comparing LAIV and TIV with placebo against influenza‐like illness
Two placebo‐controlled studies, one involving LAIV and one involving TIV, conducted in 1997–1998 provide an additional comparison of the two vaccines in adults. 15 , 16 The first study evaluated the effectiveness of LAIV in reducing febrile illness in a randomized, double‐blind, placebo‐controlled trial of 4561 healthy, working adults 18–64 years of age. 16 There was no laboratory confirmation of influenza. During 1997–1998, the predominant circulating strain was A/Sydney/05/97 (H3N2), a drifted variant of the A/Wuhan/359/95 (H3N2) vaccine strain. There was a 19% (95% CI: 7, 29) reduction in severe febrile illness and a 24% (95% CI: 13, 33) reduction in febrile upper respiratory illness among LAIV recipients. There was a limited sample size of 50‐ to 64‐year‐olds (N = 641), and effectiveness was not demonstrated in this age group in a post hoc analysis. The second study, conducted by the CDC in 1997–1998, evaluated the effectiveness of TIV in a similar population of 1184 healthy, working adults 18–64 years of age. 15 No effectiveness for TIV was observed; presumably, this was because of TIV’s reduced efficacy against mismatched strains, because effectiveness was demonstrated in the second‐season of this same study when matched strains circulated. 15
Relative efficacy of LAIV and TIV in adults ≥60 years of age
Although LAIV is not currently approved in the United States in adults ≥50 years of age, several studies have been conducted and results from these studies are presented here for completeness.
Studies comparing LAIV and TIV against natural infection with influenza
A randomized comparative study of LAIV and TIV conducted in 3009 adults ≥60 years of age did not yield meaningful results because of the low incidence of influenza during the 2002 season in South Africa (Figure 1, Table 1) 17 The incidence of confirmed influenza illness caused by closely matched strains was 0·8 and 0·5% in LAIV and TIV recipients, respectively (12/1494 versus 8/1488); the relative efficacy for LAIV versus TIV was −49% (95% CI: −259, 35). A mismatched, opposite‐lineage influenza B strain also circulated, and there was a similar incidence of this strain in each treatment group (9/1494 versus 5/1488). Among individuals with breakthrough influenza illness, there were trends toward less feverishness (LAIV, 14%; TIV, 46%; P = 0·05) and less fever (LAIV, 9%; TIV, 31%; P = 0·16) in LAIV recipients.
Studies comparing LAIV and TIV with placebo against natural infection with influenza
Given the inconclusive results from the direct comparison of LAIV and TIV in older adults, data from placebo‐controlled studies were also reviewed. Only two prospective, randomized, placebo‐controlled studies of influenza vaccine have been conducted in adults ≥60 years of age, one with TIV and one with LAIV. 18 , 19 LAIV and TIV performed similarly in these studies. A study of LAIV compared with placebo was conducted in 3242 adults ≥60 years of age and demonstrated 42% efficacy against influenza illness caused by strains matched to the vaccine; 53% efficacy was observed against A/H3N2 strains, with no efficacy against illness caused by influenza B. 18 Govaert et al. 19 conducted a placebo‐controlled study of TIV in 1838 subjects and demonstrated a 50% reduction in serologically confirmed influenza infection with TIV and a 27–47% reduction in various ILI definitions. Interpretation of this study is complicated by the lack of culture‐confirmation of influenza illness, because serologic endpoints can fail to detect breakthrough illness among TIV recipients to a greater extent than placebo or LAIV recipients. 11 , 20 Post hoc analyses of these studies found different effects of age on vaccine efficacy. For the TIV study, efficacy trended lower in those ≥70 years of age, whereas in the LAIV study, efficacy trended higher in those ≥70 years of age. However, these trends should be interpreted with caution as they could be the result of chance alone.
Studies comparing LAIV and TIV in combination against natural infection with influenza
The efficacy of LAIV in simultaneous combination with TIV has also been studied in older adults. In a double‐blind, randomized trial conducted in nursing home residents aged ≥65 years, 523 residents received TIV and at the same visit either monovalent A/H3N2 LAIV or intranasal placebo. 21 TIV + LAIV recipients experienced 61% (95% CI: 18, 82) fewer cases of laboratory‐documented influenza A compared with those who received TIV+placebo. In a subsequent study of 2215 individuals ≥50 years of age with chronic obstructive pulmonary disease, the relative efficacy of TIV plus trivalent LAIV compared with TIV plus placebo in the prevention of laboratory‐documented influenza illness was 16% (95% CI: −22, 43) for any influenza strain. 22 It was subsequently noted that TIV + LAIV recipients had improved chronic lung disease severity index scores over the course of the study. 23
Safety of LAIV and TIV
Prospective comparative studies of LAIV and TIV have generally demonstrated comparable safety of the two vaccines among individuals ≥2 years of age; most adverse effects from either vaccine are mild, transient, and of minimal clinical significance. 2 , 3 , 5 , 10 , 11 , 12 , 17 LAIV has been associated with increased rates of runny nose/nasal congestion in all ages 2 , 3 , 5 , 10 , 11 , 12 , 17 ; low‐grade fever and decreased appetite in children aged <6 years; 2 , 5 and sore throat, cough, and headache in adults. 11 , 12 , 17 TIV has been associated with increased rates of injection site reaction 2 , 3 , 5 , 10 , 11 , 12 , 17 in all ages and fever, muscle aches, and oculorespiratory syndrome in adults. 12 , 24 Although in the United States, LAIV is not recommended for use in individuals with high‐risk underlying medical conditions, some data exist in these populations. In asthmatic children 6–17 years of age, there was a statistically significant reduction in wheezing through 15 days post‐vaccination among LAIV versus TIV recipients (19·5% versus 23·8%, respectively; P = 0·02); no other significant differences in asthma symptoms were observed. 3 Additionally, among 243 HIV‐infected children 5–17 years of age receiving stable antiretroviral therapy (viral load <60 000 and CD4 count >15%), there were no unexpected toxicities, prolonged shedding, or serious adverse events associated with either LAIV or TIV. 25 Among individuals ≥50 years of age with chronic obstructive pulmonary disease, TIV + LAIV recipients reported higher rates of increased sputum, runny nose/nasal congestion, increased shortness of breath, chills, and itchiness at the intramuscular injection site compared with TIV+placebo recipients. 22
In one study, an increased rate of medically significant wheezing (MSW) and hospitalization was associated with LAIV in children <24 months of age. 5 The incidence of MSW was analyzed through 42 days after vaccination with LAIV or TIV; MSW was defined as a medical diagnosis of wheezing associated with other respiratory findings (e.g. hypoxemia, respiratory distress, or initiation of daily bronchodilator therapy). Among children 6–23 months of age, 5·9% of LAIV recipients and 3·8% of TIV recipients experienced MSW (P = 0·002). For children aged 24–59 months, rates of MSW were comparable in LAIV and TIV recipients (LAIV, 2·1%; TIV, 2·5%; P = 0·38). A review of the incidence of MSW by single‐month age cohorts revealed increased rates among LAIV recipients 6–23 months of age, with no pattern of increase for children 24–59 months of age. 26 In this same study, all‐cause hospitalization rates through 180 days post‐vaccination were higher among LAIV recipients than TIV recipients 6–11 months of age (LAIV, 6·1%; TIV, 2·6%; P = 0·002); no increase was seen with LAIV in children 12–59 months of age. Based on these observations, LAIV is not approved for use in children <24 months of age.
Conclusions
In all age groups studied, LAIV and TIV were effective in preventing influenza illness. In children, all four comparative studies of LAIV and TIV demonstrated that LAIV was more protective against culture‐confirmed influenza illness in children 6 months to 17 years of age. 2 , 3 , 5 , 7 These results are supported by the results of studies that have compared the vaccines to placebo. A meta‐analysis of six placebo‐controlled studies of LAIV calculated that the mean efficacy of two doses against matched strains of all subtypes in previously unvaccinated young children was 77% (95% CI: 72, 80) 27 ; the mean efficacy of 1 dose of LAIV in previously vaccinated children was 87% (95% CI: 81, 90). 27 Three separate meta‐analyses of the efficacy of TIV against laboratory‐confirmed influenza in children compared with placebo calculated that the mean efficacy of TIV against matched strains was 59% (95% CI: 41, 71), 28 63% (95% CI: 45, 70), 29 and 65% (95% CI: 45, 77). 30 Studies also indicate that the efficacy difference between LAIV and TIV in children increases with circulation of antigenically mismatched strains. As noted previously, LAIV is approved for eligible children 2–17 years of age because of an observed increased rate of medically attended wheezing in children 6–23 months of age.
In contrast, most comparative studies in individuals 17–49 years of age have demonstrated that LAIV and TIV were similarly efficacious or that TIV was more efficacious. A randomized, culture‐confirmed field trial conducted during three influenza seasons demonstrated that TIV was more efficacious than LAIV in healthy adults, 10 , 11 , 12 with a statistically significant difference demonstrated in 2007–2008. 10 Retrospective effectiveness studies of influenza‐like illness in the US military have demonstrated similar results in their overall population. 13 However, in the US military studies, LAIV was more protective than TIV against influenza‐like illness in new recruits of all ages and similarly effective in older non‐recruits. 14 Additionally, studies conducted in 1997–1998 suggested that LAIV was more protective against the circulating mismatched A/H3N2 strain. 15 , 16 Among individuals 17–49 years of age, the relative efficacy of LAIV and TIV appear to vary depending on the specific population studied, the type of study, and the degree of antigenic match between the study vaccines and circulating strains. Among adults ≥60 years of age, the limited available data suggest that the two vaccines are similarly effective. However, as noted previously, because of limited data, LAIV is not currently approved in the United States in adults ≥50 years of age.
Both LAIV and TIV can induce priming immune responses and boost pre‐existing anti‐influenza immunity. However, LAIV appears to be more effective than TIV as a priming vaccine, and TIV appears to be more effective in boosting pre‐existing immunity. 31 , 32 Given the annual changes in circulating strains of influenza, both types of immune responses are important for individuals of all ages. However, these differential mechanisms of action may explain the differential relative efficacy of LAIV and TIV in children and young adults. TIV efficacy may be higher in adult versus pediatric populations because adults have greater immunologic priming from multiple previous natural infections with influenza. Conversely, it has been suggested that LAIV efficacy may decline in adults because higher pre‐existing anti‐influenza immunity may limit intranasal replication of LAIV. 10 , 11 However, an analysis of multiple efficacy studies conducted in children 6 months to 17 years of age found no evidence of a decline in LAIV efficacy with increased age or with increased pre‐existing immunity to influenza within this age range. 4 In adults, no evidence has been published to directly support this hypothesis; post hoc analyses of completed studies in adults should be conducted to examine this issue.
Variations in the severity of the influenza illness measured in a study may influence relative efficacy estimates. As noted earlier and in the Table 1, studies have demonstrated that LAIV‐vaccinated children and adults who develop influenza illness tend to have less severe illness than TIV‐vaccinated individuals, perhaps because of enhanced mucosal or cellular immunity. As a result, the relative efficacy of LAIV versus TIV in children and adults may increase when measured against more severe illness. The impact of breakthrough illness severity on LAIV and TIV efficacy should be evaluated in future studies and through post hoc analyses of completed studies.
The primary limitation of the current analysis is the small number of prospective, randomized studies. Influenza vaccine efficacy studies that are appropriately powered to compare two effective vaccines require large numbers of subjects and are very costly. As a result, it is not surprising that few have been conducted. Additionally, given the annual variation in both circulating strains and the influenza attack rate, consistent results across multiple seasons and geographies are needed for robust conclusions. In children, particularly younger children, multiple randomized and observational studies as well as meta‐analyses of placebo‐controlled studies have yielded consistent results. In adults, less data are available and results have been more variable. To more fully understand the relative benefits of TIV and LAIV in adults, additional research is needed. A multi‐country, multi‐year, double‐blind, well‐powered study in individuals ≥18 years of age would be valuable. Additional studies in older children and adolescents would also be valuable. These studies should be stratified so that relative efficacy can be determined by age group. Multiple aspects of baseline anti‐influenza immunity should be evaluated to elucidate the role of pre‐existing immunity, and various influenza symptoms should be collected to describe the impact of LAIV and TIV on influenza illness of varying severity.
Acknowledgments
The authors thank Xionghua Wu, PhD of MedImmune for calculating the incidence rate ratios for several studies and Gregory Susla, PharmD, of MedImmune and Jay Bauman, PharmD, of Scientific and Technical Evaluation of Pharmaceuticals, Inc. for their assistance in compiling the data from the cited studies. Editorial assistance in preparing this manuscript (formatting of the final manuscript) was provided by Complete Healthcare Communications, Inc. (Chadds Ford, PA) and was funded by MedImmune.
Re‐use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen_Terms
References
- 1. Fiore AE, Shay DK, Broder K et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 2. Ashkenazi S, Vertruyen A, Aristegui J et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879. [DOI] [PubMed] [Google Scholar]
- 3. Fleming DM, Crovari P, Wahn U et al. Comparison of the efficacy and safety of live attenuated cold‐adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006; 25:860–869. [DOI] [PubMed] [Google Scholar]
- 4. Belshe RB, Toback SL, Yi T, Ambrose CS. Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influenza Other Respir Viruses 2010; 4:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belshe RB, Edwards KM, Vesikari T et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–696. [DOI] [PubMed] [Google Scholar]
- 6. Yang CF, Belshe RB, Kemble G et al. Genetic sequence analysis of influenza viruses and illness severity in ill children previously vaccinated with live attenuated or inactivated influenza vaccine. Vaccine 2010; 28:5128–5134. [DOI] [PubMed] [Google Scholar]
- 7. Piedra PA, Gaglani MJ, Kozinetz CA et al. Trivalent live attenuated intranasal influenza vaccine administered during the 2003‐2004 influenza type A (H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics 2007; 120:e553–e564. [DOI] [PubMed] [Google Scholar]
- 8. Halloran ME, Piedra PA, Longini IM Jr et al. Efficacy of trivalent, cold‐adapted, influenza virus vaccine against influenza A (Fujian), a drift variant, during 2003–2004. Vaccine 2007; 25:4038–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treanor JJ, Kotloff K, Betts RF et al. Evaluation of trivalent, live, cold‐adapted (CAIV‐T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild‐type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999; 18:899–906. [DOI] [PubMed] [Google Scholar]
- 10. Monto AS, Ohmit SE, Petrie JG et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009; 361:1260–1267. [DOI] [PubMed] [Google Scholar]
- 11. Ohmit SE, Victor JC, Rotthoff JR et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 2006; 355:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohmit SE, Victor JC, Teich ER et al. Prevention of symptomatic seasonal influenza in 2005‐2006 by inactivated and live attenuated vaccines. J Infect Dis 2008; 198:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Tobler S, Roayaei J, Eick A. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009; 301:945–953. [DOI] [PubMed] [Google Scholar]
- 14. Eick AA, Wang Z, Hughes H, Ford SM, Tobler SK. Comparison of the trivalent live attenuated vs. inactivated influenza vaccines among U.S. military service members. Vaccine 2009; 27:3568–3575. [DOI] [PubMed] [Google Scholar]
- 15. Bridges CB, Thompson WW, Meltzer MI et al. Effectiveness and cost‐benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA 2000; 284:1655–1663. [DOI] [PubMed] [Google Scholar]
- 16. Nichol KL, Mendelman PM, Mallon KP et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 1999; 282:137–144. [DOI] [PubMed] [Google Scholar]
- 17. Forrest BD, Steele AD, Hiemstra LA, Rappaport R, Ambrose CS, Gruber WC. A prospective, randomized, open‐label trial comparing the safety and efficacy of trivalent live attenuated and inactivated influenza vaccines in adults 60 years of age and older. Options for the Control of Influenza VII; 2010 September 3–7; Hong Kong SAR, China. [DOI] [PubMed]
- 18. De Villiers PJ, Steele AD, Hiemstra LA et al. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine 2009; 28:228–234. [DOI] [PubMed] [Google Scholar]
- 19. Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double‐blind placebo‐controlled trial. JAMA 1994; 272:1661–1665. [PubMed] [Google Scholar]
- 20. Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold‐adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis 1994; 169:68–76. [DOI] [PubMed] [Google Scholar]
- 21. Treanor JJ, Mattison HR, Dumyati G et al. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann Intern Med 1992; 117:625–633. [DOI] [PubMed] [Google Scholar]
- 22. Gorse GJ, O’Connor TZ, Young SL et al. Efficacy trial of live, cold‐adapted and inactivated influenza virus vaccines in older adults with chronic obstructive pulmonary disease: a VA cooperative study. Vaccine 2003; 21:2133–2144. [DOI] [PubMed] [Google Scholar]
- 23. Gorse GJ, O’Connor TZ, Young SL et al. Impact of a winter respiratory virus season on patients with COPD and association with influenza vaccination. Chest 2006; 130:1109–1116. [DOI] [PubMed] [Google Scholar]
- 24. Jackson L, Gaglani M, Keyserling H et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo‐controlled trial over two influenza seasons. BMC Infect Dis 2010; 10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levin MJ, Song LY, Fenton T et al. Shedding of live vaccine virus, comparative safety, and influenza‐specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV‐infected children. Vaccine 2008; 26:4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belshe RB, Ambrose C, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine 2008; 26S:D10–D16. [DOI] [PubMed] [Google Scholar]
- 27. Rhorer J, Ambrose CS, Dickinson S et al. Efficacy of live attenuated influenza vaccine in children: a meta‐analysis of nine randomized clinical trials. Vaccine 2009; 27:1101–1110. [DOI] [PubMed] [Google Scholar]
- 28. Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; CD004879. [DOI] [PubMed] [Google Scholar]
- 29. Zangwill KM, Belshe RB. Safety and efficacy of trivalent inactivated influenza vaccine in young children: a summary for the new era of routine vaccination. Pediatr Infect Dis J 2004; 23:189–197. [DOI] [PubMed] [Google Scholar]
- 30. Negri E, Colombo C, Giordano L, Groth N, Apolone G, La Vecchia C. Influenza vaccine in healthy children: a meta‐analysis. Vaccine 2005; 23:2851–2861. [DOI] [PubMed] [Google Scholar]
- 31. Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004; 59:1–15. [DOI] [PubMed] [Google Scholar]
- 32. Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold‐adapted vaccine, and inactivated vaccine. J Infect Dis 1986; 154:121–127. [DOI] [PubMed] [Google Scholar]


